Abstract
Propionibacterium acnes (P. acnes) is a Gram-positive bacterium strongly associated with acne infection. While many antimicrobial agents have been used in clinic to treat acne infection by targeting P. acnes, these existing anti-acne agents usually produce considerable side effects. Herein, we report the development and evaluation of liposomal lauric acids (LipoLA) as a new, effective and safe therapeutic agent for the treatment of acne infection. By incorporating lauric acids into the lipid bilayer of liposomes, we observed that the resulting LipoLA readily fused with bacterial membranes, causing effective killing of P. acnes by disrupting bacterial membrane structures. Using a mouse ear model, we demonstrated that the bactericidal property of LipoLA against P. acne was well preserved at physiological conditions. Topically applying LipoLA in a gel form onto the infectious sites led to eradication of P. acnes bacteria in vivo. Further skin toxicity studies showed that LipoLA did not induce acute toxicity to normal mouse skin tissues, while benzoyl peroxide and salicylic acid, the two most popular over-the-counter acne medications, generated moderate to severe skin irritation within 24 h. These results suggest that LipoLA hold a high therapeutic potential for the treatment of acne infection and other P. acnes related diseases.
Keywords: Bacterial infection, Propionibacterium acnes, Antimicrobial delivery, Nanoparticle, Free fatty acid
1. Introduction
Acne infection is a common skin disorder that affects up to 80% of individuals in their lives.[1] Acne develops as a result of blockages in follicles due to the over-production of sebum by sebaceous glands. The accumulation of sebum triggers the overgrowth of Propionibacterium acnes (P. acnes), a Gram-positive anaerobic bacterium strongly associated with acne infection.[2] Upon the rupture of follicle walls, the host immune cells react with P. acnes, leading to inflammatory acne.[2–4] Severely inflamed acne lesions can cause hyper-pigmentation and permanent skin scarring, which can be a source of embarrassment, stress, and low self-esteem among young people, therefore affecting their mental health and psychological development.[5] Many antimicrobial agents have been developed for acne treatment including adapalene, tazarotene, erythromycin, clinfamycin, benzoyl peroxide (BPO) and other antibiotics.[6–8] While these existing anti-acne agents are effective, they usually produce considerable side effects. For instance, BPO has been one of the most frequently used epicutaneous medications for acne treatment; however it produces a high incidence of erythema, scaling, burning and hair bleaching.[9, 10] Oral antibiotics, although highly effective, often pose the risk of harming the intestinal microflora and creating antibiotic-resistant P. acnes. For instance, the use of isotretinoin, a vitamin A-derived retinoid prescribed for systemic treatment of severe acne, is strictly regulated and not applicable for most acne patients owing to it strong teratogenicity.[11] Clearly, new anti-acne agents with both excellent therapeutic efficacy and negligible adverse side effects are highly desirable.
Free fatty acids (FFAs) have been known to be responsible for part of the self-antimicrobial disinfecting activity of the human skin against microbial colonization, and their bactericidal properties have been extensively investigated.[12–15] These lipid-like molecules are ubiquitous and natural, which are expected to be less harmful than the synthetic antimicrobial molecules. Various FFAs have been disclosed to possess potent antimicrobial activities against a diverse range of bacteria.[16] For example, oleic acid has been reported to inhibit the growth of Staphylococcus aureus in a mouse model,[17, 18] and lenolenic acid is able to overcome the antibiotic resistance developed by various clinically isolated strains of Helicobacter pylori.[19, 20] While the antimicrobial working mechanism of FFAs is yet fully understood, accumulating evidence suggests that they are effective in disrupting bacterial membranes and increasing membrane permeability.[16] Due to the low water solubility of FFAs, they usually need to be formulated together with other excipient materials to prepare stable and water-soluble formulations.[21] Among various FFA formulations, liposomal FFAs represent a robust and popular platform.[17, 19, 22] The amphiphilic nature of FFAs allows them to be readily incorporated into the lipid bilayer of liposomes with a high loading efficiency. The resulting liposomal formulation not only enhances the water-solubility of FFAs but also protects them from degradation in physiological conditions. Moreover, the liposomal formulation facilitates the interaction of FFAs with bacterial membranes and thus improves their antimicrobial effectiveness.[23]
We have previously reported that lauric acids had strong antibacterial activity against P. acnes without obvious cytotoxicity against SZ95 human sebocytes.[24] We have also shown that the bactericidal activity of lauric acids against P. acnes was well preserved after loading them to a liposome formulation.[22] Herein, we systematically evaluate the in vivo therapeutic efficacy and toxicity profile of liposomal lauric acids (LipoLA) for the treatment of acne infection caused by P. acnes bacteria. Using a mouse ear model, we test the bactericidal property of LipoLA against P. acnes through two administration routes, intradermal injection and topical application. Skin toxicity of LipoLA is thoroughly evaluated in comparison with two most popular over-the-counter acne care drugs, BPO and salicylic acid. The findings from this study provide more clinically related assessments of LipoLA as a new, effective, and safe anti-acne medication (Figure 1A–D).
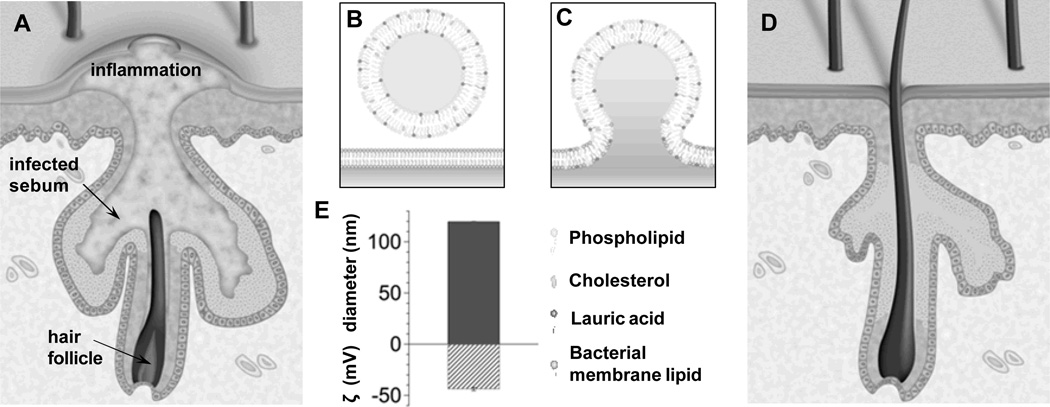
Figure 1.
Schematics of using liposomal lauric acids (LipoLA) to treat acne infection caused by Propionibacterium acnes (P. acnes) bacteria. (A) Anatomic illustration of skin with inflammatory acne. (B) Structure and composition of LipoLA consisting of phospholipid, cholesterol and lauric acid. (C) Hypothesized mechanism of action; LipoLA fusing into bacterial membranes. (D) Anatomic illustration of skin after LipoLA treatment. (E) Hydrodynamic size (diameter, nm) and surface charge (zeta potential, mV) of the prepared LipoLA.
2. Results and discussion
2.1. Interaction between LipoLA and P. acnes bacteria
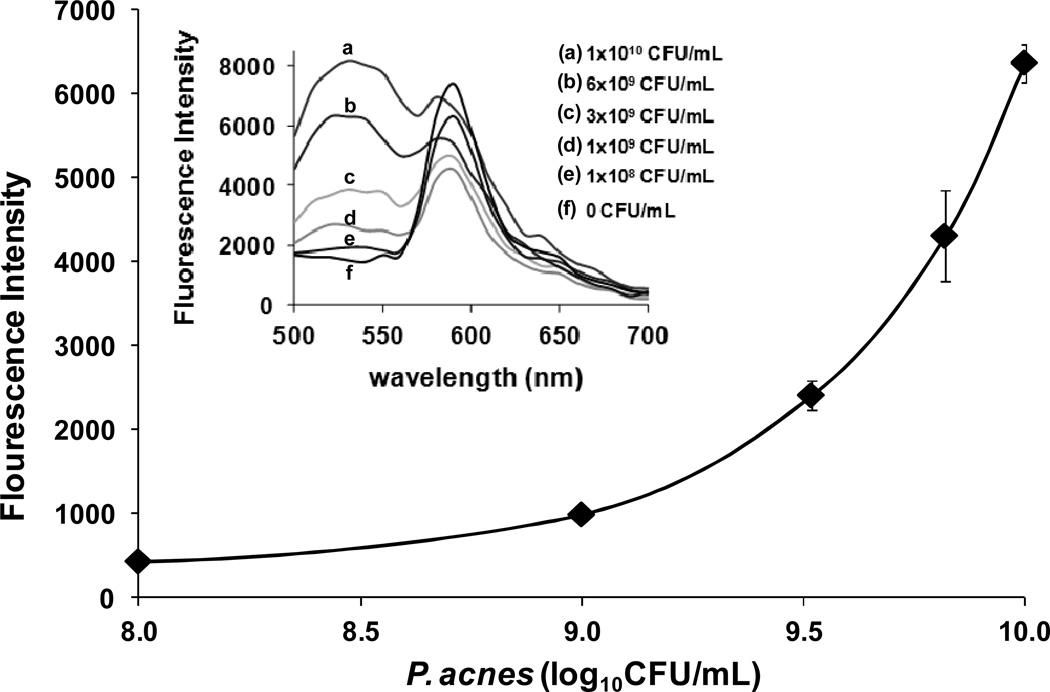
The building materials of LipoLA are all from natural sources, including hydrogenated L-a-phosphatidylcholine (EggPC) from egg yolk, cholesterol from animal fat, and lauric acid from coconut milk. With a weight ratio of 5:1:4, the mixture of EggPC, cholesterol and lauric acid were prepared to form LipoLA through a common vesicle extrusion method. The resulting LipoLA have an average diameter of 119.9 ± 0.3 nm, a polydispersity index of 0.12, and an average surface zeta potential of −43.8 ± 1.5 mV, measured by DLS (Figure 1E). The interaction between the resulting LipoLA and P. acnes bacteria were studied by FRET technique. We first incorporated 0.1 mol% of fluorescent donor C6NBD (excitation/emission = 470/520 nm) and 0.5 mol% of fluorescent acceptor DMPE-RhB (excitation/emission = 550/580 nm) into the lipid bilayer of LipoLA to prepare FRET-pair labeled LipoLA. At the used molar concentrations of the donor and the acceptor, the fluorescence emission from the donor was maximally quenched by the acceptor through a nonradiative long-range dipole-dipole coupling mechanism. By mixing the FRET-pair labeled LipoLA (0.5 mg/mL) with P. acnes at different bacterial concentrations ranging from 1×108 to 1×1010 CFU/mL for 30 min, we observed increasing emission intensity of C6NBD at 520 nm when the samples were excited at the wavelength of 470 nm (Figure 2). The rise in the emission peak of the fluorescent donor indicates the fusion of LipoLA with bacterial membranes, which causes an increase in spatial separation between the two dyes and the fluorescence recovery of the donor. Note that the emission of DMPE-RhB at 580 nm was not selected for comparison because DMPE-RhB dye could be excited by not only the FRET from C6NBD but also the excitation wavelength at 470 nm, making it difficult to make an accurate comparison.
Figure 2.
FRET measurements of the fusion between LipoLA and P. acnes bacteria. LipoLA were labeled with both a fluorescent donor (C6NBD) and a fluorescent acceptor (DMPE-RhB) at a proper molar ratio that the acceptor maximally quenched the fluorescence emission from the donor. The FRET-pair labeled LipoLA were then incubated with P. acnes at various bacterial concentrations. After removing the excess LipoLA, all samples were excited at 470 nm and the fluorescence emission spectra were recorded (Inset). A rise in emission intensity of C6NBD at 520 nm was observed, indicating the occurrence of spatial separation of C6NBD and DMPE-RhB due to the fusion between LipoLA and bacterial membranes.
2.2. In vitro antimicrobial activity and bacterial morphology
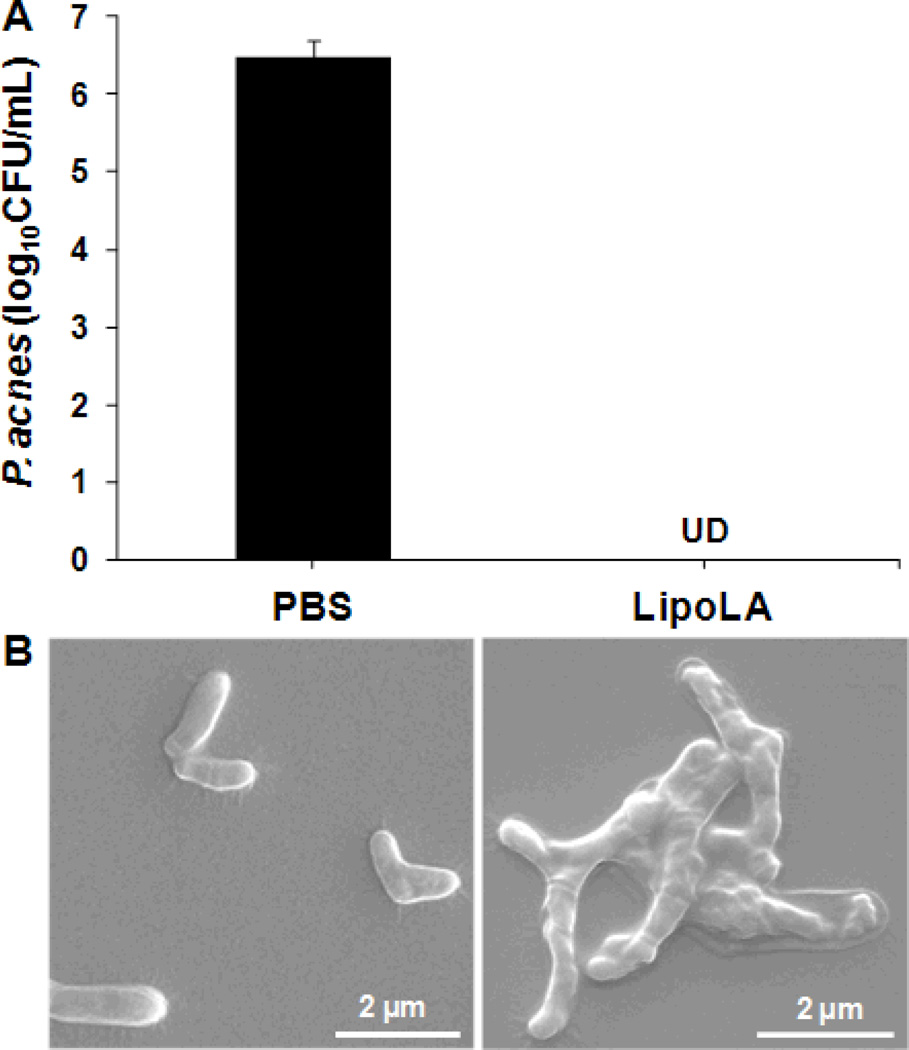
To test the antimicrobial activity of LipoLA against P. acnes in vitro, LipoLA (2 mg/mL) were incubated with P. acnes (1×107 CFU/mL) at 37°C for 5 h under anaerobic condition. The results showed that LipoLA completely killed P. acnes without detectable CFU formed on RCM agar plates while the amount of P. acnes incubated with PBS buffer (negative control) was 6.5×106 CFU/mL (Figure 3A). After quantifying the in vitro antimicrobial activity of LipoLA against P. acnes bacteria, we next investigated the effect of LipoLA on the morphology of the bacteria using SEM. P. acnes bacteria were incubated with LipoLA for 5 h, fixed with 2% glutaraldehyde, and then observed by SEM. As shown in Figure 3B, the SEM micrograph of untreated sample (i.e., incubated with PBS buffer) showed that P. acnes has a regular rod-like structure with a smooth surface and fimbriae around the organism. In contrast, bacteria treated with LipoLA exhibit clear abnormality; the bacterial surface was irregularly deformed and shrunk with the absence of the fimbriae. These results indicate that the interaction of P. acnes bacteria with LipoLA disrupts the bacterial membrane structure, suggesting a possible mechanism by which LipoLA kill the bacteria. This finding is consistent with previous report of structural change of Clostridium perfringens, another anaerobic Gram-positive bacterium, upon the treatment of FFAs.[25]
Figure 3.
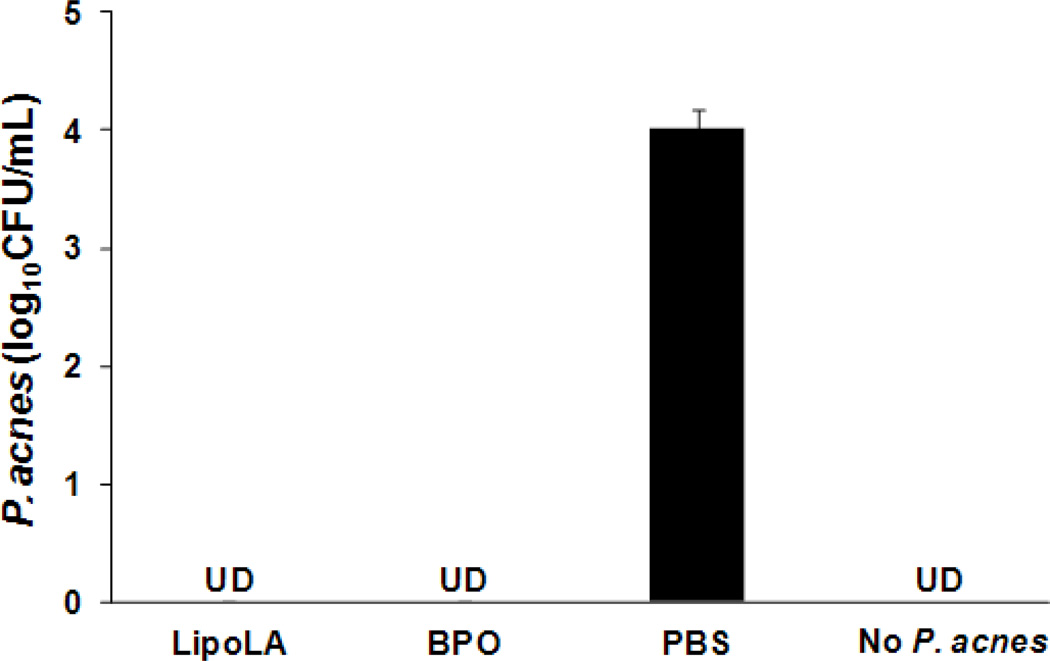
In vitro antimicrobial activity of LipoLA against P. acnes and morphology of P. acnes after LipoLA treatment. LipoLA were incubated with P. acnes for 5 h under anaerobic condition. (A) P. acnes bacteria were diluted and spotted on agar plates, and the bacterial number was quantified. LipoLA completely killed the bacteria. PBS was used as a negative control. Data represents mean ± SD of three independent experiments. UD: undetectable. (B) Following LipoLA or PBS treatment, the bacteria were fixed and imaged by scanning electron microscope (SEM). A destruction of bacterial membranes and an absence of fimbriae were observed with the LipoLA treated sample (right panel) as compared to the PBS treated sample (left panel).
2.3. In vivo antimicrobial activity
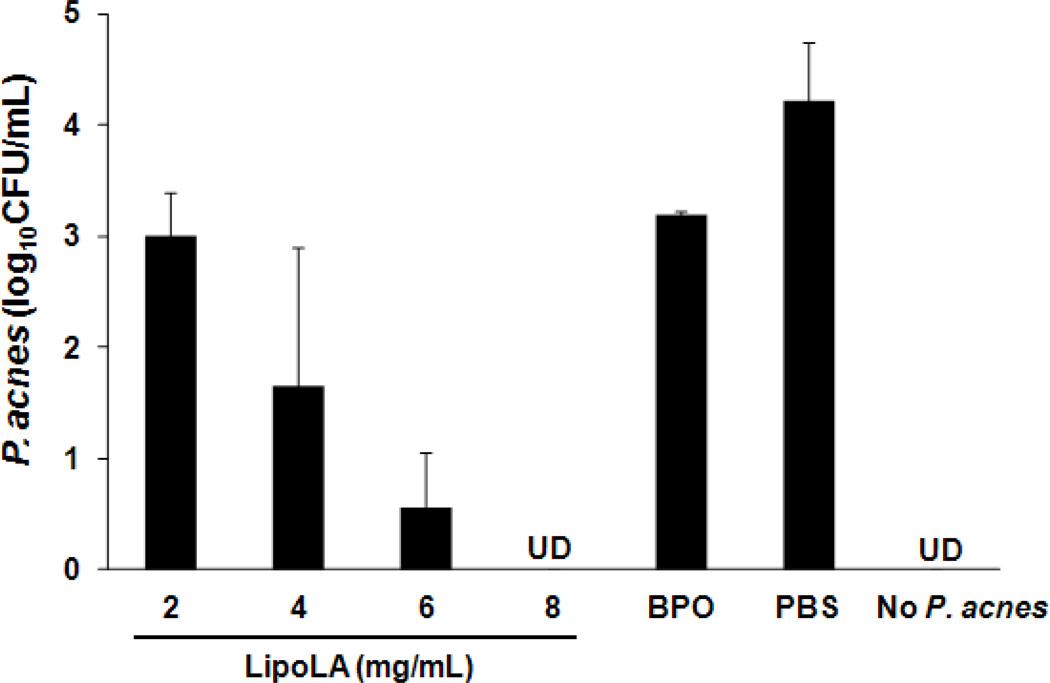
Next we tested the in vivo antimicrobial activity of LipoLA against P. acnes using an ICR mouse ear model via intradermal injection. Mouse ear was selected for this study because its confined structure can retain all inoculated bacteria at the injection area. To evaluate the antimicrobial activity of LipoLA against P. acnes in physiological environment, LipoLA (2, 4, 6, or 8 mg/mL) were mixed with P. acnes (108 CFU/mL) right before the injection. Subsequently, 10 µL of the mixture solution was intradermally injected into the mouse ear. After 24 h of injection, the ear was collected using an 8-mm biopsy punch and homogenized to quantify the remaining amount of P. acnes. In this study, BPO at a concentration of 16 mg/mL in 5% dimethyl sulfoxide (DMSO) premixed with P. acnes (108 CFU/mL) was used as a positive control, P. acnes in PBS was used as a negative control, and the injection of PBS without P. acnes was used to demonstrate that there was no contamination from other bacteria during the experiments. As shown in Figure 4, significant reductions of P. acnes were observed when treated with LipoLA. The measured CFU of P. acnes were 1.0×103, 4.4×101, 3.6, and 0 CFU/mL for 2, 4, 6, and 8 mg/mL of LipoLA, respectively. In contrast, the detected bacterial number for the negative control group (treated with PBS buffer) was 1.7×104 CFU/mL and the positive control (treated with BPO) reduced the bacterial number to 1.6×103 CFU/mL. The control group of mice injected with only PBS (without P. acnes) had no detectable bacteria, confirming no bacterial contamination during the experiments. These results confirm that LipoLA can effectively kill P. acnes bacteria in physiological environment such as in the dermis.
Figure 4.
In vivo antimicrobial activity of LipoLA against P. acnes through intradermal injection. Different concentrations of LipoLA (2, 4, 6, or 8 mg/mL) were mixed with P. acnes and immediately injected into mouse ears (n=6 per group), followed with bacteria quantification at 24 h. BPO (16 mg/mL) was used as a positive control. PBS and none P. acnes inoculation were served as negative controls. Data represents mean ± SD of three independent experiments. UD: undetectable.
2.4. In vivo therapeutic efficacy
To further evaluate the potential of LipoLA as an effective anti-acne agent, we next developed a topical model of P. acnes-induced inflamed acne infection and tested the efficacy of LipoLA against P. acnes. In the case of inflammatory acne lesion in human, chemotactic factors are secreted from P. acnes, which attract inflammatory cells into the sebaceous follicle. These cells further release other inflammatory factors, such as lysosomal enzymes, proteases, and reactive oxygen that damage follicle wall, leading to inflammatory lesions.[26] Our previous study showed that by inoculating P. acnes into the ear of ICR mouse, macrophages were attracted into the injection site, similar to P. acnes-induced inflammatory acne lesions in human.[24] Herein, 1×106 CFU of P. acnes were inoculated on the ear of the mouse that has pre-scratched wound with an area of around 10 mm2 to generate P. acnes-inducted inflammation. Following the inoculation, 2 mg/mL of LipoLA prepared in a water-based gel was applied daily onto the wound for 2 days. The gel was composed of hydroxyethyl cellulose (7.0 wt%), glycerin (5.6 wt%), and polyethylene glycol 400 (1.8 wt%). As shown in Figure 5, the 2 mg/mL LipoLA in a gel form completely killed the inoculated P. acnes bacteria. The LipoLA gel shows a comparable efficacy to the positive control of 10 wt% BPO cream purchased from a local drug store. The detected bacterial number for the negative control group (treated with PBS buffer) was 1.0×104 CFU/mL, reduced from the initially inoculated number of 1.0×106 CFU/mL. This reduction might be attributed to the clearance from the host’s immune system and tissue loss during the grinding process. The control group of mice without P. acnes inoculation had no detectable bacteria, indicating that the study groups were free from contamination of normal flora. Commercialized BPO cream has been considered as the standard treatment of mild to moderate acne due to its effective antimicrobial and oxidizing activity since the 1960s.[27] However, due to BPO’s common side effects, such as dryness, irritation, and burning, it has resulted in lowered patient compliance and unsuccessful treatment. The fact that LipoLA gel could achieve a comparable efficacy to BPO cream suggests that LipoLA may become a favorable alternative for acne treatment.
Figure 5.
In vivo therapeutic efficacy of LipoLA for the treatment of P. acnes infection through topical administration. P. acnes (106 CFU) were inoculated onto a wound generated on a mouse ear (n=6 per group), followed with topical application of LipoLA gel daily for 2 days successively. At 72 h, the ear was collected, and the remaining amount of P. acnes was quantified. Commercial BPO cream from a drug store was used as a positive control. PBS gel and none P. acnes inoculation were served as negative controls. Data represents mean ± SD of three independent experiments. UD: undetectable.
2.5 Toxicity of LipoLA to normal skin
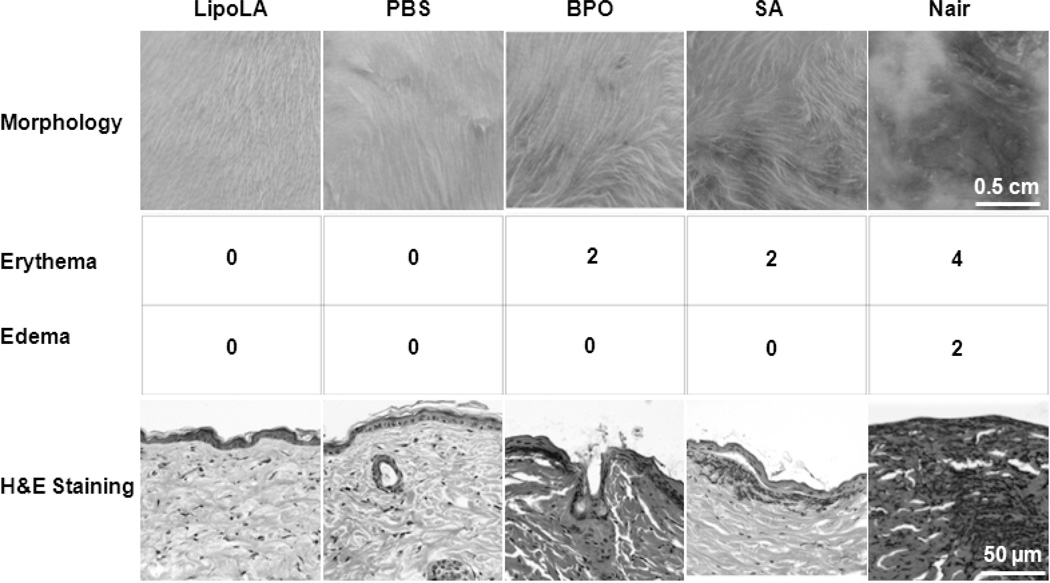
The in vivo toxicity profile of LipoLA was evaluated by examining the changes of skin morphology after topically applying LipoLA gel onto the back skin of ICR mice. The toxicity of LipoLA was compared with 10 wt% BPO, 2 wt% salicylic acid (SA), and Nair® hair removal cream. In this study, the mouse back skin was shaved 24 h prior to drug administration to allow skin to recover from any possible disturbance to the stratum corneum [28] and then moistened with PBS right before the experiments. Then the samples were topically applied onto the skin and left for 24 h, followed by removing the drugs and moistening with PBS. As shown in Figure 6, the skin treated with LipoLA gel maintained its normal structure and no erythema or edema was observed. This skin structure was similar to that treated with blank PBS gel, which served as a negative control. In contrast, both BPO cream and SA gel caused moderate erythema on the mouse skin, but no edema was observed. Nair® cream was the most irritating compound, which caused severe erythema and moderate edema throughout the application area on the mouse skin. According to the Draize’s irritation scoring system, the erythema and edema scores of LipoLA gel were 0 and 0, respectively, indicating no apparent irritation. For both BPO cream and SA gel samples, the scores were similar, which are 2 and 0 for the erythema and edema respectively, representing well defined erythema throughout the skin. In the case of Nair® cream, an erythema score of 4 indicated severe erythema with beet redness and slight eschar formation, which signified in depth injury. The average edema score from 3 Nair® cream treated mice was 2, demonstrating moderate edema with raised edges of the affected area. The in vivo toxicity of LipoLLA was further evaluated by examining the histology of the treated skin. The skin biopsy was collected and stained with hematoxylin and eosin (H&E). As shown in Figure 6 (bottom row), the LipoLA treated samples showed an undisturbed skin structure with a layer of healthy epidermal cells on top of the dermis layer, which was identical to the PBS treated skin samples. In contrast, for both BPO and SA treated samples, the epidermis layer was destroyed and disconnected and there was a significant amount of inflammatory cells in the dermis. Nair® cream caused the most severe damage to the skin structure, in which a majority of the defined epidermis layer disappeared, and many inflammatory cells accumulated in the dermis layer. These results show that within 24 h, the two most popular OTC anti-acne medications, BPO and SA, as well as Nair® hair removal cream induce significant skin toxicity on the tested mouse skin, while LipoLA do not generate any skin reaction within this time frame.
Figure 6.
Toxicity study of LipoLA on mouse back skin. LipoLA gel was topically applied onto shaved mouse back skin (n=6 per group). After 24 h, the gel was removed and the skin was analyzed. Blank PBS gel was used as a negative control. BPO, salicylic acid (SA), and Nair® cream were used as positive controls. (A) Morphology of the skin after treatment imaged by a camera. (B) Draize’s irritation scores indicating erythema. (C) Edema scores of the skin. (D) Back skin was cross-sectioned, stained with hematoxylin and eosin (H&E), and viewed by a microscope. Data is representative of three separate experiments with similar results.
3. Conclusion
In this study, we evaluated the in vivo therapeutic potential of liposomal lauric acids (LipoLA) for the treatment of acne infection. The synthesized LipoLA with a size of about 120 nm and a surface zeta potential of −43 mV were prone to fuse with bacterial membranes, thereby directly releasing a high dose of lauric acids into the membranes. Electron microscope images showed that LipoLA caused severe disruption on bacterial membrane structure and morphology including irregularly deformed surface and absent fimbriae, which were correlated with the observed killing of P. acnes bacteria. In vivo tests further confirmed that LipoLA were able to kill P. acnes bacteria through both intradermal injection and topical administration. Finally, a skin toxicity test demonstrated excellent biocompatibility of LipoLA to normal mouse skin. Overall, the results from this work indicate that LipoLA have a high potential to be a new, effective and safe therapeutic agent for the treatment of acne infection.
4. Experimental Section
Ethics statement
Animal experiments involving liposomal lauric acids and other formualtions followed protocols that were reviewed, approved and performed under the regulatory supervision of the University of California, San Diego’s institutional biosafety program and the Institutional Animal Care and Use Committee (IACUC).
Materials
Hydrogenated L-a-phosphatidylcholine (Egg PC) and cholesterol were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Lauric acic, hydroxyethyl cellulose (HEC), and glycerin were obtained from Sigma Aldrich (St Louis, MO). Brucella broth, Gas-Pak, and agar were purchased from BD (Sparks, MD). Reinforced clostridial medium was purchase from Oxoid (Hampshire, UK). Institute of Cancer Research (ICR) mice were purchased from Charles River (Wilmington, MA). Polyethylene glycol 400 (PEG 400) was purchased from USB Corporation (Cleveland, OH). Commercial Nair® cream, 10% benzoyl peroxide (Clean & Clear®), and 2% salicylic acid (Clean & Clear®) were purchased from a local drug store.
Preparation of bacteria
P. acnes (ATCC 6919) (American Type Culture Collection, Manassas, VA) was cultured on a reinforced clostridial medium (RCM) agar plate, under anaerobic condition at 37°C for 3 days. A single colony was then inoculated in RCM and cultured at 37°C till reaching approximately OD600=1.5 (logarithmic growth phase) under anaerobic condition. Note that for P. acnes, OD600 = 1 is corresponding to a bacterial concentration of 5×108 CFU/mL. The bacteria were harvested by centrifugation at 13,000 rpm for 3 min, washed with PBS, repeated 3 times, and then suspended to appropriate amount of PBS for experiment uses.
Preparation of LipoLA and LipoLA gel
LipoLA were prepared by a vesicle extrusion technique as previously reported [22]. In brief, lipid solution composed of Egg PC, cholesterol, and lauric acid (5:1:4, weight ratio) was dissolved in chloroform and evaporated under nitrogen gas. The dried lipid film was then rehydrated with sterile PBS buffer. The suspension of lipids was vortexed and then sonicated in a bath sonicator (Fisher Scientific FS30D) to produce multilamellar vesicles (MLV). The acquired MLV were further sonicated by a Ti-probe (Branson 450 sonifier) to produce small unilamellar vesicles (SUV). Lastly, the resulting SUV were extruded through a 100 nm pore-sized polycarbonate membrane to form the final product of LipoLA. The size and zeta potential of the resulting LipoLA were determined using the Malvern Zetasizer ZS (Malvern Instruments, UK), from which the mean diameter of LipoLA was measured through dynamic light scattering (DLS), and the zeta potential through electrophoretic mobility measurements. All characterization measurements were repeated three times at 25°C.
To prepare LipoLA cellulose gel, the mixture of HEC, glycerin, and PEG 400 (7.0, 5.6, and 1.8 wt%) were first swelled by 50 vol% of PBS under stirring for 15 min on a 60°C hot plate. The gel was then stirred continuously for 24 h at room temperature. Next, the pre-swelled gel was mixed with 50 vol% of LipoLA solution and vortexed until completely homogenized to obtain LipoLA gel.
Fusion study between LipoLA and P. acnes
F#x004E7;rster resonance energy transfer (FRET) study was performed to investigate the interaction between LipoLA and P. acnes. A fluorescent donor C6NBD (0.1 mol%) and a fluorescent acceptor DMPE-RhB (0.5 mol%) were concurrently incorporated into the bilayer membrane of LipoLA (0.5 mg/mL) by mixing the dyes with other lipid components prior to the preparation of LipoLA. The FRET-pair labeled LipoLA were then incubated with P. acnes at concentrations of 1×108, 1×109, 3.3×109, 6.6×109, and 1×1010 CFU/mL respectively. After 30 min of incubation at room temperature, the excess LipoLA were removed by centrifugation. The bacterial suspensions were excited at 470 nm and the fluorescence emission spectrum of C6NBD was recorded using a fluorescent spectrophotometer (BioTek Instrument, USA). Florescence intensity of all samples was subtracted with background, which was the fluorescence intensity of P. acnes solution at the corresponding bacterial concentrations. FRET-pair labeled LipoLA without P. acnes was used as a negative control.
In vitro antimicrobial activity of LipoLA against P. acnes and bacterial morphology study
The antimicrobial activity of LipoLA against P. acnes was determined by incubating P. acnes (1×107 CFU/mL) with LipoLA (2 mg/mL) in PBS at 37°C under anaerobic condition for 5 h, while P. acnes in PBS was used as a negative control. Following incubation, the samples were diluted 1:10 to 1:106 in PBS, and 10 µL of each sample was spotted on RCM agar plates. The samples were incubated at 37°C under anaerobic condition for 3 days, and then the CFU of P. acnes was quantified.
The morphology of P. acnes treated by LipoLA was visualized with scanning electron microscope (SEM). P. acnes (1×108 CFU/mL) were incubated with LipoLA (4 mg/mL) in PBS at 37 °C under anaerobic condition for 5 h. The same amount of P. acnes incubated in PBS was used as a negative control. Samples were then fixed with 2% glutaraldehyde in 1× PBS (pH=7.4) at room temperature for 30 min. Post fixing, the samples were centrifuged to remove glutaraldehyde, washed three times with water, and resuspended in 100µL water. Then 5µL of bacterial suspension was dropped onto a polished silicon wafer and allowed to dry overnight in a biosafety cabinet. The samples were then coated with chromium and imaged with an FEI XL30 Environmental SEM.
In vivo antimicrobial activity of LipoLA against P. acnes through intradermal injection
The antimicrobial activity of LipoLA against P. acnes in a physiological environment was tested using ICR mouse ears through intradermal injection. Right before injection, LipoLA (with concentrations of 2, 4, 6, and 8 mg/mL, respectively) were mixed with P. acnes (1×108 CFU/mL). The resulting solution (10 µL) was intradermally injected into the ears of mice. P. acnes mixed with BPO (16 mg/mL) in 5% DMSO was used as a positive control and P. acnes in PBS buffer was used as a negative control. The ears were collected 24 h after injection using an 8 mm biopsy punch and homogenized in 1 mL of sterile PBS (Mini-Beadbeater™). Homogenates were diluted 1:10 to 1:106 in PBS, and 10 μL of each dilution was spotted on RCM agar plates. Then, the agar plates were incubated at 37°C under anaerobic condition for 3 days, and the CFU of P. acnes was quantified. Ears without P. acnes inoculation served as a negative control to ensure that there was no contamination from other bacteria. Six mice were used for each group (n=6) and the experiment was repeated three times for statistical significance.
In vivo therapeutic efficacy of LipoLA against acne infection through topical administration
To induce P. acnes infection, the surface of the mouse ears was first scratched with a 25G needle tip to generate a wound with an area of around 10 mm2. Then, 1 μL of P. acnes (1×109 CFU/mL) was inoculated onto the wound to yield 1×106 CFU of P. acnes per ear. After 10 min of inoculation, LipoLA cellulose gel was applied topically onto the wound. The LipoLA gel was applied daily for 2 days successively. After 2 days of drug application, the ears were collected, and the same procedure as described in 2.6 was performed to quantify the CFU of P. acnes on the ear. Commercial BPO cream and PBS gel were used as a positive and a negative control, respectively. Six mice were used for each group (n=6) and the experiment was repeated three times for statistical significance.
Skin toxicity
The skin toxicity of LipoLA was tested on the back skin of ICR mice. Specifically, the back of the mice was shaved 24 h prior to the study, followed by topically administering LipoLA cellulose gel (2 mg/mL). Blank PBS gel (without LipoLA) was used as a negative control. Commercial Nair® cream, 10% PBO cream, and 2% salicylic acid (SA) gel were used as positive controls. After 24 h, the skin morphology was examined and imaged. Skin irritation was scored according to Draize’s system [28]. For histological observation, the skin was cross-sectioned by an 8 mm biopsy punch, stained with hematoxylin and eosin (H&E), and imaged with a microscope. Six mice were used for each group (n=6) and the experiment was repeated three times for statistical significance.
Acknowledgements
This work is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK095168. VF acknowledges an REU support from the National Science Foundation award CMMI 1031239.
Contributor Information
Dr. Dissaya Pornpattananangkul, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA.
Victoria Fu, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA.
Soracha Thamphiwatana, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA.
Dr. Li Zhang, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA
Michael Chen, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA.
James Vecchio, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA.
Dr. Weiwei Gao, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA
Prof. Chun-Ming Huang, Division of Dermatology, University of California, San Diego, La Jolla, California 92093, USA
Prof. Liangfang Zhang, Department of NanoEngineering and Moores Cancer Center, University of California, San Diego, La Jolla, California 92093, USA.
References
- 1.Dreno B, Poli F. Dermatology. 2003;206:7–10. doi: 10.1159/000067817. [DOI] [PubMed] [Google Scholar]
- 2.Gollnick H. Drugs. 2003;63:1579–1596. doi: 10.2165/00003495-200363150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Toyoda M, Nakamura M, Morohashi M. Eur. J. Dermatol. 2002;12:422–427. [PubMed] [Google Scholar]
- 4.Webster GF. J. Am. Acad. Dermatol. 1995;33:247–253. doi: 10.1016/0190-9622(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 5.Garg VK, Sinha S, Sarkar R. Dermatol. Surg. 2009;35:59–65. doi: 10.1111/j.1524-4725.2008.34383.x. [DOI] [PubMed] [Google Scholar]
- 6.Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, Shalita AR, Thiboutot D. J. Am. Acad. Dermatol. 2003;49:S1–S37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 7.Nyirady J, Grossman RM, Nighland M, Berger RS, Jorizzo JL, Kim YH, Martin AG, Pandya AG, Schulz KK, Strauss JS. J. Dermatolog. Treat. 2001;12:149–157. doi: 10.1080/09546630152607880. [DOI] [PubMed] [Google Scholar]
- 8.Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, Thiboutot DM, Van Voorhees AS, Beutner KA, Sieck CK, Bhushan R. J. Am. Acad. Dermatol. 2007;56:651–663. doi: 10.1016/j.jaad.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Taglietti M, Hawkins CN, Rao J. Skin Therapy Lett. 2008;13:6–8. [PubMed] [Google Scholar]
- 10.Tanghetti EA, Popp KF. Dermatol. Clin. 2009;27:17–24. doi: 10.1016/j.det.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Layton AM, Dreno B, Gollnick HP, Zouboulis CC. J. Eur. Acad. Dermatol. Venereol. 2006;20:773–776. doi: 10.1111/j.1468-3083.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 12.Drake DR, Brogden KA, Dawson DV, Wertz PW. J. Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Gallo RL, Huttner KM. J. Invest. Dermatol. 1998;111:739–743. doi: 10.1046/j.1523-1747.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- 14.Burtenshaw J. Brit.Med. Bull. 1945;3:161–169. doi: 10.1093/oxfordjournals.bmb.a071901. [DOI] [PubMed] [Google Scholar]
- 15.Nakatsuji T, Kao MC, Zhang L, Zouboulis CC, Gallo RL, Huang CM. J. Invest. Dermatol. 2010;130:985–994. doi: 10.1038/jid.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desbois AP, Smith VJ. Appl. Microbiol. Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 17.Huang CM, Chen CH, Pornpattananangkul D, Zhang L, Chan M, Hsieh MF, Zhang L. Biomaterials. 2011;32:214–221. doi: 10.1016/j.biomaterials.2010.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CH, Wang Y, Nakatsuji T, Liu YT, Zouboulis C, Gallo R, Zhang L, Hsieh MF, Huang CM. J. Microbiol. Biotechnol. 2011;21:391–399. [PubMed] [Google Scholar]
- 19.Obonyo M, Zhang L, Thamphiwatana S, Pornpattananangkul D, Fu V, Zhang L. Mol. Pharm. 2012;9:2677–2685. doi: 10.1021/mp300243w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petschow BW, Batema RP, Ford LL. Antimicrob. Agents Chemother. 1996;40:302–306. doi: 10.1128/aac.40.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prajapati HN, Dalrymple DM, Serajuddin ATM. Pharm. Res. 2012;29:285–305. doi: 10.1007/s11095-011-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Pornpattananangkul D, Nakatsuji T, Chan M, Carson D, Huang CM, Zhang L. Biomaterials. 2009;30:6035–6040. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh AJ, Kwon YJ. J.Control. Release. 2011;156:128–145. doi: 10.1016/j.jconrel.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Nakatsuji T, Kao MC, Fang JY, Zouboulis CC, Zhang L, Gallo RL, Huang CM. J. Invest. Dermatol. 2009;129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skrivanova E, Marounek M, Dlouha G, Kanka J. Lett. Appl. Microbiol. 2005;41:77–81. doi: 10.1111/j.1472-765X.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda M, Morohashi M. Med. Electron. Microsc. 2001;34:29–40. doi: 10.1007/s007950100002. [DOI] [PubMed] [Google Scholar]
- 27.Pace WE. Can. Med. Assoc. J. 1965;93:252–254. [PMC free article] [PubMed] [Google Scholar]
- 28.Draize JH, Woodard G, Calvery HO. J. Pharmacol. Exp. Ther. 1944;82:377–390. [Google Scholar]