Abstract
γ amino-butyric acid type-A receptors (GABARs) containing γ2 or δ subunits form separate pools of receptors in vivo, with distinct localization and function. We determined the rate of surface membrane insertion of native and recombinant γ2 and δ subunit-containing GABARs (γ2-GABARs and δ-GABARs). Insertion of the α-bungarotoxin binding site (BBS) tagged γ2 subunit (t-γ2)-containing GABARs in the surface membrane of HEK293 cells occurred within minutes and reached a peak by 30 min. In contrast, insertion of the BBS-tagged δ subunit (t-δ)-containing receptors required longer incubation and peaked in 120 min. Insertion of the t-γ2 subunit-containing receptors was not influenced by assembling α1 or α4 subunits. In contrast, insertion of the α4β3t-δ subunit-containing receptors was faster than those containing α1β3t-δ subunits. The rate of insertion of native GABARs in the surface membrane of cultured hippocampal neurons, determined by an antibody saturation assay, was similar to that of the recombinant receptors expressed in HEK293 cells. Insertion of the γ2-GABARs was rapid and new γ2-GABARs were detected on the surface membrane of cell soma and dendrites within minutes. In contrast, insertion of the δ-GABARs was slow and newly inserted receptors were initially present only in the surface membrane of cell soma and later also appeared over the dendrites. Thus the rate of insertion of GABARs was dependent on their subunit composition.
Introduction
The γ-amino butyric acid type-A receptors (GABARs) mediate inhibitory neurotransmission in the forebrain and spinal cord. Sixteen subunits, α1-6, β1-3, γ1-3, δ, ε, π and θ, co-assemble to form pentameric receptors (Whiting, 2003). The majority of native GABARs in the hippocampus are composed of 2α, 2β and either a γ2 or δ subunit (Sperk et al., 1997; Sun et al., 2004). Subunit composition determines the surface membrane localization, kinetics, and pharmacological properties of GABARs (Olsen and Sieghart, 2009). The γ2 subunit-containing receptors (γ2-GABARs) are expressed at the synaptic and extrasynaptic membranes, whereas the δ subunit-containing receptors (δ-GABARs) are exclusively extrasynaptic (Nusser et al., 1998; Wei et al., 2003). γ2-GABARs mediate synaptic and tonic inhibition, whereas δ-GABARs mediate only tonic inhibition (Brickley et al., 1996; Farrant and Nusser, 2005). The γ2 and δ subunits also assemble with distinct α subunits. The majority of γ2-GABARs expressed in the hippocampus contain α1 subunits, while smaller fractions contain α2, α3, α4 or α5 subunits. On the other hand, δ-GABARs expressed on hippocampal dentate granule cells contain α4 subunits, whereas those expressed on interneurons contain α1 subunits (Pirker et al., 2000; Glykys et al., 2007).
The γ2 and δ subunits do not co-assemble in vivo (Quirk et al., 1995; Araujo et al., 1998; Jechlinger et al., 1998). However, it is not known whether the characteristics of secretion of γ2- and δ-GABARs are similar or distinct. Aspects of the exocytosis of GABARs are partially understood (Chen and Olsen, 2007; Twelvetrees et al., 2010; Vithlani et al., 2011). A prior study demonstrated the rapid insertion of tagged β3 subunit-containing GABARs at the surface membrane of cultured hippocampal neurons, and all receptors were inserted at extrasynaptic sites (Bogdanov et al., 2006). The γ2-GABARs, which can diffuse between synaptic and extrasynaptic domains (Triller and Choquet, 2005), become trapped in the synapses through interaction with gephyrin (Essrich et al., 1998). These observations raise the possibility that γ2- and δ-GABARs could be inserted via the same vesicles and that δ-GABARs remain extrasynaptic, while γ-GABARs move laterally and become incorporated at the synapses. Alternately, insertion of γ2- and δ-GABARs could be distinct.
We compared the rate of surface membrane appearance of γ2- and δ-GABARs in cultured hippocampal neurons and determined the influence of co-assembled α1 or α4 subunits on their exocytosis using γ2 and δ subunits tagged with the α-bungarotoxin binding site.
Materials and methods
Materials
All the common chemicals were procured from Sigma Aldrich (St. Louis, MO). Bovine serum albumin and normal goat serum were obtained from Jackson Immuno-research (West Grove, PA).
Antibodies
A mouse monoclonal anti-γ2 subunit antibody directed against an epitope in the N-terminal extracellular domain was used at 1 μg/ml dilution. This antibody has been previously characterized for its reactivity and specificity (Joshi et al., 2011; Rannals and Kapur, 2011). A monoclonal antibody against an epitope at the extracellular N-terminal region of the δ subunit, generated in our laboratory in cooperation with Neuromab facility (clone N151/3.3), was used at 3 μg/ml dilution. The antibody was synthesized at the Lymphocyte Culture Center, University of Virginia. Immunochemical analysis performed as before (Mangan et al, 2005) revealed reactivity of this antibody with cultured hippocampal neurons (supplementary figure 1A). The immunoreactivity (IR) of anti-δ subunit antibody N151/3.3 was prominently present over the soma, and little IR was observed over the dendrites (supplementary figure 1A). This pattern of immunoreactivity was similar to that reported previously in cultured hippocampal neurons using rabbit anti-δ subunit antibody (Mangan et al, 2005)(supplementary figure 1B). The mouse monoclonal anti-δ subunit antibody also reacted with a single protein in the lysates isolated from HEK293 cells expressing a tagged δ subunit (supplementary figure 2C).
Hippocampal neuronal cultures
All animals were handled according to a protocol approved by the University of Virginia Animal Care and Use Committee, and efforts were made to minimize animal stress and discomfort. Cultures of dissociated hippocampal pyramidal neurons were made from embryonic day 18 rat fetuses as described previously (Goslin K et al., 1998; Goodkin et al., 2005). Neurons were co-cultured on glial layers for 12–14 days to allow for the formation of GABAergic synapses (Swanwick et al., 2006). Low density cultures (10,000 cells/cover glass) were used in these studies.
Antibody saturation assay
The rate of appearance of γ2- and δ-GABARs at the surface membrane was studied using an antibody saturation technique (Lu et al., 2001; Rosenberg et al., 2001). The neurons were cooled to 14°C by sequential incubation in PBS at RT for 3 min and in cold PBS at 14°C for 3 min. The neurons were then incubated with a saturating concentration of anti-γ2 or anti-δ subunit antibodies (20 μg/ml) at 14°C for 45 min. Unbound primary antibody was removed by quick repeated washes, and neurons were incubated at 37°C in culture medium for various time periods. The neurons were fixed with 4% paraformaldehyde, and non-specific sites were blocked in a blocking solution (0.1% BSA and 0.05% Normal Goat Serum in PBS). Anti-γ2 or anti-δ subunit antibodies were labeled with Alexa fluor 594 using an antibody labeling kit from Invitrogen (Carlsbad, CA), according to the manufacturer’s instructions. Neurons were then incubated with Alexafluor 594-conjugated anti-γ2 or anti-δ subunit (5 μg/ml) antibodies overnight at 4°C in the dark. In every experiment, a parallel culture was fixed immediately following incubation with unlabeled primary antibody in order to confirm the successful blockade of surface-expressed receptor epitopes at the beginning of the assay (time 0). In another parallel culture, the incubation with a saturating concentration of unlabeled antibody was omitted, and the culture was exposed to labeled primary antibody in order to determine the total number of surface-expressed receptors. Newly inserted receptors were expressed as a fraction of the total surface expressed receptors.
Construction and characterization of α-bungarotoxin-tagged γ2 and δ subunits
A 13-amino acid (WRYYESSLEPYPD) α-bungarotoxin (α-BT) binding site (BBS) was added at the N-terminus of the γ2 (t-γ2) and δ (t-δ) subunits (Supplementary fig. 2A). Both strands of cDNAs were sequenced to confirm the coding sequence. HEK293 cells were transfected with 1 μg cDNA/35 mm culture plate using a Lipotectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The expression of t-γ2 or t-δ subunits in the transfected HEK293 cells was confirmed using cell lysates corresponding to 50 μg proteins in a standard Western blotting assay. Signals of ~ 40 kD and ~ 60 kD were observed in t-γ2- and t-δ-expressing cells, respectively, and matched the predicted size of the tagged subunits (Supplementary fig. 2B and C).
Electrophysiology
Standard whole-cell patch clamp technique was used to confirm the function of t-γ2 and t-δ subunit-containing receptors expressed in HEK293 cells. Cells were transfected with cDNAs encoding α1, β3, t-γ2 or t-δ subunits along with GFP (1:1:1:0.2 μg/35 mm plate) and used 48 hrs after transfection. Recordings were performed as described previously (Saxena and Macdonald, 1994). To confirm the function of t-γ2 subunit-containing receptors, the effects of 10 μM GABA in the presence or absence of 100 nM Diazepam was studied (Supplementary fig. 2D). Diazepam augmented GABA-evoked currents in cells expressing α1β3t-γ2 and α1β3wt-γ2 subunit-containing receptors by 2.6 ± 0.4-fold (n=7) and 1.4 ± 0.06 fold (n= 12) respectively. To confirm functional expression of t-δ subunit-containing receptors, effect of allopregnanolone (30 nM) on GABA (1 um) evoked currents was also studied (Supplementary fig. 2E). Recombinant receptors containing t-δ subunits were also functional, in the cells expressing α1β3t-δ or α1β3wt-δ subunit-containing receptors, 1 μM GABA-evoked currents were augmented by 30 nM allopregnanolone 6 ± 3 fold (n= 7) and 5.1 ± 0.9 fold (n=3) respectively. These studies confirmed the ability of t-γ2 subunits to assemble with α1β3 subunits to form functional receptors.
α-BT labeling and insertion assay
HEK293 cells grown on LabTek chamber slides (Nalge Nunc International, Rochester, NY, USA) coated with 100 μg/ml poly-L-Lysine and polyethylene amine (1:500 dilution) were transfected with cDNAs encoding t-γ2 or t-δ, along with α1 or α4 and β3 subunits and GFP, as described above. The expression of GFP was used to identify transfected cells. Forty-eight hours after transfection, surface-expressed α1β3t-γ2, α4β3t-γ2, and α1β3t-δ subunit-containing receptors were detected by incubating cells with Alexafluor 594-conjugated α-BT (0.1 μg/ml) for 5 min at 14°C. α4β3t-δ subunit-containing receptors were detected by incubation in 0.5 μg/ml α-BT. Receptors containing t-γ2 and t-δ subunits but not wt-γ2 or wt-δ subunits were able to bind to α-BT (Supplementary fig. 2F and G). Therefore, although the β3 subunits of GABARs can also bind to α-BT (McCann et al., 2006), the binding observed here was due to specific interaction with t-γ2 or t-δ subunits. GFP fluorescence was evident in approximately 90% of the cells indicative of transfection efficiency. Surface α-BT fluorescence was observed in 60–65% of cells expressing t-γ2 subunit-containing receptors. On the other hand, α-BT binding was observed in only 10–15% and 2–3% of transfected cells expressing α1β3t-δ and α4β3t-δ subunit-containing receptors, respectively. This efficiency of expression of t-γ2 and t-δ subunit-containing receptors was similar to that reported previously (Saxena and Macdonald, 1994; 1996).
To determine the appearance of new recombinant receptors at the surface membrane, cells were incubated in 50 μg/ml unlabeled α-BT for 15 min at 14°C in order to block existing surface receptors at the beginning of the experiment. Cells were then incubated at 37°C to allow for the insertion of receptors; then, newly inserted receptors were labeled by incubation with Alexafluor 594-conjugated α-BT for 5 min at 14°C. As a control, in some cultures, the appearance of receptors was determined at RT (22–25°C). Receptor exocytosis was also studied in the presence of brefeldin-A (5 μg/ml), a fungal toxin known to block ER to Golgi vesicle transport.
Image acquisition and analysis
Neurons or HEK293 cells were visualized using a Nikon Eclipse TE200 fluorescent microscope equipped with a mercury lamp using a 60X, 1.4 N.A. lens as described previously (Goodkin et al., 2007). Only morphologically intact neurons were used in the study. Putative pyramidal neurons were selected from DIC images based on their morphological features described previously (Craig et al, 1993; Benson et al, 1994). The neurons with flat round cell body, dendrites with fine distal branches, and less phase-dense appearance were imaged. The interneurons, which generally have a fusiform cell body and phase-dense appearance, were omitted. The images were acquired by a Photometrics CoolSNAPcf CCD camera mounted on a Nikon Eclipse TE200 fluorescent microscope using Metamorph Imaging Software (Molecular Devices, Downington, PA). To determine immunoreactive areas, images were thresholded as described previously and then converted to binary images (Goodkin et al., 2007). The overall brightness and contrast of the images was adjusted for presentation.
6–7 neurons or HEK293 cells were imaged at each time point in each experiment, and the experiments were replicated 4–7 times. Background fluorescence, determined in the cultures incubated for “0 min”, was subtracted from the fluorescence obtained after incubation for various periods. The interaction between antigen-antibody or BBS-α-BT were stable over a period of 3 hrs (Bogdonov et al, 2006) (see results). Importantly only the last step in exocytosis, the appearance of receptors at the surface membrane was monitored in these assays. Hence, the increase in surface fluorescence over time was fitted to a single-phase exponential association equation, using the least square method, as described before (Passafaro et al., 2001; Hannan et al, 2012; Saliba et al, 2012). The R2 values ranged between 0.6–0.8 for these curves.
Statistical analysis
Each experiment was replicated 4–6 times with a total of 10–60 cells. Gaussian distribution of the data was confirmed using a Kolmogorov-Smirnov test. Values of surface fluorescence and half-life of insertion for each experiment were normally distributed. Hence all values are reported as mean ± SEM. Surface fluorescence and half-life of insertion of different receptor subtypes was compared using a t test. Significant differences are denoted wherever applicable.
Results
Insertion of t-γ2 and t-δ subunit-containing GABARs was distinct
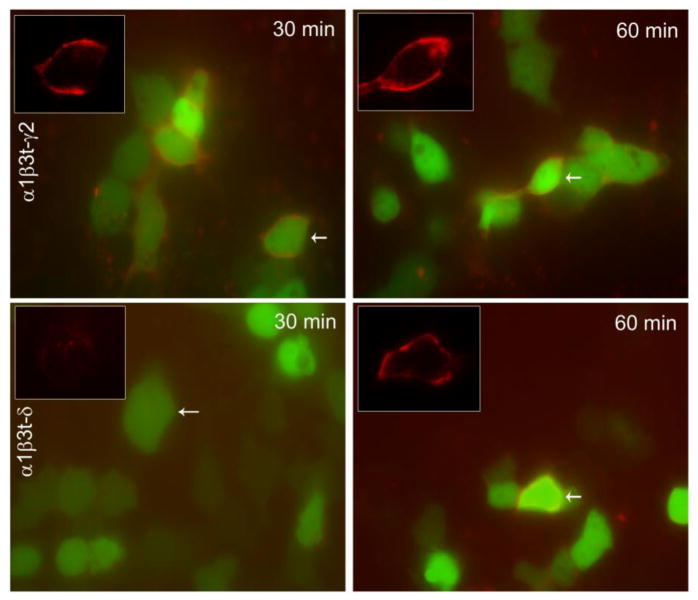
Insertion of the receptors containing t-γ2 or t-δ subunits assembled with α1β3 subunits was determined in HEK293 cells. Cells expressing these receptors were incubated with unlabeled α-BT to block the binding site (BBS) on existing surface receptors and then incubated at 37°C for 30 or 60 min. Newly inserted receptors were detected by incubation with labeled α-BT. Surface α-BT fluorescence (red) was evident in cells expressing α1β3t-γ2 subunit-containing receptors following incubation at 37°C for 30 as well as 60 min (Fig. 1). In contrast, surface α-BT fluorescence was minimal in the cells expressing α1β3t-δ subunit-containing receptors following incubation at 37°C for 30 min, but became visible after 60 min (Fig. 1). These observations raised the possibility that the time course of insertion of γ2- and δ-GABARs at the surface membrane could be distinct. In the subsequent studies the time course of insertion of γ2- and δ-GABARs was characterized.
Figure 1.
Rapid appearance of γ2-GABARs on surface membrane of HEK293 cells. Surface α-BT fluorescence in the HEK293 cells expressing α1β3t-γ2 or α1β3t-δ subunit-containing receptors. Following blockade of existing surface receptors with unlabeled α-BT, the cells were incubated at 37 °C for 30 or 60 min, and newly inserted receptors were detected by incubation with labeled α-BT (red). GFP fluorescence was used to detect transfected cells. Insets in each panel show red fluorescence of surface α-BT in single cells from each panel to highlight the differences.
Insertion of t-γ2 and t-δ subunit-containing receptors in HEK293 cells
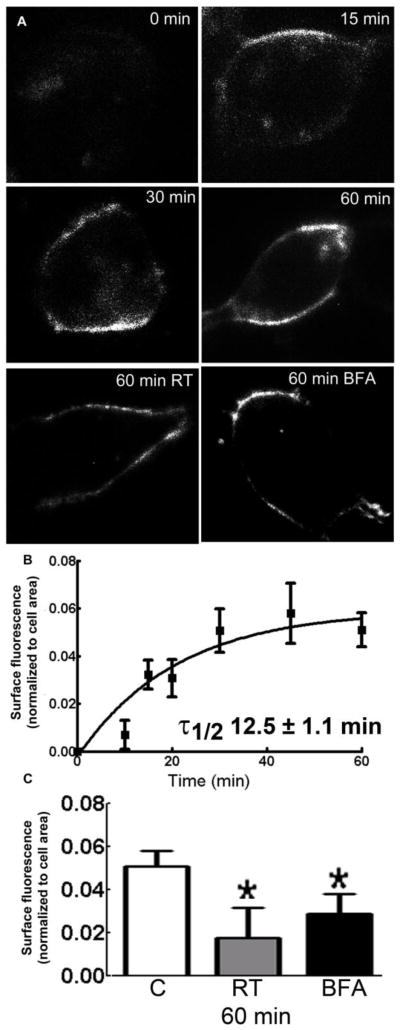
Newly inserted α1β3t-γ2 subunit-containing receptors appeared within minutes (Fig. 2A). Surface fluorescence increased with time, until it reached a plateau at 30 min (Fig. 2A, Table 1); 42 ± 6% and 43 ± 6% of all surface-expressed receptors were newly inserted within 30 and 60 min, respectively. The normalized surface fluorescent area was plotted as a function of time (Fig. 2B). Previous studies have reported that the interaction between BBS and α-BT is stable over a period of 3 hrs (Bogdonov et al, 2006), and we also found a stable interaction (data not shown). Thus the increase in surface fluorescence was due to appearance of new receptors at the surface membrane, and accordingly, the relationship was best described by a single-phase exponential association equation Y=Y0 + (Ymax−Y0)* (1−e(−k*time)), where Ymax was the IR area at the peak, Y0 was the IR area at time 0, and k was the time constant in minutes−1. The best fit of the data suggested an insertion time constant of 0.043 ± 0.007 min−1, half-time was 12.5 ± 1.1 min (Fig. 2B).
Figure 2.
Insertion of α1β3t-γ2 subunit-containing receptors in HEK293 cells. A: Representative images showing surface α-BT fluorescence in the cells incubated at 37°C for 0, 15, 30 and 60 min, or at RT (panel RT), or with brefeldin-A (5 μg/ml) for 60 min at 37°C (panel BFA). B: The α-BT surface fluorescence was normalized to the cell area, and plotted against the time of incubation at 37°C. C: As a control α-BT fluorescence corresponding to newly inserted receptors was also studied in the cells incubated at RT (gray) or with 5 μg/ml brefeldin-A (black, BFA) for 60 min. The α-BT fluorescence in the cells incubated at 37°C (white) is also plotted for comparison. *P<0.05 vs control (cells incubated at 37°C).
Table 1.
Insertion of native and recombinant γ2- and δ subunit-containing GABARs at the surface membrane.
| Receptor type | 5 min | 15 min | 30 min | 60 min | Total surface expressed |
|---|---|---|---|---|---|
| Native γ2- GABARs | 0.04 ± 0.02* | 0.085 ± 0.028* | 0.11 ± 0.03 | 0.09 ± 0.03* | 0.16 ± 0.05 |
| α1β3t-γ2 | - | 0.035 ± 0.01* | 0.050 ± 0.007* | 0.051± 0.007* | 0.11 ± 0.005 |
| α4β3t-γ2 | - | 0.027 ± 0.006* | 0.079 ± 0.011 | 0.09 ± 0.009 | 0.15 ± 0.02 |
| 30 min | 60 min | 120 min | 180 min | Total surface expressed | |
| Native δ-GABARs | 0.04 ± 0.02* | 0.08 ± 0.03* | 0.11 ± 0.02* | 0.13 ± 0.06* | 0.20 ± 0.04 |
| α1β3t-δ | 0.023 ± 0.004* | 0.024 ± 0.005* | 0.035 ± 0.005* | 0.041 ± 0.007* | 0.097 ± 0.01 |
| α4β3t-δ | 0.018 ± 0.004* | 0.033 ± 0.005 | 0.043 ± 0.008 | 0.042 ± 0.004 | 0.077 ± 0.01 |
Insertion of native receptors expressed in cultured hippocampal neurons and recombinant receptors expressed in HEK293 cells. Values represent surface immunoreactive area normalized to the cell area, mean ± SE from 30–36 cells/ 4–7 replicates each.
p<0.05 vs total surface expressed receptors.
We confirmed that the increase in surface fluorescence over time was due to the insertion of new receptors. The synthesis and insertion of receptors is mediated by temperature sensitive enzymes. Therefore incubation at room temperature is likely to slow insertion. Incubation at room temperature slowed the insertion of α1β3t-γ2 subunit-containing receptors (Fig. 2A, C). The surface α-BT fluorescence in the cells incubated at RT for 60 min was 0.036 ± 0.018 (n=20 cells). Thus, only 11 ± 6% of all surface expressed receptors were newly inserted after 60 min of incubation at RT compared to 42 ± 6% at 37°C (p<0.05).
Additionally, blockade of ER to Golgi protein transport is also expected to inhibit surface insertion of newly synthesized receptors. Fungal toxin brefeldin-A blocks protein traffic from the ER to Golgi bodies by inhibiting ADP-ribosylation factor (ARF) (Helms and Rothman, 1992). Incubation at 37 °C for 60 min in the presence of brefeldin-A (5 μg/ml) also slowed the insertion (Fig. 2A, C), the surface α-BT fluorescence in these cells was 0.049 ± 0.007 (n=15 cells), which corresponded to 22 ± 10% of all surface expressed receptors (p<0.05).
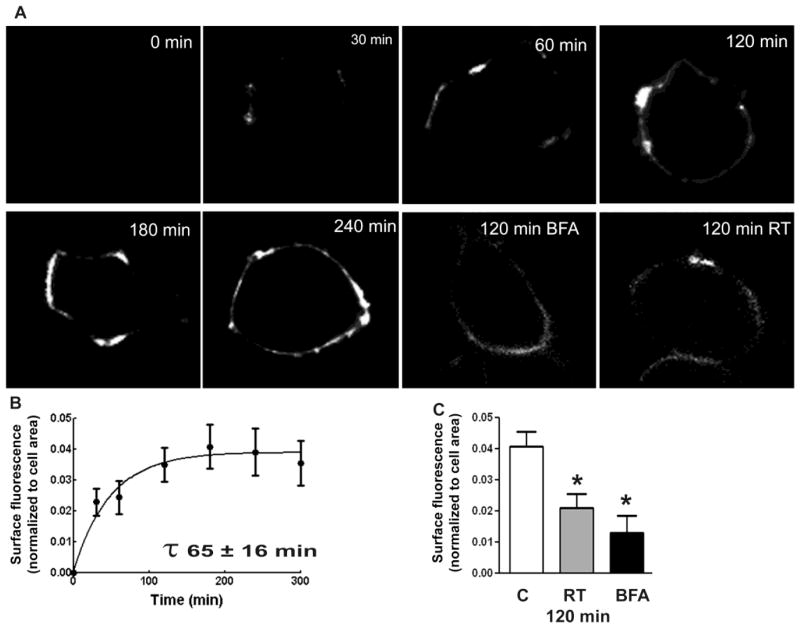
The insertion of α1β3t-δ subunit-containing receptors was also studied. Initial appearance of α-BT surface fluorescence required 30 min for α1β3t-δ subunit-containing receptors, the surface fluorescence peaked at 120 min and remained stable thereafter (Fig. 3A, Table 1). The time constant of the reaction was 0.015 ± 0.003 min−1, and the reaction half-life was 65 ± 16 min (Fig. 3B). The surface fluorescence in cells incubated at RT for 120 min was 0.023 ± 0.01 (n=10 cells) whereas that in the cells incubated for 120 min in the presence of brefeldin-A was 0.014 ± 0.008 (n=12 cells, p<0.05). During the incubation at 37 °C for 120 min, 40 ± 3%, of surface receptors were newly inserted, this number was lower in the cells incubated at RT (34 ± 17%, n=12 cells, n=10 cells) or with brefeldin-A (22 ± 8%). Thus, the insertion of α1β3t-δ subunit-containing receptors required a longer time, and this process was also temperature and brefeldin-A-sensitive (Fig. 3C).
Figure 3.
Insertion of α1β3t-δ subunit-containing receptors in HEK293 cells. A: Representative images showing surface α-BT fluorescence corresponding to newly inserted receptors after 0, 30, 60, 120, 180 and 240 min of incubation. Representative images showing surface α-BT fluorescence in the cells incubated at RT or with brefeldin-A for 120 min (BFA) are also shown. B: Surface α-BT fluorescence normalized to the cell area was plotted as a function of time. C: Surface fluorescence of newly inserted receptors normalized to the cell area in the cells expressing α1β3t-δ subunit-containing receptors, incubated at 37°C (white bars), at RT (gray bars) or at 37°C with 5 μg/ml brefeldin-A (black bars, BFA) for 120 min. * p<0.005 vs control (cells incubated at 37°C).
These studies revealed that the insertion of γ2-GABARs was significantly faster than that of δ-GABARs expressed in HEK293 cells (Fig. 2B and 3B). However in the hippocampal DGCs the δ subunits assemble with the α4 subunits and it was possible that native subunit assembly-containing GABARs were inserted more efficiently in the surface membrane. Therefore, insertion of the α4β3t-δ subunit-containing receptors was also studied in HEK293 cells.
Insertion of α4β3t-δ subunit-containing receptors was faster than that of α1β3t-δ subunit-containing receptors
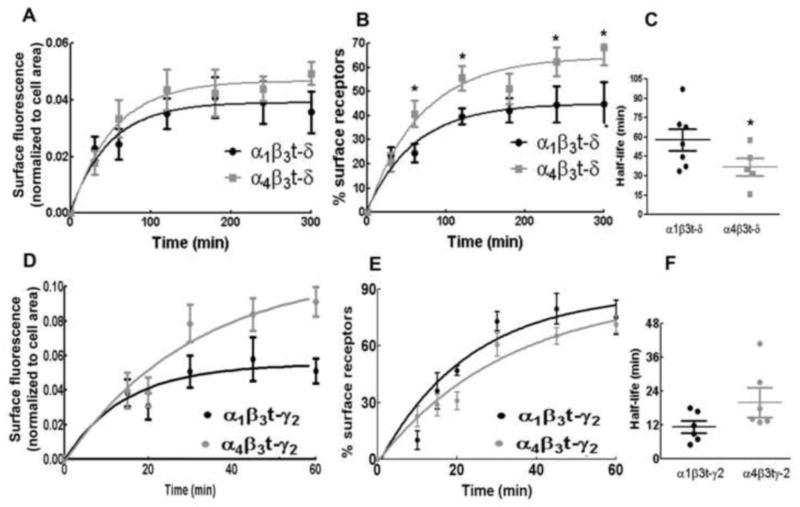
Surface α-BT labeling corresponding to newly inserted surface receptors was compared between the cells expressing α4β3t-δ and α1β3t-δ subunit-containing receptors (table 1, Fig. 4A). However this number could be influenced by the total number of surface expressed receptors. Hence α-BT fluorescence corresponding to the newly inserted receptors was expressed as a percentage of α-BT fluorescence corresponding to the total surface expressed receptors (Fig. 4B). The reaction time constant calculated from these curves revealed that insertion of α4β3t-δ subunit-containing receptors was faster than those containing α1β3t-δ subunits (Fig. 4C). The half-life of insertion of α4β3t-δ subunit-containing receptors was shorter than that of α1β3t-δ subunit-containing receptors (37 ± 7 min vs 58 ± 8 min, p<0.05). Thus α1 or α4 subunits appeared to influence the rate of insertion of δ-GABARs. Furthermore, the insertion of α4β3t-δ subunit-containing receptors was also significantly slower than that of α1β3t-γ2 subunit-containing receptors (37 ± 7 min vs 12 ± 1 min, p<0.05).
Figure 4.
Influence of α1 and α4 subunits on the insertion of γ2 or δ subunit-containing receptors. A: The time course of insertion of α1β3t-δ or α4β3t-δ subunit-containing receptors. The increase in surface α-BT fluorescence normalized to cell area was plotted as a function of time. B: Newly inserted receptors were plotted as a percentage of total surface expressed receptors over time. C: Average half-life of insertion of α1β3t-δ or α4β3t-δ subunit-containing receptors. * p<0.05. D: Comparison of the insertion of α1β3t-γ2 or α4β3t-γ2 subunit-containing receptors. E: Newly inserted receptors expressed as a percentage of total surface expressed receptors were plotted against time. F: Average half-life of insertion of α1β3t-γ2 or α4β3t-γ2 subunit-containing receptors.
We similarly determined whether α1 or α4 subunits also influenced the insertion of t-γ2 subunit-containing receptors (Fig. 4D–F). However the rate of insertion of t-γ2 subunit-containing receptors assembled with α1 or α4 subunits was similar. α4β3t-γ2 subunit-containing receptors also appeared at the surface membrane within minutes (Table 1). The half-time of the insertion of receptors containing α1β3t-γ2 or α4β3t-γ2 subunits was similar, 12 ± 1 min and 19 ± 4 min, respectively (p>0.05). The fraction of surface-expressed receptors replaced by newly inserted receptors within 30 min was also similar (42 ± 5% and 36 ± 7%, respectively). Thus α1 or α4 subunits did not influence insertion of γ2-GABARs.
Studies in HEK293 cells expressing recombinant receptors revealed that γ2 and δ subunits primarily determined the rate of insertion of GABARs. The insertion of γ2-GABARs was significantly faster than that of δ-GABARs. However, HEK293 cells may not fully express the specialized mechanisms involved in targeting and secretion of surface proteins in neurons (Kennedy and Ehlers, 2011; Vithlani et al., 2011). Therefore, in the subsequent studies the rate of insertion of native γ2-GABARs and δ-GABARs was determined in cultured hippocampal neurons.
Appearance of new γ2-GABARs on the surface membrane
The appearance of new γ2-GABARs over the surface membrane was determined in cultured hippocampal pyramidal neurons using an antibody saturation assay. All surface receptors were blocked with the unlabeled primary antibody at the beginning of the experiment, and newly inserted receptors were detected after incubation at 37°C by a labeled primary antibody directed against an epitope in the extracellular domain of the γ2- subunit.
Immunoreactivity (IR) of newly inserted γ2-GABARs appeared over the surface membrane within minutes (Fig. 5A). Surface γ2 subunit IR appeared within 5–15 min and reached a steady state in 30–45 min (Table 1). The IR appeared simultaneously over the cell soma and dendrites at all times. The normalized surface IR area was plotted as a function of time (Fig. 5B). Similar to that in HEK293 cells, the best fit of data to a single-phase exponential association equation revealed half-life of 14 ± 7 min (n=18–40 cells from 3–6 replicates). In each experiment, some cultures were incubated with fluorescently labeled primary antibody, without incubation with a blocking antibody, in order to determine the total number of surface-expressed receptors. After 30 min, newly inserted receptors constituted 62 ± 12% of the surface-expressed receptors.
Figure 5.
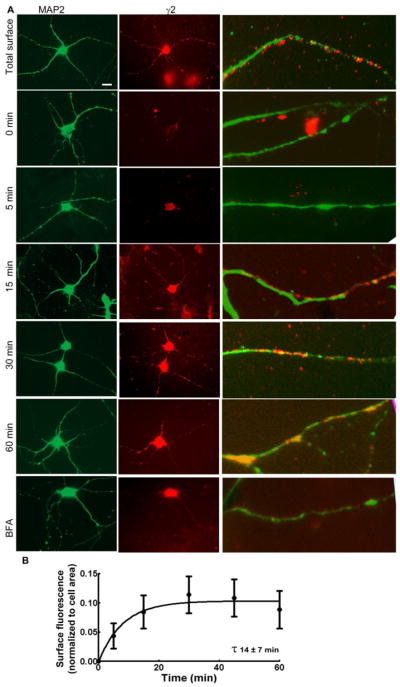
The time course of the insertion of γ2-GABARs in cultured hippocampal neurons using an antibody-saturation assay. A: Representative images showing IR of newly inserted γ2-GABARs (red) over the cell soma and dendrites after 0, 5, 15, 30 and 60 min of incubation at 37°C. Total surface expression of γ2-GABARs is shown in the panel total surface. Immunoreactivity of MAP2 (green) was used to identify the dendrites. Image BFA shows immunoreactivity of newly inserted γ2-GABARs in a representative neuron incubated with brefeldin-A (5 μg/ml) for 60 min at 37°C. The white bar in the γ2 total surface panel corresponds to 10 μm. Panels on the right are magnified portions of dendrites to show newly inserted receptors. B: Surface γ2 subunit IR was normalized with the cell area and plotted as a function of time. A single-phase association equation best fit the data. n=25–30 neurons from 4–5 replicates per time point.
Incomplete blockade of existing surface receptors by the unlabeled antibody could contribute to the observed γ2-GABAR IR. In order to control for incomplete blockade of existing surface receptors, labeled anti-γ2 subunit antibody was added immediately following incubation with unlabeled antibody. Surface IR was absent when either anti-γ2 or anti-δ subunit antibodies were used (Fig. 5A, time 0), and confirmed the complete blockade of epitopes on existing surface receptors by the unlabeled antibody.
The blocking antibody could dissociate from the receptor during incubation (at 37°C), which would confound the results. The stability of the antibody-antigen complex was confirmed by fixing cultures immediately following incubation with blocking antibodies. Fixed cultures were incubated at 37°C for 0 hr or 1 hr, and the antigen-bound antibody was detected with a fluorescent secondary antibody. If dissociation of the antibody-antigen complex occurred, then fluorescence would decline. However, the surface fluorescence in neurons incubated at 37°C for 1 hr before addition of labeled secondary antibody was 1.0 ± 0.04 (n=6), similar to that in neurons, in which the labeled secondary antibody was added immediately following fixation (1.0 ± 0.1, n=6, p>0.05); this suggested that the antibody-antigen complex was stable.
Furthermore, incubation with brefeldin-A appeared to reduce surface membrane insertion of receptors (Fig. 5A, panel BFA). Surface fluorescence in cultures incubated with brefeldin-A was lower than that in cultures incubated without brefeldin-A (0.03 ± 0.01 vs 0.07 ± 0.01, n=15 cells 3 replicates).
Time course of insertion of δ-GABARs
Appearance of new δ-GABARs over the surface membrane was studied similar to that for γ2-GABARs. However appearance of surface δ subunit IR was slow and the δ subunit IR was clearly evident on the surface membrane after 60 minutes of incubation, and remained restricted to the cell soma (Fig. 6A). The δ subunit IR increased with incubation time to reach a peak at 2 hrs (Table 1), and was visible over the cell soma as well as dendrites at this time. The data were fit to a single-phase association function (Fig. 6B). The half-life of the process was 52 ± 16 min. After 120 min, 54 ± 11 % of the surface-expressed receptors were newly inserted.
Figure 6.
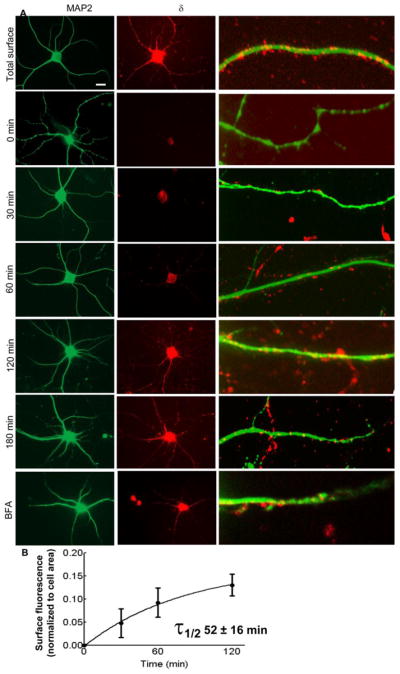
The time course of insertion of δ-GABARs in cultured hippocampal neurons. A: Representative images showing IR of newly inserted δ-GABARs (red) over the cell soma and in the dendrites of neurons incubated at 37°C for 0, 30, 60, 120 and 180 min. Total surface expression of δ-GABARs is shown in the panel total surface. Surface δ subunit IR in the neurons incubated with brefeldin-A (5 μg/ml) for 60 min is also shown (BFA). Panels on the right show δ subunit IR over the dendrites. The white bar corresponds with 10 μm. MAP2 IR (green) was used to identify dendrites. Panels on the right are magnified portions of dendrites showing δ subunit IR. B: Surface δ subunit IR was normalized to the cell area and plotted as a function of time (gray). A single-phase association equation best fit the data. n=25–30 neurons from 4–5 replicates per time point.
The δ subunit and anti-δ subunit antibody complex was also stable and the surface fluorescence in neurons incubated with anti-δ subunit antibody was stable over a 3-hour period of incubation (0.7± 0.1 vs 0.5 ± 0.1, n=6, p>0.05). Furthermore, insertion of δ-GABARs was also sensitive to blockade by Brefeldin-A (Fig. 6A). In the neurons incubated with brefeldin-A, surface δ subunit IR was lower than those incubated without brefeldin-A (0.02 ± 0.01 vs 0.06 ± 0.02, n= 10–36 cells from 4 replicates).
To ensure that differences in the expression of γ2- and δ-GABARs did not influence measurement of rate of insertion of GABARs, the fraction of newly inserted receptors was normalized to total surface expression of the receptors (Fig. 7). The data was best described by a single-phase exponential association equation with half-life 9 ± 2 min and 36 ± 7 min for γ2-GABARs and δ-GABARs respectively (p<0.05). Thus differences in the surface expression of γ2- and δ-GABARs appeared unlikely to contribute to observed differences in the half-life of insertion of these receptors.
Figure 7.
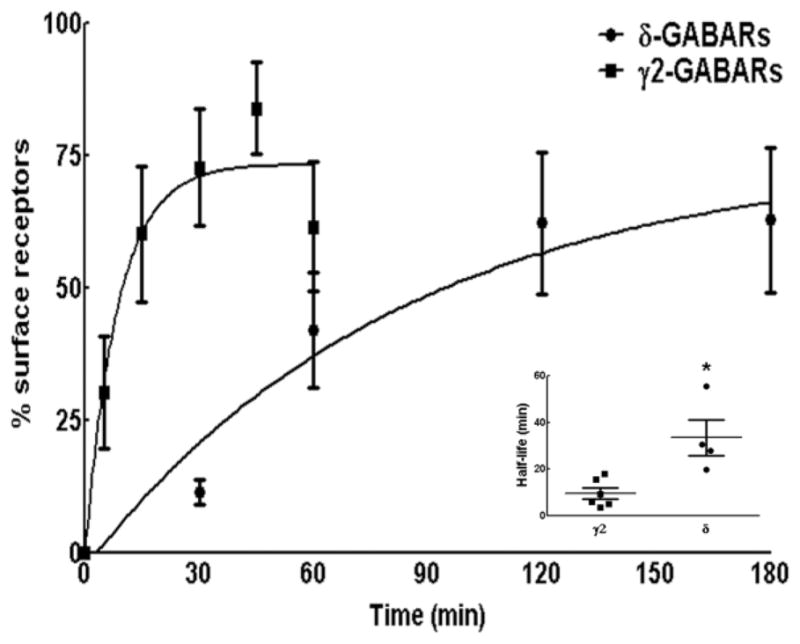
Comparison of the time course of γ2-GABAR and δ-GABAR insertion over the surface membrane. Newly inserted γ2-GABARs and δ-GABARs over time were plotted as a percentage of surface expressed receptors. n=25–30 neurons from 4–5 replicates per time point. Inset shows half-life of insertion in individual experiments with mean and SEM. N=4–6. * p<0.05.
Furthermore, the rate of insertion of native or recombinant γ2-GABARs (14 ± 7 min vs. 12 ± 3 min) and δ-GABARs (52 ± 16 vs 37 ± 7 min) were similar. Together these studies revealed distinct rate of insertion of γ2-GABARs and δ-GABARs in cultured hippocampal neurons and HEK293 cells.
Discussion
The major findings of this study are 1) in HEK293 cells and cultured hippocampal neurons, surface membrane insertion of γ2-GABARs was faster than that of δ-GABARs and 2) α1 or α4 subunits did not influence the rate of insertion of γ2-GABARs, but the insertion of α4β3δ-GABARs was faster than that of α1β3δ-GABARs.
This study used recombinant receptors containing t-γ2 and t-δ subunits expressed in HEK293 cells and an antibody saturation assay in cultured hippocampal neurons to determine the rate of insertion. The BBS derived from α subunit of nicotinic acetylcholine receptors binds to α-BT with high affinity (Scherf et al., 1997). It has been used in the past to study membrane expression and/or insertion of AMPA receptors, GABA-B receptors, and Kv4.2 channels (Sekine-Aizawa and Huganir, 2004; Wilkins et al., 2008; Moise et al., 2010), and that of GABARs via use of tagged β3 or α4 subunits (Bogdanov et al., 2006; Abramian et al., 2010; Saliba et al., 2012). In accordance with previous studies, addition of BBS was functionally silent and did not influence trafficking of GABARs. Use of t-γ2 and t-δ subunits in this study was also advantageous as only ternary receptor complexes were detected and any influence of αβ subunit-containing receptors was avoided.
An antibody saturation assay was used to detect insertion of native receptors in cultured hippocampal pyramidal neurons. Although, the cultures used in this study were a mixture of pyramidal neurons and interneurons, their morphological features are distinct (Craig et al, 1993; Benson et al, 1994). The technique of antibody saturation assay has been used to determine internalization of GABARs, and insertion of glycine and AMPA receptors (Lu et al., 2001; Rosenberg et al., 2001; Goodkin et al., 2005; 2008; Joshi and Kapur, 2009; Rannals and Kapur, 2011). Mouse monoclonal antibodies used in this study identified a single epitope and the erroneous detection of the same receptor during the second round of incubation with labeled primary antibody, which may occur with polyclonal antibodies, was avoided. The observed rate of insertion of γ2-GABARs was similar to the rate of insertion of GABARs reported previously using tagged β3 subunits (13 ± 2 min) (Saliba et al., 2012). It is possible that differences in the expression of γ2- and δ-GABARs masked the slower insertion of δ-GABARs in the study by Saliba and colleagues. The rate of insertion of GABARs could also be distinct in principal neurons and interneurons, and further studies are necessary to determine if that is the case.
The exocytosis of GABARs is a multi-step processes regulated by various proteins (Luscher et al, 2011). However, in this study only the appearance of receptors at the surface membrane was monitored. Each newly appearing receptor had a single antibody- or α-BT-binding site. Furthermore, the interactions between antibody-antigen or BBS-α-BT were stable over a period of 3 hrs. Hence the increase in fluorescence over time was solely dependent on the rate at which new receptors appeared on the surface membrane. A single-phase exponential association equation best fit the data, which was in accordance with previous studies, which used similar techniques to measure the kinetics of insertion or removal of AMPA, GABAA, and GABAB receptors (Ehlers, 2000; Passafaro et al., 2001; Goodkin et al, 2005; Wilkins et al, 2008; Joshi and Kapur, 2009; Rannals and Kapur, 2011; Hannan et al, 2012; Kuver et al, 2012; Saliba et al, 2012). In contrast, the insertion of glycine receptors appeared to follow a bi-phasic pattern due to different rate of insertion of receptors over soma and dendrites (Rosenberg et al, 2001). Further studies are necessary to determine somatic vs dendritic differences, if any, in the rate of insertion of GABARs.
The γ2 and δ subunits regulated the rate of insertion of GABARs, insertion of γ2-GABAR was much faster that that of δ-GABARs. The rate of AMPA and GABAB receptor insertion is also regulated by assembling subunits; GluR2 subunit-containing receptors are inserted faster than those containing GluR1 subunits, and similarly R1b subunit-containing GABAB receptors are inserted faster than those containing R1a subunits (Passafaro et al., 2001; Hannan et al, 2012). It was interesting to note that new γ2-GABARs appeared simultaneously over cell soma and dendrites of cultured hippocampal neurons, whereas δ-GABARs were initially evident over the cell soma and later appeared over the dendrites as well. It remains to be seen whether δ-GABARs were also inserted over dendrites but were below detection threshold at earlier time points.
ER exit is the rate-limiting step that regulates the delivery of proteins to the membrane. ER retention motifs (RXR) present on ATP-sensitive K+ channels, calcium channels, GluR2 subunits of AMPA receptors, 5-HT3B subunits of 5-hydroxytryptamine type 3 receptors, GB1 subunit of GABAB receptors, and NR2 subunits of NMDA receptors are shielded in fully assembled channels, leading to their exit from the ER (Zerangue et al., 1999; Bichet et al., 2000; Margeta-Mitrovic et al., 2000; Scott et al., 2001; Greger et al., 2002; Boyd et al., 2003). Interaction of assembled receptors with chaperon proteins also determines the rate of ER exit and transport. For example, Q/R editing of AMPA receptors leads to differential interaction with chaperon proteins SAP97 and PICK1, resulting in fast and slow ER exit respectively (Greger et al, 2002). Interaction of C terminal regions of NR1 subunit of NMDA receptors or AMPA receptor subunits with PDZ proteins also suppress their ER retention (Standley et al., 2000; Scott et al., 2001; Greger et al, 2002). Post-translational modifications such as glycosylation and phosphorylation also facilitate ER exit of AMPA receptors and N acetyl choline receptors respectively (Green et al., 1991; Greger et al, 2002). In case of GABARs, functional receptors could be formed from αβ but not αγ or βγ subunits (Angelotti et al., 1993). This suggests that αβ subunits are assembled first and γ2 or δ subunits added subsequently. The intracellular loop between TM3 and TM4 is variable between in the γ2 and δ subunits (Shivers et al., 1989), and this region interacts with various regulatory proteins (Chen and Olsen, 2007). Receptors that express the γ2 subunit with an intracellular loop from the δ subunit are not expressed at the synapses (Christie et al., 2006). Therefore, studies that concentrate on the intracellular loop could uncover potential mechanisms that underlie temporal distinctions in the secretion of γ2- and δ-GABARs.
In the neurons and neuroendocrine systems, which express some of the best studied exocytosis mechanisms, distinct kinetics have been reported based on the type of cargo carrying vesicles [see review (Martin, 2003)]. For example, calcium induced exocytosis of neurotransmitters can occur via synaptic vesicles or dense core vesicles; release via synaptic vesicles occurs within milliseconds whereas that via dense core vesicles require hundreds of milliseconds (Martin, 2003). The readily releasable pool of vesicles, which supports fast exocytosis, is replenished from the pool of slow release vesicles and both these pools are in equilibrium with the cytoplasmic vesicle pool. On the other hand, exocytosis of membrane proteins including synaptic GABARs, GABA-B, AMPA, and glycine receptors require tens of minutes (Lu et al., 2001; Passafaro et al., 2001; Rosenberg et al., 2001; Wilkins et al, 2008; Saliba et al., 2012). It is possible that observed differences in the exocytosis of γ2- and δ-GABARs are associated with distinct type of vesicles carrying them; in addition the receptors in recycling vesicles could also influence the rate of exocytosis.
Internalization, insertion and reinsertion processes regulate the number of surface expressed receptors. As the number of surface receptors remains unchanged over time, the processes of internalization, insertion and reinsertion are likely in a dynamic equilibrium. However various stimuli change surface GABAR expression. Global increase in the activity alters surface γ2-GABAR expression associated with changes in internalization (Goodkin et al, 2005; 2008; Terunuma et al., 2008; Rannals and Kapur, 2011). Alcohol-induced internalization of δ-GABARs also leads to reduced surface α4 and δ subunit expression in the hippocampus (Gonzalez et al., 2012). Furthermore, BDNF also changes surface expression of synaptic and extrasynaptic GABARs likely due to altered internalization (Connolly et al., 1999; Kittler et al., 2000; Joshi and Kapur, 2009). However whether the rate of GABAR insertion was also affected by these stimuli was not studied. Current study characterized kinetics of insertion of GABARs under basal conditions; similar assay can be used to determine stimuli that influence the rate of insertion.
Interestingly, assembling α1 or α4 subunits did not influence the insertion of γ2-GABARs, whereas insertion of α4δ subunit-containing receptors was faster than those containing α1δ subunits. Studies using concatameric subunits expressed in heterologous systems also suggest that α subunits influence the assembly of δ-GABARs but not γ2-GABARs (Baur et al., 2010). Mechanisms underlying these differences are currently unknown.
In conclusion this study demonstrated distinct rates of insertion of γ2-GABARs and δ-GABARs expressed in cultured hippocampal neurons and HEK293 cells.
Supplementary Material
Acknowledgments
This work was supported by NIH grants RO1 NS 040337 and RO1 NS044370 to JK. We thank Dr Chengsan Sun for his help in immunochemical characterization of anti-δ subunit antibody.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem. 2010;285 (53):41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelotti TP, Uhler MD, MacDonald RL. Assembly of GABAA receptor subunits: analysis of transient single-cell expression utilizing a fluorescent substrate/marker gene technique. J Neurosci. 1993;13:1418–1428. doi: 10.1523/JNEUROSCI.13-04-01418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo F, Ruano D, Vitorica J. Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol. 1998;347:347–353. doi: 10.1016/s0014-2999(98)00122-8. [DOI] [PubMed] [Google Scholar]
- 4.Benson DL, Watkins FH, Steward O, Banker G. Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol. 1994;23:279–295. doi: 10.1007/BF01188497. [DOI] [PubMed] [Google Scholar]
- 5.Baur R, Kaur KH, Sigel E. Diversity of structure and function of α1α6β3δ GABAA Receptors. J Biol Chem. 2010;285:17398–17405. doi: 10.1074/jbc.M110.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bichet D, Cornet V, Geib S, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The I-II Loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd GW, Doward AI, Kirkness EF, Millar NS, Connolly CN. Cell surface expression of 5-hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J Biol Chem. 2003;278:27681–27687. doi: 10.1074/jbc.M304938200. [DOI] [PubMed] [Google Scholar]
- 9.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- 11.Christie SB, Li RW, Miralles CP, Yang BY, De Blas AL. Clustered and non-clustered GABAA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of GABAA receptors. J Biol Chem. 1999;274:36565–36572. doi: 10.1074/jbc.274.51.36565. [DOI] [PubMed] [Google Scholar]
- 13.Craig AM, Blackstone CD, Huganir RL, Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: Postsynaptic clustering of AMPA selective subunits. Neuron. 1993;10:1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- 14.Duggan MJ, Pollard S, Stephenson FA. Immunoaffinity purification of GABAA receptor α-subunit iso-oligomers. Demonstration of receptor populations containing α1α2, α1α3, and α2α3 subunit pairs. J Biol Chem. 1991;266:24778–24784. [PubMed] [Google Scholar]
- 15.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 16.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 17.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 18.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABAA receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 19.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ subunit-containing GABAA receptors. J Neurosci. 2012;32:17874–17881. doi: 10.1523/JNEUROSCI.2535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. Cambridge MA: The MIT Press; 1998. pp. 339–370. [Google Scholar]
- 23.Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 24.Green WN, Ross AF, Claudio T. Acetylcholine receptor assembly is stimulated by phosphorylation of its γ subunit. Neuron. 1991;7:659–666. doi: 10.1016/0896-6273(91)90378-d. [DOI] [PubMed] [Google Scholar]
- 25.Hannan S, Wilkins ME, Smart TG. Sushi domains confer distinct trafficking profiles on GABAB receptors. Proc Natl Acad Sci. 2012;109:12171–12176. doi: 10.1073/pnas.1201660109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 27.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W. Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing α6 subunits. J Neurosci. 1998;18:2449–2457. doi: 10.1523/JNEUROSCI.18-07-02449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi S, Kapur J. Slow intracellular accumulation of GABAA receptor δ subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164:507–519. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi S, Sun C, Kapur J. A mouse monoclonal antibody against the γ2 subunit of GABAA receptors. Hybridoma (Larchmt) 2011;30:537–542. doi: 10.1089/hyb.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy M, Ehlers M. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuver A, Shen H, Smith SS. Regulation of the surface expression of α4β2δ GABAA receptors by high efficacy states. Brain Research. 2012;1463:1–20. doi: 10.1016/j.brainres.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu WY, Man HY, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29 (1):243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 34.Luddens H, Killisch I, Seeburg PH. More than one α variant may exist in a GABAA/benzodiazepine receptor complex. J Recept Res. 1991;11:535–551. doi: 10.3109/10799899109066426. [DOI] [PubMed] [Google Scholar]
- 35.Luscher B, Fuchs T, Kilpatrick C. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- 37.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 38.Martin TFJ. Tuning exocytosis for speed: fast and slow modes. Biochimica et Biophysica Acta (BBA) - Mol Cell Res. 2003;1641:157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 39.McCann CM, Bracamontes J, Steinbach JH, Sanes JR. The cholinergic antagonist α-bungarotoxin also binds and blocks a subset of GABA receptors. Proc Natl Acad Sci U S A. 2006;103:5149–5154. doi: 10.1073/pnas.0600847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moise L, Liu J, Pryazhnikov E, Khiroug L, Jeromin A, Hawrot E. KV4.2 channels tagged in the S1–S2 loop for alpha-bungarotoxin binding provide a new tool for studies of channel expression and localization. Channels. 2010;4:115–123. doi: 10.4161/chan.4.2.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passafaro M, Piëch V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 44.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 46.Pollard S, Thompson CL, Stephenson FA. Quantitative characterization of α6 and α1α6 subunit-containing Native GABAA receptors of adult rat cerebellum demonstrates two α subunits per receptor oligomer. J Biol Chem. 1995;270:21285–21290. doi: 10.1074/jbc.270.36.21285. [DOI] [PubMed] [Google Scholar]
- 47.Quirk K, Whiting PJ, Ragan CI, McKernan RM. Characterisation of δ subunit-containing GABAA receptors from rat brain. Eur J Pharmacol. 1995;290:175–181. doi: 10.1016/0922-4106(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 48.Rannals MD, Kapur J. Homeostatic strengthening of inhibitory synapses Is mediated by the accumulation of GABAA receptors. J Neurosci. 2011;31:17701–17712. doi: 10.1523/JNEUROSCI.4476-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg M, Meier J, Triller A, Vannier C. Dynamics of glycine receptor insertion in the neuronal plasma membrane. J Neurosci. 2001;21:5036–5044. doi: 10.1523/JNEUROSCI.21-14-05036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saliba RS, Kretschmannova K, Moss SJ. Activity-dependent phosphorylation of GABAA receptors regulates receptor insertion and tonic current. EMBO J. 2012;31:2937–2951. doi: 10.1038/emboj.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saxena NC, Macdonald RL. Properties of putative cerebellar GABAA receptor isoforms. Mol Pharm. 1996;49(3):567–569. [PubMed] [Google Scholar]
- 53.Scherf T, Balass M, Fuchs S, Katchalski-Katzir E, Anglister J. Three-dimensional solution structure of the complex of α-bungarotoxin with a library-derived peptide. Proc Natl Acad Sci U S A. 1997;94:6059–6064. doi: 10.1073/pnas.94.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekine-Aizawa Y, Huganir RL. Imaging of receptor trafficking by using α-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci USA. 2004;101:17114–17119. doi: 10.1073/pnas.0407563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 57.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 58.Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron. 2000;28:887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 59.Sun C, Sieghart W, Kapur J. Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swanwick CC, Murthy NR, Mtchedlishvili Z, Sieghart W, Kapur J. Development of GABAergic synapses in cultured hippocampal neurons. J Comp Neurol. 2006;495:497–510. doi: 10.1002/cne.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, Haydon PG, Coulter DA, Moss SJ. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28:376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAA receptors to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whiting P. GABAA receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discovery Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- 67.Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an α-bungarotoxin tag. J Biol Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.