Abstract
Recent evidence has uncovered cross-regulation of mechanisms of cell engulfment by proteins of the autophagy pathway, in what is called LC3-Associated Phagocytosis, or LAP. By LAP, lysosome fusion to phagosomes and the degradation of engulfed extracellular cargo are facilitated by autophagy proteins that lipidate LC3 onto phagosome membranes. Here we discuss the contexts where LAP is known to occur by focusing on potential roles in tumorigenesis, including predicted consequences of LAP inhibition.
Keywords: autophagy, entosis, phagocytosis, cell death, macrophage, tumor, immune
Introduction
To maintain cellular homeostasis, the continual turnover of macromolecules that are aged, damaged, or no longer needed, must be balanced by new macromolecular synthesis. Eukaryotic cells have adapted a variety of strategies to maintain homeostasis, including protein- and vesicle-based degradation and quality control systems [1]. One important homeostatic pathway is ‘macroautophagy’ (or commonly ‘autophagy’), that targets aged or damaged organelles, protein aggregates, or long-lived proteins for degradation and recycling [2, 3]. Through autophagy, intracellular substrates are sequestered into ‘autophagosome’ vesicles that fuse with lysosomes, which harbor digestive enzymes that degrade internalized cargo. By this mechanism, the autophagy pathway allows eukaryotic cells to harness the degradative power of lysosomes to turnover bulk and long-lived intracellular substrates and recycle their building blocks for use in macromolecular synthesis. For cells experiencing nutrient starvation, autophagy also represents an important mechanism of nutrient recovery that can support cell survival by allowing for self-digestion [3].
Like intracellular substrates, extracellular substrates are also constantly turned over by eukaryotic cells through endocytic mechanisms that maintain cell signaling and metabolism, and regulate cell adhesion and plasma membrane homeostasis [4]. Bulk extracellular substrates, like dying cells and pathogenic organisms, must also be cleared and degraded in order to support metazoan development, tissue homeostasis, and immunity [5]. The turnover of these various extracellular substrates, like intracellular substrates targeted by autophagy, is controlled by lysosomes that fuse with endocytic vesicles or vacuoles to degrade and recycle internalized cargo [6, 7].
While endocytosis and autophagy were once considered largely separate pathways, recent evidence has shown extensive collaboration between them in mammalian cells, including the identification of an endocytic origin for vesicles utilized for autophagosome biogenesis [8], fusion between autophagosomes and endosomes [9], and co-regulation of endocytic trafficking and autophagy by Beclin1-Vps34 protein complexes [10, 11]. It was also recently discovered that autophagy proteins control the degradation of engulfed dying cells or pathogenic organisms, in an autophagosome-independent manner, by facilitating lysosome fusion to phagosomes, in what is called LC3-Associated Phagocytosis, or LAP [12]. As links between autophagy gene dysfunction and a variety of human diseases are emerging, (for example Crohn’s disease is linked to mutations in Atg16L [13], neurodegeneration is associated with dysfunctional autophagy [14], and various cancers are associated with loss of function of autophagy genes [15]), it is important to consider that loss of the non-autophagy functions of autophagy proteins, including endocytic functions such as LAP, may also contribute to disease onset or progression. Here we consider several potential roles of autophagy proteins in cancer that are based on the recent discovery of the mechanism of LAP and the contexts where this process has been identified (see Figure).
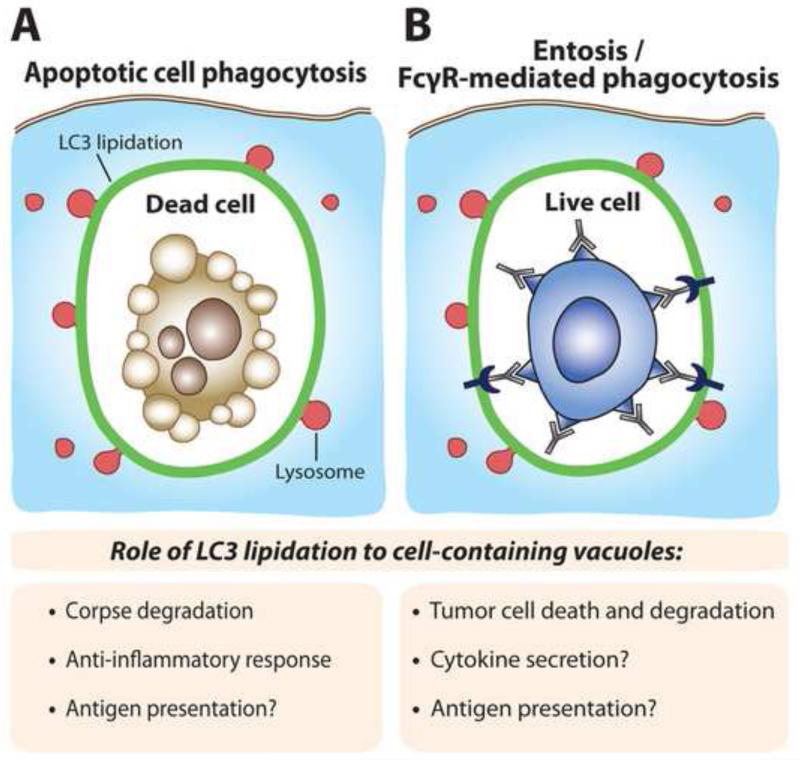
Figure. Predicted consequences of LC3 lipidation to different cell-containing vacuoles.
(A) The lipidation of LC3 (green) to phagosomes (referred to as ‘LAP’) facilitates lysosome fusion (red). For phagosomes harboring dead or dying cells, LC3 lipidation allows for efficient corpses degradation, and is required for an anti-inflammatory response mediated by secreted cytokines. For immunogenic forms of cell death, LC3 lipidation may also facilitate antigen presentation. (B) Vacuoles harboring viable cells engulfed by the cell cannibalism mechanism entosis also exhibit LC3 lipidation that facilitates lysosome fusion and the death of engulfed cells. FcγR-mediated phagocytosis of live tumor cells could also induce LC3 lipidation, which could facilitate tumor cell killing, modulate cytokine secretion or influence antigen presentation. A viable engulfed tumor cell with antibodies bound to its surface is depicted.
LC3-Associated Phagocytosis (LAP)
LAP was first identified by the recruitment of the autophagy protein microtubule-associated protein 1 light chain 3 (LC3) to phagosomes harboring engulfed microorganisms in macrophages [12]. LC3 and its homologs are ubiquitin-like molecules that are lipidated to phosphatidylethanolamine (PE) at sites of autophagosome formation, where they control elongation of autophagosome precursor membranes, or phagophores, as well as autophagosome closure, potentially by mediating vesicle-vesicle fusion events [16, 17]. LC3 proteins are conjugated to PE following a series of reactions mimicking a ubiquitination cascade, where Atg7 and Atg3 act as E1 and E2 enzymes, respectively, and the E3-like enzyme, Atg5-12:16L, is a multimeric complex involving a second ubiquitin-like molecule, Atg12. Atg12 is conjugated to Atg5 by the E1 and E2-like activities of Atg7 and Atg3, respectively, and the Atg5-12 conjugate forms a complex with Atg16L that functions as the E3 for the LC3-PE conjugation [18]. Acting upstream of these in the canonical autophagy pathway are several additional protein complexes, including two kinase complexes, one involving Beclin1 and the lipid kinase Vps34, that produces phosphatidylinositol-3-phosphate (PI(3)P) at sites of autophagosome formation, and another complex involving the Ulk1/2 kinase that is required for most forms of autophagy, potentially by facilitating the recruitment of Vps34 [18, 19].
As autophagosomes form by elongation of phagophore membrane vesicles, upon closure they have a characteristic double-membrane structure that is identifiable by transmission electron microscopy [18]. While some internalized pathogenic organisms have been found enwrapped inside of double-membrane autophagosomes [20], the engulfment by macrophages of yeast, beads coated with lipopolysaccharide (LPS) or toll-like receptor (TLR) ligands, or Escherichia coli, was associated with the acquisition of lipidated LC3 at phagosomes in a manner independent of the appearance of double-membranes structures, suggesting that LC3 was lipidated directly onto phagosome membranes [12]. The autophagy proteins Atg5 and Atg7 were required for LC3 lipidation onto phagosomes, phagosome acidification, and killing of live engulfed yeast, demonstrating a role for autophagy proteins in phagosome maturation and lysosome fusion that is distinct from the formation of double-membrane autophagosomes that mediate autophagy [12]. This autophagy-independent function of autophagy proteins was termed LC3-Associated Phagocytosis, or LAP [21].
Since the initial discovery of LAP occurring with agonists of TLR signaling [12], this non-canonical function of autophagy proteins has been shown to occur in a variety of contexts including the phagocytosis of apoptotic and necrotic cells [22, 23], FcγR-mediated engulfment of IgG-opsonized substrates [24], macropinocytic uptake of fluid-filled vacuoles [23], and the ingestion and killing of live epithelial cells by the engulfment program ‘entosis’ [23]. The variety of vacuole types that are targeted by LAP-like activity suggests that this non-canonical function of autophagy lipidation machinery may be a more general mechanism to facilitate lysosome fusion in cells than originally thought [25]. Consistent with this idea, even the fusion of lysosomes to specialized plasma membrane domains in osteoclasts that are actively engaging in resorbing bone, called ruffled borders, involves LAP-like activity of autophagy proteins that appears to facilitate secretory lysosome fusion [26]. Lipidated LC3 at these membranes may promote lysosome fusion by facilitating membrane-membrane fusion directly or by recruiting other interacting proteins, such as Rab GAPs (GTPase-Activating Proteins), to the membrane [16, 25, 27-29]. One common feature that links these LAP-associated cell systems is their occurrence in cells cultured under nutrient-replete conditions, where signaling from the mTORC1 kinase, that represses canonical autophagy, is active. Accordingly, the autophagy preinitiation complex, composed of the Ulk1/2 kinase and its binding partners, Fip200, Atg13, and Atg101, should be inactive under conditions where LAP occurs. Indeed, LAP occurring during the phagocytosis of dead cells was shown to be independent of the Ulk1 kinase that is required for most forms of starvation-induced autophagy [22]. Similarly, LAP-like activity occurring during entosis was shown to be independent of Fip200 that, like Ulk1, is required for starvation-induced autophagy [23]. Thus, LAP is a non-canonical program involving select autophagy machinery that facilitates lysosome fusion to single-membrane vacuoles or compartments in a manner that is morphologically and genetically distinct from canonical autophagy [25].
While many studies have now implicated autophagy proteins in tumor-suppressing or tumor-promoting roles, it is important to consider how roles of LAP, which occurs in many cell types, including macrophages, dendritic cells, neutrophils, epithelial cells, and breast tumor cells, could affect tumor growth or therapy in addition to canonical autophagy. Although the abovementioned studies have not investigated the direct role of LAP in phagocytes in the context of the tumor microenvironment, it can be postulated that LAP could influence tumor growth or the response of tumors to therapy. Below we consider the types of engulfments associated with LAP and discuss potential roles for LAP in cancer.
LAP-associated engulfment mechanisms that participate in cancer development or therapy
1. Phagocytosis of apoptotic tumor cells
As tumors grow, high rates of tumor cell proliferation are accompanied by cell death that occurs continuously within a tumor cell population. High rates of cell death measured by apoptotic index in fact correlate with high mitotic index, as well as with aggressive tumor characteristics such as high tumor grade and aneuploidy, and overall unfavorable disease outcome [30, 31]. Tumor cell death is also induced by anti-cancer treatments, such as irradiation or the administration of pharmacological agents. The tumor environment therefore contains, at all stages, a population of dying or dead cells that serve as ready substrates for professional phagocytes that are known to be recruited to tumors. Tumor-associated macrophages (TAMs) and other immune cells are an important fraction of the tumor microenvironment of solid tumors, with T cells representing the major immune type, and TAMs constituting approximately 20% of the total immune infiltrate in breast cancers [32]. The presence of TAMs, like apoptotic index, correlates with poor prognosis for a number of carcinomas such as breast, prostate, and other types [33, 34].
TAMs derive from circulating monocytes that recruit to the tumor microenvironment in response to a variety of chemoattractants released by tumor cells, such as macrophage colony stimulating factor (M-CSF or CSF-1), CCL2, CCL5, and others [35-37]. Once recruited, monocytes differentiate into TAMs that generally polarize toward an “alternatively activated”, or M2 state, as a result of signaling by a number of anti-inflammatory factors released from tumor cells or other immune cells in the tumor microenvironment, including IL-4, IL-10, IL-13, and TGFβ, that collaborate to polarize TAMs [35, 38, 39]. As M2-polarized cells, TAMs engage in functions normally associated with wound healing, including enhancing angiogenesis, promoting cell proliferation and invasion, remodeling matrix, and suppressing adaptive immunity, which, while beneficial for healing wounds, for cancers these functions promote disease progression [35, 39]. Interestingly, the continual rate of apoptosis that accompanies tumor progression may contribute to maintaining TAMs in an M2 state, as a direct result of anti-inflammatory molecules released from apoptotic cells [40-42], or as a consequence of phagocytic clearance, as macrophages ingesting apoptotic cells are known to secrete immunosuppressive cytokines that promote M2 polarization such as IL-10 and TGFβ[43]. Engulfment of apoptotic cells also suppresses the immunostimulatory capacity of dendritic cells by reducing their secretion of pro-inflammatory IL-12 [44]. Of note, some macrophage-polarizing cytokines have been shown to influence autophagy. For example, the M1-inducing cytokine IFN-γ, as well as LPS, induces autophagy [45-48], whereas M2-polarizing cytokines, IL-4, IL-10, and IL-13 inhibit autophagy through PI3-kinase/mTORC1 signaling or through transcription [49, 50]. While the modulation of mTORC1 signaling is not predicted to influence LAP, whether cytokine signaling, or more generally macrophage polarization, affects the ability of cells to perform LAP has not been studied.
The suppression of immune responses toward tumors by apoptotic cells could potentially be exploited therapeutically. For example, injection of mice with apoptotic cells coated with annexin V, which blocks the ‘eat-me’ signal phosphatidylserine (PS), provided immunity toward subsequent challenge to tumor growth that correlated with increased pro-inflammatory cytokine, TNF-α and IL-1β, and decreased anti-inflammatory cytokine, TGF-β, release by macrophages [51]. Similarly, the blockade of MFG-E8, a bridging molecule between PS of apoptotic cells and αvβ3 integrin on phagocytes, increased pro-inflammatory cytokine, IL-12, IL-23, and TNF-α, and reduced anti-inflammatory cytokine, IL-10, secretion by dendritic cells, contributing to an immunogenic tumor microenvironment and potentiating cytotoxic therapy-induced tumor regression in experimental models of colon carcinoma and melanoma [52]. These studies suggest potential therapeutic approaches that take advantage of the suppression of immune function by PS-dependent phagocytosis, by blocking PS-dependent signals in combination with apoptosis-inducing chemotherapeutic agents.
The engagement of apoptotic cell phagosomes by autophagy proteins during LAP facilitates the degradation of engulfed apoptotic corpses, potentially by enhancing lysosome fusion [22, 23, 25]. Like blocking PS-dependent phagocytosis, disrupting the degradation of engulfed cell components has also been shown to be pro-inflammatory; for example, macrophages in DNase-II knockout mice that ingest apoptotic cells are deficient for degrading nuclear DNA and activate innate immunity by producing TNF-α [53]. Also, loss of the lysosomal degradative enzyme lysosomal acid lipase [54], or the cholesterol trafficking protein Niemann Pick C1 [55], leads to secretion of pro-inflammatory cytokines, including IFN-β, IL-6, and IL-8, suggesting that lysosomal buildup of undigested material may be pro-inflammatory in some contexts [53]. Consistent with this idea, disrupting the degradation of engulfed cells by autophagy protein deficiency that impairs LAP also leads to the secretion of pro-inflammatory cytokines by macrophages, including IL-6 and IL-1β, and reduced secretion of the anti-inflammatory cytokines TGFβ and IL-10 [22]. Importantly, loss of the autophagy preinitiation complex protein Ulk1 had no effect on cytokine profiles, consistent with a model that LAP, rather than canonical autophagy, plays a role in the anti-inflammatory response of macrophages to ingesting apoptotic cells [22]. Autophagy proteins participating in LAP within immune cells may therefore play a role in maintaining the anti-inflammatory nature of the tumor microenvironment. Conceivably, like blocking PS-dependent phagocytosis, treatment of cancers with therapies that inhibit LAP could contribute to shifting the tumor microenvironment toward pro-inflammatory, or tumoricidal, as a result of disrupting LAP and corpse degradation within phagocytes. This may be particularly beneficial with combination therapies that induce high levels of apoptosis. Loss of Beclin1, like Atg5 and Atg7, also impairs LAP, suggesting that strategies targeting Beclin1 or Vps34 activity may be effective at inhibiting LAP and blocking corpse degradation, and shifting the cytokine profile of the tumor microenvironment.
2. FcγR-dependent phagocytosis
In recent years, monoclonal antibody-based cancer therapies have shown efficacy as targeted approaches that inhibit cell-surface oncoproteins or bind cancer-associated biomarkers. For example, treatment with the monoclonal antibody Herceptin, or Trastuzumab, that targets ErbB2 or Her2, increases overall patient survival and reduces recurrence rates when used in combination with conventional chemotherapy against Her2-overexpressing breast cancers [56]. Like Trastuzumab, a number of monoclonal antibodies are now used therapeutically including Cetuximab (Erbitux) that inhbits EGFR, and at least 12 others that are FDA-approved for the treatment of cancer [57]. While many therapeutic antibodies inhibit cell-signaling events critical for tumor cell survival or proliferation by antigen-specific binding of the antibody Fab region, the Fc region of IgG antibodies also participates in efficacy by acting in an immunostimulatory manner. The Fc region can engage the complement cascade, leading to pore formation by the membrane attack complex and death of the targeted tumor cell. And the Fc region can also activate Fcγ receptors on various immune cells leading to either Antibody-Dependent Cell Cytotoxicity (ADCC), mediated by granzyme B and perforin, or Antibody-Dependent Cell-mediated Phagocytosis (ADCP), involving engulfment and killing of antibody-opsonized tumor cells by macrophages [58, 59]. Whereas M1 macrophages express high levels of FcγRs and are competent for ADCP, some M2-differentiated macrophages have been shown to be deficient in mediating ADCP due to loss of expression of FcγRs, particularly when differentiated in the presence of IL-4 [60]. But TAMs isolated from breast tumors, or monocytes differentiated into macrophages with tumor cell-conditioned medium or M-CSF, have been shown to maintain expression of FcγRs while acquiring M2 characteristics such as the ability to facilitate of tumor cell invasion [60]. Such M2 macrophages retain competence for mediating ADCP in culture, and the tumor growth and metastasis of breast tumor cells in vivo can be blocked by administration of a tumor cell-binding IgG antibody in a manner consistent with tumoricidal function of macrophages [60]. These data suggest that macrophage-mediated ADCP can be a primary mechanism of tumoricidal activity mediated by TAMs after administration of therapeutic monoclonal antibodies. Interestingly, ADCP may also occur downstream of administration of antibodies designed to block the ‘eat-me’ signal PS, which might be predicted to inhibit apoptotic cell engulfment like Annexin V, but instead can promote engulfment through the Fc region and enable the clearance of PS-exposed cells in a pro-rather than anti-inflammatory manner [61]. Treatment of tumor-bearing mice with an anti-PS antibody has been shown to inhibit tumor growth and to induce pro-inflammatory cytokine release [61].
The opsinization of latex beads with IgG has been shown to recruit LC3 to phagosomes in macrophages and neutrophils, suggesting that engagement of FcyRs during engulfment is sufficient to activate autophagy protein machinery for LAP [62]. Similarly, the engulfment of red blood cells opsonized with IgG, or DNA-IgG complexes, induces LAP in macrophages and dendritic cells in a manner dependent on FcγR expression, which is consistent with a critical role of FcγRs in LAP [24]. These studies suggest that LAP may generally occur when engulfment is driven by FcγR engagement. If so, autophagy proteins would be predicted to play a role in ADCP by facilitating the death and degradation of engulfed cells, potentially by facilitating lysosome fusion to phagosomes. The defects in lysosome fusion and phagosome acidification observed upon LAP inhibition are indeed associated with the rescue of live engulfed microorganisms that would otherwise be killed by lysosomal enzymes [12], suggesting that in some cases a failure to mature phagosome membranes in a manner that engages autophagy machinery could completely rescue engulfed cells. Therefore, it seems plausible that tumoricidal ADCP may be inhibited by therapeutic approaches that combine inhibitors of LAP with monoclonal antibody-based therapies.
3. Phagocytosis of live tumor cells induced by CD47 blockade
Beyond ADCP that potentially underlies one aspect of the tumoricidal properties of therapeutic IgG antibodies, the manipulation of ‘eat-me’ or ‘don’t-eat-me’ signals on live cells has emerged as a potential therapeutic strategy to more directly harness the potential for phagocytes to engulf and kill tumor cells [63]. It is becoming clear that the targeting of live cells by phagocytes occurs in vivo as part of normal physiology. For example, microglia, the resident macrophages of the brain and spinal cord, when activated, can phagocytose neurons that have increased PS exposure [64]. Moreover the clearance of aged erythrocytes by macrophages in normal individuals, discussed further below, involves phagocytosis-induced death, which is thought to occur at least in part due to downregulation of the ‘don’t-eat-me’ signal CD47 on erythrocytes. These modes of cell death where phagocytes ingest and kill live cells were recently proposed as a major form of physiological programmed cell death called “phagoptosis” [65]. The engulfment of live cells can also be induced experimentally in vivo, for example the introduction of an exogenous PS analog on the surface of red blood cells induced their clearance from the peripheral circulation of syngeneic mice [66]. And the induction of DNA damage was also shown to induce αvβ3 integrin expression on the surface of tumor cells, which triggered tumor cell recognition and phagocytic uptake by dendritic cells [67]. These results demonstrate that even viable cells, if possessing the appropriate signals, can be engulfed by phagocytes, and this may represent a major mechanism of programmed cell death in vivo.
Although tumor cells in some contexts express ‘eat-me’ signals that can induce their phagocytic uptake [67], live tumor cells generally avoid being phagocytosed, and in fact many tumor cells have been found to upregulate cell surface proteins that actively block phagocytosis by acting as ‘don’t-eat-me’ signals. The best characterized of these is CD47, an immunoglobulin-like membrane protein, that can bind to SIRPα on phagocytes and signal to block phagocytosis [68]. CD47 binding to SIRPα leads to activation of tyrosine phosphatases that inhibit the accumulation of myosin at the site of the phagocytic synapse [69]. The CD47-SIRPα interaction inhibits phagocytosis and clearance of healthy erythrocytes and platelets by macrophages in vivo, as erythrocytes from CD47−/− mice are rapidly cleared from circulation in a macrophage-dependent manner [70].
Recent studies have taken advantage of the CD47-SIRPα ‘don’t-eat-me’ signaling axis to design potential therapeutic strategies to eliminate tumor cells by macrophage engulfment. Tumor cells of different types consistently express high levels of CD47 on their surface, and blocking CD47-SIRPα signaling with monoclonal anti-CD47 antibodies, or anti-SIRPα antibodies, has shown therapeutic efficacy for a variety of cancers such as acute myeloid leukemia, acute lymphoblastic leukemia, non-Hodgkin’s lymphoma, multiple myeloma, leiomyosarcoma, and also solid tumors [71-77]. In addition to inhibiting tumor growth, anti-CD47 antibody treatment can block metastasis in some studies [73, 75], suggesting that the targeting of live tumor cells for macrophage-dependent phagoptosis by blocking ‘don’t-eat-me’ signaling holds therapeutic promise. Blockade of CD47-SIRPα signaling also enhances the anti-tumor activity of therapeutic monoclonal antibodies such as Trastuzumab, as blocking SIRPα signaling can facilitate tumor cell killing in response to FcγR engagement. In support of the potential clinical significance of this interaction, the pathological response to Trastuzumab in breast cancer patients has been shown to correlate inversely with CD47 expression level [78]. But while some studies have shown that the induction of live cell-directed phagocytosis is induced by treatment with antibodies that disrupt the CD47-SIRPα interaction, and not by control anti-CD47 antibodies that are permissive for signaling or by isotype control or anti-CD45 control antibodies [72-75, 77], a recent study showed that anti-CD47 F(ab′)2 fragments, which do not engage FcγRs yet block CD47, had no tumoricidal effect independent of treatment with Trastuzumab, suggesting that at least part of the observed anti-tumor effects of CD47 blocking antibodies may also be due to FcγR engagement that drives a tumoricidal immune response, in addition to blockade of CD47-SIRPα signaling [78].
Whether autophagy proteins could play a role in tumor cell-directed phagoptosis is not known. As part of the observed tumoricidal effects of CD47 blockade may involve FcγR engagement by intact anti-CD47 IgG antibodies, one could speculate that the phagocytic ingestion of live tumor cells induced by these treatments may engage autophagy proteins to participate in phagocytosis through LAP. If so, a potential delay in lysosome fusion or tumor cell killing activity by inhibition of autophagy proteins, in therapeutic settings where phagoptosis is argued to occur, may affect therapeutic efficacy. Whether LAP is involved in the ingestion of live tumor cells by these methods, and whether LAP inhibition could rescue engulfed cells from death, are important topics for future studies. Conceivably, even a delay in the degradation of live engulfed cells by LAP inhibition could alter the tumor microenvironment by changing the cytokine milieu, as occurs with a failure of LAP during the phagocytosis of apoptotic cells.
4. Entosis and Homotypic Cell Cannibalism (HoCC)
While LAP mediated by immune cells in the tumor microenvironment may participate in tumor growth or therapeutic responses indirectly, the engulfment of tumor cells by their neighbors, commonly referred to as tumor cell cannibalism, has also been shown to engage autophagy machinery in a LAP-like activity that induces tumor cell death [23]. In this manner, autophagy proteins may participate in tumor growth directly through a LAP-like mechanism.
Tumor cell cannibalism has been reported for many years in breast, colon, lung, and liver carcinoma, melanoma, and other tumor types, but only recently have potential mechanisms underlying this activity been investigated [79]. One mechanism proposed to account for the appearance of cannibalistic, or “cell-in-cell” structures in human tumors is called entosis [80]. By entosis, tumor cells utilize the machinery of cell-cell adhesion to ingest and kill their neighbors [80]. Cells ingested by entosis are engulfed alive, and in fact they participate actively in their own engulfment [80]. But the majority of engulfed cells eventually undergo cell death, by a non-cell-autonomous mechanism, as they are killed by the neighboring cells into which they internalize. Internalized cells are killed by lysosomal enzymes following a LAP-like mechanism that induces lysosome fusion to entotic vacuoles [23]. Like LAP described in macrophages, the lipidation of LC3 to entotic vacuoles is not associated with double-membrane structures and lipidation occurs in a manner dependent on Atg5 and Atg7, but independent of the autophagy preinitiation complex protein Fip200 [23].
Entosis may be a tumor-suppressive mechanism, like other forms of cell death, as high rates of entosis and entotic cell death are associated with the inhibition of transformed growth [23, 80]. In this context, autophagy proteins involved in LAP could suppress tumor growth. Conversely, entosis has also been shown to induce ploidy changes that are known to promote tumor progression, due to the disruption of cell division by live engulfed cells [81]. The cannibalistic cell structures resembling those formed by entosis occur most frequently in high-grade, aggressive breast tumors, suggesting that entosis may promote tumor progression in the long-term [81, 82]. How LAP-like activity in this context could relate to the potential of entosis to promote tumor progression is unclear, as it is the formation of cell-in-cell structures, rather than the induction of cell death, that promotes division failure. Conceivably, an inability to kill internalized cells could increase the persistence of individual cell-in-cell structures and increase the chance for division failure. In this manner, autophagy proteins involved in entotic cell death could act tumor-suppressively by inducing cell death and also by suppressing the ability of entosis to promote ploidy changes. The role of LAP-like activity in this context awaits further demonstration of the tumor-suppressive or -promoting roles of entosis.
Another mechanism recently proposed to underlie the formation of cell-in-cell structures is called homotypic cell cannibalism, or HoCC [83]. Cannibalistic cell structures resembling those formed by HoCC occur in human pancreatic tumors and their frequency correlates with increased metastasis-free survival, suggesting that HoCC could inhibit tumor progression [83]. Cell cannibalism by HoCC is proposed to occur by a phagocytosis-like mechanism induced by TGFβ signaling and involving a number of phagocytosis-related genes including Cdc42. The knockdown of the autophagy protein Atg5 was actually shown to increase the frequency of cannibalism by HoCC in cultured cells, but whether the loss of Atg5 affects the fate of engulfed cells, which appear to die by apoptosis in this system, was not examined [83]. Conceivably, an increased frequency of cannibalism upon Atg5 knockdown could reflect either an increased rate of engulfment, as proposed, or also longer persistence of individual cell-in-cell structures within a cell population. Nevertheless, whereas autophagy proteins have been shown to facilitate entotic cell death by acting in a LAP-like manner, no such role for autophagy machinery has yet been identified for HoCC.
Further considerations and concluding remarks
In this review, we have discussed several potential implications of the recently identified LC3-Associated-Phagocytosis mechanism, or LAP, in cancer development and therapy. The various cellular contexts where autophagy machinery has been found to facilitate lysosome fusion to single-membrane vacuoles, in an apparently autophagosome-independent manner, allow for several predictions to be made regarding how this mechanism could influence cancer. Perhaps the most straightforward prediction concerns the homotypic cell cannibalism mechanism entosis, where, if entosis participates in cancers by inducing tumor cell death, then core autophagy proteins such as Atg5 and Atg7, Beclin1, and Vps34, are predicted to act as tumor suppressors through this non-canonical mechanism. It is far less straightforward to predict how the modification of phagosomes harboring dying or dead cells by autophagy proteins may influence tumor progression. While LAP of apoptotic cells may participate in anti-inflammatory cytokine secretion in the tumor microenvironment, the cytokines potentially affected by LAP act in vivo within highly complex and heterogeneous tumor microenvironments, where multiple cell types function in a delicate balance, making the actual role of LAP, and the effects of LAP inhibition, difficult to predict. In addition to controlling the degradation of engulfed apoptotic cells, LAP was also recently implicated in facilitating antigen presentation from engulfed material onto MHC class II, in the context of TLR signaling [84, 85], which may also have implications for immunogenic forms of cell death that are associated with the release or exposure of danger-associated molecular patterns (DAMPs) that activate TLRs [86, 87]. Cytokine secretion may also be affected by LAP in this context, where the LAP-dependent fusion of lysosomes to phagosomes harboring DAMPs could engage TLR signaling pathways that control pro-inflammatory cytokine production, as shown recently during antibody-DNA complex-dependent stimulation of TLR9 [24]. Autophagy proteins likely also affect engulfment mechanisms by a variety of LAP-independent mechanisms; for example, the canonical autophagy pathway was previously implicated in exposure of the ‘eat-me’ signal PS by acting within apoptotic cells to generate ATP [88], and Beclin1 and Atg7 were recently reported to regulate apoptotic corpse engulfment by supporting the activity of Rac1 that is required for phagocytosis [89, 90]. These functions of autophagy proteins are also predicted to contribute to cell engulfment mechanisms that participate in cancer development or therapeutic response.
While some autophagy genes act as tumor suppressors to inhibit tumor formation, there is accumulating evidence that autophagy is also required for tumor progression or therapy resistance after lesions have initiated [91, 92]. The inhibition of autophagy has therefore emerged as a potential therapeutic strategy that may inhibit tumor progression or metastasis, or increase cell death in combination therapies [93, 94]. It is reasonable to assume that most autophagy-inhibiting therapies, at least in the near future, will block canonical autophagy and LAP simultaneously, and inhibition of the canonical functions of autophagy proteins will have profound influence over the effects on tumor growth that are observed upon administration of autophagy pathway inhibitors. The only currently available autophagy pathway inhibitor approved for clinical use is hydroxychloroquine, which does not block autophagy induction but rather inhibits lysosome function. While a block in LAP might be predicted to be pro-inflammatory, chloroquine is a known anti-inflammatory compound, potentially due to stabilization of the glucocorticoid receptor that is normally degraded by lysosomes [95]. Potential therapies aimed at targeting the lipid kinase Vps34 or its binding partner Beclin1 will also inhibit canonical autophagy and LAP, and will likely have other pleiotropic effects including multiple downstream consequences on phagosome maturation and endocytic trafficking [96]. Therefore, while the hypothetical scenarios considered here suggest that LAP may influence tumor progression, and that LAP inhibition may affect therapeutic responses, such effects will occur in concert with many other cellular changes resulting from disruption of the autophagic or endocytic pathways. Another kinase that is critical to the initiation of most, but not all [97], forms of autophagy is Ulk1/2. Potential inhibitors of this kinase would not be predicted to inhibit LAP, which may allow for more direct assessment of the effects of autophagy inhibition on tumor progression or therapeutic intervention. Moreover, Ulk1/2 inhibition may block canonical autophagy while leaving several potentially tumoricidal LAP-related processes, including entosis and perhaps ADCP, intact.
Acknowledgements
We would like to thank members of the Overholtzer laboratory for discussions, and Dr. Oliver Florey for reading the manuscript. This work was financially supported by a grant from the National Cancer Institute (CA154649; M.O.), the Louis V. Gerstner, Jr. Young Investigators Fund (M.O.), and the Benjamin Friedman Research Fund (M.O.). These sponsors had no involvement in the writing of the manuscript or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement:
The authors declare that there are no conflicts of interest.
References
- [1].Wong E, Cuervo AM. Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol. 2010;2:a006734. doi: 10.1101/cshperspect.a006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hughes T, Rusten TE. Origin and evolution of self-consumption: autophagy. Adv Exp Med Biol. 2007;607:111–8. doi: 10.1007/978-0-387-74021-8_9. [DOI] [PubMed] [Google Scholar]
- [3].Mizushima N. Autophagy in protein and organelle turnover. Cold Spring Harb Symp Quant Biol. 2011;76:397–402. doi: 10.1101/sqb.2011.76.011023. [DOI] [PubMed] [Google Scholar]
- [4].Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20:256–75. doi: 10.1038/cr.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189:1059–70. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fairn GD, Grinstein S. How nascent phagosomes mature to become phagolysosomes. Trends Immunol. 2012;33:397–405. doi: 10.1016/j.it.2012.03.003. [DOI] [PubMed] [Google Scholar]
- [8].Ravikumar B, Moreau K, Jahreiss L, Puri C, Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol. 2010;12:747–57. doi: 10.1038/ncb2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun. 1988;151:40–7. doi: 10.1016/0006-291x(88)90556-6. [DOI] [PubMed] [Google Scholar]
- [10].Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–62. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim HJ, Zhong Q, Sheng ZH, Yoshimori T, Liang C, Jung JU. Beclin-1-interacting autophagy protein Atg14L targets the SNARE-associated protein Snapin to coordinate endocytic trafficking. J Cell Sci. 2012;125:4740–50. doi: 10.1242/jcs.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- [13].Naser SA, Arce M, Khaja A, Fernandez M, Naser N, Elwasila S, et al. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J Gastroenterol. 2012;18:412–24. doi: 10.3748/wjg.v18.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–11. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–62. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- [16].Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29:1792–802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weidberg H, Shpilka T, Shvets E, Abada A, Shimron F, Elazar Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev Cell. 2011;20:444–54. doi: 10.1016/j.devcel.2011.02.006. [DOI] [PubMed] [Google Scholar]
- [18].Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–68. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Curr Biol. 2012;22:R540–5. doi: 10.1016/j.cub.2012.06.001. [DOI] [PubMed] [Google Scholar]
- [21].Sanjuan MA, Milasta S, Green DR. Toll-like receptor signaling in the lysosomal pathways. Immunol Rev. 2009;227:203–20. doi: 10.1111/j.1600-065X.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- [22].Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011;108:17396–401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Florey O, Kim SE, Sandoval CP, Haynes CM, Overholtzer M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat Cell Biol. 2011;13:1335–43. doi: 10.1038/ncb2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, et al. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–97. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 2012;22:374–80. doi: 10.1016/j.tcb.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. 2011;21:966–74. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Itoh T, Kanno E, Uemura T, Waguri S, Fukuda M. OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J Cell Biol. 2011;192:839–53. doi: 10.1083/jcb.201008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Popovic D, Akutsu M, Novak I, Harper JW, Behrends C, Dikic I. Rab GTPase-activating proteins in autophagy: regulation of endocytic and autophagy pathways by direct binding to human ATG8 modifiers. Mol Cell Biol. 2012;32:1733–44. doi: 10.1128/MCB.06717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lipponen P, Aaltomaa S, Kosma VM, Syrjanen K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer. 1994;30A:2068–73. doi: 10.1016/0959-8049(94)00342-3. [DOI] [PubMed] [Google Scholar]
- [31].Liu S, Edgerton SM, Moore DH, 2nd, Thor AD. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 2001;7:1716–23. [PubMed] [Google Scholar]
- [32].Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett. 2013;332:3–10. doi: 10.1016/j.canlet.2013.01.024. [DOI] [PubMed] [Google Scholar]
- [34].Nonomura N, Takayama H, Nakayama M, Nakai Y, Kawashima A, Mukai M, et al. Infiltration of tumour-associated macrophages in prostate biopsy specimens is predictive of disease progression after hormonal therapy for prostate cancer. BJU Int. 2011;107:1918–22. doi: 10.1111/j.1464-410X.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- [35].Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80:705–13. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- [36].DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–85. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- [38].Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–66. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 20122012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weigert A, Tzieply N, von Knethen A, Johann AM, Schmidt H, Geisslinger G, et al. Tumor cell apoptosis polarizes macrophages role of sphingosine-1-phosphate. Mol Biol Cell. 2007;18:3810–9. doi: 10.1091/mbc.E06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tomimori Y, Ikawa Y, Oyaizu N. Ultraviolet-irradiated apoptotic lymphocytes produce interleukin-10 by themselves. Immunol Lett. 2000;71:49–54. doi: 10.1016/s0165-2478(99)00163-7. [DOI] [PubMed] [Google Scholar]
- [42].Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–25. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- [43].Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen X, Doffek K, Sugg SL, Shilyansky J. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–94. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- [45].Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–44. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Waltz P, Carchman EH, Young AC, Rao J, Rosengart MR, Kaczorowski D, et al. Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy. 2011;7:315–20. doi: 10.4161/auto.7.3.14044. [DOI] [PubMed] [Google Scholar]
- [47].Li P, Du Q, Cao Z, Guo Z, Evankovich J, Yan W, et al. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1) Cancer Lett. 2012;314:213–22. doi: 10.1016/j.canlet.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Matsuzawa T, Kim BH, Shenoy AR, Kamitani S, Miyake M, Macmicking JD. IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol. 2012;189:813–8. doi: 10.4049/jimmunol.1102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–17. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- [50].Park HJ, Lee SJ, Kim SH, Han J, Bae J, Kim SJ, et al. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway. Mol Immunol. 2011;48:720–7. doi: 10.1016/j.molimm.2010.10.020. [DOI] [PubMed] [Google Scholar]
- [51].Bondanza A, Zimmermann VS, Rovere-Querini P, Turnay J, Dumitriu IE, Stach CM, et al. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med. 2004;200:1157–65. doi: 10.1084/jem.20040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jinushi M, Sato M, Kanamoto A, Itoh A, Nagai S, Koyasu S, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206:1317–26. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–30. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- [54].Lian X, Yan C, Yang L, Xu Y, Du H. Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L801–7. doi: 10.1152/ajplung.00335.2003. [DOI] [PubMed] [Google Scholar]
- [55].Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, et al. Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci. 2007;27:1879–91. doi: 10.1523/JNEUROSCI.5282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Del Mastro L, Lambertini M, Bighin C, Levaggi A, D’Alonzo A, Giraudi S, et al. Trastuzumab as first-line therapy in HER2-positive metastatic breast cancer patients. Expert Rev Anticancer Ther. 2012;12:1391–405. doi: 10.1586/era.12.107. [DOI] [PubMed] [Google Scholar]
- [57].Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- [58].Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- [59].Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Grugan KD, McCabe FL, Kinder M, Greenplate AR, Harman BC, Ekert JE, et al. Tumor-associated macrophages promote invasion while retaining Fc-dependent anti-tumor function. J Immunol. 2012;189:5457–66. doi: 10.4049/jimmunol.1201889. [DOI] [PubMed] [Google Scholar]
- [61].Beck AW, Luster TA, Miller AF, Holloway SE, Conner CR, Barnett CC, et al. Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer. 2006;118:2639–43. doi: 10.1002/ijc.21684. [DOI] [PubMed] [Google Scholar]
- [62].Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, et al. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci U S A. 2009;106:6226–31. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fadeel B. Programmed cell clearance. Cell Mol Life Sci. 2003;60:2575–85. doi: 10.1007/s00018-003-3145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol. 2011;186:4973–83. doi: 10.4049/jimmunol.1003600. [DOI] [PubMed] [Google Scholar]
- [65].Brown GC, Neher JJ. Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci. 2012;37:325–32. doi: 10.1016/j.tibs.2012.05.002. [DOI] [PubMed] [Google Scholar]
- [66].Schroit AJ, Madsen JW, Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem. 1985;260:5131–8. [PubMed] [Google Scholar]
- [67].Jinushi M, Chiba S, Baghdadi M, Kinoshita I, Dosaka-Akita H, Ito K, et al. ATM-mediated DNA damage signals mediate immune escape through integrin-alphavbeta3-dependent mechanisms. Cancer Res. 2012;72:56–65. doi: 10.1158/0008-5472.CAN-11-2028. [DOI] [PubMed] [Google Scholar]
- [68].Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- [69].Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- [71].Kikuchi Y, Uno S, Kinoshita Y, Yoshimura Y, Iida S, Wakahara Y, et al. Apoptosis inducing bivalent single-chain antibody fragments against CD47 showed antitumor potency for multiple myeloma. Leuk Res. 2005;29:445–50. doi: 10.1016/j.leukres.2004.09.005. [DOI] [PubMed] [Google Scholar]
- [72].Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr., et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118:4890–901. doi: 10.1182/blood-2011-02-338020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci U S A. 2012;109:6656–61. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–84. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, et al. CD47-signal regulatory protein-alpha (SIRPalpha) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A. 2011;108:18342–7. doi: 10.1073/pnas.1106550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Overholtzer M, Brugge JS. The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol. 2008;9:796–809. doi: 10.1038/nrm2504. [DOI] [PubMed] [Google Scholar]
- [80].Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- [81].Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, et al. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–30. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Krajcovic M, Overholtzer M. Mechanisms of ploidy increase in human cancers: a new role for cell cannibalism. Cancer Res. 2012;72:1596–601. doi: 10.1158/0008-5472.CAN-11-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cano CE, Sandi MJ, Hamidi T, Calvo EL, Turrini O, Bartholin L, et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol Med. 2012;4:964–79. doi: 10.1002/emmm.201201255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ma J, Becker C, Lowell CA, Underhill DM. Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J Biol Chem. 2012;287:34149–56. doi: 10.1074/jbc.M112.382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, et al. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–39. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- [87].Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- [88].Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–46. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- [89].Konishi A, Arakawa S, Yue Z, Shimizu S. Involvement of Beclin 1 in engulfment of apoptotic cells. J Biol Chem. 2012;287:13919–29. doi: 10.1074/jbc.M112.348375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Teplova I, Lozy F, Price S, Singh S, Barnard N, Cardiff RD, et al. ATG proteins mediate efferocytosis and suppress inflammation in mammary involution. Autophagy. 2013:9. doi: 10.4161/auto.23164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Debnath J. The multifaceted roles of autophagy in tumors-implications for breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:173–87. doi: 10.1007/s10911-011-9223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sosa MS, Bragado P, Debnath J, Aguirre-Ghiso JA. Regulation of tumor cell dormancy by tissue microenvironments and autophagy. Adv Exp Med Biol. 2013;734:73–89. doi: 10.1007/978-1-4614-1445-2_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–66. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30:671–8. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].He Y, Xu Y, Zhang C, Gao X, Dykema KJ, Martin KR, et al. Identification of a lysosomal pathway that modulates glucocorticoid signaling and the inflammatory response. Sci Signal. 2011;4:ra44. doi: 10.1126/scisignal.2001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Miller S, Oleksy A, Perisic O, Williams RL. Finding a fitting shoe for Cinderella: searching for an autophagy inhibitor. Autophagy. 2010;6:805–7. doi: 10.4161/auto.6.6.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–6. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]