Abstract
Importance
BRAF V600E is a prominent oncogene in papillary thyroid cancer (PTC), but its role in PTC-related patient mortality has not been established.
Objective
To investigate the relationship between BRAF V600E mutation and PTC-related mortality.
Design, Setting, and Participants
Retrospective study of 1849 patients (1411 women and 438 men) with a median age of 46 years (interquartile range, 34–58 years) and an overall median follow-up time of 33 months (interquartile range, 13–67 months) after initial treatment at 13 centers in 7 countries between 1978 and 2011.
Main Outcomes and Measures
Patient deaths specifically caused by PTC.
Results
Overall, mortality was 5.3% (45/845; 95% CI, 3.9%–7.1%) vs 1.1% (11/1004; 95% CI, 0.5%–2.0%) (P<.001) in BRAF V600E–positive vs mutation-negative patients. Deaths per 1000 person-years in the analysis of all PTC were 12.87 (95% CI, 9.61–17.24) vs 2.52 (95% CI, 1.40–4.55) in BRAF V600E–positive vs mutation-negative patients; the hazard ratio (HR) was 2.66 (95% CI, 1.30–5.43) after adjustment for age at diagnosis, sex, and medical center. Deaths per 1000 person-years in the analysis of the conventional variant of PTC were 11.80 (95% CI, 8.39–16.60) vs 2.25 (95% CI, 1.01–5.00) in BRAF V600E–positive vs mutation-negative patients; the adjusted HR was 3.53 (95% CI, 1.25–9.98). When lymph node metastasis, extrathyroidal invasion, and distant metastasis were also included in the model, the association of BRAF V600E with mortality for all PTC was no longer significant (HR, 1.21; 95% CI, 0.53–2.76). A higher BRAF V600E–associated patient mortality was also observed in several clinicopathological subcategories, but statistical significance was lost with adjustment for patient age, sex, and medical center. For example, in patients with lymph node metastasis, the deaths per 1000 person-years were 26.26 (95% CI, 19.18–35.94) vs 5.93 (95% CI, 2.96–11.86) in BRAF V600E–positive vs mutation-negative patients (unadjusted HR, 4.43 [95% CI, 2.06–9.51]; adjusted HR, 1.46 [95% CI, 0.62–3.47]). In patients with distant tumor metastasis, deaths per 1000 person-years were 87.72 (95% CI, 62.68–122.77) vs 32.28 (95% CI, 16.14–64.55) in BRAF V600E–positive vs mutation-negative patients (unadjusted HR, 2.63 [95% CI, 1.21–5.72]; adjusted HR, 0.84 [95% CI, 0.27–2.62]).
Conclusions and Relevance
In this retrospective multicenter study, the presence of the BRAF V600E mutation was significantly associated with increased cancer-related mortality among patients with PTC. Because overall mortality in PTC is low and the association was not independent of tumor features, how to use BRAF V600E to manage mortality risk in patients with PTC is unclear. These findings support further investigation of the prognostic and therapeutic implications of BRAF V600E status in PTC.
Papillary thyroid cancer (PTC) is the most common endocrine malignancy and accounts for 85% to 90% of all thyroid cancers. There are several variants of PTC, the majority of which are conventional PTC and follicular variant PTC, with the former typically showing papillary structures and the latter follicular structures in addition to the characteristic nuclear features of PTC. The overall 5-year patient survival rate for PTC is 95% to 97%.2 A major clinical challenge is how to reliably distinguish patients who need aggressive treatments to reduce mortality from those who do not. This represents a widely controversial issue in thyroid cancer medicine, particularly because of the low overall mortality of this cancer. The issue has become even more challenging given the high annual incidence of PTC.1,2 Several clinicopathological risk factors have been used in the stratification of PTC, including older age of patients at diagnosis, larger tumor size, cervical lymph node metastasis (LNM), extrathyroidal invasion, distant metastasis, and high levels on disease staging.3–5 Although these factors are known to be associated with a higher risk of progression of PTC, they often lack accuracy in helping tailor the extent of treatment of PTC to balance treatment-associated benefit and risk.
The T1799A nucleotide transversion in the BRAF gene (NM_004333) is a prominent oncogenic mutation in PTC6–11 and occurs, on average, in 45% of cases.12 This mutation causes a valine–to–glutamic acid change in codon 600 of the BRAF protein, resulting in BRAF V600E, which possesses elevated serine/threonine protein kinase activities and constitutively activates the mitogen-activated protein kinase signaling pathway in human cancer.13 Many studies have shown an association of the BRAF V600E mutation with aggressive clinicopathological characteristics of PTC, including LNM, extrathyroidal invasion, loss of radioiodine avidity, and, hence, failure of radioiodine treatment and disease recurrence.14,15 Consequently, the BRAF V600 Emutation has drawn considerable attention and interest as a potential prognostic factor for PTC. However, the clinical significance of this mutation in PTC-related mortality has not been established. We undertook the present multi-center study to examine and define the association between the BRAF V600 Emutation and PTC-related mortality.
The Box contains a glossary of terms used in this article.
Box. Glossary of Terms.
Methylation
Covalent attachment of methyl groups to DNA, usually at cytosine bases. Methylation can reduce transcription from a gene and is a mechanism in X-chromosome inactivation and imprinting.
Oncogene
A gene, 1 or more forms of which is associated with cancer. Many oncogenes are involved, directly or indirectly, in controlling the rate of cell growth.
Transversion
The substitution of a purine for a pyrimidine nucleotide or vice versa (eg, an A for a C or T) in a DNA sequence.
For a complete list of genomic terms, see the Appendix in this issue.
METHODS
This study was conducted at 13 medical centers in 7 countries, including the Johns Hopkins Medical Institutions, University of Pittsburgh Medical Center, and Memorial Sloan-Kettering Cancer Center in the United States; medical centers at the University of Pisa, University of Perugia, University of Milan, and University of Padua in Italy; Kanagawa Cancer Center, Yokohama, Japan; Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology in Poland; medical centers at Griffith Medical School and University of Sydney in Australia; Hospital La Paz Health Research Institute in Spain; and the Institute of Endocrinology, Prague, Czech Republic.
Study Patients
All patients had been treated and followed up for PTC at the participating institutions and their collaborating medical centers. Patients at each center were consecutively selected from different periods at the 13 centers, which overall spanned 1978–2011. All patients were treated with total thyroidectomy for PTC, and therapeutic neck dissection was performed in patients with standard indications. Standard pathological diagnoses of PTC were based on World Health Organization criteria and documented in peer-reviewed publications.16–29 Postoperative treatments included, as guided by standard criteria, conventional thyrotropin suppression at appropriate levels and radioiodine I 131 ablation (eTable 1; available at http://www.jama.com), except for Kanagawa Cancer Center, where no radioiodine I 131 treatment was used for thyroid cancer patients. Follow-up or survival time was defined as the time from the initial surgical treatment to patient death due to PTC or to the most recent clinic visit.
Study Design
This was a retrospective study approved by the institutional review boards of each center, with written informed patient consent obtained where required; patient consent was waived in some cases following institutional review board–approved procedures in the collection of pathological data. The study involved the use of only thyroid tumor tissues and clinicopathological information of patients. The BRAF V600E mutation status of primary PTC tumors was determined after surgical and medical (eg, radioiodine) treatments in all cases and did not affect decisions on selection of treatments. Genomic DNA isolated from primary PTC tumors was used to analyze the sequence of exon 15 of the BRAF gene for BRAF V600E as described in published studies.16–29 Clinicopathological information was obtained from the medical records using a uniform protocol designed for this study. Papillary thyroid cancer–specific death was defined as death that occurred as a result of incurable advanced PTC disease that invaded and compromised vital organs. Patient data from the 13 centers were pooled for the analysis of the relationship of BRAF V600E with PTC-specific mortality in various clinicopathological categories.
Statistical Analyses
Papillary thyroid cancer–specific mortality was calculated by dividing the number of deaths due to PTC by the total number of patients. The Fisher exact test was used to compare mortality by BRAF V600E mutation status. Rates per person-year were calculated by dividing the number of PTC-specific deaths by the total follow-up time, and Poisson regression was used to calculate the 95% confidence intervals. Kaplan-Meier survival curves and log-rank tests, censoring patients at the time of last follow-up or 12 years, and Cox proportional hazards regression analyses, censoring patients at the time of last follow-up, were used to compare PTC-specific survival by BRAF V600E mutation status. Proportional hazards regressions were adjusted for age at diagnosis, sex, and medical center. A second model was also used to additionally adjust for LNM, extrathyroidal invasion, and distant metastasis. The covariates were tested for the proportional hazards assumption using the “assess” statement in SAS version 9.3 (SAS Institute Inc). As medical centers violated the proportional hazards assumption, stratified models were used. Subgroup analyses were not adjusted for multiple comparisons and should be considered exploratory. Additive interactions of BRAF V600E mutation status with other factors on the crude death rates were tested using the synergy index and 95% confidence intervals described by Hosmer and Lemeshow.30 Exact binomial confidence intervals for mortality percentages were calculated using Stata/IC version 12.1 (Stata Corp). All other analyses were performed using SAS version 9.3. All reported P values are 2-sided and significance was set at P<.05. The P values from the log-rank tests comparing each stratum with the lowest risk stratum were adjusted for multiple comparisons using the Dunnett test.
RESULTS
Relationship Between BRAF V600E and PTC-Related Mortality
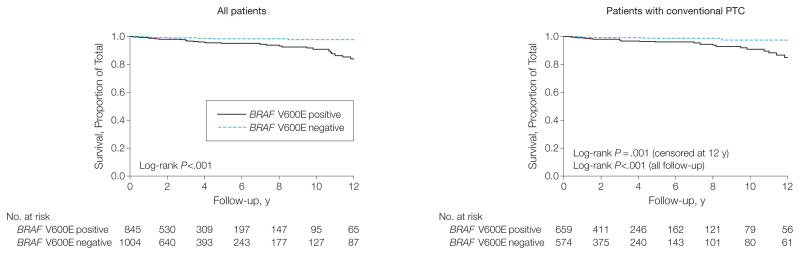
The number, sex, and age of patients from each center and country are summarized in Table 1. A total of 1849 patients (1411 women and 438 men) with a median age of 46 years (interquartile range [IQR], 34–58 years) were included, with an overall median follow-up time of 33 months (IQR, 13–67 months) after the initial treatment. Median follow-up time for surviving patients did not differ between BRAF V600E–positive patients (30 [IQR, 14–63] months) and BRAF V600E–negative patients (36 [IQR, 12–67] months) (P=.30). The overall prevalence of BRAF V600E was 45.7% (845/1849; 95% CI, 43.4%–48.0%) (Table 1), which is within the range of published BRAF V600E mutation rates.12,14,15 There were 56 PTC-related deaths among the 1849 patients, representing an overall mortality of 3.0% (95% CI, 2.3%–3.9%), which is consistent with the general mortality rate of PTC.2 Among these deaths, 45 cases (80.4%) were positive for BRAF V600E. Mortality percentages and deaths per 1000 person-years for different types of PTC are reported in Table 2. The overall mortality of all PTC cases was 5.3% (45/845; 95% CI, 3.9%–7.1%) in BRAF V600E–positive patients vs 1.1% (11/1004; 95% CI, 0.5%–2.0%) in mutation-negative patients (P<.001). The total follow-up for all PTC cases was 7856.75 person-years. Deaths per 1000 person-years on the analysis of all PTC cases were 12.87 (95% CI, 9.61–17.24) vs 2.52 (95% CI, 1.40–4.55) in BRAF V600E–positive vs mutation-negative patients; the hazard ratio (HR) was 2.66 (95% CI, 1.30–5.43) after adjustment for age at diagnosis, sex, and stratification by medical center. Deaths per 1000 person-years for patients with the conventional variant of PTC were 11.80 (95% CI, 8.39–16.60) vs 2.25 (95% CI, 1.01–5.00) in BRAF V600E–positive vs mutation-negative patients; the adjusted HR was 3.53 (95% CI, 1.25–9.98) (Table 2). No significant result was observed for the follicular variant PTC group, which had low numbers of cases and patient deaths (adjusted HR, 1.67; 95% CI, 0.06–47.49). When the aggressive tumor features of LNM, extrathyroidal invasion, and distant metastasis were also included in the model, the association of BRAF V600E with mortality was no longer statistically significant (for all PTC, HR, 1.21 [95% CI, 0.53–2.76]; for conventional PTC, HR, 1.51 [95% CI, 0.50–4.57]). Kaplan-Meier survival curves for all PTC and conventional PTC cases are shown in Figure 1. BRAF V600E–positive patients had significantly poorer survival in each analysis.
Table 1.
Demographic Characteristics, BRAF V600E Mutation, and Follow-up Time of Patients by Medical Center and Country
| No. of Patients | Age at Diagnosis, Median (IQR), y | Male, No. (%) | BRAF V600E Mutation, No. (%) | PTC-Related Deaths, No. (%)
|
Follow-up, Median (IQR), mo
|
||||
|---|---|---|---|---|---|---|---|---|---|
| All | BRAF V600E– Positive | BRAF V600E– Negative | All Patients | Survivors | |||||
| By medical center | |||||||||
| Johns Hopkins Hospital | 387 | 45 (35–57) | 101 (26.1) | 151 (39.0) | 8 (2.1) | 8 (5.3) | 0 | 12 (1–30) | 12 (1–28) |
|
| |||||||||
| University of Pittsburgh | 169 | 52 (38–63) | 42 (24.8) | 101 (59.8) | 1 (0.6) | 1 (1.0) | 0 | 19 (11–26) | 19 (11–26) |
|
| |||||||||
| Memorial Sloan-Kettering Cancer Center | 135 | 50 (35–63) | 44 (32.6) | 64 (47.4) | 11 (8.2) | 10 (15.6) | 1 (1.4) | 96 (1–144) | 90 (1–144) |
|
| |||||||||
| University of Pisa | 189 | 38 (28–51) | 47 (24.9) | 65 (34.4) | 9 (4.8) | 6 (9.2) | 3 (2.4) | 72 (24–180) | 84 (24–180) |
|
| |||||||||
| University of Perugia | 117 | 49 (37–59) | 32 (27.4) | 76 (65.0) | 5 (4.3) | 2 (2.6) | 3 (7.3) | 22 (6–39) | 22 (6–40) |
|
| |||||||||
| University of Milan | 110 | 42 (34–55) | 24 (21.8) | 38 (34.6) | 1 (0.9) | 0 | 1 (1.4) | 48 (24–64) | 48 (24–64) |
|
| |||||||||
| University of Padua | 135 | 48 (39–57) | 32 (23.7) | 87 (64.4) | 1 (0.7) | 1 (1.2) | 0 | 26 (22–30) | 26 (22–30) |
|
| |||||||||
| Kanagawa Cancer Center | 49 | 55 (41–65) | 16 (32.6) | 33 (67.4) | 9 (18.4) | 7 (21.2) | 2 (12.5) | 68 (31–78) | 65 (33–76) |
|
| |||||||||
| Maria Sklodowska-Curie Memorial Cancer Centre and Institute of Oncology | 99 | 49 (33–59) | 10 (10.1) | 42 (42.4) | 1 (1.0) | 1 (2.4) | 0 | 48 (42–53) | 48 (43–53) |
|
| |||||||||
| Griffith Medical School | 76 | 40 (34–56) | 20 (26.3) | 34 (44.7) | 0 | 0 | 0 | 42 (4–82) | 42 (4–82) |
|
| |||||||||
| University of Sydney | 95 | 44 (34–59) | 20 (21.0) | 55 (57.9) | 5 (5.3) | 5 (9.1) | 0 | 103 (63–135) | 104 (64–137) |
|
| |||||||||
| Hospital La Paz, Health Research Institute | 66 | 42 (32–54) | 11 (16.7) | 28 (42.4) | 2 (3.0) | 1 (3.6) | 1 (2.6) | 41 (30–57) | 42 (30–57) |
|
| |||||||||
| Institute of Endocrinology, Prague | 222 | 47 (31–60) | 39 (17.6) | 71 (32.0) | 3 (1.4) | 3 (4.2) | 0 | 50 (30–85) | 50 (30–85) |
|
| |||||||||
| By country | |||||||||
| United States | 691 | 47 (36–59) | 187 (27.1) | 316 (45.7) | 20 (2.9) | 19 (6.0) | 1 (0.3) | 17 (2–36) | 16 (2–32) |
|
| |||||||||
| Italy | 551 | 44 (34–56) | 135 (24.5) | 266 (48.3) | 16 (2.9) | 9 (3.4) | 7 (2.5) | 33 (20–70) | 34 (20–72) |
|
| |||||||||
| Japan | 49 | 55 (41–65) | 16 (32.6) | 33 (67.4) | 9 (18.4) | 7 (21.2) | 2 (12.5) | 68 (31–78) | 65 (33–76) |
|
| |||||||||
| Poland | 99 | 49 (33–59) | 10 (10.1) | 42 (42.4) | 1 (1.0) | 1 (2.4) | 0 | 48 (42–53) | 48 (43–53) |
|
| |||||||||
| Australia | 171 | 43 (34–57) | 40 (23.4) | 89 (52.0) | 5 (2.9) | 5 (5.6) | 0 | 75 (32–118) | 76 (33–118) |
|
| |||||||||
| Spain | 66 | 42 (32–54) | 11 (16.7) | 28 (42.4) | 2 (3.0) | 1 (3.6) | 1 (2.6) | 41 (30–57) | 42 (30–57) |
|
| |||||||||
| Czech Republic | 222 | 47 (31–60) | 39 (17.6) | 71 (32.0) | 3 (1.4) | 3 (4.2) | 0 | 50 (30–85) | 50 (30–85) |
|
| |||||||||
| Overall | 1849 | 46 (34–58) | 438 (23.7) | 845 (45.7) | 56 (3.0) | 45 (5.3) | 11 (1.1) | 33 (13–67) | 33 (13–65) |
Abbreviation: IQR, interquartile range.
Table 2.
Papillary Thyroid Cancer–Related Mortality, Person-Years of Follow-up, and Hazard Ratios for BRAF V600E Mutation-Positive vs Mutation-Negative Patients
| Type of Papillary Thyroid Cancer | Mortality, No./Total (%)
|
Person- Years of Follow-up | Deaths per 1000 Person-Years (95% CI)
|
Hazard Ratio (95% CI)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | BRAF V600E– Positive | BRAF V600E– Negative | P Value | BRAF V600E– Positive | BRAF V600E– Negative | Unadjusted | Adjusteda | ||
| All types | 56/1849 (3.0) | 45/845 (5.3) | 11/1004 (1.1) | <.001 | 7856.75 | 12.87 (9.61–17.24) | 2.52 (1.40–4.55) | 5.31 (2.74–10.30) | 2.66 (1.30–5.43) |
|
| |||||||||
| Conventional | 39/1233 (3.2) | 33/659 (5.0) | 6/574 (1.0) | <.001 | 5466.75 | 11.80 (8.39–16.60) | 2.25 (1.01–5.00) | 5.63 (2.34–13.51) | 3.53 (1.25–9.98) |
|
| |||||||||
| Follicular variant | 6/411 (1.5) | 4/82 (4.9) | 2/329 (0.6) | .02 | 1572.25 | 11.21 (4.21–29.86) | 1.65 (0.41–6.58) | 6.02 (1.10–32.96) | 1.67 (0.06–47.49) |
Proportional hazards regression model adjusted for patient sex and age at diagnosis and stratified by medical center.
Figure 1.
Kaplan-Meier Survival Curves of PTC-Specific Survival by BRAF V600E Mutation Status
Comparison of patient survival, represented by log-rank P values in each panel, was performed between BRAF V600E–negative and BRAF V600E–positive groups for all patients and for patients with conventional papillary thyroid cancer (PTC). Follow-up time is truncated at 12 years.
Papillary thyroid cancer–related mortality, total person-years, and rates by BRAF V600E mutation status in various clinicopathological subcategories are presented in Table 3. Higher mortality percentages and deaths per 1000 person-years were seen with BRAF V600E within most of the categories, including among patients with distant metastasis and advanced American Joint Committee on Cancer stage IV disease, in which the highest mortality percentages and deaths per 1000 person-years were seen. However, after adjustment for age, sex, and medical center, the HRs were no longer statistically significant in some of these stratified categories. The association of BRAF V600E with mortality among patients with disease stages I, II, and III was not statistically significant. When tumors were stratified by size, the absolute magnitude of mortality increased from smaller to larger tumors, particularly in the BRAF V600E–positive groups. BRAF V600E had a significant association with mortality percentages of micro-PTC (≤1.0 cm), but the absolute mortality was low and the adjusted HRs were not significant (Table 3).
Table 3.
Papillary Thyroid Cancer–Related Mortality, Person-Years of Follow-up, and Hazard Ratios for BRAF V600E Mutation-Positive vs Mutation-Negative Patients in Various Clinicopathological Categories
| Category | Mortality, No./Total (%)
|
Person- Years of Follow-up | Deaths per 1000 Person-Years (95% CI)
|
Hazard Ratio (95% CI)
|
||||
|---|---|---|---|---|---|---|---|---|
| BRAF V600E–Positive | BRAF V600E–Negative | P Value | BRAF V600E–Positive | BRAF V600E–Negative | Unadjusted | Adjusteda | ||
| All patients | 45/845 (5.3) | 11/1004 (1.1) | <.001 | 7856.75 | 12.87 (9.61–17.24) | 2.52 (1.40–4.55) | 5.31 (2.74–10.30) | 2.66 (1.30–5.43) |
|
| ||||||||
| Age, y | ||||||||
| <45 | 5/346 (1.4) | 2/530 (0.4) | .12 | 4037.67 | <.19 (1.33–7.66) | 0.81 (0.20–3.24) | 3.85 (0.74–20.02) | 1.20 (0.13–10.97) |
|
| ||||||||
| ≤45 | 40/499 (8.0) | 9/474 (1.9) | <.001 | 3819.08 | 20.75 (15.22–28.29) | 4.76 (2.48–9.14) | 4.59 (2.22–9.48) | 3.38 (1.59–7.21) |
|
| ||||||||
| <60 | 14/617 (2.3) | 5/819 (0.6) | .009 | 6400.00 | 5.27 (3.12–8.89) | 1.34 (0.56–3.21) | 4.09 (1.46–11.45) | 1.85 (0.58–5.91) |
|
| ||||||||
| ≤60 | 31/228 (13.6) | 6/185 (3.2) | <.001 | 1456.75 | 37.03 (26.04–52.65) | 9.68 (4.35–21.56) | 3.80 (1.58–9.10) | 3.76 (1.49–9.52) |
|
| ||||||||
| Lymph node metastasis | ||||||||
| No | 4/410 (1.0) | 3/623 (.5) | .45 | 4363.83 | 2.43 (0.91–6.47) | 1.97 (0.38–10.35) | 2.17 (0.48–9.78) | 1.97 (0.38–10.35) |
|
| ||||||||
| Yes | 39/351 (11.1) | 8/307 (2.6) | <.001 | 2834.58 | 26.26 (19.18–35.94) | 5.93 (2.96–11.86) | 4.43 (2.06–9.51) | 1.46 (0.62–3.47) |
|
| ||||||||
| Extrathyroidal invasion | ||||||||
| No | 12/497 (2.4) | 2/770 (.3) | <.001 | 5734.83 | 5.24 (2.98–9.23) | 0.58 (0.15–2.32) | 9.38 (2.09–42.00) | 7.90 (1.65–37.69) |
|
| ||||||||
| Yes | 32/336 (9.5) | 9/209 (4.3) | .03 | 1999.33 | 27.02 (19.11–38.21) | 11.04 (5.75–21.22) | 2.12 (1.00–4.49) | 0.91 (0.39–2.11) |
|
| ||||||||
| Distant metastasis | ||||||||
| No | 11/772 (1.4) | 3/944 (.3) | .01 | 7210.33 | 3.54 (1.96–6.39) | 0.73 (0.24–2.27) | 4.28 (1.18–15.57) | 4.17 (1.06–16.40) |
|
| ||||||||
| Yes | 34/66 (51.5) | 8/44 (18.2) | <.001 | 635.42 | 87.72 (62.68–122.77) 32.28 (16.14–64.55) | 2.63 (1.21–5.72) | 0.84 (0.27–2.62) | |
|
| ||||||||
| Multifocality | ||||||||
| No | 30/495 (6.1) | 8/630 (1.3) | <.001 | 4935.33 | 14.01 (9.80–20.04) | 2.86 (1.43–5.73) | 5.09 (2.33–11.14) | 1.87 (0.79–4.43) |
|
| ||||||||
| Yes | 14/345 (4.1) | 3/355 (.8) | .006 | 2815.00 | 10.43 (6.18–17.61) | 2.04 (0.66–6.32) | 5.14 (1.48–17.91) | 5.03 (1.26–20.11) |
|
| ||||||||
| Stage IV disease | ||||||||
| No | 6/700 (0.9) | 1/893 (.1) | .048 | 6836.75 | 2.08 (0.93–4.62) | 0.25 (0.04–1.80) | 9.48 (1.10–81.56) 10.38 (1.02–105.55) | |
|
| ||||||||
| Yes | 38/121 (31.4) | 10/77 (13.0) | .004 | 851.92 | 69.97 (50.91–96.16) | 32.38 (17.42–60.18) | 1.93 (0.96–3.91) | 1.66 (0.73–3.77) |
|
| ||||||||
| Stage | ||||||||
| I | 1/443 (0.2) | 1/664 (0.2) | >.99 | 4926.83 | 0.52 (0.07–3.67) | 0.33 (0.05–2.37) | 1.51 (0.09–24.14) | 3.17 (0.11–92.20) |
|
| ||||||||
| II | 1/77 (1.3) | 0/127 | .38 | 828.58 | 3.48 (0.49–24.71) | 0 | ||
|
| ||||||||
| III | 4/180 (2.2) | 0/102 | .30 | 1081.33 | 5.99 (2.25–15.96) | 0 | ||
|
| ||||||||
| Tumor size, cm | ||||||||
| ≤1.0 | 4/168 (2.4) | 0/267 | .02 | 1701.83 | 6.55 (2.46–17.46) | 0 | ||
|
| ||||||||
| 1.0–2.0 | 6/392 (1.5) | 3/417 (0.7) | .33 | 3596.25 | 3.69 (1.66–8.21) | 1.52 (0.49–4.72) | 2.52 (0.63–10.18) | 0.74 (0.12–4.32) |
|
| ||||||||
| 2.0–3.0 | 13/237 (5.5) | 3/236 (1.3) | .02 | 2179.00 | 13.32 (7.73–22.93) | 2.49 (0.80–7.73) | 5.51 (1.56–19.40) | 2.51 (0.61–10.41) |
|
| ||||||||
| 3.0–4.0 | 9/120 (7.5) | 4/154 (2.6) | .08 | 1170.17 | 17.54 (9.13–33.71) | 6.09 (2.28–16.22) | 2.81 (0.86–9.14) | 1.38 (0.35–5.46) |
|
| ||||||||
| ≥4.0 | 13/99 (13.1) | 5/137 (3.6) | .01 | 1021.75 | 29.20 (16.96–50.29) | 8.67 (3.61–20.83) | 3.31 (1.18–9.32) | 1.96 (0.30–12.84) |
Proportional hazards regression model adjusted for patient sex and age at diagnosis and stratified by medical center, except for age categories, which were adjusted only for sex and medical center.
We found that the therapeutic doses of radioiodine used in the treatment of patients were comparable between the BRAF V600E–positive and mutation-negative groups, except in some centers where the BRAF V600E group received higher doses (eTable 1).
Interaction of BRAF V600E With Conventional Clinicopathological Risk Factors
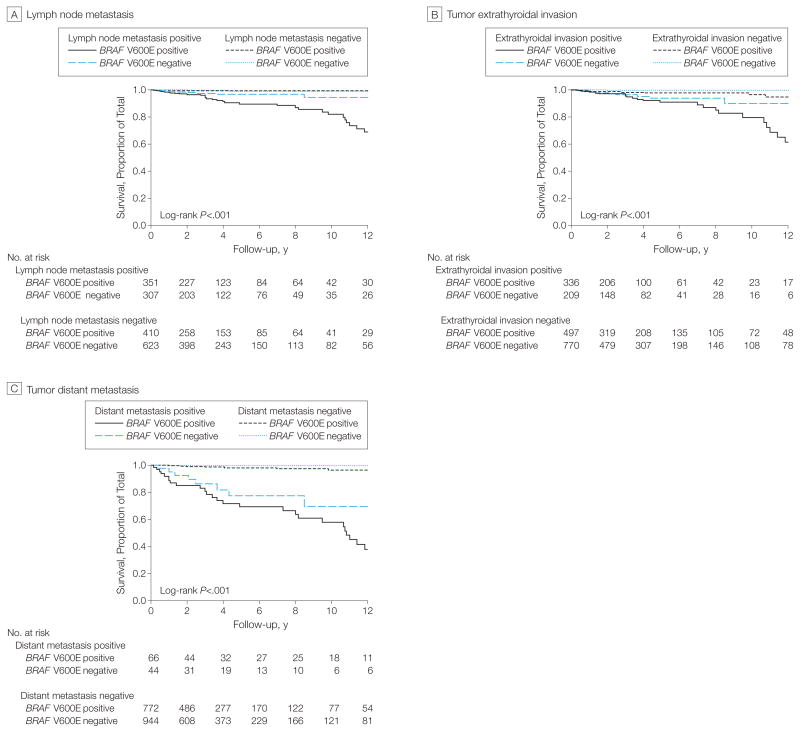
We observed a significant additive interaction of BRAF V600E with several conventional clinicopathological risk factors in affecting PTC-related mortality, as reflected by a significant synergy index (eTable 2). These included LNM, distant metastasis, stage IV disease, and patient age at diagnosis. The synergy index was not statistically significant for extrathyroidal invasion. As shown in Table 3, deaths per 1000 person-years for coexisting LNM and BRAF V600E were 26.26 (95% CI, 19.18–35.94), whereas they were 5.93 (95% CI, 2.96–11.86) in LNM-positive but BRAF V600E–negative patients and 2.43 (95% CI, 0.91–6.47) in LNM-negative but BRAF V600E–positive patients. Deaths per 1000 person-years for coexisting distant metastatic disease and BRAF V600E were 87.72 (95% CI, 62.68–122.77), whereas they were 32.28 (95% CI, 16.14–64.55) in distant metastasis–positive but BRAF V600E–negative patients and 3.54 (95% CI, 1.96–6.39) in distant metastasis–negative but BRAF V600E–positive patients. Similarly, with coexistence of stage IV disease and BRAF V600E, deaths per 1000 person-years were 69.97 (95% CI, 50.91–96.16), whereas they were 32.38 (95% CI, 17.42–60.18) in BRAF V600E–negative patients with stage IV disease and 2.08 (95% CI, 0.93–4.62) in BRAF V600E-positive patients without stage IV disease. The common pattern of these relationships is that the mortality associated with coexistence of BRAF V600E and a conventional risk factor was higher than the addition of the 2 types of mortality associated with either alone, further supporting the synergistic additive interactions of BRAF V600E with these risk factors demonstrated by the synergy index test (eTable 2). This pattern of interaction of BRAF V600E with clinicopathological factors in affecting PTC-related mortality was also reflected in the Kaplan-Meier survival curves (Figure 2).
Figure 2.
Kaplan-Meier Survival Curves of the Interaction of BRAF V600E Mutation With Clinicopathological Risk Factors in Affecting Disease-Specific Survival of Patients With Papillary Thyroid Cancer
In all panels, follow-up time is truncated at 12 years. In each panel, P values are from the log-rank test adjusted for multiple comparisons comparing each stratum with patients negative for both the BRAF V600E mutation and the indicated clinicopathological factor.
BRAF V600E and Patient Age in PTC-Related Mortality
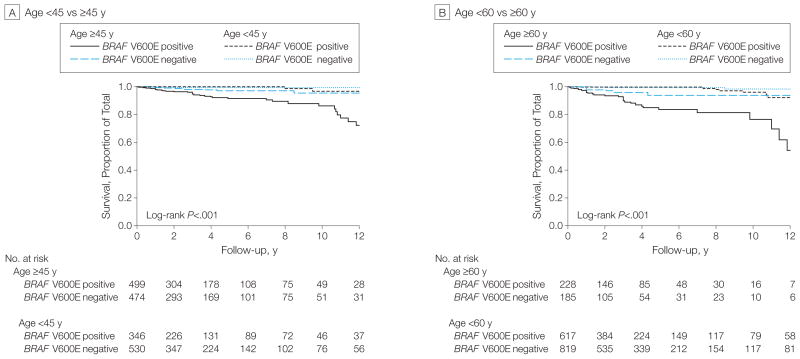
As shown in Table 3, in both BRAF V600E–positive and mutation-negative patients, mortality increased with age, and this was particularly evident in BRAF V600E–positive patients. Specifically, deaths per 1000 person-years in BRAF V600E–positive patients younger than 45 years and 45 years or older were 3.19 (95% CI, 1.33–7.66) and 20.75 (95% CI, 15.22–28.29), respectively, vs 0.81 (95% CI, 0.20–3.24) and 4.76 (95% CI, 2.48–9.14) in BRAF V600E–negative patients in these age groups with a significant synergistic interaction (synergy index, 3.15; 95% CI, 1.37–7.27) (eTable 2). Deaths per 1000 person-years in BRAF V600E–positive patients younger than 60 years and 60 years or older were 5.27 (95% CI, 3.12–8.89) and 37.03 (95% CI, 26.04–52.65), respectively, vs 1.34 (95% CI, 0.56–3.21) and 9.68 (95% CI, 4.35–21.56) in BRAF V600E–negative patients in these age groups, with a significant synergistic interaction (synergy index, 3.40; 95% CI, 1.52–7.62). Thus, these results showed a significant additive interaction of BRAF V600E with older patient age on mortality due to PTC. This positive interaction of BRAF V600E with patient age in affecting PTC mortality was also shown in Kaplan-Meier survival curves (Figure 3).
Figure 3.
Kaplan-Meier Survival Curves of the Interaction of BRAF V600E Mutation With Age in Affecting Disease-Specific Survival of Patients With Papillary Thyroid Cancer
In all panels, follow-up time is truncated at 12 years. In each panel, P values are from the log-rank test adjusted for multiple comparisons comparing each stratum with patients negative for both the BRAF V600E mutation and younger than 45 years (panel A) or younger than 60 years (panel B).
When analyzing patients with the conventional PTC variant, BRAF V600E was similarly associated with higher patient mortality within various clinicopathological risk categories (eTable 3).
Mortality in BRAF V600E Mutation-Negative Conventionally Low-Risk Patients
As shown in Table 3, the overall mortality was low in conventionally low-risk patients; ie, those with tumor size of 1.0 cm or smaller, stage I to III diseases, or age younger than 45 years. Mortality was lowest in the BRAF V600E–negative patients of these groups, ranging from 0 to 0.81 (95% CI, 0.20–3.24) deaths per 1000 person-years. A uniform 0 mortality was observed in BRAF V600E–negative patients in these groups when the analysis was restricted to only conventional PTC (eTable 3). A moderate increase in mortality was seen in the presence of BRAF V600E in some of these groups but was not statistically significant (Table 3 and eTable 3).
DISCUSSION
In this multicenter study, we found a significant association of BRAF V600E mutation with PTC-related mortality, both in patients with all types of PTC and in patients with conventional PTC. The majority of the mortality cases (80.4%) harbored this mutation. These results suggest the importance of BRAF V600E in PTC-related mortality. We also observed a significant additive interaction between BRAF V600E and several conventional clinicopathological factors affecting the magnitude of PTC-related mortality, including older patient age at diagnosis, LNM, distant metastasis, and advanced disease (stage IV). Most of these factors alone had only a modest mortality risk, which was significantly increased by coexisting BRAF V600E. Thus, the widely known mortality risk associated with the conventional high-risk clinicopathological factors of PTC is closely related to the coexisting BRAF V600E mutation.
The significance of the association of BRAF V600E with mortality needs to be interpreted from the perspective of absolute risk. For example, as shown in Table 3, BRAF V600E in patients without distant metastasis was associated with an increase in mortality from 0.3% (3/944) to 1.4% (11/772) (P=.01), whereas it was increased in patients with distant metastasis from 18.2% (8/44) to 51.5% (34/66) (P<.001). Although the difference was statistically significant in both situations, there was only 1 additional patient death in the former vs 33 additional deaths in the latter associated with BRAF V600E in 100 patients. In some of the conventionally low-risk categories, such as tumors of 1.0 cm or smaller, BRAF V600E was also associated with mortality, consistent with previous findings that this mutation could be associated with aggressive tumor features even in conventionally low-risk patients.14,18,31 In such low-risk categories, however, the absolute mortality rate is low. It is also clinically important to note that absence of the BRAF V600E mutation was associated with a mortality of nearly 0% in conventionally low-risk patients.
The explanation for a role of BRAF V600E in PTC-related mortality likely lies in the molecular mechanisms by which BRAF V600E promotes aggressive molecular patho genesis of PTC. For example, BRAF V600E causes de differentiation of PTC, resulting in the loss of expression of thyroid genes involved in thyroid iodide concentration and, hence, failure of radioiodine treatment.14,15 BRAF V600E strongly up-regulates many classic angiogenic and tumor-promoting molecules (eg, vascular endothelial growth factor, matrix metalloproteinases, c-MET, and nuclear transcription factor κB)and is associated with hypermethylation and, hence, inactivation of tumor suppressor genes (eg, tissue inhibitor of matrix metalloproteinase 3, death-associated protein kinase, and SLC5A8)14,31 as well as extracellular protumor microenvironmental changes.32 BRAF V600E causes genome-wide alterations in methylation and, hence, aberrant expression of prominent genes in thyroid cancer33 as well as in melanoma.34 There are also other molecular derangements and signaling path-way aberrations caused by BRAF V600E in thyroid cancer.35
It is likely that through these unique molecular mechanisms and others as yet unknown, BRAF V600E promotes aggressive tumor behaviors such as LNM, tumor invasion, and distant metastasis; silences thyroid iodide-metabolizing genes and renders the tumor resistant to radioiodine treatment; and expedites tumor progression, hence aggravating the risk of PTC-related mortality, which is ultimately caused by these aggressive tumor behaviors. Thus, BRAF V600E cannot be independent of such tumor behaviors in affecting patient mortality. In fact, when we adjusted for these tumor behaviors, the association with BRAF V600E was no longer statistically significant, indicating that these tumor behaviors may lie in a causal pathway. In contrast, stratification by center and adjustment for patient age and sex did not remove the significant association of BRAF V600E with mortality in either the overall analysis of all PTC or the analysis of conventional PTC.
The large number of cases and multicenter design with worldwide geographic reach represent a major strength of this study. The treatments, including total thyroidectomy, therapeutic neck dissection, and appropriate postoperative thyrotropin suppression, were pursued following accepted standards at the participating centers, and the pathological diagnoses of tumors were formally documented.16–29 Although patient follow-up durations after the initial treatment varied between centers, this was not different by BRAF V600E status within centers and in the overall analysis. Moreover, we used Cox proportional hazards regression and Kaplan-Meier survival analysis as well as person-year mortality rates to account for different durations. Most of the known relationship patterns of conventional clinicopathological risk factors with PTC-related mortality were accurately reproduced in the present study, supporting its validity. At some centers, BRAF V600E patients received higher doses of radioiodine treatments when retrospectively analyzed after BRAF V600E testing. This likely reflects that BRAF V600E patients tended to present with more aggressive clinicopathological behaviors of PTC, prompting more aggressive radioiodine treatment. This may have caused an underestimate of the association of BRAF V600E with PTC mortality in the present study, as radioiodine treatment has been associated with decreased mortality of thyroid cancer in conventionally high-risk patients,3 which, as the present study showed, is where BRAF V600E has the most significant association with mortality.
There are a few limitations of the present study. First, the low number of PTC-specific deaths, as is generally seen for PTC, reduced the power to find associations and resulted in wide confidence intervals for some of the subcategory estimates. This was particularly an issue in the stratified analyses. In fact, adjusted HRs lost significance in some stratified subcategories (Table 3). This, however, partially reflects that BRAF V600E synergistically interacts with patient age in its association with mortality and the effect of BRAF V600E would therefore be attenuated when patient age was adjusted in the model. Additionally, stratified analyses were performed with no adjustment for multiple comparisons because of a relatively small number of cases. Therefore, these stratified analyses should be considered exploratory and hypothesis generating. Second, many patients had a relatively short clinical follow-up. This may have led to an incomplete representation by the present study of the natural mortality course of PTC and, hence, an in accurate picture of the relationship of BRAF V600E and PTC-related mortality. This seems to be suggested by the observation that the association became clearer after longer follow-up. Another limitation is that we captured only PTC-specific deaths in our data, censoring patients who died of other causes at the time of last follow-up. Therefore, we could not look at all-cause mortality.
In summary, in this multicenter study, the presence of the BRAF V600E mutation was significantly associated with increased cancer-related mortality among patients with PTC. However, overall mortality in PTC is low, and the association was not independent of tumor behaviors. Therefore, how to use BRAF V600E for the management of mortality risk among patients with PTC is not clear. These findings support further investigation of the prognostic and therapeutic implications of BRAF V600E status in PTC.
Supplementary Material
Acknowledgments
Funding/Support: This project was supported by National Institutes of Health (NIH) grants R01CA134225 and R01CA113507 to Dr Xing. The statistical effort of Ms Carson for this project was supported by grant UL1RR025005 from the National Center for Advancing Translational Sciences and the NIH Roadmap for Medical Research. In addition, the studies at individual centers were supported as follows: the Ministry of Science and Higher Education Research grants N N403 194340 and N N401 612440 to Drs Czarniecka and Jarzab, respectively(Poland); grant NIDCR/NCI SPORE P50DE019032 to Dr Sindransky (United States); grants BFU2010–16025, RD06/0020/0060-RD12/0036/0030 FIS, ISCIII, and S2011/BMD-2328TIRONET to Dr Santisteban (Spain); grant NIH-R01-CA50706 and Byrne Foundation funding to Dr Fagin (United States); grants from Fondazione Cassa di Risparmio di Perugia and Associazione Italiana per la Ricerca sul Cancro (IG 9338) (Italy) and the Beadle Family Foundation (San Antonio, Texas) to Dr Puxeddu; grant IGA MHCRNT13901–4 to Drs Sykorova and Bendlova (Czech Republic); and grants from the New South Wales Cancer Institute to Dr O’Neill and from the Cancer Council of New South Wales to Dr Clifton-Bligh (Australia).
Role of the Sponsor: The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Online-Only Material: The 3 eTables are available at http://www.jama.com.
Author Contributions: Dr Xing had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Xing
Acquisition of data: Xing, Alzahrani, Viola, Elisei, Bendlova, Mian, Vianello, Tuttle, Robenshtok, Fagin, Puxeddu, Fugazzola, Czarniecka, Jarzab, O’Neill, Sywak, Lam, Riesco-Eizaguirre, Santisteban, Nakayama, Clifton-Bligh, Sykorova.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Xing.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Xing, Carson.
Obtained funding: Xing.
Administrative, technical, or material support: All authors.
Study supervision: Xing.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Drs Xing and Sidransky reported receiving royalties as coholders of a licensed US patent related to BRAF V600E mutation in thyroid cancer. Dr Elisei reports board membership, consultancy, and/or payment for lectures/speakers bureaus for AstraZeneca, Bayer, Exelixis, Genzyme, and Roche. Dr Fagin reports that he is lead investigator in clinical trials with an AZ MEK inhibitor and receives grant support for preclinical studies with this and other compounds in BRAF mutant thyroid cancer models. Dr Puxeddu reports consultancy for Euro 7000. Dr Jarzab reports consultancy for AstraZeneca, employment by AstraZeneca, Novartis, Pfizer, Bayer, Roche, Eisai, OxiGene, and Exelixis for clinical trials, payment for lecturing and development of educational presentations by Novartis and Ipsen, payment for manuscript preparation by Molecular and Cellular Endocrinology, and payment for travel/meeting expenses by Novartis, Sanofi, and Ipsen. Dr Tufano reports consultancy for Ethicon Endosurgery and Medtronic. Dr Clifton-Bligh reports advisory board membership (money to his institution) for Amgen, Merck Sharp & Dohme, Ipsen, and Genzyme. No other disclosures were reported.
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH or the funding entities of the individual centers participating in this study.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) Bethesda, MD: National Cancer Institute; Apr, 2012. [Accessed March 15, 2013]. http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 3.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM, Ball DW, Byrd D, et al. National Comprehensive Cancer Network. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. doi: 10.6004/jnccn.2010.0093. [DOI] [PubMed] [Google Scholar]
- 5.Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer. 2011;2:193–199. doi: 10.7150/jca.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95(8):625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 7.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63 (7):1454–1457. [PubMed] [Google Scholar]
- 8.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22(29):4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63(15):4561–4567. [PubMed] [Google Scholar]
- 10.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88(9):4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22(41):6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 12.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 13.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28(7):742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 15.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118(7):1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 16.Puxeddu E, Moretti S, Elisei R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89(5):2414–2420. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- 17.Fugazzola L, Mannavola D, Cirello V, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf) 2004;61(2):239–243. doi: 10.1111/j.1365-2265.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 18.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90 (12):6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 19.Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13(1):257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama H, Yoshida A, Nakamura Y, et al. Clinical significance of BRAF (V600E) mutation and Ki-67 labeling index in papillary thyroid carcinomas. Anticancer Res. 2007;27(5B):3645–3649. [PubMed] [Google Scholar]
- 21.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 22.Xing M, Clark D, Guan H, et al. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27(18):2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip L, Nikiforova MN, Carty SE, et al. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146(6):1215–1223. doi: 10.1016/j.surg.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69(11):4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sykorova V, Dvorakova S, Ryska A, et al. BRAF V600E mutation in the pathogenesis of a large series of papillary thyroid carcinoma in Czech Republic. J Endocrinol Invest. 2010;33(5):318–324. doi: 10.1007/BF03346593. [DOI] [PubMed] [Google Scholar]
- 26.Czarniecka A, Rusinek D, Stobiecka E, et al. Occurrence of BRAF mutations in a Polish cohort of PTC patients—preliminary results. Endokrynol Pol. 2010;61(5):462–466. [PubMed] [Google Scholar]
- 27.O’Neill CJ, Bullock M, Chou A, et al. BRAF (V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery. 2010;148(6):1139–1145. doi: 10.1016/j.surg.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Pelizzo MR, Boschin IM, Barollo S, et al. BRAF analysis by fine needle aspiration biopsy of thyroid nodules improves preoperative identification of papillary thyroid carcinoma and represents a prognostic factor: a mono-institutional experience. Clin Chem Lab Med. 2011;49(2):325–329. doi: 10.1515/CCLM.2011.031. [DOI] [PubMed] [Google Scholar]
- 29.Smith RA, Salajegheh A, Weinstein S, Nassiri M, Lam AK. Correlation between BRAF mutation and the clinicopathological parameters in papillary thyroid carcinoma with particular reference to follicular variant. Hum Pathol. 2011;42(4):500–506. doi: 10.1016/j.humpath.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321(1):86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nucera C, Lawler J, Parangi S. BRAF(V600E) and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res. 2011;71 (7):2417–2422. doi: 10.1158/0008-5472.CAN-10-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou P, Liu D, Xing M. Genome-wide alterations in gene methylation by the BRAF V600E mutation in papillary thyroid cancer cells. Endocr Relat Cancer. 2011;18(6):687–697. doi: 10.1530/ERC-11-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou P, Liu D, Dong J, Xing M. The BRAF(V600E) causes widespread alterations in gene methylation in the genome of melanoma cells. Cell Cycle. 2012;11 (2):286–295. doi: 10.4161/cc.11.2.18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.