Summary
Lineage conversion of differentiated cells in response to hormonal feedback has yet to be described. To investigate this we studied the adrenal cortex, which is composed of functionally distinct concentric layers that develop postnatally, the outer zona Glomerulosa (zG) and the inner zona Fasiculata (zF). These layers have separate functions, are continuously renewed in response to physiological demands and are regulated by discrete hormonal feedback loops. Their cellular origin, lineage relationship and renewal mechanism, however, remain poorly understood. Cell fate mapping and gene deletion studies using zG-specific Cre expression demonstrate that differentiated zG cells undergo lineage conversion into zF cells. In addition, zG maintenance is dependent on the master transcriptional regulator Steroidogenic Factor 1 (SF-1) and zG-specific Sf-1 deletion prevents lineage conversion. These findings demonstrate that adrenocortical zonation and regeneration result from lineage conversion and may provide a paradigm for homeostatic cellular renewal in other tissues.
Introduction
Proper development and function of the adrenal gland is paramount for organism survival. During early postnatal life the adrenal cortex in mice undergoes the process of zonation in which two concentric and functionally discrete layers, the zG and the zF, are formed (Figure 1A) (Kim et al., 2009). The morphologically distinct outer layer, the zG, is comprised of differentiated cells that produce mineralocorticoids, essential for sodium and potassium homeostasis. In contrast, differentiated cells within the inner layer, the zF, produce glucocorticoids, critical for diverse processes including stress response, glucose homeostasis, vascular tone and immune regulation. Both layers are continuously renewed throughout life and undergo dynamic hormonal feedback regulation. Despite the functional importance of these separate layers, surprisingly little is known about the developmental mechanisms underlying their formation. Two hypotheses have been proposed to explain postnatal adrenocortical zonation, the model of centripetal migration (Salmon and Zwemer, 1941) and the zonal model of lineage development (Deane and Greep, 1946). In the centripetal migration model, undifferentiated progenitor cells in the capsule or subcapsular region give rise to terminally differentiated mineralocorticoid-producing zG cells. These cells then migrate centripetally and are thought to undergo lineage conversion into glucocorticoid-producing zF cells before undergoing apoptosis at the corticomedullary junction (Kim et al., 2009). In contrast, the zonal model argues that each zone develops and is maintained independently by zone-specific progenitor cells. Recent advances using lineage-tracing to map the cell fate of Shh and Gli1 expressing progenitor cells demonstrate radial stripes that appear to migrate through the zG into the zF, providing support for the model of centripetal migration (King et al., 2009). Definitive proof for this model, however, is lacking given these studies were not designed to test whether zG cells directly contribute to the zF. Whether additional mechanisms are required for tissue homeostasis remains to be determined.
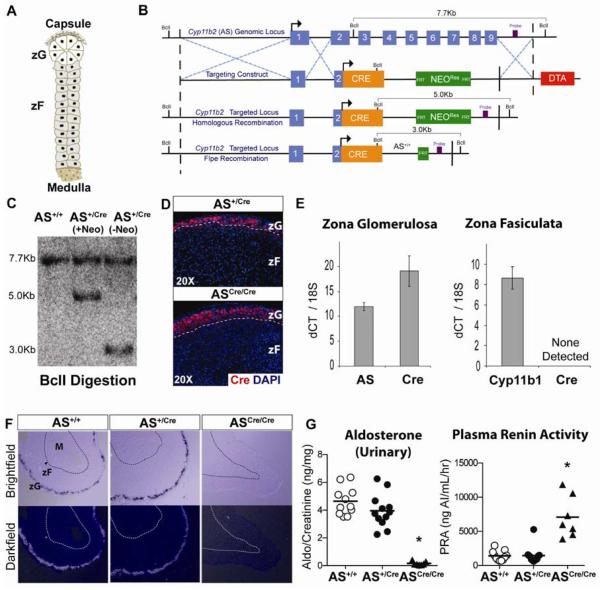
Figure 1. Zona Glomerulosa is normally regulated in AS+/Cre mice.
(A) Schematic of adrenal cortex showing the relationship of zG to zF cells. (B) Cre recombinase was targeted to the Cyp11b2 (AS) genomic locus by homologous recombination in mouse ES cells. The resultant transgenic mice were crossed with FLP deleter mice to excise the FRT-flanked NeomycinRes cassette. (C) Southern blot analysis confirmed homologous recombination. (D) Cre recombinase is restricted to the zG demonstrated by immunostaining in both AS+/Cre and ASCre/Cre mice. (E) Laser capture microdissection and isolation of zG and zF RNA, followed by qRT-PCR analysis, confirmed restriction of Cre expression to zG cells, Mean±SEM. (F) In Situ Hybridization demonstrates comparable AS expression in wild-type AS+/+ and heterozygous AS+/Cre adrenals and the lack of AS expression in homozygous ASCre/Cre adrenals. (G) Aldosterone and PRA levels are unchanged in AS+/Cre mice compared with controls. ASCre/Cre mice show undetectable levels of aldosterone and a compensatory rise in PRA levels (Mean, * p<0.0001). See also Figure S1.
Lineage conversion of one fully differentiated cell type to another without passing through an undifferentiated state has been described following the over-expression of select transcription factors, reviewed in (Sancho-Martinez et al., 2012). First demonstrated in 1987, studies showed that expression of MyoD was sufficient to convert fibroblasts into myoblasts (Davis et al., 1987). A more recent example involves the conversion of pancreatic exocrine cells into insulin-producing endocrine cells (Zhou et al., 2008). Taken together, these studies highlight the role of master transcriptional regulators in cell fate determination. Direct conversion between cell types also occurs spontaneously, in vivo, during early development, chronic injury and regenerative states (Sisakhtnezhad and Matin, 2012), though the underlying mechanisms responsible for lineage conversion remain poorly understood.
Steroidogenic Factor 1 (SF-1, also known as NR5A1 and AD4BP) is a master regulator essential for adrenal development (Luo et al., 1994). In the adult, SF-1 regulates steroidogenic gene expression and is required for adrenocortical homeostasis following unilateral adrenalectomy (Beuschlein et al., 2002). SF-1 can also convert embryonic and mesenchymal stem cells into steroid producing cells (Crawford et al., 1997; Sakai et al., 2008), suggesting that SF-1 plays a role in lineage conversion.
Using lineage-tracing studies we demonstrate that adrenocortical zonation occurs when differentiated zG cells undergo lineage conversion into zF cells during postnatal development, consistent with the model of centripetal migration. Our studies also show this mechanism is recapitulated during adrenal regeneration in older animals following dexamethasone suppression. Furthermore, zG maintenance is dependent on SF-1. Surprisingly, following loss of SF-1 in zG cells, a fully functional zF is formed and maintained long-term, demonstrating that a zG-independent mechanism also exists. Together these results establish that lineage conversion between terminally differentiated cells gives rise to adrenal zonation during development and regeneration and provide insight into the cellular and molecular mechanisms underlying tissue homeostasis.
Results
Generation and Validation of Aldosterone Synthase-Cre Mice
The steroidogenic enzyme aldosterone synthase (AS) is required for the final steps of aldosterone synthesis and its gene expression is restricted to terminally differentiated cells in the zG (Kim et al., 2009), making it a highly specific zG marker. To determine whether lineage conversion occurs within the adrenal and is responsible for cortical zonation via centripetal migration, we generated AS-Cre mice by introducing Cre recombinase into the genetic locus for AS (official gene symbol: Cyp11b2), using homologous recombination in ES cells (Figures 1B–C). Following breeding to germ-line heterozygosity, Cre expression was confirmed to be restricted to the zG by immunostaining (Figure 1D) as well as by gene expression analysis of zG and zF regions isolated by laser capture microdissection (Figures 1E, S1C). To ensure that disruption of the AS allele in AS+/Cre mice did not result in haploinsufficiency we measured AS levels by in situ hybridization, which showed no change in gene expression (Figure 1F). We also measured the production of aldosterone, the principal hormone produced by the zG, as well as plasma renin activity (PRA), an essential component of the renin-angiotensin system (RAS). Both levels were unchanged between wild type and AS+/Cre mice (Figure 1G), indicating normal feedback regulation. Therefore, AS+/Cre mice can be used to investigate the role of zG cells in adrenal zonation under normal physiologic conditions. In contrast, homozygous ASCre/Cre mice are AS null (Figures 1F, S1A) and show up regulation of the RAS (Figure 1G), in agreement with a previous report (Lee et al., 2005). Despite disruption of both AS alleles in ASCre/Cre mice, Cre expression remains restricted to the zG region (Figure 1D).
Zona Fasciculata cells arise from the Zona Glomerulosa during postnatal development and regeneration
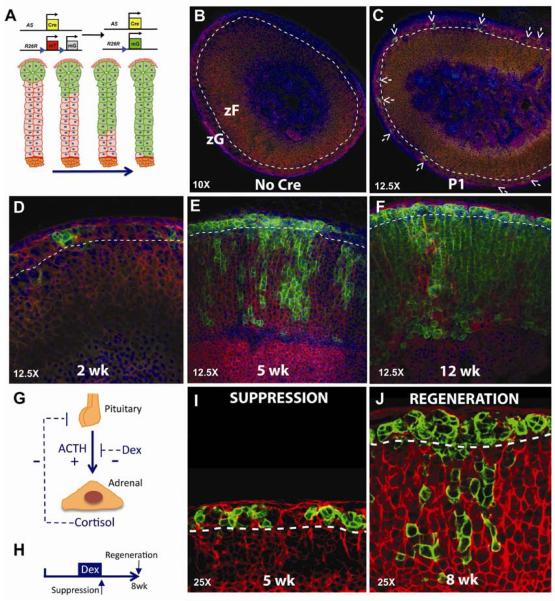
To investigate the lineage relationship between differentiated zG and zF cells and the origins of adrenal zonation we generated AS+/Cre ∷ R26R+/mTmG bigenic mice (Figure 2A) and performed a series of lineage-tracing experiments. As expected, given AS expression begins before birth (Wotus et al., 1998), confocal analysis of adrenal glands at day e16.5 (Figure S1B) and at postnatal day 1 (Figure 2B) revealed single GFP+ cells were restricted to the subcapsular region and not present in controls (Figure 2B). During subsequent weeks, the zG is progressively marked by GFP (Figure 2D), which over time arises in the zF in a radial fashion (Figure 2E) ultimately remodeling the entire zF by ~12 weeks of age (Figures 2F, S2A). Specificity of zF marking was confirmed by laser capture micro-dissection and co-immunofluorescent analyses (Figures 1E, S1C, S2B, C). In addition, the age-dependent increases in GFP+ cells as well as the presence of marked zF cells were validated using flow cytometry (Figure S1D). To investigate whether zG to zF lineage conversion also functions during adrenal regeneration, five-week old AS+/Cre ∷ R26R+/mTmG mice were treated with dexamethasone, a potent synthetic glucocorticoid, for 2 weeks to suppress the zF through negative feedback on the hypothalamus and pituitary gland and then allowed to recover for 3–5 weeks (Figure 2G, H). Dexamethasone treatment resulted in a marked reduction in the zF and loss of GFP+ cells, with no change in GFP+ zG cells (Figure 2I). Following dexamethasone withdrawal the zF expanded with a return of zG to zF lineage conversion (Figure 2J), indicating that regeneration recapitulates development in the adult. Together, these data establish that differentiated zG cells give rise to zF cells through a process of direct lineage conversion during postnatal adrenocortical zonation and regeneration, consistent with the model of centripetal migration.
Figure 2. zF cells arise from zG cells through lineage conversion.
(A) Schematic of genetic loci for AS+/Cre and the R26RmT/mG reporter along with the expected result for centripetal migration over time. (B–F) Confocal analysis of mTomato and mGFP expression from AS+/Cre ∷ R26RmT/mG mice from birth (P1) through 12 weeks. Arrows demarcate GFP+ cells. (G) Schematic showing positive (+) and negative (−) feedback regulation. (H) Mice received dexamethasone from 3–5 weeks of age and adrenals were analyzed at the peak of suppression (I) and following adrenal regeneration (J). See also Figure S2.
Accelerated lineage marking in AS null mice
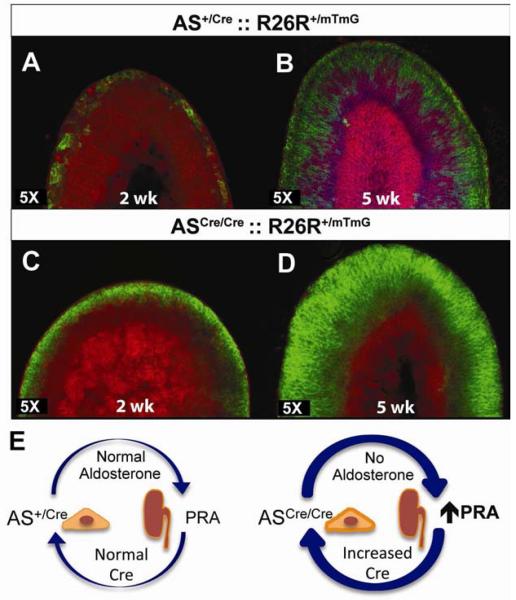
Our lineage-tracing studies indicate that complete marking of the zF takes up to 12 weeks (Figure 2), consistent with the notion that adrenocortical turnover in the adult is a relatively slow process. To investigate whether up regulation of the RAS, as occurs in AS null mice (Figure 1G), would alter lineage marking we generated ASCre/Cre ∷ R26R+/mTmG mice. Compared with AS+/Cre mice (Figure 3A), the zG from ASCre/Cre mice was fully labeled with GFP (Figure 3C) by 2 weeks of age, consistent with increased Cre expression and presumed increased recombination efficiency. Remarkably, at 5 weeks of postnatal development the entire zF was GFP+ (Figure 3D) in contrast to AS+/Cre mice (Figure 3B). In addition, the zG was disorganized, as previously described for AS-null mice (Lee et al., 2005), although Cre expression remained restricted to this region (Figures S3, 1D). These data indicate that lineage marking is accelerated in ASCre/Cre mice (Figure 3E) and further support lineage conversion via centripetal migration as the cellular mechanism for adrenocortical zonation.
Figure 3. Accelerated lineage marking in AS deficient mice.
(A–D) Confocal analysis of adrenals from AS+/Cre ∷ R26R+/mTmG (A, B) and ASCre/Cre ∷ R26R+/mTmG (C, D) mice at 2 (A,C) and 5 (B,D) weeks of age. Female adrenal that retains the X-Zone (bright pink) is shown in B. (E) Schematic showing aldosterone deficiency leads to secondary elevation of PRA levels through hormonal feedback. See also Figure S3.
SF-1, but not Dax-1, is required for zG maintenance and subsequent lineage conversion
To investigate the molecular mechanism(s) regulating lineage conversion in the adrenal and to identify genes involved in zonation we performed a series of zG-specific gene deletion studies. SF-1, a master transcriptional regulator of adrenal development, is expressed throughout the cortex in the adult. To investigate whether SF-1 plays a critical role in zG growth and maintenance, we generated AS+/Cre ∷ Sf-1fl/fl mice. Morphological analysis of young and old mice revealed the zG to be disorganized with pleomorphic nuclei, while the zF appeared normal (Figure 4F, G). Consistent with the observed morphology, gene expression studies for zG-specific steroidogenic enzymes (AS and Hsd3b6) showed levels to be decreased in AS+/Cre ∷ Sf-1fl/fl mice, raising the possibility of dedifferentiation, while zF-specific steroidogenic enzymes (Cyp11b1 and Hsd3b1) were unchanged (Figure S4A). To investigate the effect of zG-specific Sf-1 deletion on lineage conversion, we analyzed AS+/Cre ∷ R26R+/mTmG ∷ Sf-1fl/fl mice. Remarkably, deletion of Sf-1 within zG cells resulted in a complete absence of GFP expression within the zF (Figure 4H), even by 6 months of age (data not shown). In contrast, loss of only one copy of Sf-1 did not impact lineage conversion (data not shown). To confirm deletion of Sf-1 we performed co-immunofluorescent analysis for SF-1 and GFP using AS+ /Cre ∷ R26R+/mTmG ∷ Sf-1fl/fl mice. In contrast to control mice, in which ~90% of zG cells co-expressed SF-1 (Figures 4E, S4E), only ~20% of GFP-positive zG cells co-expressed SF-1 in these mice (Figures 4J, S4E–F). A similar decrease was observed for AS marking of zG cells (Figures 4I, S4D), further implying dedifferentiation of zG cells in the absence of SF-1. Analysis of zF cells in AS+ /Cre ∷ R26R+/mTmG ∷ Sf-1fl/fl mice revealed they were SF-1+ and GFP− (Figure 4J), indicating these cells were not derived from the SF-1 deficient zG cells. Since the ASCre/Cre model demonstrates increased efficiency of zG labeling we generated ASCre/Cre ∷ R26R+/mTmG ∷ Sf-1fl/fl mice to confirm that the presence of a normal zF was not simply due to inefficient Cre recombination. Consistent with the prior analysis, SF-1 deficient zG cells were unable to contribute to zF cells (Figure S4B,G). To assess the functional capacity of zF cells in mice harboring zG-specific SF-1 deficiency, we analyzed P450SCC expression, an essential steroidogenic enzyme, by immunohistochemistry (Figure 4K) as well as plasma corticosterone levels (Figure S4C), which revealed entirely normal zF functional activity. In contrast, functional analysis of the zG revealed decreased P450SCC expression and a state of compensated hypoaldosteronism, indicated by normal aldosterone levels and a nearly 3-fold increase in the levels of plasma renin (Figure 4L). The presence of a morphologically normal zF in these mice suggests that an alternate zG-independent pathway exists for zF formation and maintenance.
Figure 4. SF-1 is required for zG maintenance and subsequent lineage conversion into zF.
Histological and confocal analyses of 12 week old AS+/Cre ∷ Sf-1+/+ (A–C) and AS+/Cre ∷ Sf-1fl/fl (F–H) adrenal glands. Analysis of SF-1 expression in GFP+ zG cells revealed lack of co-expression in the majority of cells (J) compared with control zG cells (E). Despite deletion of Sf-1 in GFP+ zG cells, zF cells retained SF-1 expression (J) and showed normal levels of P450scc, in contrast to zG cells, which showed decreased P450scc (K). (L) Analysis of plasma aldosterone levels in 7 week old mice from both groups were unchanged, however, PRA levels were increased ~3 fold in mice with Sf-1-deficient zG cells (Mean±SEM,* p<0.05). (M) Schematic illustration showing zG-dependent and independent models of adrenal lineage development. See also Figure S4.
To gain insight into the mechanisms underlying SF-1's role in zG cell maintenance we assessed whether the SHH and Wnt signaling pathways were altered in the adrenals of zG-specific Sf-1 knock-out mice. These pathways were chosen because they have been implicated in both adrenocortical homeostasis and zonation. Analysis of gene expression showed changes in both SHH signaling (decreased Shh and increased Gli1) and Wnt signaling (decreased APCDD1, increased Sfrp2 and Sfrp3) (Figure S4H). These results further support a role for these pathways in adrenal maintenance and suggest that important changes in homeostatic feedback regulation occur following loss of SF-1 in zG cells.
To investigate whether DAX-1 (also known as NR0B1), a transcription factor important for adrenal development and maintenance (Scheys et al., 2011), plays a role in lineage conversion we generated AS+/Cre ∷ R26R+/mTmG ∷ Dax-1fl/Y mice. Compared with control mice, loss of DAX-1 had no obvious impact on the ability of zG cells to contribute to the zF (Figure S4I), even by 8 months of age, demonstrating that postnatal zonation and lineage conversion is not DAX-1 dependent.
Discussion
We have established that functional zonation of the adrenal results from direct lineage conversion between terminally differentiated cells during postnatal development and during regeneration, consistent with the model of centripetal migration. Following zG-specific deletion of Sf-1, zG cells can no longer contribute to zF cells, presumably due to an inability to differentiate, although remarkably a functional zF is maintained throughout adult life providing evidence for a zG-independent mechanism of lineage development (Figure 4M). These findings underscore the plasticity of this vital organ and raise the possibility that lineage conversion within other tissues, e.g. pancreatic alpha to beta cell conversion (Thorel et al., 2010), may occur in response to hormonal signals.
Cellular mechanisms underlying adrenocortical homeostasis
Tissue homeostasis during postnatal development is thought to result from either differentiation of tissue-specific progenitor/stem cells (e.g., intestinal epithelium) or from self-duplication of differentiated cells (e.g., pancreatic beta cells). In response to regenerative stress, however, additional mechanisms have been proposed to play a role, including cellular dedifferentiation, reprogramming and direct lineage conversion (Jopling et al., 2011; Sancho-Martinez et al., 2012). In the adult adrenal the mechanisms responsible for tissue maintenance are just beginning to be elucidated. For example, recent evidence demonstrates that undifferentiated progenitor cells present in the capsule and subcapsular region, marked by Gli1 and Shh expression, contribute to the multiple differentiated lineages (King et al., 2009). Down-regulation of Shh signaling, possibly in response to morphogen gradients, leads to the differentiation of these progenitors into zG cells. Our lineage-tracing data establish that differentiated zG cells then undergo direct lineage conversion and migrate to become morphologically distinct zF cells. Furthermore, co-immunostaining for CYP11B1 and CYP11B2 led to the identification of occasional double positive cells present at the zG-zF boundary (Figure S2D, arrows), suggesting that an intermediate transition state may exist. Interestingly, double positive cells were not detected in zG-specific Sf-1 knock out adrenals (Figure S2E) where the zG-zF boundary is more evident.
Molecular mechanisms underlying adrenal lineage conversion
To explore the molecular mechanisms underlying lineage conversion we performed zG-specific gene deletion of candidate genes involved in adrenal development. SF-1 is a master regulator of adrenocortical development with embryonic loss resulting in adrenal agenesis and perinatal death from adrenal insufficiency (Luo et al., 1994). During postnatal life, SF-1 is important for the homeostatic proliferation of subcapsular progenitor cells and mice haploinsufficient for SF-1 have smaller adrenals, show impaired growth potential and demonstrate decreased stress responsiveness (Beuschlein et al., 2002; Bland et al., 2000). Analysis of AS+/Cre ∷ Sf-1+/fl mice demonstrated normal lineage conversion, suggesting haploinsufficiency does not impact this step. Our data demonstrate that zG-specific deletion of Sf-1 results in morphological changes consistent with zG dedifferentiation, including a decrease in AS and P450SCC expression. Similar results were recently described using P450SCC-Cre to delete Sf-1 in steroidogenic tissues (Buaas et al., 2012). In addition, our results demonstrate that Shh expression is decreased and Gli1 is increased (Figure S4H) implying that feedback regulation exists between differentiated zG cells and both Shh and Gli1 expressing progenitor cells. Also, analysis of the Wnt signaling pathway revealed a decrease in Wnt-downstream target genes such as APCDD1, while the soluble Wnt inhibitors Sfrp3 and Sfrp2 were increased (Figure S4H) indicating a general repression of this pathway. Together, these findings support a role for both signaling pathways in zG homeostasis and subsequent lineage conversion.
Alternative mechanisms of adrenal homeostasis
Our data demonstrate that lineage conversion of zG into zF cells is the preferred pathway during postnatal adrenocortical remodeling. However, under conditions where the zG does not function normally (such as in the absence of SF-1) an alternate pathway for zF formation and maintenance appears to exist, underscoring the importance of continued glucocorticoid production for organismal survival. The cellular origin of this fully functional (and zG independent) zF raises important questions about the plasticity and regenerative strategies employed by this tissue (Figure 4M). It is possible that a facultative zF-specific progenitor population is activated to maintain the zF, consistent with the zonal model of lineage development, when the zG is not able to populate the zF. Alternatively, a progenitor/stem cell residing in the subcapsular region, whose fate is restricted to the zF lineage, may be able to transit through the zG en route to the zF without expressing zG-specific enzymes, such as AS. Another possibility involves putative X-zone progenitor cells. The X-zone is a region of unknown function in mice located between the zF and the medulla, which originates from the fetal zone before zG cells are first evident (Zubair et al., 2008). Our studies indicate that during normal postnatal development the X-zone is not maintained via lineage conversion of zG cells (Figure S4J). However, following zG-specific Sf-1 deletion, X-zone progenitors could contribute to zF cells given the capacity for X-zone regeneration following gonadectomy (Hershkovitz et al., 2007), deletion of Inhibin (Beuschlein et al., 2003), adrenal specific deletion of Prkar1a (Sahut-Barnola et al., 2010) and elimination of SF-1 sumoylation (Lee et al., 2011). Finally, it remains possible that prenatally derived zF cells are maintained through self-duplication.
Our finding that lineage conversion of terminally differentiated cells occurs during postnatal development and regeneration provides insight into the cellular mechanisms underlying tissue remodeling. Our results demonstrate both a preferred pathway, via lineage conversion of differentiated cells, and an alternate pathway for tissue remodeling and raise the possibility that other tissues, e.g., pancreatic islets, may use similar mechanisms for development and renewal.
Experimental Procedures
Mouse Experiments
Analysis Gene Expression
RNA was purified from adrenals cleaned of adherent fat and homogenized in TRI®Reagent as recommended (Sigma). For zone specific gene expression analysis, the zG and zF regions were isolated by laser capture microdissection (Arcturus) and RNA was isolated using the RNAqueous-Micro Kit (Ambion). Further processing of total RNA involved treatment with DNAse (Promega) and reverse transcription into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Gene expression analysis was performed by qPCR on 5–20 ng of cDNA as template with primers listed in Table 1 using Fast SYBR Green Master Mix (Roche) on an iQ5 Thermocycler (Bio-Rad). 18S was used as an internal control and data are expressed using the 2−ddCt method.
Tissue preparation and microscopy
Mouse adrenals were fixed in 4% paraformaldehyde for 1 hour at 4°C, embedded in 2% low melting SeaPlaque®Agarose (Lonza, Rockland, USA) and cut into 100 μM sections using a vibratome. Sections were stained with DAPI and mounted in Prolong Gold Mount Solution (Molecular Probes). Images were captured with a laser scanning confocal microscope LSM 700 (Zeiss) and processed using ZEN (Zeiss) or ImageJ software. Details of immunohistochemical staining and antibodies used are included in Supplemental Procedures.
Hormone measurements and treatments
Samples were collected using either rapid retro-orbital sampling (plasma) or metabolic cages (urine) followed by RIA. See Supplemental Procedures for details. Dexamethasone (Intensol 1mg/mL) was administered in drinking water at a concentration of 0.2 mg/dL.
Statistics
Student's t-Test was used for comparisons between groups of two and One-Way ANOVA and Bonferonni post-hoc analysis was used for comparisons between groups of three or more. Data are presented as Mean±SEM. Statistical significance was set at p<0.05.
Supplementary Material
Highlights
Adult homeostasis and regeneration both involve transfating of differentiated cells
Glomerulosa cell maintenance is dependent on the core transcriptional regulator SF1
An alternative pathway maintains zF cell production following loss of SF1 in zG cells
Acknowledgments
We thank C. Richmond and other members of the Breault laboratory, Q. Zhou and T. Serwold for helpful discussions and M. Thompson for help with ES cell work. We also thank J. L. Jameson, Y. Weinstein, E. Laufer, R. Auchus and the late K. Parker for their generous gifts of mouse strains and antibodies. This research was supported by T32 DK007699, PES Research Award and EFF Award (to BDF and DTB), R01 HL036977 and R01 HL089717 (to PQB) and PES Career Development Award, K08 DK 066305, R01 DK 084056 and HSCI Junior Faculty Award (to DTB). Also supported by the Timothy Murphy Fund and the IDDRC Center Grant P30 HD18655.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beuschlein F, Looyenga BD, Bleasdale SE, Mutch C, Bavers DL, Parlow AF, Nilson JH, Hammer GD. Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Molecular and cellular biology. 2003;23:3951–3964. doi: 10.1128/MCB.23.11.3951-3964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, Hammer GD. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143:3122–3135. doi: 10.1210/endo.143.8.8944. [DOI] [PubMed] [Google Scholar]
- Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci U S A. 2000;97:14488–14493. doi: 10.1073/pnas.97.26.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–4570. doi: 10.1242/dev.087247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Molecular and cellular biology. 1997;17:3997–4006. doi: 10.1128/mcb.17.7.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Deane HW, Greep RO. A morphological and histochemical study of the rat's adrenal cortex after hypoph ysectomy, with comments on the liver. Am J Anat. 1946;79:117–145. doi: 10.1002/aja.1000790104. [DOI] [PubMed] [Google Scholar]
- Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148:976–988. doi: 10.1210/en.2006-1100. [DOI] [PubMed] [Google Scholar]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, et al. In search of adrenocortical stem and progenitor cells. Endocrine reviews. 2009;30:241–263. doi: 10.1210/er.2008-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106:21185–21190. doi: 10.1073/pnas.0909471106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FY, Faivre EJ, Suzawa M, Lontok E, Ebert D, Cai F, Belsham DD, Ingraham HA. Eliminating SF-1 (NR5A1) sumoylation in vivo results in ectopic hedgehog signaling and disruption of endocrine development. Developmental cell. 2011;21:315–327. doi: 10.1016/j.devcel.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Makhanova N, Caron K, Lopez ML, Gomez RA, Smithies O, Kim HS. Homeostatic responses in the adrenal cortex to the absence of aldosterone in mice. Endocrinology. 2005;146:2650–2656. doi: 10.1210/en.2004-1102. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Sahut-Barnola I, de Joussineau C, Val P, Lambert-Langlais S, Damon C, Lefrancois-Martinez AM, Pointud JC, Marceau G, Sapin V, Tissier F, et al. Cushing's syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS genetics. 2010;6:e1000980. doi: 10.1371/journal.pgen.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Terami H, Suzuki S, Haga M, Nomoto K, Tsuchida N, Morohashi K, Saito N, Asada M, Hashimoto M, et al. Identification of NR5A1 (SF-1/AD4BP) gene expression modulators by large-scale gain and loss of function studies. The Journal of endocrinology. 2008;198:489–497. doi: 10.1677/JOE-08-0027. [DOI] [PubMed] [Google Scholar]
- Salmon TN, Zwemer RL. A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. The Anatomical Record. 1941;80:421–429. [Google Scholar]
- Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nature cell biology. 2012;14:892–899. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152:3430–3439. doi: 10.1210/en.2010-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisakhtnezhad S, Matin MM. Transdifferentiation: a cell and molecular reprogramming process. Cell Tissue Res. 2012;348:379–396. doi: 10.1007/s00441-012-1403-y. [DOI] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotus C, Levay-Young BK, Rogers LM, Gomez-Sanchez CE, Engeland WC. Development of adrenal zonation in fetal rats defined by expression of aldosterone synthase and 11beta-hydroxylase. Endocrinology. 1998;139:4397–4403. doi: 10.1210/endo.139.10.6230. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.