Abstract
Objective
Optimal management of renal artery stenosis (RAS) remains unclear. Recent randomized controlled trails have shown no clear benefit with angioplasty (with and without stenting) (PTRA/S) over medical management. We hypothesize that interventions for RAS are decreasing nationally.
Methods
The Nationwide Inpatient Sample, 1988-2009, was used to identify patients with a diagnosis of renal artery atherosclerosis undergoing open surgical repair (bypass or endarterectomy) or PTRA/S. The rate of interventions, in-hospital death, and perioperative outcomes were analyzed over time. Additionally, we used individual State Inpatient and Ambulatory databases in order to better understand the influence of outpatient procedures on current volume and trends.
Results
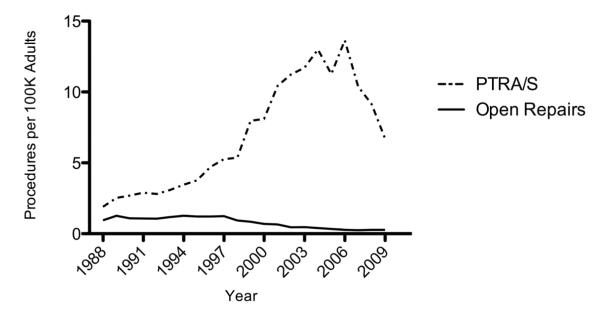
We identified 308,549 PTRA/S and 33,147 open surgical repairs. PTRA/S increased from 1.9/100K adults in 1988 to 13.7 in 2006 followed by a decrease to 6.7 in 2009. Open surgical repair steadily decreased from 1.3/100K adults in 1988 to 0.3 in 2009. In 2009, PTRA/S procedures (6.4/100K adults) greatly outnumbered procedures done by open repair alone (0.1/100K), combined open renal and aortic repair (0.2/100K), and combined PTRA/S and EVAR (0.3/100K). From 2005-2009 33,953 patients underwent PTRA/S in states NJ, MD, FL, and CA combined. The total number of PTRA/S performed in the outpatient setting remained stable from 2005 (3.8/100K) to 2009 (3.7/100K), while the total number of inpatient procedures mirrored the national trend, declining from 2006 (7.9/100K) to 2009 (4.2/100K). PTRA/S had lower in-hospital mortality (0.9% vs. 4.1%, P < .001) compared to open repair. PTRA/S patients were more likely to be discharged home (86.2% vs. 76.3%, P < .001) and had a shorter length of stay (4.4 days vs. 12.3 days, P < .001). Mortality was higher after combined open renal and open aortic surgery compared to open repair alone (6.5% vs. 4.1%, P < .001). Mortality was similar for combined PTRA/S + EVAR compared to PTRA/S alone (1.2% vs. 0.9%, P = .04).
Conclusions
The performance of PTRA/S procedures for the management of RAS has decreased significantly after 2006. An increasing proportion of these procedures are performed in the outpatient setting. PTRA/S remains the dominant revascularization procedure for RAS with lower in-hospital mortality and morbidity than surgery.
Introduction
Patient selection for renal artery stenosis (RAS) intervention remains unclear largely because of the results of several prospective randomized controlled trials (RCTs). 1-5 These RCTs, which included trials performed both before and after the shift from angioplasty alone to angioplasty with stenting (PTRA/S), evaluated the outcome of RAS treated medically or by PTRA/S. Inclusion criteria varied for each study, but included criteria such as diastolic blood pressure, creatinine clearance, and the percentage of renal artery lumen diameter reduction. Outcome variables in these studies included mortality, blood pressure control, required dosage of antihypertensive medications, renal function, and procedural complications. The data from these trials showed that there was no clear benefit for interventional treatments over medical management.
Regardless, the number of renal artery interventions had increased after the publication of these trials. National inpatient data have shown a 173% increase in catheter-based procedures from 1988 to 2001. 6 The rate of inpatient and outpatient percutaneous renal artery intervention among Medicare beneficiaries increased 2.4-fold from 1996 to 2000. 7 With the initial RCTs on interventional treatment versus medical management of RAS published over ten years ago and the continuing lack of evidence regarding the optimal treatment for RAS, we wondered whether RAS treatment has plateaued, declined, or continued to rise.
The purpose of this study was to evaluate national trends in intervention for RAS using PTRA/S or open surgery with and without aortic surgery. RAS encompasses both renal atherosclerosis and fibromuscular dysplasia and treatment of these are generally considered distinct, so separate evaluation of these two disease processes was performed.
Methods
Databases
The Healthcare Cost and Utilization Project is a family of health care databases including the Nationwide Inpatient Sample (NIS) and State Inpatient and Ambulatory Databases. The NIS is a 20% all-payer sample of hospital stays and contains sampling weights to allow for calculation of total population estimates. NIS data are collected from hospital discharge abstracts which include information about all procedures performed during a given hospital admission as well as patient characteristics, comorbidities, in-hospital mortality, and length of stay. The NIS currently consists of over 8 million annual hospitalizations from 1,050 hospitals in 44 states, representing approximately 95% of all hospitalizations. It is the largest all-payer inpatient care database in the United States.8
Many endovascular procedures have shifted to the outpatient surgery setting and data regarding outpatient procedures are not available in the NIS. However, New Jersey, Maryland, Florida, and California are a few states that make these data available in both their State Inpatient Database (SID) and their State Ambulatory Surgery Database (SASD). These databases represent a 100% sample of hospital discharges and ambulatory procedures, respectively, within each state. We analyzed the inpatient and ambulatory surgery data in these states, years 2005 to 2009, to determine the extent to which the trends seen nationally are simply a reflection of the changes in utilization of outpatient care for RAS patients.
Data Retrieval – Nationwide Inpatient Sample
Patients were selected in the NIS, years 1988-2009, using diagnosis and procedure codes from the International Classification of Diseases, 9th Revision (ICD-9). Two groups of patients were studied, 1) those with a diagnosis of renal atherosclerosis and 2) those with a diagnosis of fibromuscular dysplasia (FMD).
For renal atherosclerosis, patients were required to have a primary diagnosis code for renal artery atherosclerosis (440.1) or renovascular hypertension (405.01, 405.11, 405.91) combined with a procedural code for open renal artery revascularization [endarterectomy (38.16), resection of vessel with anastomosis (38.36), resection of vessel with replacement (38.46), aorto-renal bypass (39.24), and reimplantation of renal artery (39.55)] or angioplasty with or without stenting (PTRA/S) [angioplasty or atherectomy of other non-coronary vessels (39.50), other repair of vessel (39.59) or insertion of non-drug-eluting peripheral vessel stent (39.90)]. Patients with a concomitant diagnosis code for mesenteric ischemia (55.70, 55.71) or vascular trauma (902.xx) were excluded. Patients with a concomitant code for renovascular hypertension and FMD were excluded from the renal atherosclerosis group and instead classified in the FMD group.
For FMD, patients were required to have a primary diagnosis code for fibromuscular dysplasia of the renal artery (447.3) combined with a procedural code for open renal artery revascularization or PTRA/S. Patients with a concomitant diagnosis code for mesenteric ischemia, carotid stenosis (433.10, 433.11, 433.31), or vascular trauma were excluded.
We also investigated combined procedures such as open renal artery repair with aortic surgery and PTRA/S with endovascular aneurysm repair (EVAR). Aortic surgery procedure codes included endarterectomy (38.14), resection of vessel with anastomosis (38.34), resection of vessel with replacement (38.44, 38.45), shunt or vascular bypass (39.25), and other abdominal bypass (39.26). The EVAR procedure code was 39.71. The adult population of the United States for each year was determined from US Census Bureau data.9
Data Retrieval – State Databases
The inpatient and ambulatory databases of states New Jersey, California, Maryland, and Florida were examined. Patients were identified using ICD-9 diagnosis codes for renal artery atherosclerosis, renovascular hypertension, and FMD. With the exception of the state ambulatory databases of California and Maryland, ICD-9 procedure codes were used to identify all patients undergoing open renal artery revascularization or PTRA/S. In the state ambulatory databases of California and Maryland, Current Procedural Terminology (CPT) codes were used to identify open renal artery revascularization procedures (35341, 35536, 35636, 35560, and 35631) and PTRA/S (37205, 37207, 35450, 35471, 35480, and 35490).
Physician Specialty Determination
For PTRA/S, we were interested in volume trends with the following specialties: vascular surgeons (VS), interventional cardiologists (IC), and interventional radiologists (IR). NIS provides unique physician identifiers per state that allows tracking of procedures performed by that physician during that specific year. Of the available states, 27 provided 2 unique physician identifiers, of them 22 states specifically stated which identifier correlated with the primary procedure performed. For the remaining 5 states, the identifiers were only used when both were identical to make sure that it involved the physician that performed the primary procedure (the identifiers were identical in 75% of cases). We composed a list of specialty specific procedures that we used to identify the specialty of each physician, which is included in a supplemental appendix. Similar approaches have been previously reported. 10-12 Subsequently we created a hierarchical model: each physician that performed >10 interventional cardiac procedures was labeled an IC; physicians with >10 interventional radiology procedures were identified as IR; the remaining physicians that performed 75%-100% vascular surgery procedures (of vascular and general surgery codes combined) and >10 in number were classified as VS. As these identifiers became available from 2001 onwards, specialty related analyses were restricted to that period.
Outcomes
Our primary outcome was procedure utilization over time. Secondary outcomes included in-hospital death, postoperative complications [including stroke (997.02), cardiac complications (997.1), respiratory complications (997.3), digestive complications (997.4), genitourinary complications (997.5), vascular (997.71, 997.72, 997.79), peripheral vascular (997.2), wound disruption (998.3), post-op infection (998.5), and hemorrhage/hematoma (998.11, 998.12)], length of stay, and discharge disposition. We excluded patients with combined procedures when analyzing outcomes after open renal artery repair alone and PTRA/S alone.
Statistical Analysis
Comparisons between cohorts were done using the t test for parametric continuous data, and the Pearson χ2 test for categorical and numeric data. Continuous data are presented as mean ± standard deviation (SD). Groups were stratified by diagnosis (renal atherosclerosis vs. FMD) and by procedural method (open surgical repair vs. PTRA/S). Queries were performed using SAS 9.1 statistical software (SAS Institute, Cary, NC). Statistical analyses were performed using PASW 18.0 (SPSS Inc, Chicago, IL). Population estimates are calculated by applying the sampling weight for each observation. Statistical significance was assigned at P<.05.
Results
Patient Characteristics
From 1988 to 2009 there were 308,549 PTRA/S and 33,147 open surgical repairs for patients with renal atherosclerosis and 6,706 PTRA/S and 595 open surgical repairs for patients with FMD. The mean age was higher for patients receiving PTRA/S compared to open repair for both renal atherosclerosis (70.8 vs. 66.8, P<.001) and FMD (58.8 vs. 48.7, P<.001) (Table 1). Patients undergoing intervention for FMD were younger than those undergoing intervention for renal atherosclerosis (mean age 59.4 vs. 70.9, P<.001) and FMD patients were more likely to be female (87.0% vs. 58.0%, P<.001).
Table 1.
Baseline characteristics of patients diagnosed with renal atherosclerosis or fibromuscular dysplasia undergoing endovascular repair or open repair of renal artery stenosis from 1988-2008.
| RAS | FMD | |||||
|---|---|---|---|---|---|---|
| PTRA/S | Open | P | PTRA/S | Open | P | |
| N | 308,549 | 33,147 | 6,706 | 595 | ||
| Age (mean ± SD) | 70.8 ± 10.4 | 66.8 ± 9.9 | < .001 | 58.8 ± 15.4 | 48.7 ± 12.8 | < .001 |
| Female | 56.2 | 47.3 | < .001 | 88.9 | 83.6 | < .001 |
| Hypertension | 74.8 | 54.9 | < .001 | 78.2 | 36.7 | < .001 |
| PVD | 67.9 | 79.2 | < .001 | 29.5 | 38.5 | < .001 |
| COPD | 16.2 | 21.8 | < .001 | 7.2 | 9.9 | 0.02 |
| Prior MI | 8.2 | 6.5 | < .001 | 2.9 | 0.7 | < .01 |
| CHF | 14.1 | 12.4 | < .001 | 3.6 | 1.7 | 0.01 |
| Valvular Disease | 8.1 | 4.7 | < .001 | 5.6 | 4 | 0.11 |
| Diabetes w/o comp | 22.0 | 10.1 | < .001 | 8.0 | 5.2 | 0.02 |
| Diabetes w/ comp | 4.8 | 2.0 | < .001 | 0.7 | 0.0 | 0.04 |
| Chronic renal disease | 8.8 | 3.0 | < .001 | 2.6 | 0.0 | < .001 |
| Obesity | 3.2 | 1.6 | < .001 | 3.3 | 0.0 | < .001 |
Values are in % unless otherwise indicated.
Abbreviations: RAS, renal atherosclerosis; FMD, fibromuscular dysplasia; PTRA/S, percutaneous transluminal angioplasty/stenting; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; CHF, congestive heart failure.
Renal Artery Atherosclerosis
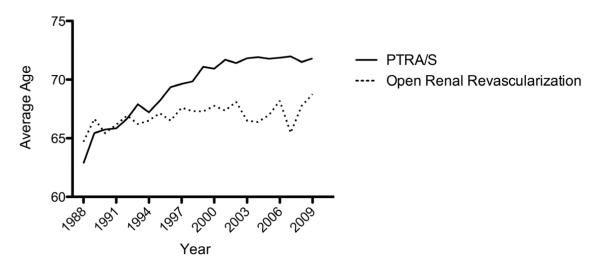
Over the study period, there were consistently more PTRA/S repairs in patients with a diagnosis of renal atherosclerosis. PTRA/S interventions increased substantially from 1988 to 2006 (1.9 to 13.7 procedures per 100K adults), followed by a marked decrease from 2006 to 2009 (13.7 to 6.7 procedures per 100K adults), whereas the number of open repairs gradually decreased throughout the study period (1.3 to 0.3 procedures per 100K adults) (Figure 1). Compared to open repair, patients undergoing PTRA/S had higher rates of comorbidities except for peripheral vascular disease and chronic obstructive pulmonary disease (COPD) (Table 1). The mean age for PTRA/S intervention increased from 62.9 years in 1988 to 71.8 years in 2009 for patients with renal atherosclerosis (P<.001) (Figure 2). No significant trend was seen for the mean age of open treatment of renal atherosclerosis.
Figure 1.
Annual number of inpatient procedures per 100K adults for treatment of renal artery atherosclerosis from 1988-2009: percutaneous transluminal angioplasty with or without stenting (PTRA/S) compared with open repair.
Figure 2.
Change in mean age of patients undergoing inpatient PTRA/S or open renal artery revascularization for a diagnosis of renal artery atherosclerosis from 1988-2009.
From 2005-2009 20,759 patients in the state inpatient databases (of NJ, MD, FL, and CA) and 13,194 patients in the state ambulatory surgery databases underwent PTRA/S for a diagnosis of renal artery atherosclerosis. The number of PTRA/S performed in the outpatient setting remained stable from 2005 (3.8/100K) to 2009 (3.7/100K), while the total number of inpatient procedures declined from 2006 (7.9/100K) to 2009 (4.2/100K) (Figure 3). The percentage of outpatient procedures increased from 36% in 2005 to 47% in 2009. There was no variability between states as all four states showed a decrease in total interventions after 2006.
Figure 3.
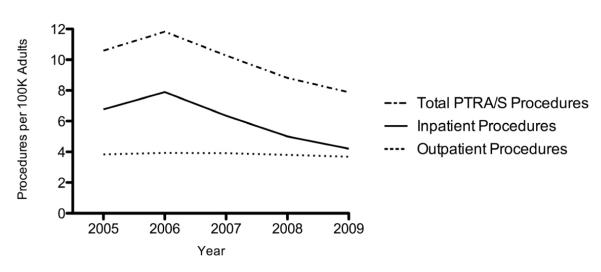
Annual number of PTRA/S procedures per 100K adults performed in NJ, MD, FL, and CA from 2005 to 2009 in patients with a diagnosis of renal atherosclerosis.
PTRA/S had lower overall in-hospital mortality compared to open repair (0.9% vs. 4.1%, P<.001) (Table 2). There was no significant change in the year-to-year in-hospital mortality trend from 1988-2009. The proportion of patients discharged home was higher (86.2% vs. 76.3%, P<.001) and the length of stay was shorter (4.4 days vs. 12.3 days, P<.001) after PTRA/S. PTRA/S had fewer postoperative complications compared to open repair in all categories except for hemorrhage and hematoma.
Table 2.
Perioperative complications and length of stay after PTRA/S and open repair for patients with a diagnosis of renal atherosclerosis.
| PTRA/S | Open Repair | P | |
|---|---|---|---|
| Mortality | 0.9 | 4.1 | < .001 |
| Discharged home | 86.2 | 76.3 | < .001 |
| LOS, mean (SD) | 4.4 (6.3) | 12.3 (11.4) | < .001 |
| Stroke | 0.2 | 0.7 | < .001 |
| Cardiac | 0.3 | 6.1 | < .001 |
| Respiratory | 0.3 | 6.1 | < .001 |
| Digestive | 0.2 | 3.9 | < .001 |
| Urinary | 0.7 | 4.3 | < .001 |
| Vascular | 0.1 | 0.4 | 0.87 |
| Peripheral Vascular | 0.9 | 1.5 | < .001 |
| Wound Disruption | 0.1 | 0.8 | < .001 |
| Postoperative Infection | 0.2 | 1.6 | < .001 |
| Hemorrhage/hematoma | 5.5 | 5.5 | 1.00 |
Values are in %, unless otherwise indicated.
Abbreviations: PTRA/S, percutaneous transluminal angioplasty/stenting; LOS, length of stay; SD, standard deviation.
Renal Fibromuscular Dysplasia
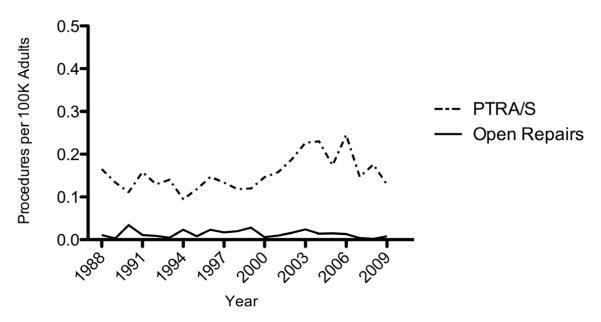
The change in the rate of treatment for patients with FMD was similar to that seen for patients with renal atherosclerosis. The number of PTRA/S interventions increased from 1994 to 2006 (0.1 to 0.3 procedures per 100K adults) and decreased from 2006 to 2009 (0.3 to 0.1 procedures per 100K adults) (Figure 4). Although the number of open repairs fluctuated over the study period, it consistently remained lower than PTRA/S interventions per year. Similar to patients with renal atherosclerosis, patients undergoing PTRA/S had higher rates of comorbidities except for peripheral vascular disease and COPD (Table 1).
Figure 4.
Annual number of procedures per 100K adults for treatment of renal fibromuscular dysplasia from 1988 to 2009; PTRA/S compared with open repair.
From 2005-2009, 357 patients in the state inpatient databases (of NJ, MD, FL, and CA) and 422 patients in the state ambulatory surgery databases underwent PTRA/S for a diagnosis of FMD. The number of PTRA/S performed in the outpatient setting increased from 2005 (0.12/100K) to 2007 (0.14/100K), and declined from 2007 to 2009 (0.11/100K). The trend in inpatient procedures mirrored the trend in outpatient procedures, increasing from 2005 (0.11/100K) to 2007 (0.12/100K), and declining from 2007 to 2009 (0.07/100K). There was no variability between states as all four states showed a decrease in total interventions after 2007.
In hospital mortality was significantly lower after PTRA/S compared to open repair (1.6% vs. 7.8%, P<.001) (Table 3). The proportion of patients discharged home was higher (95.8% vs. 92.6%, P<.001) and length of stay was shorter (2.8 vs. 7.9, P<.001) after PTRA/S. PTRA/S resulted in significantly lower complication rates of respiratory (0.8% vs. 12.5%, P<.001), digestive (0.2% vs. 23.5%, P<.001), urinary (1.2% vs. 7.7%, P<.001) and wound disruption (0.9% vs. 4.8%, P<.05).
Table 3.
Perioperative complications and length of stay after PTRA/S and open repair for patients with a diagnosis of renal fibromuscular dysplasia
| PTRA/S | Open Repair | P | |
|---|---|---|---|
| Mortality | 1.6 | 7.8 | < .001 |
| Discharge Home | 95.8 | 92.6 | < .001 |
| LOS, mean (SD) | 2.8 (4.0) | 7.9 (5.6) | < .001 |
| Stroke | 2.3 | 0 | 0.30 |
| Cardiac | 1.4 | 2.7 | 0.06 |
| Respiratory | 0.8 | 12.5 | < .001 |
| Digestive | 0.2 | 23.5 | < .001 |
| Urinary | 1.2 | 7.7 | < .001 |
| Vascular | 1.1 | 0 | 0.21 |
| Peripheral Vascular | 1.3 | 9 | 0.11 |
| Wound Disruption | 0.9 | 4.8 | < .01 |
| Postoperative Infection | 0 | 0 | 1.00 |
| Hemorrhage/hematoma | 5.8 | 5.2 | 0.56 |
Values are in %, unless otherwise indicated.
Abbreviations: PTRA/S, percutaneous transluminal angioplasty/stenting; LOS, length of stay; SD, standard deviation.
Combined Procedures
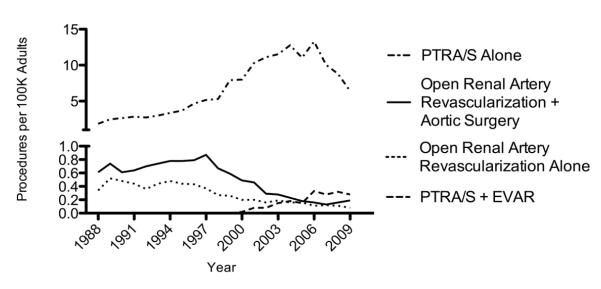
From 1988 to 2009, PTRA/S was the predominant procedure for treatment of renal artery atherosclerosis in the United States (Figure 5). In this outcome analysis, patients with combined open aortic and renal artery procedures and combined endovascular aortic and renal artery procedures were excluded from the categories of open renal artery repair alone and PTRA/S alone, respectively. PTRA/S alone greatly outnumbered the procedures done by open repair alone, combined open renal and open aortic surgery, and combined PTRA/S and EVAR. Combined open renal and open aortic surgery procedures outnumbered that of open repair alone throughout the study period. Procedures done by combined open renal and open aortic surgery decreased with a trend similar to procedures done by open repair alone.
Figure 5.
Annual number of inpatient procedures per 100K adults for treatment of renal atherosclerosis from 1988 to 2009 by PTRA/S alone, open repair alone, open repair combined with open aortic surgery, and PTRA/S combined with endovascular aneurysm repair (EVAR) of the aorta.
The frequency of PTRA/S + EVAR continued to increase steadily after 2000, reaching a plateau between 2006 and 2009 at 0.3 procedures per 100K adults. PTRA/S + EVAR surpassed both the number of procedures done by open repair alone and procedures done by combined open renal and open aortic surgery in 2006.
Overall mortality was higher after combined open renal and open aortic surgery compared to open renal repair alone (6.5% vs. 4.1%, P<.001) (Table 4). The proportion of patients discharged home was significantly higher after open repair alone (76.3% vs. 69.2%, P<.001). However, length of stay was similar. There were significantly more cardiac, respiratory, and urinary complications after combined open renal and open aortic surgery.
Table 4.
Perioperative complications and length of stay after open repair alone (OR), open repair combined with aortic surgery (OR + AS), PTRA/S alone, and PTRA/S combined with endovascular aortic aneurysm repair (PTRA/S + EVAR) for patients with a diagnosis of renal atherosclerosis.
| OR | OR+AS | P | PTRA/S | PTRA/S + EVAR |
P | |
|---|---|---|---|---|---|---|
| Mortality | 4.1 | 6.5 | < .001 | 0.9 | 1.2 | 0.04 |
| Discharge home | 76.3 | 69.2 | < .001 | 86.2 | 77.1 | < .001 |
| LOS, mean (SD) | 12.3 (11.4) | 12.7 (45.1) | 0.33 | 4.4 (6.3) | 4.2 (5.3) | < .001 |
| Stroke | 0.8 | 0.6 | 0.40 | 0.2 | 0.3 | 0.08 |
| Cardiac | 5.5 | 6.8 | < .001 | 0.8 | 2.1 | < .001 |
| Respiratory | 6.2 | 7.9 | < .001 | 0.3 | 0.3 | 0.76 |
| Digestive | 3.9 | 4.4 | 0.05 | 0.2 | 0.9 | < .001 |
| Urinary | 4.3 | 5.7 | < .001 | 0.7 | 1.5 | < .001 |
| Vascular | 0.1 | 0.1 | 0.58 | 0.1 | 0.7 | < .001 |
| Periph Vasc | 1.6 | 1.3 | 0.04 | 0.9 | 1.0 | 0.56 |
| Wound Disruption | 0.5 | 0.8 | < .01 | 0.1 | 0.4 | < .001 |
| Postop Infection | 1.6 | 1.3 | 0.07 | 0.2 | 0.4 | < .01 |
| Hemorrhage | 5.5 | 4.0 | < .001 | 5.5 | 4.5 | < .01 |
Values are in %, unless otherwise indicated.
Abbreviations: OR, open repair; AS, aortic surgery; PTRA/S, percutaneous transluminal angioplasty/stenting; EVAR, endovascular aortic aneurysm repair; LOS, length of stay; SD, standard deviation.
Although discharge home was less frequent after PTRA/S + EVAR compared to those undergoing PTRA/S alone (77.1% vs. 86.2%, P<.001), overall mortality remained similar (1.2% vs. 0.9%, P=.04). PTRA/S + EVAR had significantly higher complications compared to patients undergoing PTRA/S alone.
Physician Specialty Analysis
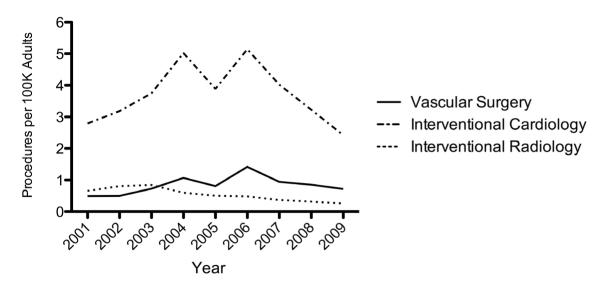
In 2001, interventional cardiologists (IC) performed the majority (70.8%) of all PTRA/S in patients with renal artery atherosclerosis, followed by interventional radiologists (IR) (16.7%) and vascular surgeons (VS) (12.5%). In 2009, IC continued to perform the majority of interventions (71.3%), followed by VS (21.1%) and IR (7.6%). IC performed an increasing number of PTRA/S between the years of 2001 (2.8/100K) and 2006 (5.1/100K) followed by a steep decrease to 2.4/100K in 2009 (Figure 6). Similarly, VS performed an increasing number of PTRA/S between 2001 (0.5/100K) and 2006 (1.4/100K) followed by a decline to 0.7/100K in 2009. IR experienced a steady decrease in procedures from 2001 (0.7/100K) to 2009 (0.3/100K).
Figure 6.
PTRA/S procedures per 100K adults performed by vascular surgeons, interventional cardiologist, and interventional radiologists from 2001-2009
Discussion
After a rapid increase in the number of PTRA/S procedures performed for the management of RAS between 1988 and 2006 a significant decrease in the number of PTRA/S procedures followed. This decrease was only seen in the inpatient setting, as the number of outpatient procedures remained constant. The management of FMD was similar compared to the intervention trend for renal atherosclerosis, but on smaller scale. The increase in mean age for patients undergoing PTRA/S from 1988-2009 may reflect an increase in the use of medical management early in renal atherosclerotic disease prior to intervention. The number of open repair procedures with or without open aortic surgery repair consistently decreased throughout the study period, reflecting a shift towards less invasive procedures. The trend towards minimally invasive procedures is further supported by an increase in the number of combined PTRA/S and EVAR procedures beginning in 2000.
The rise in the number of number of PTRA/S procedures performed prior to 2006 has previously been appreciated. Studies using NIS years 1988-2001 6 and Medicare data (1996, 1998, 2000) 7 have previously shown a similar rapid increase in percutaneous renal artery interventions prior to 2001, but our study now reveals a reversal of this trend with a decline in percutaneous renal artery therapy after 2006. This decrease in the number of interventional treatments for RAS may be explained by a lack of demonstrated benefit of procedural intervention in recent RCTs.
Three small RCTs, all with less than 136 total patients, were published between 1998 and 2000, comparing PTRA without stenting and medial management.1-3 In 1998, the Essai Multicentrique Medicaments vs Angioplastie (EMMA) study found that patients treated with angioplasty experienced more complications, but had better blood pressure control with half as many antihypertensive agents. However, BP response after 6 months and renal function was not different between the treatment groups.1 Similarly, the Scottish and Newcastle Renal Artery Stenosis Collaborative Group (SNRASCG) study found no difference in blood pressure or renal function between angioplasty and medical management.3 In 2000, the Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial reported no blood pressure or renal function difference between the two treatment groups using intention-to-treat analysis, but found the average daily drug dose was significantly lower in the angioplasty group.2 These trials were limited by factors such as small sample size, infrequent use of stenting, inclusion of patients with only mild-moderate RAS (>50%-60%), and high crossover rates from medical to revascularization treatments.
The demonstration of success with renal stenting 13 over angioplasty alone in 1999 spearheaded a number of trials to compare renal angioplasty with stenting and medical management. In 2009, the STAR trial, which included 140 patients with RAS > 50%, creatinine clearance < 80 mL/min, and a controlled BP < 140/90 mmHg, concluded that renal stenting had no benefit over medical therapy. 5 However, this study has been criticized because a large number of patients with mild-moderate renal disease and controlled hypertension were included, which under normal clinical circumstance, would not have been treated by renal artery stenting. Additionally, the criterion of renal artery stenosis of >50% may include patients who do not have hemodynamically significant stenoses.
In 2009, the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial, enrolling a total of 806 patients, found no difference in mortality, systolic and diastolic blood pressure, vascular events, renal function, or adverse renal events between the two treatment options. 4 However, the ASTRAL trial has also been under scrutiny and criticism; 41% of patients included in the trial had < 70% stenosis, patients who the treating clinician believed would benefit from renal stenting were excluded, and there was no defined treatment regimen for medical therapy. 14 The results from three other large RCTs have yet to be published; the RAVE trial, the RADAR study, and the CORAL trial. 15 Of these trials, the CORAL trail is unique in that it is the largest and most scientifically rigorous of all the current trials. 16 The weaknesses and criticisms for RCTs evaluating management of RAS are reflected in the 2005 ACC/AHA’s lack of definitive treatment guidelines for RAS treatment and the 2006 AHRQ analysis conclusion for a need of higher-quality evidence studies for the treatment of RAS. 17,18 These management guidelines combined with the mounting lack of evidence from RCTs to support PTRA/S likely contributed to the sharp drop in the number of PTRA/S starting in 2006, well before the reported results from the ASTRAL or STAR trials.
The number of open repairs, with and without aortic surgery, steadily declined throughout our study, reflecting the movement towards less invasive therapies. A retrospective study, evaluating renal angioplasty, stent placement, and bypass grafting, concluded that these procedures were all equally efficacious for control of renovascular hypertension, but the initial treatment cost for bypass grafting was significantly higher than that for angioplasty and stenting. 19 However, the ACC/AHA guidelines do recommend the use of surgical intervention in certain clinical situations. This includes patients with FMD exhibiting macroaneurysms or complex disease that extends into the segmental arteries, patients with atherosclerotic RAS exhibiting multiple small renal arteries or early primary branching of the main renal artery, and patients with atherosclerotic RAS in combination with pararenal aortic reconstruction (in treatment of aortic aneurysms or severe aortoiliac occlusive disease). 17 Despite the various situations where open revascularization with or without open aortic surgery has been indicated, our data have shown that the majority of interventions for RAS have shifted away from open repair.
Due to the growing popularity in EVAR procedures, we hypothesized that there would be a growing movement towards combined PTRA/S and EVAR procedures (PTRA/S + EVAR) for patients with both renal artery and aortic disease. The first two EVAR stent grafts to be approved by the US Food and Drug Administration (FDA) occurred in September 1999 and the ICD-9 code for EVAR was subsequently introduced in 2000. Thereafter, the number of combined PTRA/S + EVAR procedures has continued to rise. Our results demonstrate that PTRA/S + EVAR procedures resulted in higher postoperative complications compared to PTRA/S alone, as expected due to the increased procedural complexity and disease severity for patients undergoing combined procedures. We expect that the number of combined minimally invasive procedures will continue to increase in the future.
Interventional cardiologists, interventional radiologists, and vascular surgeons have all been involved with the procedural treatment of RAS. We found a decrease in the number of PTRA/S procedure performed for all three specialities after 2006, reflecting a universal movement away from PTRA/S. However, it is clear that interventional cardiologists continue to perform the largest number of PTRA/S for RAS.
The limitations to this study are inherent to studies using administrative data, such as the accuracy of hospital coding of comorbidities and complications. Alterations in coding methods in the NIS are therefore a potential for error, and in some instances, makes it difficult to differentiate perioperative complications from pre-existing conditions. Diagnosis codes may have been selected by clinical or financial importance, thereby explaining the fact that a lower proportion than expected of patients, particularly patients with open surgery intervention, had a diagnosis code for hypertension. There was no randomization between treatment options in this study and the selection of treatment method was at the treating physician’s discretion. However, our primary objective was to document changes in utilization of PTRA/S and open surgery for renal artery stenosis. In this regard the NIS is an ideal tool. We had access to outpatient data from 4 states that may not accurately reflect the entirety of the United States. However, the results in these diverse states were similar and the inpatient utilization represented by the NIS was corroborated by inpatient data from these states.
The NIS allows for evaluation of a large patient sample. Therefore, we are able to draw strong conclusions in the trend of RAS treatment in the United States. We ultimately conclude that interventions for RAS have declined since 2006, likely due in part to a lack of demonstrated benefit in RCTs. Analysis of the State Inpatient and Ambulatory Surgery databases have shown that this decrease in PTRA/S seen nationally is only found in the inpatient setting while outpatient procedures have remained stable. Nevertheless, as interventions have decreased nationally, PTRA/S remains the dominant procedural option compared to open interventions due to its lower rate of mortality and morbidity.
Supplementary Material
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Supplemental Appendix: ICD-9 procedure codes used to identify the physician specialty of vascular surgeons, general surgeons, interventional cardiologists, and interventional radiologists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures: Drs Schermerhorn, Hamdan, and Wyers are consultants for Endologix, Dr Wyers is a consultant for Boston Scientific, and Dr Schermerhorn is a consultant for Medtronic. For all other authors none were reported.
This paper was presented at the annual meeting of the SCVS in Las Vegas 2012
References
- 1.Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension. 1998 Mar;31(3):823–829. doi: 10.1161/01.hyp.31.3.823. [DOI] [PubMed] [Google Scholar]
- 2.van Jaarsveld BC, Derkx FH, Krijnen P, Pieterman H, Man in’t Veld AJ, Woittiez AJ, et al. ‘Hypertension resistant to two-drug treatment’ is a useful criterion to select patients for angiography: the ‘Dutch Renal Artery Stenosis Intervention Cooperative’ (DRASTIC) study. Contrib Nephrol. 1996;119:54–58. [PubMed] [Google Scholar]
- 3.Webster J, Marshall F, Abdalla M, Dominiczak A, Edwards R, Isles CG, et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens. 1998 May;12(5):329–335. doi: 10.1038/sj.jhh.1000599. [DOI] [PubMed] [Google Scholar]
- 4.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009 Nov 12;361(20):1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 5.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009 Jun 16;150(12):840–848. W150–841. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 6.Knipp BS, Dimick JB, Eliason JL, Cowan JA, Henke PK, Proctor MS, et al. Diffusion of new technology for the treatment of renovascular hypertension in the United States: surgical revascularization versus catheter-based therapy, 1988-2001. J Vasc Surg. 2004 Oct;40(4):717–723. doi: 10.1016/j.jvs.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TP, Soares G, Kim M. Increase in utilization of percutaneous renal artery interventions by medicare beneficiaries, 1996-2000. AJR Am J Roentgenol. 2004 Sep;183(3):561–568. doi: 10.2214/ajr.183.3.1830561. [DOI] [PubMed] [Google Scholar]
- 8.The Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS) [Accessed July 6, 2011]; http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 9.United States Census Bureau [Last accessed August 8, 2011]; http://www.census.gov/popest/
- 10.Csikesz NG, Simons JP, Tseng JF, Shah SA. Surgical specialization and operative mortality in hepato-pancreatico-biliary (HPB) surgery. J Gastrointest Surg. 2008 Sep;12(9):1534–1539. doi: 10.1007/s11605-008-0566-z. [DOI] [PubMed] [Google Scholar]
- 11.Schipper PH, Diggs BS, Ungerleider RM, Welke KF. The influence of surgeon specialty on outcomes in general thoracic surgery: a national sample 1996 to 2005. Ann Thorac Surg. 2009 Nov;88(5):1566–1572. doi: 10.1016/j.athoracsur.2009.08.055. discussion 1572-1563. [DOI] [PubMed] [Google Scholar]
- 12.Vogel TR, Dombrovskiy VY, Carson JL, Haser PB, Graham AM. Lower extremity angioplasty: impact of practitioner specialty and volume on practice patterns and healthcare resource utilization. J Vasc Surg. 2009 Dec;50(6):1320–1324. doi: 10.1016/j.jvs.2009.07.112. discussion 1324-1325. [DOI] [PubMed] [Google Scholar]
- 13.Bax L, Mali WP, Buskens E, Koomans HA, Beutler JJ, Braam B, et al. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol. 2003 Nov-Dec;16(6):807–812. [PubMed] [Google Scholar]
- 14.Sarac TP. Influence and critique of the ASTRAL and CORAL Trials. Semin Vasc Surg. 2011 Sep;24(3):162–166. doi: 10.1053/j.semvascsurg.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Fleming SH, Hansen KJ. Randomized clinical trials regarding management of atherosclerotic renovascular disease. Semin Vasc Surg. 2010 Sep;23(3):156–164. doi: 10.1053/j.semvascsurg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Seddon M, Saw J. Atherosclerotic renal artery stenosis: review of pathophysiology, clinical trial evidence, and management strategies. Can J Cardiol. 2011 Jul-Aug;27(4):468–480. doi: 10.1016/j.cjca.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006 Mar 21;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 18.Balk E, Raman G, Chung M, Ip S, Tatsioni A, Alonso A, et al. Comparative Effectiveness of Management Strategies for Renal Artery Stenosis. Rockville (MD): 2006. [PubMed] [Google Scholar]
- 19.Xue F, Bettmann MA, Langdon DR, Wivell WA. Outcome and cost comparison of percutaneous transluminal renal angioplasty, renal arterial stent placement, and renal arterial bypass grafting. Radiology. 1999 Aug;212(2):378–384. doi: 10.1148/radiology.212.2.r99au20378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.