Abstract
Prevalence of neuropathic pain is high after major surgeries. However, effective treatment for preventing neuropathic pain is lacking. Here we report that peri-surgical treatment of Neuroprotectin D1/protectin D1 (NPD1/PD1), derived from docosahexaenoic acid, prevents nerve injury-induced mechanical allodynia and ongoing pain in mice. Intrathecal post-treatment of NPD1/PD1 also effectively reduces established neuropathic pain and produces no apparent signs of analgesic tolerance. Mechanistically, NPD1/PD1 treatment blocks nerve injury-induced long-term potentiation, glial reaction, inflammatory responses, and reverses synaptic plasticity in the spinal cord. Thus, NPD1/PD1 and related mimetics might serve as a new class of analgesics for preventing and treating neuropathic pain.
Introduction
Neuroprotectin D1/protectin D1 (NPD1/PD1) is biosynthesized from omega-3 fatty acid docosahexaenoic acid (DHA). It was first identified in resolving inflammatory exudates from mouse peritonitis and brain1,2 as well as human leukocytes2. NPD1/PD1 was also identified in murine brain with stroke3 and its 10,17-docosatriene structure and potent actions were confirmed via its total organic synthesis.4 NPD1/PD1 (10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z hexaenoic acid) exhibits potent neuroprotective actions. Recently, we found that NPD1/PD1 potently inhibited inflammatory pain by blocking TRPV1 and TNF-α-mediated enhancement of spinal cord synaptic transmission.5 However, nerve injury-induced neuropathic pain, as results of major surgeries, is very resistant to current treatments.6,7 In the present study, we evaluated whether pre- and post-treatment of NPD1/PD1 would prevent or reverse neuropathic pain symptoms in the established neuropathic pain models.
Materials and Methods
Reagents
We purchased clonidine, gabapentin, streptozotocin, and lipopolysaccharide from Sigma, TNF-α from R & D, and DHA from Cayman Chemical. NPD1/PD1 and RvE1 were gifts from Resolvyx Pharmaceutical Inc.
Animals
Adult CD1 mice (male, 25-35 g) were used for behavioral and pharmacological studies. All the animal procedures were approved by the Institutional Animal Care & Use Committee (IACUC) of Duke University and Harvard Medical School.
Statistical analyses
All data were expressed as mean ± SEM. Behavioral data were analyzed using student's t-test or two-way ANOVA followed by Bonferroni post hoc test. Electrophysiology data were analyzed using paired or unpaired student's t-test. LTP data were tested using two-way ANOVA. The criterion for statistical significance was P<0.05.
Details of all experimental procedures are described in Supplementary Methods.
Results
We first examined whether pretreatment of NPD1/PD1 would protect nerve injury-induced neuropathic pain in two traumatic nerve injury models. Chronic construction injury (CCI) of the sciatic nerve produced long-lasting (>4 weeks) mechanical allodynia, a cardinal feature of neuropathic pain, elicited by normally innocuous low-threshold mechanical stimuli (Fig. 1A). Local delivery of NPD1/PD1 (300 ng) to the injury site of the sciatic nerve, at the time of surgery, completely prevented CCI-induced mechanical allodynia for 4 weeks (Fig. 1A). Local delivery of PD1 also prevented spinal nerve ligation (SNL) -induced mechanical allodynia (Supplementary Fig. 1A). As a comparison, treatment of resolvin E1 (RvE1, 5S,12R,18R-trihydroxyeicosa-6Z,8E,10E,14Z,16E-pentaenoic acid, 300 ng), another potent lipid analgesic for inflammatory pain,8 only transiently prevented nerve injury-induced allodynia (Supplementary Fig 1A and B). Of note, systemic injections of NPD1/PD1 via i.v. route (600 ng, 3, 24, and 48 hours after CCI) also prevented CCI-induced allodynia and heat hyperalgesia (Supplementary Fig 2A and B). It is suggested that peri-surgical treatment, even beginning a few hours after the surgery, is still effective in preventing neuropathic pain development.
Figure 1. NPD1/PD1 protects evoked and ongoing neuropathic pain symptoms after nerve injury in mice.
(A, B) Local peri-surgical pretreatment of NPD1/PD1 (300 ng per mouse, peri-sciatic nerve) prevents CCI-induced mechanical allodynia (A) and ongoing pain (B). (A) The two-way ANOVA showed significant overall between-group differences between the NPD1 and vehicle groups, F(1,113) = 118, p < 0.0001, and between sham and vehicle groups, F(1,80) = 98.77, p < 0.0001. Post hoc analysis of each time point revealed significant differences between NPD1- and vehicle-treated mice from 3 to 28 days. *P<0.05, compared to sham control, #P<0.05, compared to corresponding vehicle (PBS) control, n = 5-12 mice. (B) Spinal clonidine (10 μg, i.t.)-induced chamber preference in CCI mice, as indicated by time spent in each chamber, is eliminated by the NPD1/PD1 pretreatment. $P<0.05, n = 5-9 mice. (C-E) Local peri-surgical pretreatment of NPD1/PD1 (300 ng/mouse, peri-sciatic nerve) protects axotomy-induced autotomy and stump pain. Incidence (C) and score (D) of autotomy in vehicle and NPD1/PD1-treated mice. *P<0.05, compared to vehicle, n = 7 mice. (E) Stump pain (mechanical allodynia) induced by a von Frey hair (0.07 g) in vehicle and NPD1/PD1-treated mice. *P<0.05, compared to sham control, #P<0.05, compared to corresponding vehicle control, n = 5-7 mice.
Next, we tested whether NPD1/PD1 would also affect nerve injury-induced ongoing pain. Negative reinforcement was shown to unmask ongoing neuropathic pain in a 2-chamber conditioned place preference (CPP) test.9 Using the same test, we found mice spent more time in the chamber paired with the analgesic clonidine treatment (i.t., 10 μg) than in the chamber paired with vehicle injection (Fig. 1B), indicating that mice developed ongoing pain after CCI. Notably, the CCI-induced ongoing pain was abolished by the NPD1/PD1 pretreatment (Fig. 1B).
Amputation is known to cause phantom pain, as a result of deafferentation of the amputated limb.10,11 Transection of the sciatic nerve (axotomy) mimics deafferentation and also elicits autotomy in rodents, a self-mutation behavior by chewing nails and digits on the affected side,12 which is associated with neuropathic pain.13 In saline-treated mice, 85% mice developed autotomy within 2 weeks. However, only 30% mice with NPD1/PD1 pretreatment (300 ng, peri-sciatic) had autotomy (Fig. 1C). Further, the degree of autotomy (autotomy score) and stump pain induced by axotomy were substantially reduced in NPD1/PD1-treated mice (Fig 1D and E). As recently reported,13 axotomized mice also developed ongoing pain (Supplementary Fig. 3A). NPD1/PD1 pretreatment not only abrogated the ongoing pain but also reduced the mass of neuroma (Supplementary Fig 3A and 3B). Our results suggest that autotomy might be associated with phantom pain, which can be significantly alleviated by NPD1/PD1.
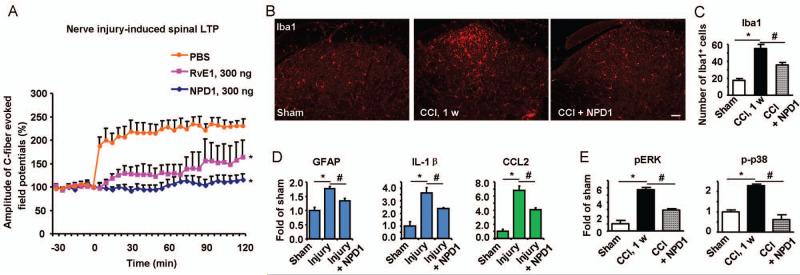
To explore the mechanisms by which peripheral application of NPD1/PD1 protects neuropathic pain, we examined the electrophysiological and biochemical changes in the dorsal horn following nerve injury. Long-term potentiation (LTP) and activation of glial cells (microglia and astrocytes) in the spinal cord have been implicated in the genesis of neuropathic pain.7,14 Spinal LTP (sLTP) is typically induced by high-frequency titanic stimulation.14 However, transection of the sciatic nerve, in the absence of titanic stimulation, also elicited sLTP of C-fiber-evoked field potentials in vivo for > 2 hours (Fig. 2A). The nerve injury-induced sLTP was completely prevented by peri-sciatic application of NPD1/PD1 (300 ng). For comparison, RvE1 (300 ng) only partially prevented nerve injury-induced sLTP (Fig. 2A). Nerve injury also elicited robust microglia reaction (Iba1 up-regulation, Fig 2B and C) and astroglial reaction (GFAP up-regulation, Fig. 2D) in the dorsal horn, and both were blocked by NPD1/PD1 pretreatment (Fig 2B to D). The activated spinal glial cells produce pro-inflammatory cytokines (e.g., IL-1β) and chemokines (e.g., CCL2), via activation of MAP kinase pathways, to promote neuropathic pain.7 Nerve injury-induced expression of IL-1β and CCL2 and phosphorylation of p38 and ERK in the dorsal horn were also reduced by the NPD1/PD1 pretreatment (Fig 2D and E; Supplementary Fig 4). NPD1/PD1 could also directly modulate the activity of microglia and astrocytes, by inhibiting the cytokine and chemokine expression in glial cultures (Supplementary Fig 5). Down-regulation of isolectin IB4 and up-regulation of the transcription factor ATF-3 in DRG neurons are commonly used markers of axonal injury.15 CCI-induced IB4 down-regulation and ATF-3 up-regulation in DRGs were normalized by the NPD1/PD1 pre-treatment (Supplementary Fig 6A and B). Further, nerve injury-induced infiltration of inflammatory macrophages to DRGs was also prevented by NPD1/PD1 (Supplementary Fig 6C). Thus, NPD1/PD1 further protects DRG neurons against nerve injury.
Figure 2. Local NPD1/PD1 prevents nerve injury-induced LTP, glial reaction, and cytokine/chemokine expression in the spinal cord dorsal horn.
(A) LTP of the sciatic nerve transection-evoked field potentials (C-fiber stimulation) in the dorsal horns of anesthetized mice after pretreatment of vehicle (PBS), NPD1/PD1 (300 ng), and RvE1 (300 ng). The two-way ANOVA showed significant overall between-group differences between the NPD1 and vehicle groups, F(1,240) = 34.49, p =0.0004, and between RvE1 and vehicle groups, F(1,240) = 8.421, p=0.0198. *P<0.05 vs. PBS, n=5 mice. (B) Iba1 immunostaining in the dorsal horns of sham control and CCI mice (1 week) with pretreatment of PBS or NPD1/PD1. Scale, 50 μm. (C) Number of Iba1+ cells in the dorsal horn. *P<0.05, compared to sham, #P<0.05, compared to corresponding vehicle control, n=6 mice. (D) Expression of GFAP, IL-1β, and CCL2 mRNAs in the dorsal horns of sham control mice and SNL mice (3 days) with pretreatment of vehicle or PD1. *P<0.05, compared to sham, #P<0.05, compared to corresponding vehicle control, n=4 mice. (E) Expression of p-p38 and pERK in the dorsal horn of sham mice and CCI mice (1 week) with pretreatment of vehicle or PD1. *P<0.05, compared to sham, #P<0.05, compared to corresponding vehicle control, n=3-5 mice. All the results are mean ± SEM.
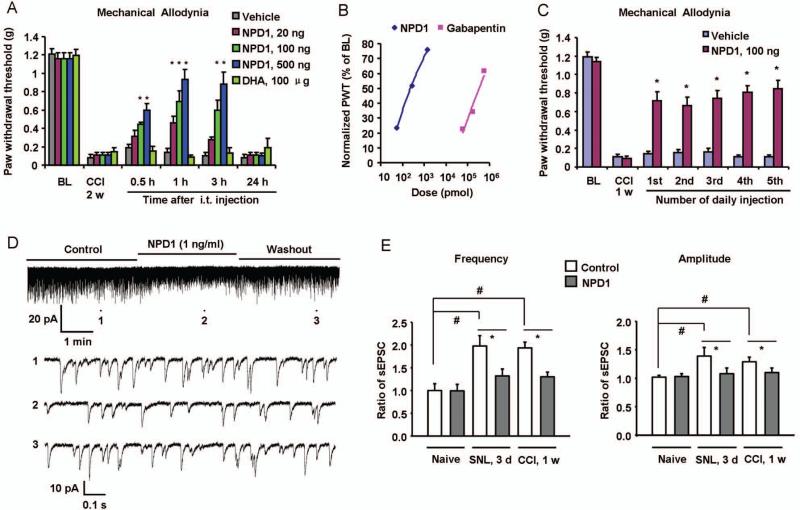
We next determined whether and how NPD1/PD1 attenuates established neuropathic pain. Post-treatment of NPD1/PD1 via spinal intrathecal route (20-500 ng), 2 weeks after nerve injury, dose-dependently reduced CCI-induced mechanical allodynia and heat hyperalgesia for >3 h (Fig 3A and Supplementary Fig 7A). In contrast, DHA, the upstream n-3 EFA precursor of NPD1/PD1, had no effect on allodynia even at a very high dose (100 μg, i.t., Fig 3A). We also compared the analgesic efficacy of NPD1/PD1 to gabapentin, a common treatment for clinical neuropathic pain.16 Gabapentin effectively reduced mechanical allodynia, but with much higher dose range (10-100 μg, i.t.) (Fig 3B, Supplementary Fig 7B). Repeated injections of opioids are known to produce antinoiciceptive tolerance and even paradoxical hyperalgesia. In contrast, NPD1/PD1-initated antinociception was fully maintained after repeated injections (i.t., once a day) for 5 days, without showing apparent signs of antinoiciceptive tolerance (Fig 3C).
Figure 3. Spinal post-treatment of NPD1/PD1 reduces nerve injury-induced neuropathic pain and normalizes spinal cord synaptic plasticity.
(A) Intrathecal injection of NPD1/PD1 (20, 100, 500 ng), 2 weeks after nerve injury, dose-dependently reduces CCI-induced mechanical allodynia. The two-way ANOVA showed significant overall between-group differences between the vehicle and NPD1 groups, F(1,60) = 6.039, p =0.0302 for 20 ng group, F(1,60) = 16.23, p =0.0017 for 100 ng group, and F(1,60) = 42.72, p<0.0001 for 500 ng group. However, there's no significant difference between the vehicle and DHA (100 μg) group, F(1,55) = 0.1275, p =0.7278. Bonferroni post hoc tests revealed that CCI-induced mechanical allodynia was significantly reduced by NPD1 treatment in a dose-dependent manner. *P<0.05, compared to corresponding vehicle (PBS) control, n = 6~7 mice. BL, baseline before nerve injury. (B) Dose-dependent inhibition of CCI-induced mechanical allodynia by NPD1/PD1 (i.t., 20-500 ng ≈ 0.06-1.4 nmol) and gabapentin (i.t., 10-100 μg ≈ 58.4-584.1 nmol). Mechanical allodynia was tested 3 h after the drug injection. PWT, paw withdrawal threshold. Note that the effective doses of gabapentin are much higher than that of NPD1/PD1. (C) Repeated injections of NPD1/PD1 (100 ng, i.t., once a day for 5 days) produces sustained reduction of CCI-induced mechanical allodynia, tested 1 h after each injection. The two-way ANOVA showed significant difference between the NPD1 and vehicle group, F(1,60) = 81.12, p<0.0001. Post hoc tests revealed that CCI-induced mechanical allodynia was significantly reduced by each NPD1 treatment. *P<0.05, compared to corresponding vehicle (PBS) control, n = 6 mice. (D) Traces of spontaneous excitatory postsynaptic currents (sEPSCs) in lamina II neurons of spinal cord slices of CCI mice (1 week) before and after NPD1/PD1 perfusion. Trace 1, 2, and 3 (top panel) show sEPSCs before NPD1/PD1 treatment, during NPD1/PD1 treatment, and after washout, respectively. Low panels are enlargements of traces (1, 2, and 3) in the up panel. (E) sEPSC frequency and amplitude in spinal cord slices from SNL and CCI mice. Note that nerve injury-induced increases in sEPSC frequency and amplitude are normalized by NPD1. *P<0.05, #P<0.05, n=5-10 neurons. Results are mean ± SEM.
Nerve injury-induced spinal cord synaptic plasticity is important for the genesis of neuropathic pain.17 We found that CCI and SNL-induced synaptic plasticity retained in spinal cord slices ex vivo, as indicated by increased frequency and amplitude of spontaneous excitatory postsynaptic currents (sEPSCs) in lamina II neurons (Fig 3D). Superfusion of spinal cord slices with NPD1/PD1, at a very low concentration (1 ng/ml, i.e. 2.8 nM), reversed the SNL- and CCI-induced increases in sEPSC frequency and amplitudes (Fig 3D and E). Thus, spinal NPD1/PD1 could reduce neuropathic pain via both fast actions (modulation of synaptic plasticity) and slow actions (inhibition of glial activation).
In addition to nerve trauma-induced neuropathic pain, we also tested the effect of NPD1/PD1 in another neuropathic pain condition, produced by streptozotocin (STZ)-induced diabetic neuropathy. As previously reported,18 systemic STZ (75 mg/kg) induced marked mechanical allodynia, which was largely prevented by NPD1/PD1 pretreatment (Supplementary Fig 8).
Discussion
In summary, these results demonstrate that NPD1/PD1 effectively protects neuropathic pain from nerve trauma (CCI, SNL, and axotomy/amputation). Local peri-surgical NPD1/PD1 treatment, started around the time of surgery, was sufficient to prevent both nerve injury-induced evoked pain (mechanical allodynia) and ongoing pain (negative reinforcement and autotomy behavior). Notably, systemic NPD1/PD1 treatment, started a few hours after the nerve trauma, also eliminated neuropathic pain. Intrathecal post-treatment of NPD1/PD1 further reduced established neuropathic pain, without producing antinociceptive tolerance. Mechanistically, NPD1/PD1 attenuated neuropathic pain by (i) preventing nerve injury-induced spinal LTP, (ii) preventing nerve injury-induced spinal glial reaction and pro-inflammatory responses, (iii) protecting nerve injury responses of DRG neurons, and (iv) reversing nerve injury-induced spinal cord synaptic plasticity. Although growth factors GDNF and artemin can effectively protect nerve injury responses in DRG neurons, they may not fully prevent nerve trauma-induced neuropathic pain.15,19
Prevalence of neuropathic pain is very high after chest surgeries (e.g., thoracotomy) and amputation.6,10,17 Despite improved perioperative pain management, phantom limb pain still occurs in 50-80% amputees.11 NPD1/PD1 appears to possess multiple beneficial features for treating neuropathic pain, including (i) high potency, (ii) anti-inflammation, (iii) pro-resolution, (iv) normalization of neural plasticity, and (v) neuroprotection. A recent study shows that NPD1/PD1 also protects from infection and improves severe influenza.20 Unfortunately, the currently used analgesic drugs only offer some of these beneficial features. Thus, peri-surgical treatment of NPD1/PD1 and its related mimetics could be useful in reducing or/and preventing surgery-induced neuropathic pain. Post-treatment of NPD1/PD1 and related mimetics may also be helpful leads toward alleviating chronic neuropathic pain.
Although NPD1/PD1 inhibited neuropathic pain at much low doses than gabapentin, we do not intend to conclude that NPD1 is more effective than gabapentin in treating neuropathic pain. Therapeutic index is more appropriate to determine the efficacy of a drug. Nevertheless, given the safety profile of the endogenous lipid mediators and well-known side effects of gabapentin, NPD1/PD1 and related mimetics could have great potentials. It remains to be investigated whether NPD1/PD1 can protect nerve degeneration and promote axonal regeneration as growth factors in neuropathic pain conditions as results of chemotherapy and diabetes.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants R01DE17794 and R01DE22743 to R.R.J., P01GM095467 to C.N.S., R21NS082985 to Z.Z.X. and Transformative R01NS67686 to R.R.J & C.N.S.
REFERENCES
- 1.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong S, Gronert K, Devchand PR, et al. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 3.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemiareperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 5.Park CK, Lu N, Xu ZZ, et al. Resolving TRPV1- and TNF-a-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 7.Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–642. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- 8.Xu ZZ, Zhang L, Liu T, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King T, Vera-Portocarrero L, Gutierrez T, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flor H, Nikolajsen L, Staehelin JT. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- 11.Giummarra MJ, Moseley GL. Phantom limb pain and bodily awareness: current concepts and future directions. Curr Opin Anaesthesiol. 2011;24:524–531. doi: 10.1097/ACO.0b013e32834a105f. [DOI] [PubMed] [Google Scholar]
- 12.Wall PD, Devor M, Inbal R, et al. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7:103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- 13.Qu C, King T, Okun A, et al. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruscheweyh R, Wilder-Smith O, Drdla R, et al. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Guo W, Ossipov MH, et al. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- 16.Rose MA, Kam PC. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 17.Von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–652. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alessandri-Haber N, Dina OA, Joseph EK, et al. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci. 2008;28:1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardell LR, Wang R, Ehrenfels C, et al. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- 20.Morita M, Kuba K, Ichikawa A, et al. The lipid mediator protectin d1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153(1):112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.