Abstract
Background
The treatment of patients with extensive thoracic aortic disease involving the arch and descending thoracic/thoracoabdominal aorta is often performed using the frozen elephant trunk technique (FET). We retrospectively analyzed our results of the FET operation.
Methods
A total of 51 consecutive patients underwent total aortic arch surgery with the FET technique between January 2006 and August 2013. For all patients, the E-vita hybrid open stent-graft (Jotec, Hechingen, Germany) was used. The patients had a mean age of 64±13 years, with 51.1% being female. Degenerative or atherosclerotic aneurysm was the indication for surgery in 62.7% of patients. Another 15.7% and 13.7% suffered from acute Type A, and Type B aortic dissection, respectively.
Results
The in-hospital and 30-day mortality was 7.8%. Stroke occurred in 11.8% (n=6), and new-onset paraplegia in 19.6% (n=10) of patients. The core body temperature ≥28 °C during circulatory arrest, in combination with a prolonged circulatory arrest time of more than 45 minutes, was an independent predictor of permanent spinal cord injury [odds ratios (OR), 4.8; 95% confidence intervals (CI), 1.1-21; P=0.04]. The estimated 1- and 5-year survival was (80.2±5.5)% and (59.7±10.2)%, respectively, with a mean survival time of 3.4±0.4 years. The estimated mean freedom from endovascular intervention was 4.2±0.4 years. The unadjusted 1- and 5-year freedom from thoracic endovascular aortic repair (TEVAR) was (84.9±5.9)% and (69.2±11.2)%, respectively.
Conclusions
The FET procedure for extensive thoracic aortic disease is associated with an acceptable early and medium term mortality rate. This procedure is associated with a high incidence of perioperative spinal cord injury. In order to prevent the above complication, deep hypothermia is strongly recommended in patients with expected prolonged circulatory arrest time.
Keywords: Aortic arch surgery, elephant trunk
Introduction
The standard surgical technique for patients with extensive pathology of the thoracic aorta is still the conventional elephant trunk technique, developed by Borst in 1983 (1). However, this procedure is associated with increased operative medium term mortality, and also with a high incidence of neurological complications (2). In recent years many new technological solutions based on endovascular methods have been introduced into clinical practice, one of them being a hybrid procedure, the frozen elephant trunk technique (FET) (3).
The current study shows our 7-year institutional experience of using the FET technique for the treatment of patients with extensive thoracic aortic disease. The objectives of this study were (1) to evaluate clinical characteristics, and early, and mid-term results, and (2) to determine predictors of clinical and neurological outcome, particularly, spinal cord injury after aortic arch surgery using FET.
Patients and methods
A total of 51 consecutive patients underwent total aortic arch surgery with FET implantation between January 2006 and August 2013 and were included in our analysis. Data were prospectively collected in a database, and retrospective review was approved by the local Ethics Committee. Individual patient consent was waived.
Patients were followed-up annually with a mailed questionnaire or, when needed, by contacting the referring cardiologist or general practitioner. Follow-up was 100% complete.
Operative technique
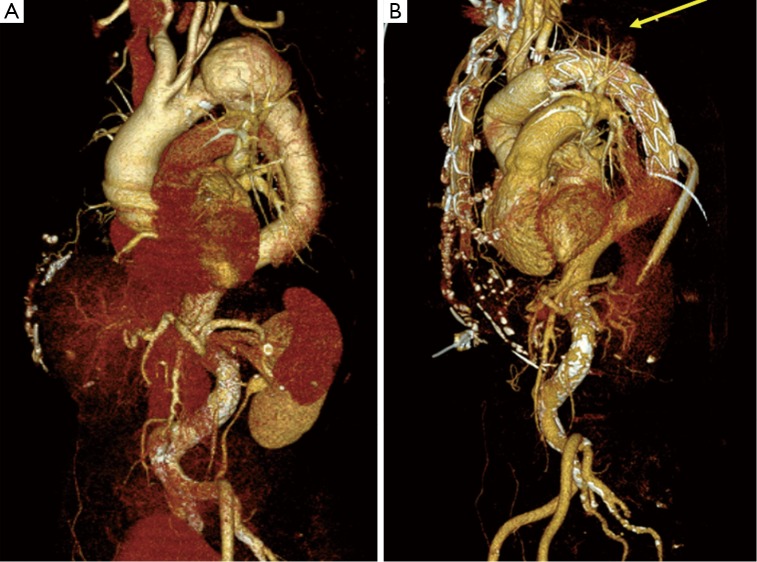
The FET technique has been performed in our hospital since 2006. In all patients the E-vita hybrid open stent-graft (Jotec, Hechingen, Germany) was used (Figure 1). The surgical technique and indication were as previously described (4).
Figure 1.
Preoperative CT scan of patient with distal aortic arch aneurysm (A) and after aortic arch surgery using FET technique (B) with completely excluded aneurysm (yellow arrow)
Definitions
In accordance with STS guidelines, early mortality was defined as all-cause mortality at 30 days. Operations were considered emergent if performed within 24 hours of hospital admission for cardiovascular instability, and urgent if performed during the same hospital admission.
All patients with suspected neurological complications on physical examination underwent computerized tomography or magnetic resonance imaging. Spinal cord injury was defined as new-onset transient or permanent paraparesis or paraplegia. Neurological complications were defined as permanent neurological deficit (PND) for patients with stroke or paraplegia, and temporary neurological deficit (TND) for patients with reversible deficits (2).
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (Chicago, IL). Categorical variables were compared using the Chi-square test or Fisher’s exact test. Independent continuous variables were compared by unpaired Student’s t-test for comparison of normally distributed data between two groups or Kruskal-Wallis test for the comparison of more than two groups as appropriate. Continuous variables are expressed as mean ± SD, and categorical data as proportions throughout the manuscript.
Dichotomous adverse outcome events were analyzed using an uni- and multivariate logistic regression model with backwards stepwise elimination, and were expressed as odds ratios (OR) with 95% confidence intervals (CI). Event-free survival was calculated by Kaplan-Meier methods with 95% CI. Independent predictors of medium-term survival were determined with Cox proportional hazards analysis. P<0.05 was considered statistically significant.
Results
A total of 51 consecutive patients underwent total replacement of the aortic arch using the FET technique during the study period. The majority (62.7%) suffered from degenerative aortic aneurysm. Acute Type A and Type B aortic dissection was the indication for surgery in 15.7% and 13.7% of patients, respectively (Table 1).
Table 1. Demographics and preoperative clinical characteristics.
| FET n=51 (%) | |
|---|---|
| Age (years) | 69±10 |
| Female | 24 (51.1) |
| COPD | 6 (11.8) |
| Diabetes | 9 (17.6) |
| Hypertension | 27 (52.9) |
| Cerebral vasculopathy | 2 (3.9) |
| Aortic disease | |
| Degenerative aneurysm | 32 (62.7) |
| Acute Type A aortic dissection | 8 (15.7) |
| Acute Type B aortic dissection | 7 (13.7) |
| Chronic Type A aortic dissection | 1 (2) |
| Chronic Type B aortic dissection | 1 (2) |
| Downstream aneurysm following Acute Type A aortic dissection |
2 (3.9) |
| Previous surgery | 9 (17.6) |
| CABG | 1 (2.0) |
| Valve | 3 (5.9) |
| Root | 1 (2) |
| Abdominal aorta | 1 (2) |
| Thoracic aorta | 6 (11.8) |
| Aortic diameter (mm) | |
| Ascending | 46±11 |
| Arch | 53±16 |
| Descending | 43±12 |
| Abdominal | 31±6 |
Data are presented as numbers of cases unless otherwise indicated. COPD, chronic obstructive pulmonary disease; TEVAR, thoracic endovascular aortic repair; CABG, coronary artery bypass grafting
Operative details and post-operative complications
All operative details are presented in Table 2. Mean core body temperature was (26±2) °C. The mean prosthesis size was 33.7±4.8 mm (24-40 mm). Prosthesis over-sizing was observed in 14% (n=7) patients. The distal prosthesis landing zone was T7 and lower in 19.6% (n=9), between T8 and T9 in 52.2% (n=24), and more than T10 in 26.1% (n=12) of patients.
Table 2. Operation data and postoperative clinical characteristics.
| FET n=51 (%) | |
|---|---|
| Operation | |
| CPB time (min) | 213±66 |
| Cross clamp time (min) | 98±38 |
| Circulatory arrest time (min) | 50±14 |
| ASCP | 51 (100) |
| ASCP time (min) | 47±14 |
| Minimum rectal temperature (°C) | 26±2 |
| Axillary artery cannulation | 38 (74.5) |
| Reimplantation of supraaortic vessels | |
| Island | 40 (78.4) |
| Separate | 11 (21.6) |
| Aortic valve/root intervention | |
| ARR | 6 (11.8) |
| AV Reimplantation (David) | 1 (2) |
| AV Reconstruction (Yacoub) | 1 (2) |
| AVR | 1 (2) |
| Concomitant surgery | |
| CABG | 10 (19.6) |
| Postoperative outcome | |
| PND | 6 (11.8) |
| TND | 5 (9.8) |
| Paraplegia | 10 (19.6) |
| Respiratory failure | 19 (37.3) |
| Renal failure | 13 (25.5) |
| Reoperation for bleeding | 7 (13.7) |
| 30-day mortality | 4 (7.8) |
| In hospital mortality | 4 (7.8) |
Data are presented as number of cases unless otherwise indicated. CPB, cardiopulmonary bypass; ASCP, antegrade selective cerebral perfusion; AVR, aortic valve replacement; AV, aortic valve; ARR, aortic root replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; PND, permanent neurologic deficit; TND, temporary neurological deficit
Postoperative outcome
The 30-day and in-hospital mortality for the entire cohort of patients was 7.8% (n=4). PND occurred in 11.8% (n=26) of patients (Table 2). The incidence of PND and TND was 11.8% and 9.8%, respectively. The overall rate of paraplegia was 19.6% (n=10). Multivariate analysis identified a core body temperature of 28 °C or higher during circulatory arrest in combination with duration of circulatory arrest greater than 45 minutes, as an independent predictor of permanent spinal cord injury (OR 4.8; 95% CI, 1.1-21; P=0.04).
Predictors of medium-term survival and freedom from thoracic endovascular aortic repair (TEVAR)
The estimated mean follow-up time for all patients was 3.4±0.4 years (0-5.1 years) with a total follow-up of 58.8 patient years. The unadjusted 1- and 5-year survival for the entire group was 80.2±5.5%, and 59.7±10.2%, respectively.
During follow-up TEVAR was performed in 8 patients. The estimate mean freedom from endovascular intervention was 4.2±0.4 years. The unadjusted 1- and 5-year freedom from TEVAR was (84.9±5.9)%, and (69.2±11.2)%, respectively (Figure 2). One patient underwent re-operative thoracoabdominal aortic surgery 5.5 years after initial surgery due to progression of extensive thoracoabdominal disease.
Figure 2.
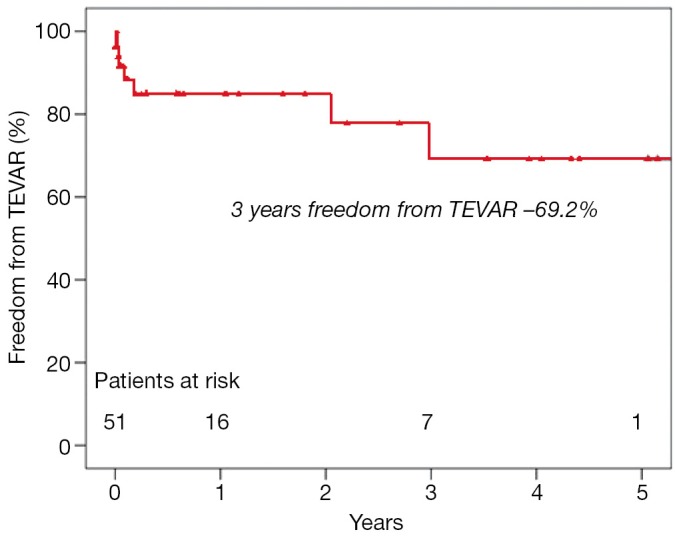
Freedom from TEVAR procedure after initial FET implantation
Discussion
The current study summarizes our 7-year institutional experience in aortic repair with “FET” for patients with extensive aortic pathology, a technique, which was introduced into clinical practice at our institution in 2006.
The 30-day mortality of 7.8%, observed in our study, is comparable to other case series reported in the literature. The in-hospital mortality, reported in 2011 by the International E-vita Open Registry, is 15% (5). The lower mortality rate in the present study must be interpreted with caution, because a lower number of patients suffered from acute type A aortic dissection. This disease has previously been identified as an independent predictor of 30-day mortality in patients undergoing aortic arch surgery (2,6).
The neurological status of patients after extended aortic arch surgery has an important impact on postoperative outcome. The most devastating complications associated with reduced quality of life and long-term survival in these patients are PND and spinal cord injury (6-8).
Permanent or transient spinal cord injury is a rare complication after extended aortic arch surgery with conventional elephant trunk implantation, with incidence rates ranging from 0.4% to 2.8% (9-11). As opposed to that, in patients who received FET, this complication was significantly higher. A multicenter study, including 240 patients with FET, reported a 9% incidence of this dreaded complication (12). Single center studies with relatively small numbers of patients have reported incidences as high as 21-24% after FET implantation (13-15). The factors influencing paraplegia are extended length of circulatory arrest (13,14), and level of the distal landing zone beyond T7 (15). Flores and colleagues identified a distal landing zone of T7 or lower, and a history of previous abdominal aortic aneurysm repair as a predictor of spinal cord injury. This observation suggests an important role of distal inflow to the paraspinous arterial collateral network via the hypogastric arteries (15). The Collateral Network Concept, investigated by Griepp’s group (10), has been used to explain and understand this phenomenon. The FET technique has the potential to impact both Collateral Network inflow pathways simultaneously: the segmental artery by insertion of the stented part of the hybrid graft into the descending aorta, and the vertebral artery due to lack of perfusion during circulatory arrest. This can explain the increased incidence of spinal cord injury to patients who underwent the FET procedure compared to those who underwent conventional elephant trunk implantation. In the current study, the incidence of spinal cord injury was 21.4%. A core body temperature of 28 °C or higher during circulatory arrest, in combination with circulatory arrest time greater than 45 minutes, was identified as an independent predictor of spinal cord injury. Five out of twelve patients, who suffered ischemic spinal cord injury, had circulatory arrest times greater than 45 minutes during which the core body temperature was 28 °C or warmer. Similar to observations of Pacini et al. (12), the core body temperature or duration of circulatory arrest alone do not have a significant impact on the incidence of postoperative spinal cord injury. In the current study we also failed to find a significant correlation between the distal FET landing zone level and the incidence of acute ischemic spinal cord injury. The landing zone of the distal end of the stent-graft was at or even proximal to the T8 level in six of ten patients with postoperative spinal cord injury.
The incidence of PND in patients undergoing aortic arch surgery has been reported to range between 1.1% and 9.8% (8,16,17). Age, acute type A aortic dissection (2), urgent status (7,16), renal insufficiency (16), operation time (16) and, prolonged circulatory arrest (2,16) were factors that influenced the occurrence of PND after surgery. PND was observed in 11.8% of our patients and was nearly twice as high among patients who presented with acute type A aortic dissection.
In conclusion, the FET technique can be performed with a relatively low mortality in patients with extensive pathology of the thoracic aorta. However, this procedure is associated with a significantly increased incidence of postoperative permanent paraplegia due to ischemic spinal cord injury. The independent predictor of paraplegia is the combination of prolonged circulatory arrest times (≥45 minutes) with mild hypothermia (≥28 °C). The FET procedure should therefore be performed using deep hypothermia, particularly in patients with prolonged aortic arch surgery, in order to prevent complications due to ischaemic spinal cord injury.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg 1983;31:37-40 [DOI] [PubMed] [Google Scholar]
- 2.Misfeld M, Leontyev S, Borger MA, et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8 [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:II188-93 [PubMed] [Google Scholar]
- 4.Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J Cardiothorac Surg. 2013 doi: 10.1093/ejcts/ezt252. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Jakob H, Tsagakis K, Pacini D, et al. The International E-vita Open Registry: data sets of 274 patients. J Cardiovasc Surg (Torino) 2011;52:717-23 [PubMed] [Google Scholar]
- 6.Misfeld M, Leontyev S, Borger MA, et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8 [DOI] [PubMed] [Google Scholar]
- 7.Goldstein LJ, Davies RR, Rizzo JA, et al. Stroke in surgery of the thoracic aorta: incidence, impact, etiology, and prevention. J Thorac Cardiovasc Surg 2001;122:935-45 [DOI] [PubMed] [Google Scholar]
- 8.Immer FF, Moser B, Krähenbühl ES, et al. Arterial access through the right subclavian artery in surgery of the aortic arch improves neurologic outcome and mid-term quality of life. Ann Thorac Surg 2008;85:1614-8; discussion 1618 [DOI] [PubMed] [Google Scholar]
- 9.Safi HJ, Miller CC 3rd, Estrera AL, et al. Optimization of aortic arch replacement: two-stage approach. Ann Thorac Surg 2007;83:S815-8; discussion S824-31. [DOI] [PubMed]
- 10.Etz CD, Plestis KA, Kari FA, et al. Staged repair of thoracic and thoracoabdominal aortic aneurysms using the elephant trunk technique: a consecutive series of 215 first stage and 120 complete repairs. Eur J Cardiothorac Surg 2008;34:605-14; discussion 614-5 [DOI] [PubMed] [Google Scholar]
- 11.Palma JH, Almeida DR, Carvalho AC, et al. Surgical treatment of acute type B aortic dissection using an endoprosthesis (elephant trunk). Ann Thorac Surg 1997;63:1081-4 [DOI] [PubMed] [Google Scholar]
- 12.Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: a multicenter experience. Ann Thorac Surg 2011;92:1663-70; discussion 1670. [DOI] [PubMed]
- 13.Miyairi T, Kotsuka Y, Ezure M, et al. Open stent-grafting for aortic arch aneurysm is associated with increased risk of paraplegia. Ann Thorac Surg 2002;74:83-9 [DOI] [PubMed] [Google Scholar]
- 14.Mizuno T, Toyama M, Tabuchi N, et al. Stented elephant trunk procedure combined with ascending aorta and arch replacement for acute type A aortic dissection. Eur J Cardiothorac Surg 2002;22:504-9 [DOI] [PubMed] [Google Scholar]
- 15.Flores J, Kunihara T, Shiiya N, et al. Extensive deployment of the stented elephant trunk is associated with an increased risk of spinal cord injury. J Thorac Cardiovasc Surg 2006;131:336-42 [DOI] [PubMed] [Google Scholar]
- 16.Khaladj N, Shrestha M, Meck S, et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg 2008;135:908-14 [DOI] [PubMed] [Google Scholar]
- 17.LeMaire SA, Carter SA, Coselli JS. The elephant trunk technique for staged repair of complex aneurysms of the entire thoracic aorta. Ann Thorac Surg 2006;81:1561-9; discussion 1569 [DOI] [PubMed] [Google Scholar]