Abstract
This paper addresses sources contributing to the differences in the degree of recovery from extinction observed with different renewal paradigms. In two lick suppression experiments with rats, we assessed the role of the associative status of the acquisition context in both the weakness of AAC renewal and the sometimes observed weaker renewal resulting from an ABC design relative to an ABA design. In Experiment 1, we observed that AAC renewal relative to an AAA control group was small unless Context A had undergone associative deflation (i.e., extinction of Context A). Deflation of Context A not only decreased behavioral control by the CS in the AAA condition, but increased it in the AAC condition, thereby implicating a comparator process in addition to associative summation between the CS and test context. In Experiment 2, an excitatory acquisition context was found to enhance the difference between ABC and ABA renewal. Associative deflation of the acquisition context decreased ABA renewal more than ABC renewal. Thus, the associative value of the acquisition context (A) was more positively related to the level of renewal when the target CS (X) was tested in this context than when it was tested in a neutral but equally familiar context (C), consistent with the frequently observed greater renewal in an ABA condition than an ABC condition arising from associative summation of the CS and test context. These findings demonstrate that the excitatory status of the acquisition context influences the observed degree of renewal.
Keywords: extinction, renewal, context
1. Introduction
Renewal can be behaviorally defined as the recovery of an extinguished response when testing occurs in a context different from that in which the extinction treatment took place. This behavioral definition is not to be confused with one that is based on a specific underlying mechanism. There are a number of different procedures to implement a context shift between extinction and testing. A classic example is a preparation in which acquisition, extinction, and testing each occur in separate contexts (i.e., ABC renewal, wherein the first letter denotes the acquisition context, the second letter denotes the extinction context, and the third letter denotes the test context; e.g., Bouton & Bolles, 1979). Additionally, robust renewal has been observed when acquisition and testing occur in the same context, but extinction occurs in a second context (i.e., ABA renewal; e.g., Bouton & King, 1983). Although ABC and ABA renewal often produce similar levels of recovery, ABC renewal tends to result in a nominally weaker conditioned response (e.g., Thomas et al., 2003). Finally, renewal can sometimes be observed when acquisition and extinction take place in the same context, and testing occurs in a different context (i.e., AAC renewal; e.g., Bouton & Ricker, 1994).
Although each of these paradigms can produce behavioral renewal, it is generally accepted that the magnitude of recovery varies across the different renewal designs. The AAC (also referred to as AAB) renewal paradigm has been shown to produce the weakest amount of recovery from extinction when compared to either ABC or ABA renewal, and sometimes recovery may not be observed at all (e.g., Laborda, Witnauer, et al., 2011; Tamai & Nakajima, 2000; Tamai et al., 2001; Thomas et al., 2003). Interpretation of a comparison between AAC renewal and either ABC or ABA renewal is complicated because AAC renewal does not involve a context shift between acquisition and extinction, whereas ABC and ABA renewal do. A direct comparison between ABC and ABA renewal is simpler to interpret as the two designs differ only in the change in context at the time of testing. This can also be seen in the fact that both ABA and ABC designs ordinarily make use of the same control condition (i.e., ABB), whereas AAC renewal usually employs a different control condition (i.e., AAA).
Context-specific learning during extinction treatment
Several accounts of renewal have been suggested based on the view that the test context modulates retrieval of the memory produced by extinction (e.g., Bouton, 1993; Miller & Escobar, 2002; Rosas et al., 2006). Bouton proposed that extinction treatment produces an inhibitory association between the conditioned stimulus (CS) and the unconditioned stimulus (US). This inhibitory association is assumed to be more context specific than the excitatory CS-US association (either because it is inhibitory or because it is second learned; e.g., Bouton, 1993, 1997; Nelson, 2002). This context specificity of inhibitory associations results in behavior indicative of excitation when testing occurs outside the context of extinction treatment. In this framework, the extinction context serves as a negative occasion setter (Holland, 1989) that modulates the expression of the inhibitory relationship. Miller & Escobar’s and Rosas et al.’ accounts of renewal are variants of Bouton’s explanation, but make the same predictions with respect to the present research. Hence, we will not speak further to Miller and Escobar’s and Rosas et al.’s accounts here. Critically, Bouton’s account by itself, although well able to explain the basic renewal effect, seemingly incorrectly anticipates equal degrees of renewal across the three types of renewal designs. However, one might argue that learning that the extinction context is a negative occasion setter should be less effective in an AAC design than an ABC or ABA design because in the AAC design the extinction context was also the acquisition context, making the extinction context a less effective discriminative stimulus. Thus, Bouton’s account, with this minor added feature, may be viewed as anticipating AAC renewal to be weaker than the other two renewal designs. But there is nothing in Bouton’s account to explain why ABC renewal is often somewhat weaker than ABA renewal.
Associative summation of the test context with the CS
The most frequently cited alternative to Bouton’s (1993) account of renewal is based on the view that renewal is the result of direct associations between the various contexts and US. One potential example of this sort of association is that the extinction context could become inhibitory due to presentations of the excitatory CS occurring within it in the absence of the US (i.e., in an ABB control condition; see Polack et al., 2012, for supporting data). This inhibitory potential of the extinction context could both protect the CS from unlearning of the CS-US association during extinction treatment (McConnell & Miller, 2010; Rescorla, 2003) and reduce responding to the CS during a test of the CS in the extinction context, as in an ABB control group. If the extinction context functions as a conditioned inhibitor during testing in the extinction context, then responding to the target CS would be expected to recover outside of the extinction context. Thus, this account is able to explain the basic renewal effect. Granted there are many demonstrations of failures to observe any inhibitory status of the context, suggesting that direct inhibition of the US by the extinction context is at most only one of several sources of renewal (Bouton & King, 1983; Bouton & Swartzentruber, 1986; Nelson et al. 2011; Rescorla 2008). However, this rejection of the view that the extinction context becomes inhibitory is, by its nature, predicated on null results. Additionally, there is a bias in the renewal literature toward using parameters that encourage contextual modulation of memories of extinction, rather than those parameters that encourage modulation of responding to the target cue by an inhibitory extinction context (in contrast, Polack et al. used parameters biased to enhance the inhibitory value of the extinction context). Accounts of renewal that focus on the associative status of the test context not only emphasize the possibility that the extinction context is inhibitory, but that the acquisition context may be excitatory. An excitatory acquisition context could result in associative summation of the CS and the test context if testing occurs in the acquisition context, as in an ABA renewal condition. This sort of associative summation could explain part or all of ABA renewal and is at least a plausible contender in explaining why ABA renewal is often more robust than ABC renewal, in which testing occurs in a neutral context. Moreover, due to Context A’s initial potential for excitatory value from CS-US acquisition occurring in Context A, Context A may be slow to acquire inhibitory value during extinction when extinction occurs in the acquisition context. This could result in low contextual inhibition of responding to the CS when the CS is tested in the acquisition context (i.e., an AAA condition). The resultant heightened (i.e., uninhibited) responding to the CS in Context A would diminish the difference between an AAC renewal condition and its AAA control. Thus, consideration of the associative status of the extinction and test contexts provide a potential account not only of the basic renewal effect, but the observed differences in degree of recovery among the three types of renewal designs (i.e., AAC < ABC ≤ ABA). However, accounts of renewal based on associative summation with the extinction and/or test contexts are challenged by considerable evidence that renewal can occur even when the associative status of the contexts in the renewal and control conditions have been equated (e.g., Harris et al., 2000). Thus, accounts based on potential differences in the associative status of the contexts alone are not able to account for all reports of renewal.
In addition to these two families of mechanisms that may contribute to renewal effects, there are other models (e.g., Larrauri & Schmajuk, 2008). But these models tend to invoke a large number of independent mechanisms (and hence a large number of free parameters) that preclude their making unambiguous a priori predictions although they can often be made to fit the data post hoc. Hence, we will not pursue such models here.
Although the basic renewal effect can be interpreted in terms of either modulation of responding to the target by the test context or the associative status of the test context, neither account is fully adequate. ABA renewal being more robust than ABC renewal challenges the completeness of the contextual modulation account, and evidence of renewal even when the associative histories of the different contexts have been equated challenges the completeness of accounts dependent on the associative status of the contexts. However, these two roles of the context are not mutually exclusive. Holland (1985) and others have established that an occasion setter can simultaneously be a Pavlovian CS. The intent of the present research was to examine the contribution of associations between the test context and the US in producing the differences observed in the magnitude of the different types of renewal.
The question addressed in Experiment 1 was whether the acquisition context-US association contributes to the frequently observed weakness of AAC renewal. During acquisition (i.e., CS-US pairings), the acquisition context (i.e., A) presumably enters into an excitatory association with the US which may then summate with the residual excitatory strength of the extinguished stimulus when testing occurs in that context, as in the AAA control condition to which AAC renewal is ordinarily compared. Thus, responding at test in the AAA control condition may reflect not just responding to the extinguished CS, but responding to the conjoint stimulus and the excitatory context. This increased responding in the AAA control group due to associative summation could reduce the difference in responding between recovery from extinction in an AAC renewal group and its AAA control group. Several renewal demonstrations have directly assessed the associative value of the acquisition context and found that this context alone did not elicit a conditioned response (e.g., Bouton & King, 1983); however, the context used by Bouton and King may have had a low associative strength that was merely below the threshold required to observe a conditioned response. Any subthreshold response potential of the context could still be available to summate with the residual response potential of the extinguished CS (see Reberg, 1972). A similar account may be proffered for the comparison between ABC and ABA renewal effects to explain why ABA renewal is often slightly more robust than ABC renewal. That is, at test for an ABA group the associative strength of the test context (A) could summate with the residual associative strength of the target CS, whereas for an ABC group an excitatory context is absent during CS testing. In Experiment 2, we examined this possibility. Several clever designs have attempted to completely rule out the potential contribution of direct context associations by perfectly matching experience between test contexts in order to highlight the contribution to renewal of occasion setting by the extinction context (e.g., Harris et al., 2000; Rescorla, 2008). Renewal seen in these studies makes clear that associations of the US to the test context are not adequate to account for all instances of renewal. Although controlling for associative experience between test contexts is an excellent strategy for studying the potential occasion setting properties of the extinction context, such designs eliminate other potential contributing mechanisms that may be involved in settings that are more ecologically valid in modeling relapse from exposure therapy (e.g., Laborda, McConnell, et al., 2011). The present manuscript serves to highlight the relevance of some additional contributing mechanisms; however, we are not prepared to suggest that the present parameters have more clinical relevance than others. The potential contribution of various renewal mechanisms would depend on the nature of acquisition, treatment, and testing conditions specific to an individual patient, the etiology of the disorder, and the particular clinical setting.
2. Experiment 1
This experiment was designed to determine whether the relatively small increase in responding to the CS seen in an AAC renewal group, relative to an AAA control group which is the conventional comparison group for AAC renewal, can be explained in terms of the associative status of Context A. That is, might responding, seemingly to the CS, in the AAA control condition reflect not only the residual associative strength of the CS but associative summation of the CS with Context A? Two factors were orthogonally manipulated to answer this question. The first factor was the context in which testing took place: Context A (the context of acquisition and extinction [AAA]) or Context C (a neutral context [AAC]), thereby allowing AAC renewal to be assessed relative to an AAA control condition. The second factor was the excitatory status of Context A. Subjects were given either additional exposure to Context A (i.e., context extinction [CtxExt]) following CS extinction, or did not receive this exposure. Without the additional context exposure, Context A was expected to remain excitatory due to the US presentations administered in Context A during the CS-US pairings of acquisition (NoCtxExt). This allowed us to evaluate responding to the X-Context A compound in the AAA condition as a function of Context A’s associative strength (i.e., with and without extinction of the Context A-US association) relative to responding in the AAC condition (with and without extinction of the Context A-US association). Presumably extinction of the Context A-US association would not diminish the occasion setting properties of Context A as mere exposure to an occasion setter is known not to reduce the occasion setting potential of the occasion setter (i.e., Holland, 1989).The excitatory status of Context A was directly assessed prior to the test of the extinguished CS X in order to validate the effectiveness of the intended extinction of the Context A-US association.
2.1 Methods
2.1.1 Subjects
The subjects were 24 male and 24 female, experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 263 – 372 g for males and 206 – 271 g for females. Subjects were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. Sex differences were not found to influence responding in this preparation, nor did it significantly interact with any of our experimental manipulations; therefore, we pooled data across sexes and omitted mention of this factor from the subsequent results section. It is likely that we lacked power to be sensitive to any potential role of sex in these experiments because including sex as a factor would have appreciably decreased group size (ns = 6). The animals were individually housed in standard hanging stainless-steel wire-mesh cages in a vivarium maintained on a 16/8-hr light/dark cycle.
Experimental manipulations occurred near the middle portion of the light phase. The animals received free access to Purina Lab Chow, whereas water availability was limited to 30 min per day following a progressive deprivation schedule initiated one week prior to the start of the study. From the time of weaning until the start of the study, all animals were handled for 30 s, three times per week.
2.1.2 Apparatus
Six identical copies of each of two different types of experimental chambers were used. Chamber V was a 27-cm long box in a vertical truncated-V shape (29.5-cm height, 21.5-cm wide at top, and 5.5-cm wide at bottom). The floor was comprised of two 27-cm long, 2-cm wide stainless steel plates, with a 1.5-cm gap between the two plates. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the sidewalls were stainless steel. A 0.8-mA, 0.5-s constant-current footshock, produced by a high voltage AC circuit in series with a 1.0-MΩ resistor could be delivered through the metal walls and floor of the chamber. Each of six copies of Chamber V was housed in a separate sound- and light-attenuating environmental isolation chest. The chamber was illuminated by a 7-W (nominal at 120 VAC, but driven at 50 VAC) light bulb, which was mounted on the inside wall of the environmental enclosure, approximately 30-cm from the center of the experimental chamber. The light entered the chamber primarily by reflection from the ceiling of the environmental chest.
Chamber R was rectangular, measuring 24.0 × 9.0 × 12.5 cm (l × w × h). The walls and ceiling of Chamber R were clear Plexiglas, and the floor was comprised of stainless steel rods measuring 0.5-cm diameter, spaced 1.3-cm apart (center to center). The rods were connected by NE-2 bulbs, which allowed for the delivery of a 0.8-mA, 0.5-s constant-current footshock. Each of six copies of Chamber R was housed in separate light- and sound-attenuating environmental isolation chambers. Each chamber was dimly illuminated by a 2-W (nominal at 120 VAC, but driven at 50 VAC) incandescent house light mounted on an inside wall of the environmental chest located approximately 30 cm from the animal enclosure.
All chambers could be equipped with a water-filled lick tube that extended 1-cm into a cylindrical niche, which was 4.5 cm in diameter, left right centered on the narrow walls of the chamber, with its bottom 1.75-cm above the floor of the apparatus and 5.0 cm deep. There was a photobeam detector 1-cm in front of the lick tube that was broken whenever a subject licked the tube. A 45-Ω speaker on the inside walls of each isolation chest could deliver a click train (6 Hz) 6 dB (C-scale) above background. Ventilation fans in each enclosure provided a constant 76-dB (C-scale) background noise. The light intensities inside the two illuminated chambers were approximately equal due to the difference in opaqueness of the walls of Chambers V and R.
The click train served as conditioned stimulus X and was 15 s in duration during acquisition and CS extinction. The footshock served as the US. The physical identity of Contexts A and C were counterbalanced between Chambers R and V within groups.
2.1.3 Procedure
See Table 1. Within each of the two context extinction conditions, exposure to the two test contexts was equated across groups in each phase of treatment.
Table 1.
Design of Experiment 1
| Groups | Acquisition Day 2 |
Extinction Day 3 |
Ctx Ext Days 4–6 |
Test Ctx Days 7–8 |
Test X Day 9 |
|---|---|---|---|---|---|
| AAA-NoCtxExt | (6 X+)A / (−)C | (24 X−)A / (−)C | Handling | (−)A | XA |
| AAC-NoCtxExt | (6 X+)A / (−)C | (24 X−)A / (−)C | Handling | (−)A | XC |
| AAA-CtxExt | (6 X+)A / (−)C | (24 X−)A / (−)C | (−)A / (−)C | (−)A | XA |
| AAC-CtxExt | (6 X+)A / (−)C | (24 X−)A / (−)C | (−)A / (−)C | (−)A | XC |
Note: AAA = acquisition, extinction and testing all occurred in the same context; AAC = acquisition and extinction occurred in the same context, but testing occurred in a different context; CtxExt = context extinction treatment; X = Clicks; + = unconditioned stimulus presentation, − = no nominal stimulus presentation; A = Context A; C = Context C.
2.1.3.1 Acclimation
On Day 1, all subjects were acclimated to drinking in Context A and Context C during two 30-min sessions. The order of context acclimation was counterbalanced within groups. During the acclimation phase, subjects had free access to water-filled lick tubes. There were no presentations of any nominal stimuli during this phase. At the end of acclimation, the water tubes were removed until testing.
2.1.3.2 Phase 1 (acquisition)
On Day 2, all subjects received a single conditioning training session in Context A and an equal amount of exposure to Context C without presentation of any nominal stimulus. Subjects received six presentations of X with X coterminating with the US. The reinforced trials were initiated 15, 45, 80, 110, 135 and 160 s into the 180-s session; thus, the acquisition trials were highly massed. This was done to minimize context extinction during the intertrial intervals. Alternatively stated, we used parameters that were designed to leave Context A excitatory at the end of Phase 1. The order of the sessions in Contexts A and C was counterbalanced within groups and the time between sessions was approximately 220 min.
2.1.3.3 Phase 2 (extinction of X)
On Day 3, all subjects received one 8-min extinction session in Context A in which they received 24 presentations of CS X alone with an average ITI of 5-s; thus, the extinction trials were highly massed. This was done to minimize context extinction during the intertrial intervals. All subjects also received 8-min of exposure to Context C without the presentation of any nominal stimulus. This was done to equate Phase 2 exposure to the test contexts.
2.1.3.4 Phase 3 (context extinction)
On Days 4–6, rats in Condition CtxExt (i.e., Groups AAA-CtxExt and AAC-CtxExt) received 180-min of exposure per day to Context A and Context C during two separate daily sessions (one daily session in each context). Enclosures were opened every 30-min to ensure that the rats were awake. Subjects in Condition NoCtxExt received handling equivalent to those in the CtxExt condition but were not placed in either context. The order of these sessions was counterbalanced within groups.
2.1.3.5 Testing
On Days 7 and 8, all subjects were tested for conditioned lick suppression to Context A during a 5-min session (i.e., no punctuate CS was present) on one day and received comparable exposure in Context C on the other day. The short test session was intended to minimize extinction of any existing Context A-US association. The test measured time to complete an initial five cumulative seconds of drinking in the context. Half of the rats were tested on Day 7 and the other half on Day 8, whereas they received exposure to Context C on the opposing day in order to counterbalance the order of exposure to Context A and Context C within groups. All subjects were given 30-min of water shortly after each exposure to control for differences in water deprivation on subsequent testing as a result of initial testing. By virtue of the session length, a ceiling score of 5-min was imposed on the time to complete five cumulative seconds of drinking. On Day 9, all subjects were tested for conditioned lick suppression to CS X. Subjects in Condition AAA were tested in Context A, whereas, subjects in Condition AAC were tested in Context C. To match testing conditions on Day 9 with that of the context tests on Days 7–8, CS X was presented immediately at the start of the test trial. The time to complete an initial five cumulative seconds of licking was recorded. The test session for X was 10 min in duration. By virtue of the session length, a ceiling score of 10 min was imposed on the time to complete five cumulative seconds of drinking.
2.1.4 Results and discussion
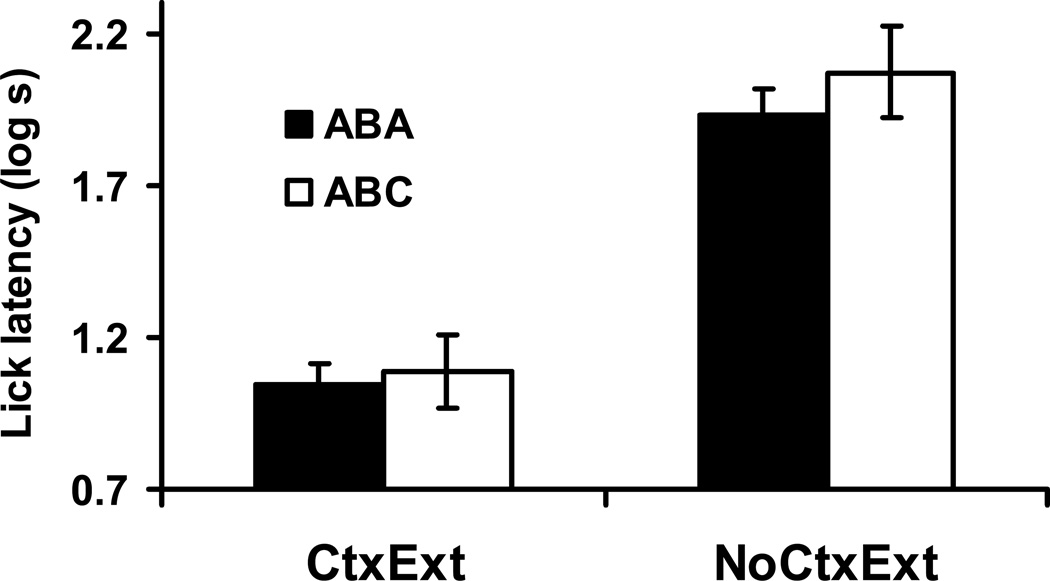
A log (base 10) transformation of all recorded times to complete five cumulative seconds of licking was conducted to satisfy the assumptions of normality and homogeneity of variance for parametric data analysis. During testing of Context A, we expected subjects in Condition CtxExt to have lower overall lick suppression scores than subjects in Condition NoCtxExt. That is, the additional exposure to Context A on Days 4–6 was expected to extinguish most or all observable excitatory value of that context. We conducted a 2 (Test Context: A vs. C) × 2 (Context Treatment: CtxExt vs. NoCtxExt) analysis of variance (ANOVA) on log latencies to complete the first five cumulative seconds of drinking in Context A on Day 7 or 8. Test Context was included as a factor to determine if the AAC and AAA conditions interacted with the context extinction manipulation. Figure 1 depicts the group means for the test of Context A. Shorter latencies indicate less fear of the context. We observed a main effect of Context Treatment F(1, 44) = 12.37, p < .05, MSE = .17, Cohen’s f = .49 (CtxExt < NoCtxExt), but no effect of Test Condition, F < 1, and no interaction was observed between these two factors, F < 1. Therefore, we can conclude that our context extinction treatment produced observable differences in the associative value of Context A. The lack of a Test Context effect is consistent with the fact that at the time of the context test, the two Test conditions had not yet experienced any differential treatment (see Table 1).
Figure 1. Context Test (Experiment 1).
Bars indicate mean log time to complete five cumulative seconds of licking upon being placed in Context A in Experiment 1. Higher lick latency scores reflect more fear to the context. Brackets reflect standard error of the mean (SEM). 0.7 log s corresponds to completion of five cumulative seconds of licking in 5 s, the lowest score possible.
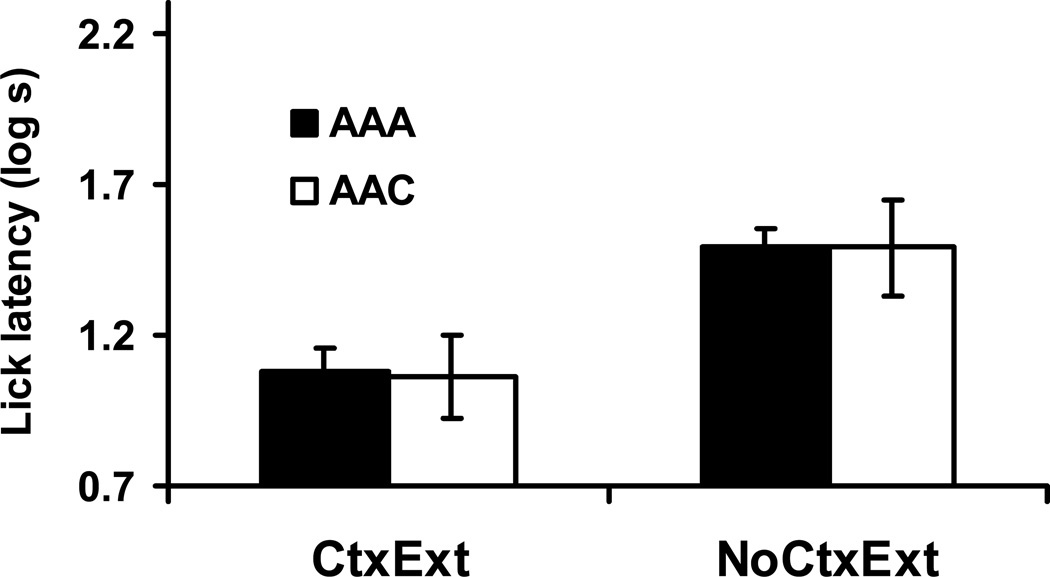
For the test of X, we conducted an identical 2 × 2 ANOVA on the log lick latencies to complete the first five cumulative seconds of drinking during the test session of Day 9 (see Figure 2). Shorter latencies indicate less fear of the CS. We expected to see a small or negligible higher level of conditioned suppression in Group AAC-NoCtxExt than in Group AAA-NoCtxExt, that is, AAC renewal with any summative contribution of Context A working against the renewal effect by increasing conditioned suppression in Group AAA-NoCtxExt. Furthermore, we expected less suppression in Group AAA-CtxExt than in Group AAA-NoCtxExt due to attenuation of any summative contribution from the putative Context A-US association in Group AAA-CxtExt. Additionally we expected to observe the typical recovery-from-CS-extinction effect with testing in a context different from that of extinction when Context A received extinction treatment. In other words, we expected Group AAC-CtxExt to exhibit more conditioned suppression than Group AAA-CtxExt.
Figure 2. Renewal Test (Experiment 1).
Bars indicate mean log time to complete five cumulative seconds of licking upon being placed in the test context with the CS immediately present in Experiment 1. Higher lick latency scores reflect more fear to the stimulus and context compound. Brackets reflect standard error of the mean (SEM). 0.7 log s corresponds to completion of five cumulative seconds of licking in 5 s, the lowest score possible.
We observed a marginal main effect of Context Treatment, F(1, 44) = 3.88, p < .06, MSE = .12, Cohen’s f = .24, and a main effect of Test Context, F(1, 44) = 4.31, p < .05, Cohen’s f = .26. More important, an interaction between these two variables was also detected, F( 1, 44) = 21.89, p < .05, Cohen’s f = .66. Planned contrasts using the error term from the ANOVA were conducted in order to determine the source of the interaction. Group AAA-CtxExt displayed less conditioned suppression than Group AAC-CtxExt, F(1, 44) = 22.82, p < .05, indicating that robust AAC renewal was observed following context extinction. In the NoCtxExt condition, Group AAA-NoCtxExt exhibited more suppression than Group AAC-NoCtxExt, although this numerical difference was not statistically significant, F(1, 44) = 3.38, p < .08. Thus, a robust AAC renewal effect was observed only after Context A was extinguished. This confirmed that AAC renewal is more difficult to observe when Context A is excitatory. We also observed a marginal difference between responding in Group AAA-CtxExt and Group AAA-NoCtxExt, F(1, 44) = 3.67, p < .07, suggesting that part of this difference in sensitivity is due to associative summation of CS X with the test context.
Surprisingly, Group AAC-CtxExt displayed more conditioned suppression than Group AAC-NoCtxExt, F(1, 44) = 22.10, p < .05. This difference is explicable neither in terms of modulation of responding to the CS by the test context nor summation of associative strengths of the CS and test context. However, this finding is consistent with the suggestion of Laborda, Witnauer, et al. (2011) that the weak responding to the CS seen in an AAC renewal group relative to an ABC renewal group is partly due to a comparator effect at test between the extinguished CS and Context A. Laborda, Witnauer, et al. observed that the absolute level of responding to a CS in an AAC renewal group (as distinct from its difference from an AAC control group) could be increased by giving extensive post-extinction exposure to Context A alone (i.e., context extinction; see also Witnauer & Miller, 2009). The findings of Laborda, Witnauer, et al. identified one mechanism that can contribute to AAC renewal being weaker than ABC renewal when these two conditions are directly compared to each other (as opposed to their appropriate controls, AAA and ABB, respectively, with the differences between the renewal groups and their controls being compared). A potential weakness of the Laborda, Witnauer, et al. studies is that their AAC renewal and ABC renewal preparations were not evaluated relative to the usual AAA and ABB control conditions. These controls were omitted by Laborda, Witnauer, et al. because their focus was on the difference between the absolute magnitude of responding following AAC and ABC renewal procedures rather than on the magnitude of each type of renewal relative to its appropriate control condition. Laborda, Witnauer, et al. interpreted their results within the framework of the comparator hypothesis (SOCR; e.g., Stout & Miller, 2007; also see Miller & Matzel, 1988), which suggests that cue comparison between the target CS (X) and Context A at the time of test (even when testing occurs outside Context A) can influence responding in an AAC renewal preparation. Specifically, SOCR assumes that conditioned responding to X is the result of a comparison at test between the representation of the US directly activated by presentation of X and the representation of the US indirectly activated through an X-comparator stimulus-US associative linkage. This indirect activation of the US representation is proportional to the product of the within-compound associations between X and its so-called comparator stimuli and the associations between these comparator stimuli and the US. (SOCR explains conventional cue competition through the same cue comparison mechanism.) In an AAC renewal design, during acquisition, a within-compound association between X and Context A is formed in addition to X-US and Context A-US associations. When extinction treatment also occurs in Context A, this phase serves to further strengthen the within-compound association between X and Context A. Little extinction of the Context A-US association in the form of unlearning (i.e., erasure) is ordinarily anticipated during the CS extinction treatment because the unlearning mechanism for extinction within SOCR plays a secondary role to response reduction resulting from comparator processes (i.e., strengthening of the X-Context A association). Laborda, Witnauer, et al. suggested that differences in cue comparisons between CS X and Context A may be in part responsible for the weaker recovery from extinction seen in AAC renewal (i.e., AAC vs. AAA) than ABC renewal (i.e., ABC vs. ABB) because of the stronger X-Context A association in the AAC condition than the ABC condition. That is, cue comparisons should play a greater role in determining responding in AAC renewal compared to ABC renewal. Comparator models of learning like SOCR are not good candidates as explanations for basic renewal because renewal clearly is by definition dependent on the test context, whereas SOCR focuses on a role for the acquisition context even when testing occurs in a different context. But comparator processes may also be at play in a renewal situation. Clearly SOCR explains the present difference between Groups AAC-CtxExt and AAC-NoCtxExt. Ideally one model of learning would explain all phenomena observed in learning situations. But research has repeatedly demonstrated that no contemporary model (e.g., comparator models like SOCR, total-error reduction models like Rescorla & Wagner [1972], and attentional models like Mackintosh [1975] and Pearce & Hall [1980]) is this successful. Each of many models appears to describe processes that conjointly contribute to behavior.
The preceding digression concerning the observed difference in conditioned suppression between the two AAC groups was demanded by the data, but the focal interest of the present experiment was on the role of the associative status of the acquisition context (A) on the magnitude of AAC renewal (i.e., AAC condition vs. AAA control condition). Considering that our primary interest was how the associative value of Context A influences conditioned suppression to X under different test conditions (i.e., testing in Context A vs. in a neutral context such as Context C), we conducted a separate analysis to determine whether the relationship between responding during the test of Context A alone and responding to X changed as a function of the test context. Although this is suggested by the data analysis above, a more direct test of this would be to determine whether responding to Context A could be used to predict responding to X in the different groups. Specifically, if the associative value of Context A summates with the associative value of X when X is tested in Context A, then responding to Context A alone should be positively related to responding to X in Context A. However, cue comparisons at test due to a comparator process may also play a role, which would be expected to produce a negative relationship between responding to Context A and responding to X. When X was tested in Context C (Condition AAC), the associative strength of Context A was unable to summate with the associative strength of X because Context A was absent. Consequently, only the negative relationship anticipated by SOCR between the associative status of Context A and responding to X would be expected. Thus, we expected a different relationship between test trial suppression to Context A alone and testing on X depending on whether the test of X occurred in Context A or C. The amount of responding to X in Context C was expected to result in a negative relationship to suppression to Context A alone, whereas responding to X in Context A should yield a less negative (maybe even positive) relationship with suppression to Context A alone due to the direct contribution of excitation from Context A (i.e., associative summation) in the latter case.
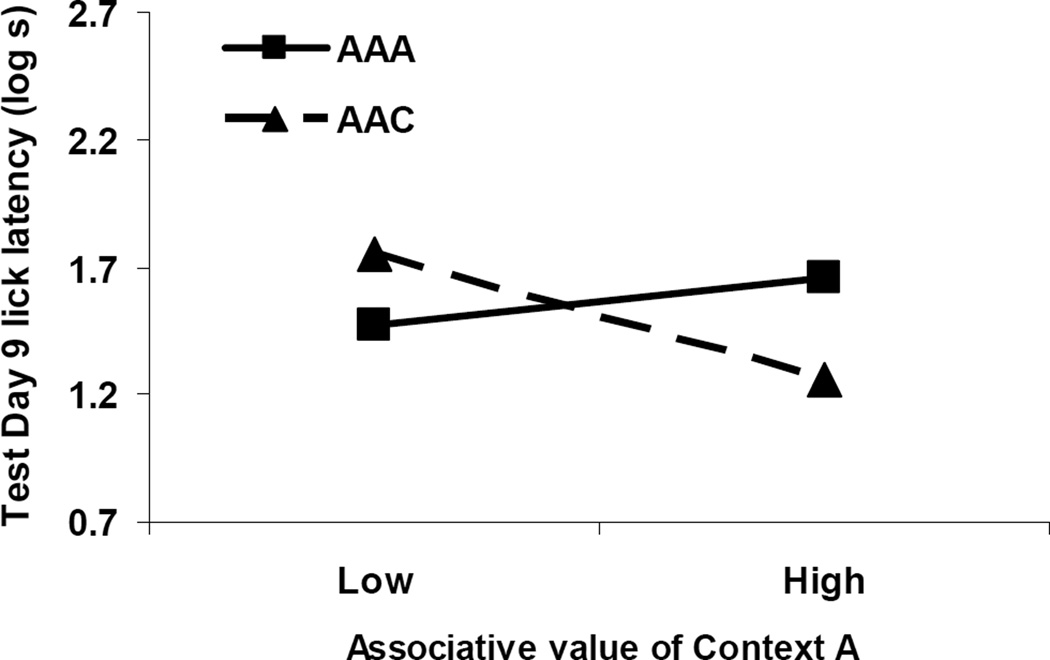
To further test the influence of Context A’s associative strength on suppression to X, we conducted an ANCOVA on the log cumulative seconds recorded during Test Day 9 using Test Context as a predictive variable and the log lick latencies to Context A alone as the covariate. Context Extinction was removed as a factor because it was previously demonstrated to predict the covariate (i.e., suppression to Context A alone). We included an interaction term between our qualitative variable (Test Context) and our continuous covariate because our concern was whether the covariate was differentially related to suppression to X depending on the context of testing (see Myers & Well, 2003, Chapter 15; Tabachnick & Fidell, 2007, p. 212, for a description of the evaluation of covariates). An effect of Test Context was detected, F(1, 44) = 7.50, p < .05, but not of suppression to Context A, F(1, 44) < 1. Critically, we observed the predicted interaction, F(1, 44) = 4.40, p < .05, with the measure of the associative value of Context A being more positively related to conditioned suppression to X in Context A and more negatively related to suppression to X in Context C (Figure 3 illustrates this interaction by blocking the upper and lower quartiles of suppression to Context A alone into high and low responders, respectively). This procedure clearly violates the assumption of homogeneity of regression which is necessary for the typical use of ANCOVA because the interaction specifically tells us that the regressions differ; however, testing our particular prediction is not a typical use of ANCOVA. Immediately, we are not interested in using the covariate to increase sensitivity of our predictor variable, but rather as a method to test the specific interaction between our covariate and the predictor variable. Figure 3 depicts the expected crossover interaction. A negative relationship between suppression to Context A (discretely categorized as Low vs. High for the visualization) and suppression to X was observed when testing occurred in a neutral context (Condition AAC), indicating cue comparison as anticipated by SOCR, whereas a more positive relationship was observed when testing occurred in Context A (Condition AAA), suggesting the occurrence of associative summation.
Figure 3. Covariate Analysis (Experiment 1).
The present graph depicts the relationship between responding to Context A alone and responding to CS X in Experiment 1. Subjects were categorized as Low if they were in the bottom 25% of responders from the test of Context A alone, whereas animals were categorized as having High associative value of Context A if they were in the top 25% of responders during the test of Context A alone. 0.7 log s corresponds to completion of five cumulative seconds of licking in 5 s, the lowest score possible.
3. Experiment 2
Experiment 2 was designed to determine whether the difference in robustness of recovery between ABC and ABA renewal procedures can be partially explained in terms of the difference in the associative status of Contexts A and C. Specifically, we asked whether associative summation between the CS and the test context enhances responding in an ABA renewal group. Experiment 2 was similar to Experiment 1, with the exception that Phase 2 (CS Extinction Treatment) was conducted in a separate context (Context B) from acquisition or testing. As ABC renewal and ABA renewal use the same control condition (ABB), we did not include the control condition as it would have been identical for these two types of renewal. Paralleling Experiment 1, a 2 (Context treatment: CtxExt vs. NoCtxExt) × 2 (Test context: ABA vs. ABC) experimental design was used (see Table 2). Because this extinction treatment occurred in Context B, we expected a limited role of cue comparison (SOCR) between the CS and Context A due to a relatively weak (and equivalent) X-Context A association in all groups. Moreover, an effect of cue comparison should take the form of lower suppression in the NoCtxExt condition than the CtxExt condition, with cue comparison’s contribution being equal across the ABC and ABA conditions.
Table 2.
Design of Experiment 2
| Groups | Acquisition Day 2 |
Extinction Day 3 |
Ctx Ext Days 4–6 |
Test Ctx Days 7–8 |
Test X Day 9 |
|---|---|---|---|---|---|
| ABA-NoCtxExt | (6 X+)A / (−)C | (24 X−)B | Handling | (−)A | XA |
| ABC-NoCtxExt | (6 X+)A / (−)C | (24 X−)B | Handling | (−)A | XC |
| ABA-CtxExt | (6 X+)A / (−)C | (24 X−)B | (−)A / (−)C | (−)A | XA |
| ABC-CtxExt | (6 X+)A / (−)C | (24 X−)B | (−)A / (−)C | (−)A | XC |
Note: ABC = acquisition, extinction and testing all occurred in separate contexts; ABA = acquisition and testing occurred in the same context, but extinction of the CS occurred in a different context; CtxExt = context extinction treatment; X = Clicks; + = unconditioned stimulus presentation; − = no nominal stimulus presentation; A = Context A; B = Context B; C = Context C.
3.1 Methods
3.1.1 Subjects
The subjects were 24 male and 24 female, experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Body-weight ranges were 226 – 305 g for males and 214 – 284 g for females. Subjects were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. Again, sex did not significantly influence our results (all ps > .14). The animals were housed as described in Experiment 1.
3.1.2 Apparatus
The apparatus consisted of the same R and V chambers that were used in Experiment 1. However, now three contexts (A, B, and C) were needed rather than the two contexts (A and C) of Experiment 1. Chamber R was modified in order to create Context B. These modifications included the insertion of a Plexiglas floor, the houselight being turned off, and the application of an odor (methyl salicylate). Additionally, for each subject an instance of Chamber R was used that differed from that used for either Context A or Context C.
3.1.3 Procedure
See Table 2. As in Experiment 1, exposure to the two test contexts was equated within the two context extinction conditions in each phase of treatment.
3.1.3.1 Acclimation and Phase 1 (acquisition)
All subjects were given acclimation to Contexts A and C (Day 1) and acquisition treatment in Context A with equivalent exposure to Context C (Day 2) identical to those of Experiment 1.
3.1.3.2 Phase 2 (extinction of X)
On Day 3, all subjects received 24 presentations of X alone. These were administered in Context B, but otherwise were in all aspects identical to the extinction treatment in Experiment 1.
3.1.3.3 Phase 3 (context extinction)
On Days 4–6, rats in Condition CtxExt received 180-min exposure per day to Context A and Context C as in Experiment 1, while rats in Condition NoCtxExt received equivalent handling.
3.1.3.4 Testing
On Days 7 and 8, all subjects were tested for conditioned lick suppression to Context A and exposed to Context C as in Experiment 1. On Day 9, all subjects were tested for conditioned lick suppression to CS X. Subjects in Condition ABA were tested in Context A, whereas subjects in Condition ABC were tested in Context C. All other aspects of the testing phase were identical to testing in Experiment 1.
3.1.4 Results and discussion
The log transformed data from both tests satisfied assumptions of normality and homogeneity of variance for parametric data analysis. As expected on the test of Context A, Condition CtxExt produced lower mean lick suppression scores than Condition NoCtxExt due to extinction treatment of Context A. Figure 4 depicts the group means for the test of Context A. A 2 (Test Context: ABA vs. ABC) × 2 (Context Treatment: CtxExt vs. NoCtxExt) ANOVA on the log latency to complete the first five cumulative seconds of licking in Context A yielded a main effect of Context Treatment, F(1, 43) = 66.89, MSE = .15, Cohen’s f = 1.17, but no effect of Test Condition, F < 1, and no interaction was observed between these two factors, F < 1. Thus, extensive exposure to the acquisition context (A) produced observable differences in the associative value of Context A. Note that in the NoCtxExt condition suppression to Context A was smaller in Experiment 1 than Experiment 2 (compare Figures 1 and 4). This difference may have been the result of the additional 8-min of context exposure to Context A in Experiment 1 (during extinction of X in Context A) which was absent in Experiment 2. But this explanation is challenged by the observation that context extinction typically requires much longer exposure to be effective (see Laborda, Witnauer, et al., 2011, for rate of context extinction).
Figure 4. Context Test (Experiment 2).
Bars indicate mean log time to complete five cumulative seconds of licking upon being placed in Context A in Experiment 2. Higher lick latency scores reflect more fear to the Context. Brackets reflect SEM. 0.7 log s corresponds to completion of five cumulative seconds of licking in 5 s, the lowest score possible.
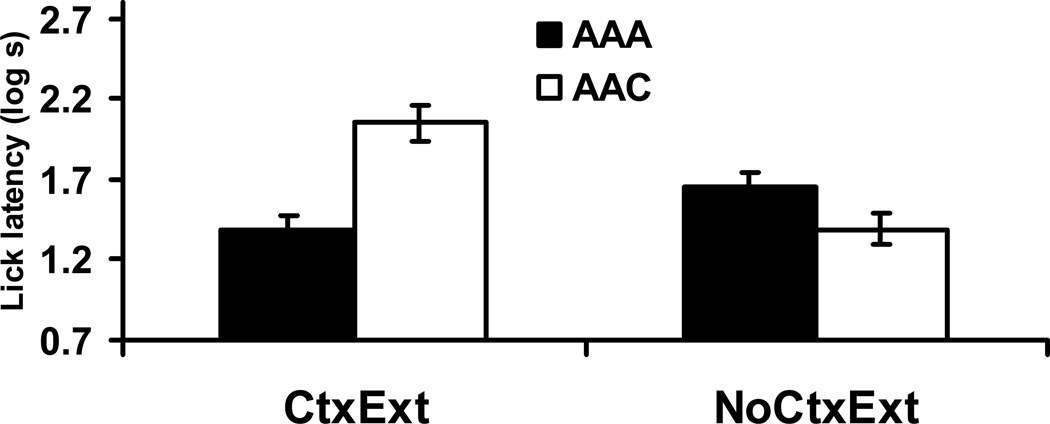
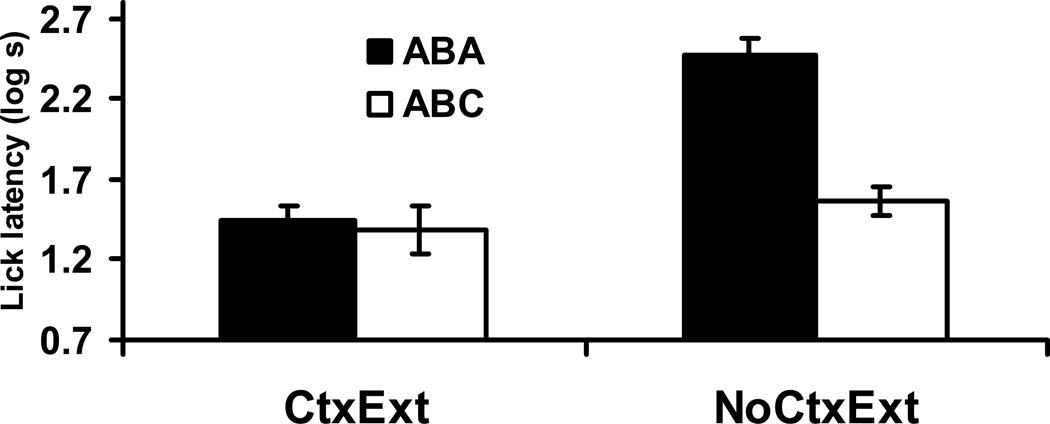
An identical 2 × 2 ANOVA was conducted on the log lick latencies to drink for the first five cumulative seconds in the presence of the target stimulus (see Figure 5). One animal from Group ABC-CtxExt was excluded from the analysis due to equipment failure. Main effects of Context Treatment, F(1, 43) = 28.71, p < .05, MSE = .15, Cohen’s f = .76, and Test Condition, F(1, 43) = 17.85, p < .05, Cohen’s f = .59, as well as an interaction between these two factors, F(1, 43) = 14.38, p < .05, Cohen’s f = .53, were detected. Planned contrasts were conducted in order to determine the source of the interaction. As expected, Group ABA-NoCtxExt exhibited longer latencies than Group ABC-NoCtxExt, F(1,43) = 32.87, p < .05, indicating enhanced responding to X (and the test context) when tested in an excitatory context. This observation seems to be best explained through associative summation of the CS and Context A, with the associative strength of Context A having been effectively reduced by extinction of Context A during Phase 2. Additionally, Groups ABA-CtxExt and ABC-CtxExt did not differ, F(1,43) < 1, suggesting that ABA and ABC renewal procedures produced the same results when the test contexts are similarly low in associative status (i.e., Context C was never paired with the US and the Context A-US association was low due to context extinction during Phase 2). Longer latencies were also detected in Group ABA-NoCtxExt than in Group ABA-CtxExt, F(1,43) = 42.81, p < .05, further supporting the conclusion that the excitatory status of Context A (as directly observed during the context test) can summate with the residual CS-US association.
Figure 5. Renewal Test (Experiment 2).
Bars indicate mean log time to complete five cumulative seconds of licking upon being placed in the test context with the CS immediately present in Experiment 2. Higher lick latency scores reflect more fear to the stimulus and context compound. Brackets reflect standard error of the mean (SEM). 0.7 log s corresponds to completion of five cumulative seconds of licking in 5 s, the lowest score possible.
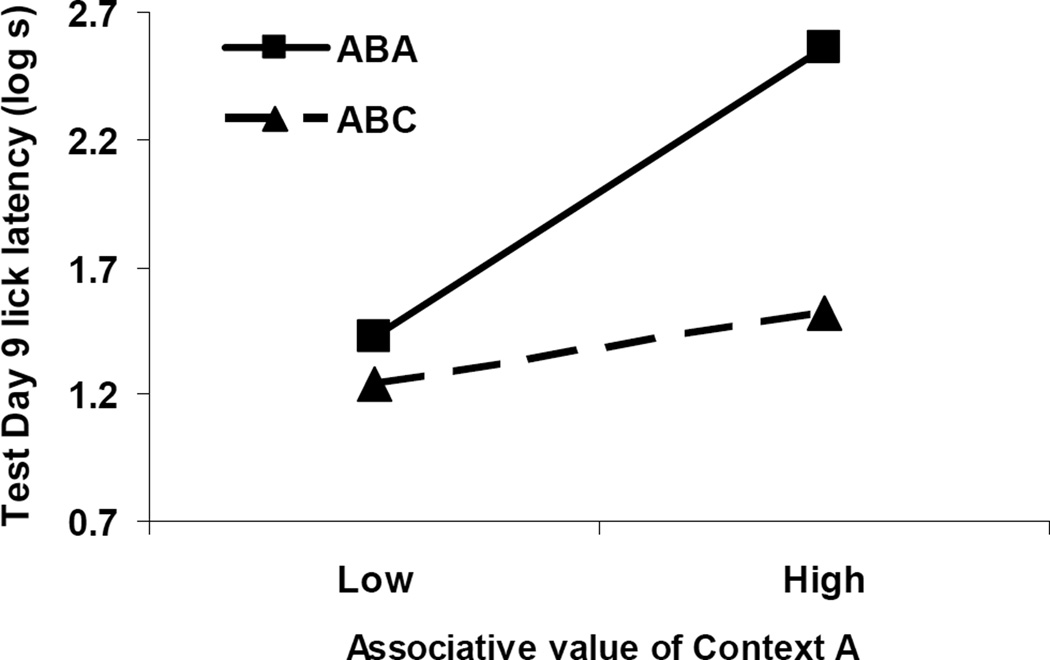
Here again we were interested in whether a predictive relationship between suppression to Context A and suppression to X could be observed. As in Experiment 1, covariation between suppression to Context A alone on Day 7 and suppression to X on Day 9 was expected. We conducted an ANCOVA on the log cumulative seconds recorded during Test Day 9 using Test Context (i.e., ABC vs. ABA) as a predictive variable and the log lick latencies to Context A alone as the covariate. Context Extinction was removed as a factor because it was previously demonstrated to predict the covariate (i.e., suppression to Context A alone). We sought an interaction between our qualitative variable (Test Context) and our continuous covariate because our hypothesis was that the covariate is differentially related to X depending on the context of testing. We did not find an effect of Test Context, F(1, 43) = 1.46, p > .23, but a main effect of suppression to Context A was observed, F(1, 43) = 22.53, p < .05. We also detected the predicted interaction, F(1, 43) = 11.04, p < .05, with the measure of the associative value of Context A being more positively related to suppression to X in Context A than suppression to X in Context C. Figure 6 illustrates this interaction by blocking the upper and lower quartiles of suppression to Context A alone into high and low responders, respectively. Again we can see that testing X in Context A (i.e., ABA) resulted in a more positive correlation between conditioned suppression to Context A alone and suppression to CS X compared to testing CS X in Context C (i.e., ABC).
Figure 6. Covariate Analysis (Experiment 2).
The present graph depicts the relationship between responding to Context A alone and responding to CS X in Experiment 2. Subjects were categorized as Low if they were in the bottom 25% of responders from the test of Context A alone, whereas animals were categorized as having High associative value of Context A if they were in the top 25% of responders during the test of Context A alone. 0.7 log s corresponds to completion of five cumulative seconds of licking in 5 s, the lowest score possible.
4. General Discussion
The present experiments demonstrate the influence of an excitatory Context A on the differences in the degree of renewal observed between AAC, ABC, and ABA renewal preparations. We should note that, to enhance factors that may contribute to differences in magnitude between the different types of renewal, we used somewhat unusual parameters, specifically, massed trials in both acquisition and extinction and onset of the CS at the very beginning of the CS test trials. These parameters were selected to ensure that the acquisition context was clearly excitatory for illustrative purposes. We argue for the perspective that these observable influences likely still contribute to apparent responding to the CS even when direct measures of the associative value of the acquisition context detect no excitation (e.g., Reberg, 1972). Additionally, similar processes, albeit with reduced effect size, are suggested to be at work with more conventional parameters (e.g., Laborda, Witnauer, et al 2011). Experiment 1 replicated the prior finding of Laborda, Witnauer, et al. (2011) that renewal in an AAC condition is stronger after the associative value of Context A has been extinguished, and this time did so relative to AAA control groups. Moreover, in an AAA control condition the associative status of Context A had a significant effect on conditioned suppression to the X-Context A compound (i.e., testing of X in Context A). This demonstration illustrates one mechanism that can attenuate the observation of recovery from CS extinction in an AAC renewal condition relative to an AAA control condition. That is, associative summation with the test context can elevate conditioned responding by the AAA control group, thereby reducing the apparent recovery in the AAC renewal group. In Experiment 2, associative summation between Context A and the extinguished CS was found to be one source of ABA renewal design producing more robust responding than an ABC renewal. Testing of the CS in Context A (i.e., Group ABA) allowed the excitatory potential of Context A that was acquired during the initial CS-US pairings to summate with the residual excitatory value of the CS. In contrast, testing the CS in Context C (i.e., Group ABC) provided a test context devoid of excitatory potential, so there was nothing to summate with the residual excitatory potential of the CS. However, associative summation of the CS with Context A is only part of the story concerning differing degrees of renewal across the different renewal paradigms. This was evident in the AAC condition of Experiment 1 in which extinction of the acquisition context (A) increased conditioned suppression to the CS despite associative summation being precluded by test of the CS occurring in a neutral context (C). Seemingly, a comparator-like process was responsible for this difference when testing occurred in a neutral context.
Although the present research provides clear evidence of associative summation between the test context and the CS, Harris et al. (2000; Experiment 2) demonstrated that enhanced responding resulting from associative summation between a target stimulus and the acquisition context can be specific to the excitor trained in that context. Testing a second excitor in that acquisition context resulted in less responding. Because of the equivalent experience of the test contexts, it is difficult to suggest that direct US associations played a strong role in that demonstration. Clearly something other than direct summation was involved; potentially the acquisition context functioned as a positive occasion setter for the cue (or vice versa). However, this effect may have arisen from generalization decrement (Pearce, 1987). It would have been interesting to see whether the excitatory status of the context alone would have predicted the amount of summation in Harris et al. (2000), as we observed in the present experiments using modified ANCOVAs.
In summary, it is becoming ever clearer that several different mechanisms underlie the basic renewal effect (i.e., all three types of renewal), with the relative contributions of these different mechanisms being dependent upon both procedures and parameters. Testing the CS outside of the extinction context eliminates both negative occasion setting by the extinction context and the extinction context serving as a conditioned inhibitor, each of which appear able to contribute to the basic renewal effect (e.g., Harris et al., 2000; Polack et al., 2012). Turning from the basic renewal effect to differences in magnitude of the three types of renewal, in Experiment 1 we saw that associative summation elevated responding in an AAA control condition, whereas under select circumstances comparator effects such as posited by Stout and Miller (2007) can contribute to the relatively weak responding that is observed in an AAC group. Either of these factors alone or the two together could explain the relatively small effect size of AAC renewal. In Experiment 2 we saw that differential associative values of the test context readily speak to the greater magnitude of ABA renewal compared to ABC renewal. That is, testing in the acquisition context (as in ABA renewal) can result in any existing association between the acquisition context and the US summating with the residual CS-US association to produce apparent heightened responding to the CS relative to an ABC renewal group.
Recently, it has been suggested that the term renewal should be reserved for recovery from extinction in the absence of direct associations with the test context (Nelson et al., 2011). This definition invites confusion between one particular mechanism that may contribute to renewal and the behavioral phenomenon of recovery from extinction when testing occurs outside the extinction context. Considering that the findings of Harris et al. (2000) and others highlight one factor that contributes to renewal effects (i.e., negative occasion setting by the extinction context), whereas the present results highlight several other factors, it is important to remember that renewal preparations are often meant to model clinical settings which are more likely to involve more than one isolated mechanism. Given this consideration, there may be some merit in researchers moving away from renewal designs that emphasize one particular mechanism by eliminating the potential contribution of other possible mechanisms and instead beginning to identify the relative contributions of various processes in more ecologically valid procedures. This criticism certainly applies to the present experiments as well as many others investigating renewal. Additionally, the labels we give to contexts depend on whether the experimenter views the contexts as sufficiently different from one another (Bouton & Swartzentruber, 1986, Experiments 1a & 1b). Contexts may not always be as distinct as we think they are and conversely we can often find some features of presumably different contexts that are similar (e.g., Todd et al., 2012). It is important to consider all of the potential determinants of ABA, ABC and AAC renewal because real world contexts are not likely to be as cleanly defined as the putatively distinguishable contexts in laboratory experiments. At best we might hope to claim that a particular situation is more ABA-like or relatively ABC-like.
Highlights.
-
>
Differences in degree of recovery from extinction among the different renewal designs arise from multiple factors.
-
>
The excitatory training context can play a role in renewal as both a summative excitor and a comparator stimulus.
-
>
Renewal effects are determined by a number of contributing mechanisms.
Acknowledgments
National Institute of Mental Health Grant 33881 supported this research. Mario A. Laborda was partially funded by Program U-Apoya, University of Chile. The authors thank Cara Burney, Henry X. Cham, Lisa Mash, and Gonzalo Miguez for their comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Signals for whether versus when an event will occur. In: Bouton ME, Fanselow MS, editors. Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles. Washington DC: American Psychological Association; 1997. pp. 385–409. [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn. Motiv. 1979;10:445–466. [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. J. Exp. Psychol. Anim. Behav. Process. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, Ricker ST. Renewal of extinguished responding in a second context. Anim. Learn. Behav. 1994;22:302–308. [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. J. Exp. Psychol. Anim. Behav. Process. 1986;12:333–350. [Google Scholar]
- Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual control over conditioned responding in an extinction paradigm. J. Exp. Psychol. Anim. Behav. Process. 2000;26:174–185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- Holland PC. The nature of conditioned inhibition in serial and simultaneous feature negative discriminations. In: Miller RR, Spear NE, editors. Information processing in animals: Conditioned inhibition. Hillsdale, NJ: Erlbaum; 1985. pp. 267–297. [Google Scholar]
- Holland PC. Transfer of negative occasion setting and conditioned inhibition across conditioned and unconditioned stimuli. J. Exp. Psychol. Anim. Behav. Process. 1989;15:311–328. [PubMed] [Google Scholar]
- Laborda MA, McConnell BL, Miller RR. Behavioral techniques to reduce relapse after exposure therapy: Applications of studies of experimental extinction. In: Schachtman TR, Reilly S, editors. Associative learning and conditioning theory: Human and non-human applications. Oxford, UK: Oxford University Press; 2011. pp. 79–103. [Google Scholar]
- Laborda MA, Witnauer JE, Miller RR. Contrasting AAC and ABC renewal: The role of contexts associations. Learn. Behav. 2011;39:46–56. doi: 10.3758/s13420-010-0007-1. [DOI] [PubMed] [Google Scholar]
- Larrauri JA, Schmajuk NA. Attentional, associative, and configural mechanisms in extinction. Psychol. Rev. 2008;115:640–676. doi: 10.1037/0033-295X.115.3.640. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychol. Rev. 1975;82:276–298. [Google Scholar]
- McConnell BL, Miller RR. Protection from extinction provided by a conditioned inhibitor. Learn. Behav. 2010;38:68–79. doi: 10.3758/LB.38.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Escobar M. Associative interference between cues and between outcomes presented together and presented apart: An integration. Behav. Process. 2002;57:163–185. doi: 10.1016/s0376-6357(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Myers JL, Well AD. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. Research design and statistical analysis. [Google Scholar]
- Nelson JB. Context specificity of excitation and inhibition in ambiguous stimuli. Learn. Motiv. 2002;33:284–310. [Google Scholar]
- Nelson JB, Sanjuan MC, Vadillo-Ruiz S, Pérez J, León SP. Experimental renewal in human participants. J. Exp. Psychol. Anim. Behav. Process. 2011;37:58–70. doi: 10.1037/a0020519. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychol. Rev. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not unconditioned stimuli. Psychol. Rev. 1980;82:532–552. [PubMed] [Google Scholar]
- Polack CW, Laborda MA, Miller RR. Extinction context as a conditioned inhibitor. Learn. Behav. 2012;40:24–33. doi: 10.3758/s13420-011-0039-1. [DOI] [PubMed] [Google Scholar]
- Reberg D. Compound tests for excitation in early acquisition and after prolonged extinction of conditioned suppression. Learn. Motiv. 1972;3:246–258. [Google Scholar]
- Rescorla RA. Protection from extinction. Learn. Behav. 2003;31:124–132. doi: 10.3758/bf03195975. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Within-subject renewal in sign tracking. Q. J. Exp. Psychol. 2008;61:1793–1802. doi: 10.1080/17470210701790099. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York, NY: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rosas JM, Callejas-Aguilera JE, Ramos-Álvarez MM, Abad MJF. Revision of retrieval theory of forgetting: What does make information context-specific. Int. J. Psy. Psychol. T. 2006;6:147–166. [Google Scholar]
- Stout SC, Miller RR. Sometimes competing retrieval (SOCR): A formalization of the extended comparator hypothesis. Psychol. Rev. 2007;114:759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston, MA: Pearson Education; 2007. [Google Scholar]
- Tamai N, Nakajima S. Renewal of formerly conditioned fear in rats after extensive extinction training. Int. J. Comp. Psychol. 2000;13:137–147. [Google Scholar]
- Tamai N, Nakajima S, Kitaguchi K, Imada H. Renewal of extinguished fear by context-shifting in rats conditioned lick suppression. Jpn. J. Psychol. 2001;71:493–497. [Google Scholar]
- Thomas BL, Larsen N, Ayres JB. Role of context similarity in ABA, ABC, and AAB renewal paradigms: Implications for theories of renewal and for treating human phobias. Learn. Motiv. 2003;34:410–436. [Google Scholar]
- Todd TP, Winterbauer NE, Bouton ME. Effects of the amount of acquisition and contextual generalization on the renewal of instrumental behavior after extinction. Learn. Behav. 2012;40:145–157. doi: 10.3758/s13420-011-0051-5. [DOI] [PubMed] [Google Scholar]
- Witnauer JE, Miller RR. Contrasting overexpectation and extinction effects. Behav. Process. 2009;81:322–327. doi: 10.1016/j.beproc.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]