Abstract
Acetaminophen (APAP) overdose is a major cause of hepatotoxicity and acute liver failure in the US, but the pathophysiology is incompletely understood. Despite evidence for apoptotic signaling, hepatic cell death after APAP is generally considered necrotic in mice and in humans. Recent findings suggest that the receptor interacting protein kinase 3 (RIP3) acts as a switch from apoptosis to necrosis (programmed necrosis). Thus, the aim of the current investigation was to determine if RIP3 is involved in APAP-induced liver cell death. APAP (200–300 mg/kg) caused glutathione depletion and protein adduct formation, oxidant stress, mitochondrial release of apoptosis inducing factor, and nuclear DNA fragmentation resulting in centrilobular necrosis in C57Bl/6J mice. Inhibiting RIP3 protein induction with anti-sense morpholinos in wild-type animals or using RIP3-deficient mice had no effect on protein adduct formation but attenuated all other parameters, including necrotic cell death, at 6h after APAP. In addition, cultured hepatocytes from RIP3-deficient mice showed reduced injury compared to wild-type cells after 24h. Interestingly, APAP-induced mitochondrial translocation of dynamin-related protein 1 (Drp1), the initiator of mitochondrial fission, was inhibited by reduced RIP3 protein expression and the Drp1 inhibitor MDIVI reduced APAP-induced cell death at 24h. All of these protective effects were lost after 24h in vivo or 48h in vitro. Conclusion: RIP3 is an early mediator of APAP hepatotoxicity, involving modulation of mitochondrial dysfunction and oxidant stress. Controlling RIP3 expression could be a promising new approach to reduce APAP-induced liver injury, but requires complementary strategies to control mitochondrial dysfunction for long-term protection.
Keywords: Programmed Necrosis, Drp1, RIP1, Mitochondrial Fission
INTRODUCTION
Acetaminophen (APAP) is a safe analgesic and antipyretic drug at therapeutic levels. However, APAP overdose is the most common cause of acute liver failure in the US and UK.1 The toxicity is mediated by the metabolism of APAP to the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which forms adducts on cellular proteins, especially in the mitochondria.2 This leads to mitochondrial oxidant stress, which activates the c-jun N-terminal kinase (JNK), resulting in its translocation to the mitochondria3 and amplification of the oxidant stress.4 The extensive mitochondrial oxidant stress eventually causes the opening of the mitochondrial membrane permeability transition pore5 and necrotic cell death.6,7 Early bax translocation to the mitochondria triggers release of intermembrane proteins,8 including apoptosis inducing factor (AIF) and endonuclease G which translocate to the nucleus and are responsible for nuclear DNA fragmentation.9,10 Interestingly, smac/DIABLO and cytochrome c are also released8 but this does not induce caspase activation or apoptotic cell death.6,11,12 The reason for the virtual absence of apoptotic cell death after APAP overdose in mice,6 in mouse hepatocytes,5,7,13 in a human hepatocyte cell line (HepaRG)14 and in humans,15,16 despite conditions that would support the initiation of a mitochondrial pathway of apoptosis (cytochrome c and smac/DIABLO release), remains unclear. It was argued that declining ATP levels due to inhibition of mitochondrial respiration17 or lack of glycogen in starved mice18 may prevent apoptosis; however, this explanation may not be sufficient.19
Recent data indicate that necrosis can also be a programmed form of cell death (“necroptosis”) and unique molecular pathways involved in necrotic cell death are being defined.20 The receptor interacting protein kinase 3 (RIP3) has been identified by RNA interference screens as an essential mediator of necrotic cell death induced by TNF-α and other ligands.21,22 Necrotic signaling by RIP3 requires its interaction with the receptor interacting protein kinase 1 (RIP1) and FADD (Fas associated death domain), but unlike RIP1, RIP3 is not involved in NF-κB or apoptotic signaling.22 RIP3 has also been shown to switch TNF-α-induced cell death from apoptosis to necrosis,23 suggesting that it is a unique molecular regulator which dictates necrotic cell death. Recent studies have shown that downstream cellular signaling induced by TNF-α-mediated activation of RIP3 involves the mixed lineage kinase domain-like protein (MLKL),24,25 as well as the mitochondrial phosphatase PGAM5.26 These mediators in turn have been shown to regulate activation of JNK24 as well as changes in mitochondrial dynamics.26 Given the central role of mitochondria and JNK activation in the pathophysiology of APAP-induced necrotic cell death,27 the objective of the current investigation was to assess a potential role of RIP3 in the pathophysiology of APAP-induced cell death using gene knock-down approaches and genetically deficient mice.
MATERIALS AND METHODS
Animals
Male C57Bl/6J mice (Jackson Laboratories, Bar Habor, Maine) with an average weight of 20g were used in this study. RIP3-deficient mice (C57Bl/6J background) were kindly provided by Dr. Vishva Dixit (Genentech, Inc). All animals were housed in an environmentally controlled room with 12 h light/dark cycle and allowed free access to food and water. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center and followed the criteria of the National Research Council for the care and use of laboratory animals in research.
In vivo morpholino treatment
All vivo-morpholinos were obtained from Gene Tools, LLC (Philomath, OR, USA). Details are described in the supplemental material.
Experimental design
Mice were injected ip with 200 mg/kg or 300 mg/kg APAP (dissolved in warm saline) after overnight fasting. The animals were killed 6 or 24h after APAP treatment. Plasma alanine aminotransferase (ALT) activities and liver GSH and glutathione disulfide (GSSG) levels were measured as described in the supplemental material.
Histology and immunohistochemistry
Sections of formalin-fixed tissue were stained with hematoxylin and eosin (H&E) for evaluation of necrosis,6 the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay for DNA strand breaks and nitrotyrosine as described in the supplemental material.
Measurement of APAP protein adducts
APAP protein adducts were measured using high pressure liquid chromatography with electrochemical detection.28
Quantitative real-time polymerase chain reaction (qRT-PCR)
Expression of the RIP3 gene was quantified using qRT-PCR analysis as previously described19 using the following primers – Fwd: 5’-TCCCAATCTGCACTTCAGAAC-3’, Rev: 5’-GACACGGCACTCCTTGGTAT-3’.
Primary hepatocyte isolation
Primary hepatocytes were isolated as previously described.13 Cell treatment is described in the supplemental material.
Isolation of subcellular fractions and western blotting
Mitochondria and cytosolic fractions were isolated as described.8 Western blotting was carried out as described in detail8 using the following antibodies: a rabbit anti-RIP3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit anti-AIF antibody (Abcam, Cambridge, MA) and a rabbit anti-Drp1 polyclonal antibody (Cell signaling Technology, Danvers, MA). A horseradish peroxidase-coupled anti-rabbit IgG (Santa Cruz) was used as secondary antibody.
Statistics
All results were expressed as mean ± SE. Comparison between two groups were performed with Student’s t-test. One-way analysis of variance (ANOVA) was used to assess statistical significance between three or more groups. When a difference was detected, the Student Newman-Keul’s test was used for multiple comparisons. For non-normally distributed data, the Kruskal-Wallis test was used. P<0.05 was considered significant.
RESULTS
APAP-induced RIP3 expression
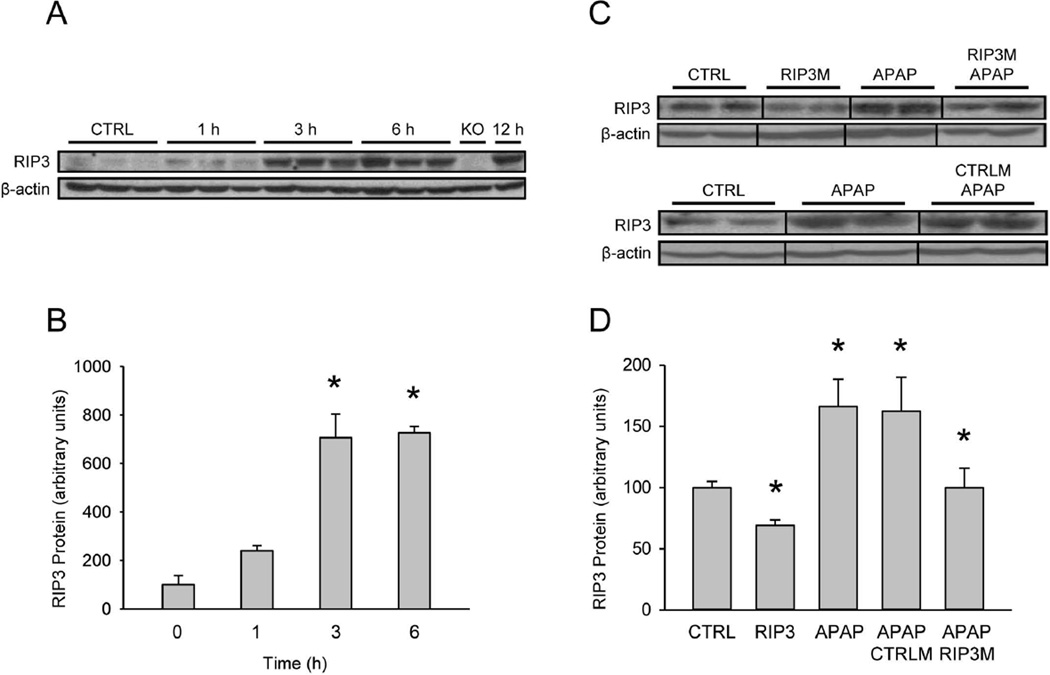
To evaluate the role of RIP3 in APAP-induced hepatotoxicity, we began by measuring the time course of RIP3 protein expression after APAP overdose. Treatment of mice with 300 mg/kg APAP revealed a mild response as early as 1h with the largest increase between 1 and 3h (Figure 1A,B). Enhanced RIP3 protein levels were also observed after a lower dose of APAP (200 mg/kg) (Figure 1C,D), which was accompanied by a 6-fold increase in mRNA at 6h (data not shown).
Figure 1. Acetaminophen induces liver RIP3 expression.
(A) RIP3 protein over time after treatment with 300 mg/kg acetaminophen (APAP), and (B) densitometry. (C) RIP3 protein after treatment with 200 mg/kg APAP with or without control morpholino (CTRLM) or RIP3 morpholino (RIP3M) pretreatment, and (D) densitometry. Data represent mean ± SE of n=3. *P<0.05 (compared to CTRL).
Functional significance of RIP3 in APAP toxicity
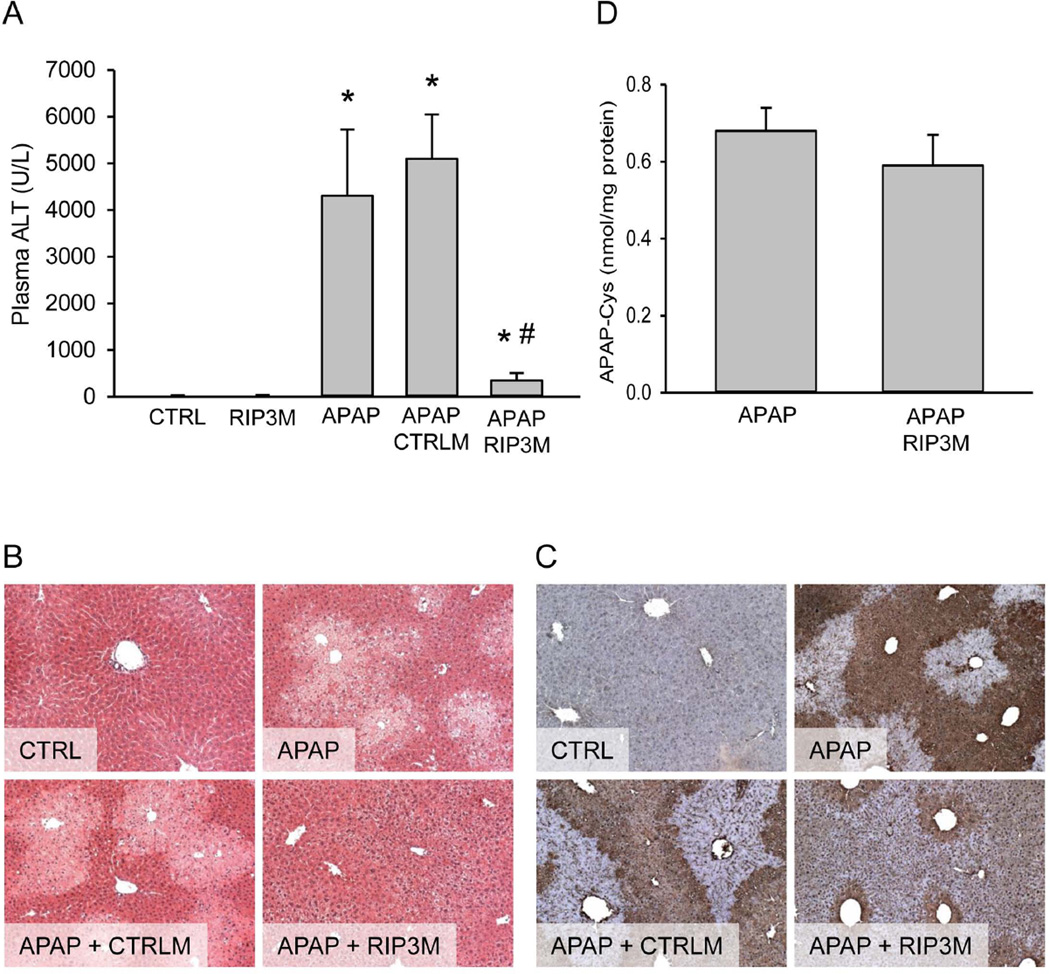
To evaluate whether or not the changes in RIP3 protein levels were the cause or merely a consequence of APAP hepatotoxicity, experiments were designed to knock down the protein. Administration of in vivo morpholinos targeting RIP3 resulted in a significant decrease in RIP3 protein in mice (Figure 1C,D). Although RIP3 morpholinos only partially reduced the expression of constitutive RIP3 protein, it completely prevented RIP3 protein upregulation subsequent to 200 mg/kg APAP at 6h. Treatment with control morpholinos did not prevent the APAP-mediated induction of RIP3 (Figure 1C,D). Measurement of plasma ALT as indicator of liver cell death demonstrated that treatment with a low overdose of 200 mg/kg APAP resulted in significant liver injury by 6h (Figure 2A). Administration of control morpholinos prior to APAP had no effect on this elevation. However, preventing the upregulation of RIP3 by treatment with RIP3 morpholinos resulted in a significant protection against APAP-induced liver injury as seen by the dramatically lower ALT values in RIP3 morpholino-treated animals (Figure 2A). This was supported by histology, where the characteristic centrilobular necrosis after APAP overdose is evident in animals treated with APAP alone or control morpholinos before APAP, while treatment with RIP3 morpholinos resulted in significantly less cellular necrosis (Figure 2B). In order to assess oxidant stress and peroxynitrite formation, tissue sections were stained for nitrotyrosine (NT) protein adducts (Figure 2C). Previous studies showed that NT reflects events in mitochondria.27,29 APAP caused extensive NT staining in untreated and control morpholino-treated animals. RIP3 morpholinos extensively reduced NT staining suggesting a substantially lower mitochondrial oxidant stress in these animals (Figure 2C). Measurement of protein-derived APAP-cysteine adducts showed similar levels of protein binding with or without RIP3 morpholino treatment (Figure 2D). Thus, the protection of the RIP3 morpholinos against APAP-induced liver injury was not caused by an effect on metabolic activation of APAP.
Figure 2. Inhibition of RIP3 induction protects against acetaminophen hepatotoxicity at 6h.
Mice were treated with 200 mg/kg acetaminophen (APAP), with or without control morpholino (CTRLM) or RIP3 morpholino (RIP3M) pretreatment. (A) Plasma ALT and (B) H&E staining. (C) Liver APAP-protein adducts. (D) Nitrotyrosine staining. Data represent mean ± SE of n=4. *P<0.05 (compared to CTRL). #P<0.05 (compared to APAP)
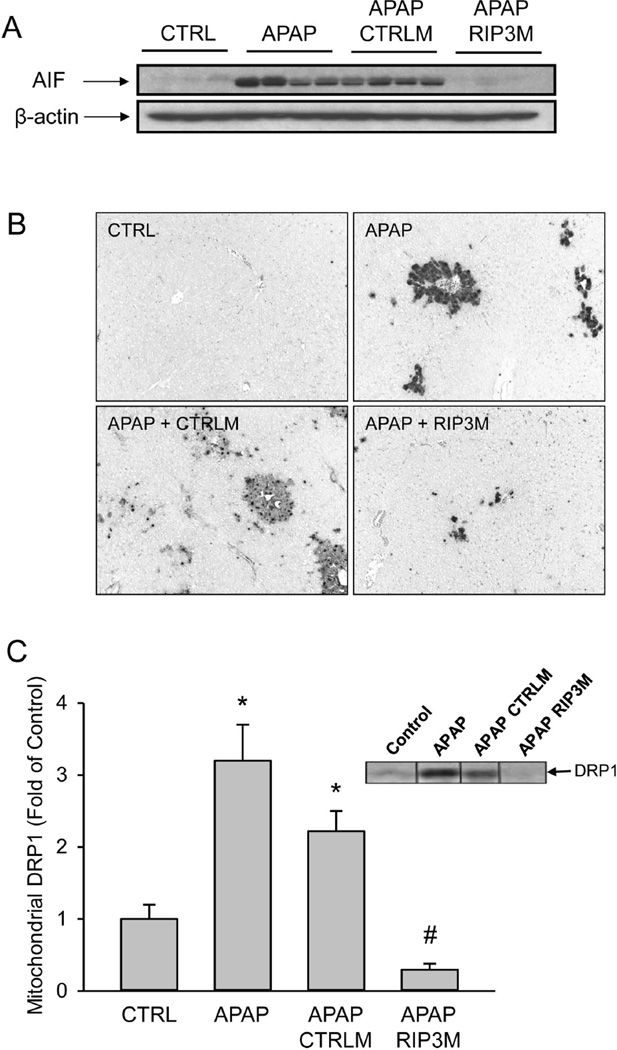
RIP3 has been suggested to induce mitochondrial generation of free radicals.23 Mitochondrial oxidant stress followed by activation of the mitochondrial permeability transition and release of mitochondrial proteins such as AIF are known to play critical roles in APAP induced liver injury.10 To determine if the APAP-induced induction of RIP3 played a role in mitochondrial dysfunction, the release of AIF into the cytosol was measured (Figure 3A). As expected, APAP overdose caused AIF release into the cytosol, an effect which was not altered by pretreatment with control morpholinos. Preventing RIP3 induction with the RIP3 morpholinos however, resulted in almost complete elimination of AIF release into the cytosol (Figure 3A). This suggests that RIP3 was involved in the mitochondrial dysfunction after APAP overdose. Mitochondrial AIF and endonuclease G are known to cause DNA fragmentation in APAP hepatotoxicity.9,10 Consistent with the AIF data (Figure 3A), RIP3 morpholinos strongly reduced nuclear DNA fragmentation as indicated by the TUNEL assay (Figure 3B). It has recently been shown that RIP3 controls mitochondrial dynamics by modulating localization of the dynamin related protein 1 (Drp1), which localizes to mitochondria to induce mitochondrial fission, an event that occurs during cell necrosis.26 Therefore, mitochondrial Drp1 levels were evaluated by western blotting. APAP treatment induced significant translocation of Drp1 to mitochondria independent of pretreatment with control morpholinos (Figure 3C). However, mitochondrial Drp1 translocation was completely prevented in animals treated with RIP3 morpholinos.
Figure 3. Effect of RIP3 knockdown on mitochondrial AIF release and Drp1 translocation.
(A) Apoptosis-inducing factor (AIF) in cytosolic fractions from control and APAP-treated animals with or without RIP3 (RIP3M) or control (CTRLM) morpholinos.(B) TUNEL staining for nuclear DNA fragmentation. (C) Drp1 in mitochondrial fractions from control and APAP-treated animals with or without RIP3M or CTRLM and densitometry. Data represent mean ± SE of n=4. *P<0.05 (compared to CTRL). #P<0.05 (compared to APAP).
APAP toxicity in RIP3-deficient mice
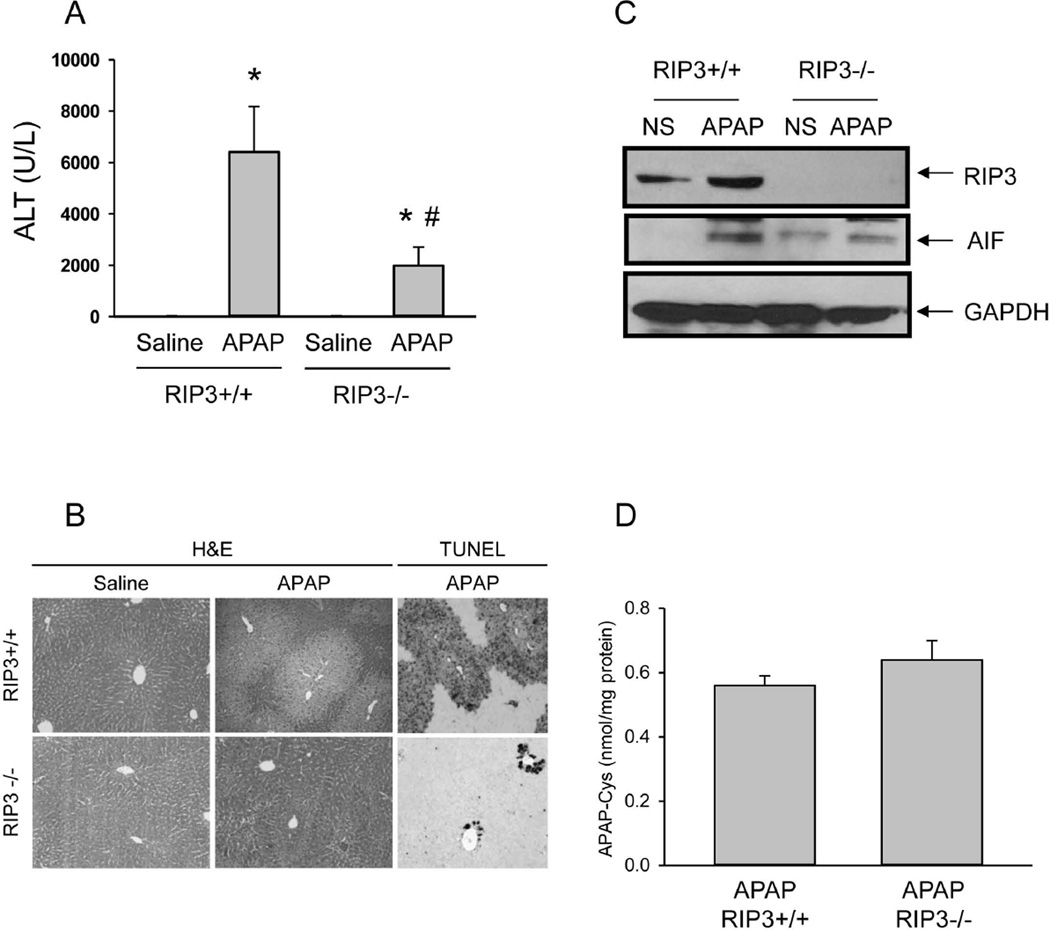
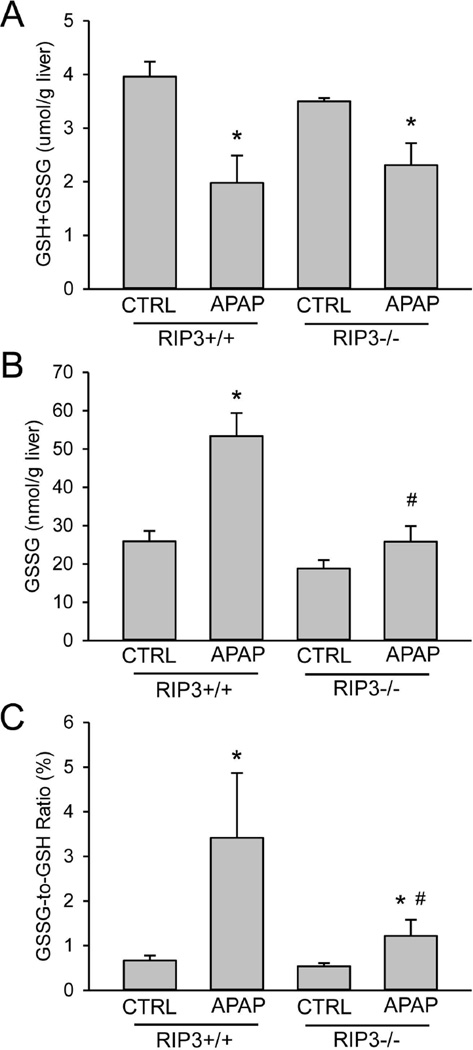
In order to support the RIP3 morpholino findings, similar experiments were performed in RIP3-deficient mice using a higher overdose of APAP (300 mg/kg). Treatment of wild type (RIP3+/+) and RIP3−/− mice with APAP resulted in severe injury in the RIP3+/+ animals as indicated by the increase in plasma ALT activities (Figure 4A), and extensive necrosis and DNA fragmentation as indicated by the centrilobular TUNEL staining (Figure 4B). RIP3−/− mice developed substantially less injury as indicated by the 70% reduction in ALT values (Figure 4A) and the smaller areas of necrosis and less DNA fragmentation (Figure 4B). Western blot analysis of RIP3 confirmed the induction after APAP treatment in wild type mice and the lack of the protein in the RIP3−/− animals (Figure 4C). In addition, the AIF increase in the cytosol in wild type animals was prevented in RIP3−/− mice (Figure 4C). Importantly, there was no significant difference in APAP protein adducts, suggesting that the reduced injury in RIP3−/− mice was not caused by inhibition of metabolic activation (Figure 4D). Hepatic glutathione levels, although partially recovered, were still significantly lower than controls in both RIP3+/+ and RIP3−/− mice 6h after APAP treatment (Figure 5A). However, GSSG levels and the GSSG-to-GSH ratio were significantly increased in wild-type animals, reflecting an increased mitochondrial oxidant stress in these livers (Figure 5B,C). In contrast, GSSG levels in RIP3−/− mice were not different from control values and the GSSG-to-GSH ratio was only modestly elevated, demonstrating significantly less APAP-induced oxidant stress in RIP3-deficient animals (Figure 5B,C).
Figure 4. RIP3-deficient mice are protected against acetaminophen hepatotoxicity at 6h.
Wild type (RIP3+/+) and RIP3−/− mice were treated with 300 mg/kg acetaminophen (APAP) or saline. (A) Plasma ALT and (B) H&E and TUNEL staining 6h after APAP. (C) Apoptosis-inducing factor (AIF) protein in cytosol fractions from liver (pooled samples from 3–5 animals per group). (D) Hepatic APAP-protein adducts. All data are expressed as mean ± SE of n=3–5. *P<0.05 (compared to CTRL). #P<0.05 (compared to RIP3+/+ APAP).
Figure 5. Hepatic glutathione levels in wild type and RIP3-deficient mice.
Wild type (RIP3+/+) and RIP3−/− mice were treated with 300 mg/kg APAP or saline. At 6 h after APAP, total glutathione (GSH+GSSG) (A) and GSSG (B) levels were determined and the GSSG-to-GSH ratio (C) was calculated. Data represent means ± SE of n= 3–5. *P<0.05 (compared to CTRL). #P<0.05 (compared to RIP3+/+ APAP).
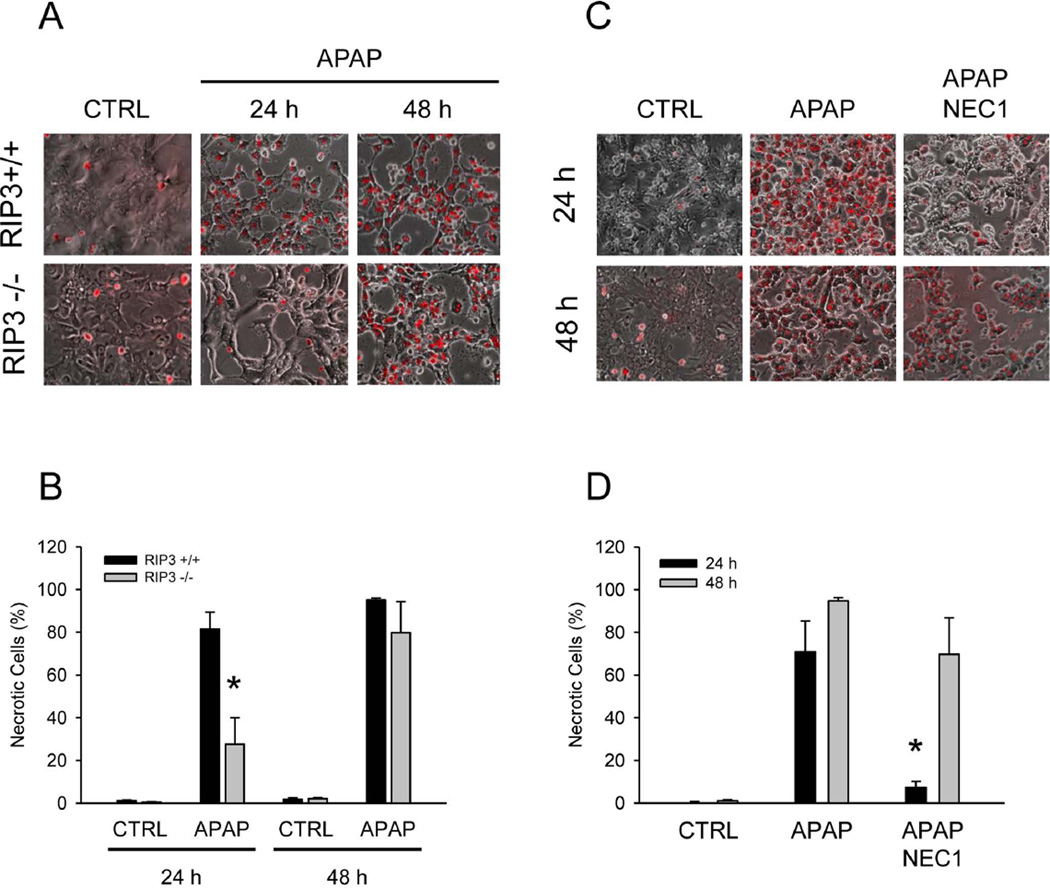
Role of RIP3 in cultured hepatocytes
To further confirm the protective effect of RIP3-deficiency, cultured hepatocytes isolated from RIP3+/+ and RIP3−/− mice were exposed to 10 mM APAP. As indicated by PI staining, there was extensive necrotic cell death in hepatocytes from wild type mice, which was reduced by 65% in RIP3−/− hepatocytes at 24h (Figure 6A,B). However, the protective effect was lost by 48h. Because previous reports indicated a protective effect of the RIP1 inhibitor necrostatin after 20h of APAP exposure,30 we repeated these experiments for up to 48h. Similar to the RIP3−/− hepatocytes, necrostatin reduced cell death by 90% at 24h but did not protect at 48h (Figure 6C,D). To determine if the effect of RIP3 is more stable in vivo, we assessed liver injury at 24h after APAP in RIP3 morpholino-treated animals (200 mg/kg) and in RIP3−/− mice (300 mg/kg). Similar to the observation in cultured cells, the protective effect of RIP3 elimination was lost at 24h (Supplemental Figure 1). Likewise, there was no difference in oxidant stress (GSSG) between the groups (data not shown).
Figure 6. RIP3-deficient and necrostatin-treated cultured hepatocytes are protected against APAP toxicity at early time points.
(A) Propidium iodide (PI) fluorescence in wild-type (RIP3 +/+) and RIP3 −/− mouse hepatocytes after treatment with 10 mM acetaminophen (APAP) and (B) quantification. (C) PI fluorescence in wild-type mouse hepatocytes after treatment with APAP or APAP + Necrostatin 1 (NEC1) and (D) quantification. For quantitation, more than 300 cells were counted in each experiment. Data represent means ± SE from three independent experiments. *P<0.05 (compared to CTRL). #P<0.05 (compared to RIP3+/+ APAP).
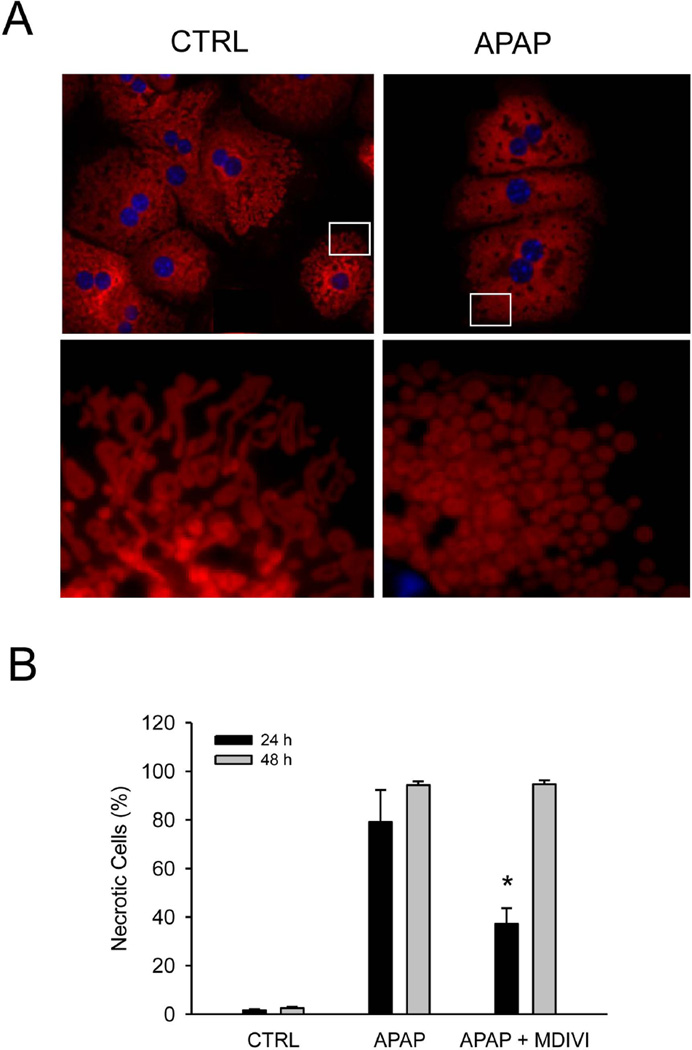
Because there is evidence for mitochondrial translocation of Drp1 (Figure 4), the effect of APAP on mitochondrial morphology was investigated. As shown in Figure 7A, significant changes were evident in mitochondria 6h after APAP treatment. Control cells had a significant number of filamentous mitochondria (Figure 7A lower left panel) while almost all mitochondria in APAP treated cells were fragmented (Figure 7A lower right panel). These data suggest that APAP-induced RIP3 induction affects mitochondrial morphology by stimulating Drp1-mediated mitochondrial fission. To test if there is a functional significance to mitochondrial Drp1 translocation, hepatocytes from wild type mice were treated with 50 µM of the Drp1 inhibitor MDIVI.31 Compared to control hepatocytes, APAP-induced necrotic cell death in MDIVI-treated cells was reduced by 53% at 24h; however, the protective effect of MDIVI was lost at 48h (Figure 7B).
Figure 7. Morphological changes in mitochondria during APAP-induced cell injury.
(A) Mitotracker fluorescence in primary hepatocytes 6h after 10 mM acetaminophen (APAP). Lower images show higher magnifications of indicated areas (white rectangles). Representative images from three independent experiments are shown. (B) Primary cultured wild type hepatocytes were treated with 10 mM APAP in the presence or absence of 50 µM MDIVI for 24 h. Necrotic cells were quantified from a total of more than 300 cells in each experiment. Data represent mean ± SE from three independent experiments. *P<0.05 (compared to CTRL).
DISCUSSION
The objective of this investigation was to assess the presence and functional significance of RIP3 in APAP-induced cell death in vitro and liver injury in vivo. Using three different experimental approaches, our data consistently showed that RIP3 is a critical component of the signaling cascade of APAP-induced cell death independent of the dose, at least during the early injury phase.
RIP3 expression and APAP-induced cell death
RIP3 protein expression in livers of untreated C57Bl/6J mice and hepatocytes was low, but was significantly induced after APAP treatment. RIP3 morpholinos were only modestly effective in reducing the baseline levels of RIP3 but prevented APAP-induced transcriptional activation of RIP3. In contrast to control morpholinos, which did not affect RIP3 induction or APAP-induced liver injury, RIP3 morpholinos were highly effective in reducing APAP-induced cell death. These data suggest that the early transcriptional induction of RIP3 is critical for APAP-induced necrosis. The importance of RIP3 in APAP-induced liver injury was confirmed in RIP3-deficient mice and in cultured hepatocytes, suggesting that RIP3 expression in parenchymal cells is involved in cell death. The mode of cell death after APAP is generally considered to be necrosis due to characteristics such as cell swelling and massive cell contents release, the absence of active caspases and the lack of a protective effect of caspase inhibitors in this model.6,7,11,12,17 The current findings that RIP3 is critical for the injury combined with recent reports on the beneficial effect of the RIP1 inhibitor necrostatin30,32 indicate that early APAP-induced hepatocyte cell death fits many characteristics of programmed necrosis (necroptosis).20 Interestingly, in the absence of RIP3 the remaining cell death is still necrotic based on the TUNEL assay, which shows the typical features of necrotic DNA fragmentation.6 Although the most studied initiator of necroptosis is TNF-α and TNF receptor 1 signaling,20 TNF-α is unlikely the initiator in the context of APAP hepatotoxicity. First, there is very limited TNF-α protein formation in response to APAP overdose.33,34 Second, TNF-deficient or TNF receptor type 1-deficient mice are not protected against APAP hepatotoxicity35,36 and anti-TNF-α antibodies or soluble TNF-α receptor treatment did not affect APAP-induced liver injury.34 Third, treatment with a low dose of endotoxin, which produces large amounts of TNF-α, did not enhance APAP-induced liver injury.37 Together these data suggest that TNF receptor 1 signaling is probably not responsible for the formation of the necrosome and initiation of programmed necrosis during APAP hepatotoxicity. Although TNF receptor signaling is the best studied initiating event for necroptosis, it is well established that there are multiple ways to trigger this mode of cell death.38 Further studies are needed to identify potential activators of this pathway in APAP hepatotoxicity.
Downstream events of RIP3 activation
A critical question is what downstream events are triggered by RIP3 activation, which may lead to cell death. Mitochondrial dysfunction has emerged as the central and most critical event in the pathophysiology of APAP toxicity in experimental animals27 and humans.15 Inhibition of respiration, a selective mitochondrial oxidant stress, mitochondrial DNA damage, nuclear DNA fragmentation dependent on mitochondrial dysfunction, and eventual MPT pore opening with collapse of the membrane potential and ATP depletion are all hallmarks of APAP-induced cell death.27 Our current data indicate that absence of RIP3 or prevention of RIP3 induction can eliminate mitochondrial AIF release and attenuate nuclear DNA fragmentation and the oxidant stress which is almost exclusively located in the mitochondria.29 Recent insight into the development of the mitochondrial oxidant stress suggests the presence of an amplification loop. The initial metabolic stress, presumably protein adducts in mitochondria, induces a moderate mitochondrial oxidant stress, which triggers JNK phosphorylation through activation of MLK330 and apoptosis signal-regulating kinase 1 (ASK1).39 P-JNK translocates to the mitochondria3 and potentiates the oxidant stress,4 which eventually triggers the MPT and cell necrosis.5,40 The fact that RIP3-deficiency prevented mitochondrial dysfunction and oxidant stress suggests that RIP3 acted upstream of JNK activation. Consistent with this conclusion is the observation that the RIP1 inhibitor necrostatin reduced APAP-induced liver injury by inhibiting JNK activation.31 In addition, overexpression of ARC (apoptosis repressor with caspase recruitment domain), which interacts with JNK downstream of the necrosome, attenuated JNK activation, oxidant stress and liver cell injury.32 Thus, the emerging evidence from our data in combination with recently published findings strongly suggests that RIP3 is involved in regulating the mitochondrial oxidant stress through JNK activation.
In addition to modulation of the APAP-induced oxidant stress by RIP3, we observed the mitochondrial translocation of Drp1, which is a protein involved in mitochondrial fission.41 Mitochondria are now recognized to be dynamic organelles, which undergo continuous cycles of fusion and fission to maintain function. Mitochondrial fission has been shown to occur during cell death, predominantly in models of apoptosis.41 It was recently shown that RIP3-mediated necrotic cell death can also occur through activation of mitochondrial fission.26 The molecular control of mitochondrial fission is mediated by Drp1, which polymerizes and constricts mitochondria to facilitate organelle division.42 Our data provide evidence that mitochondrial fission seems to be a feature of APAP-induced hepatocyte necrosis and this is controlled by RIP3-mediated Drp1 translocation to the mitochondria. The Drp1 inhibitor MDIVI31 prevented cell death, suggesting that Drp1 translocation and mitochondrial fission are critical events in APAP-induced cell death. Mitochondrial fission has been associated with production of ROS.43 Thus, RIP3 can affect the critical mitochondrial oxidant stress by controlling JNK activation and mitochondrial fission during APAP hepatotoxicity.
Despite convincing evidence for the involvement of RIP3 in the signaling mechanisms of cell death early after APAP (6h in vivo and 24h in vitro), the protective effect of RIP3 elimination was lost at later time points (24h in vivo and 48h in vitro). Interestingly, the recently reported protection by the RIP1 inhibitor necrostatin was confirmed in our study; however, this protection was also lost at 48h in vitro, as was the reduced cell death with the Drp1 inhibitor MDIVI. Although it is beyond the scope of the present study to elucidate the mechanistic details of this effect, there are some similarities to our previous findings on the role of mitochondrial bax translocation in APAP hepatotoxicity. Bax deficiency was found to prevent the outer mitochondrial membrane permeability, DNA fragmentation, and cell necrosis at 6h.8 However, this effect was lost by 12h because of continued mitochondrial oxidant stress, which eventually became the dominant injury mechanism.8 Our data suggest that the initiation of cell death by RIP3-dominant programmed necrosis signaling is eventually overtaken by a secondary event. The fact that protein adduct formation is unaffected by RIP3 but the downstream mitochondrial dysfunction and oxidant stress is drastically reduced indicates that RIP3 is one factor that controls JNK activation and the mitochondrial oxidant stress. However, if this pathway is eliminated, the effect on mitochondria is delayed but not prevented. More studies are needed to elucidate the delicate network of MAP kinase regulation of the mitochondrial oxidant stress, which may be the ultimate deciding factor in cell death after APAP overdose.27
Summary and Conclusion
Our data identified a unique molecular mediator for early APAP-induced hepatocyte necrosis, namely RIP3. APAP overdose triggers the transcriptional activation of RIP3 and the newly expressed RIP3 is critical for cell necrosis by acting upstream of mitochondrial dysfunction and oxidant stress, which is controlled by JNK activation and mitochondrial translocation of P-JNK. We have also shown that mitochondrial dynamics are relevant to the mechanism of APAP-induced cell death and are controlled by activation of RIP3, which induces translocation of Drp1 from the cytosol to the mitochondria. The resulting mitochondrial fission and oxidant stress lead to activation of the mitochondrial permeability transition, release of AIF, nuclear DNA fragmentation and hepatocyte necrosis. However, despite its prominent role in the cell death signaling pathway, elimination of RIP3 does not cause long-term protection, most likely due to alternate pathways of amplification of the mitochondrial oxidant stress. Controlling RIP3 induction or modulating function could be a promising new therapeutic approach to prevent the early APAP-induced liver injury, but requires complementary strategies to control the mitochondrial dysfunction for long-term protection.
Supplementary Material
(A) Mice were treated with 200 mg/kg acetaminophen (APAP) with or without control (CTRLM) or RIP3 (RIP3M) morpholino pretreatment and plasma ALT was measured. (B) Wild-type (RIP3 +/+) and RIP3−/− mice were treated with 300 mg/kg and plasma ALT was measured. Data represent mean ± SE of n=3–6.
Acknowledgments
Financial Support: This investigation was supported in part by National Institutes of Health Grants AA12916 and DK070195 (to H.J.), AA020518 (to WX.D.) and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. M.R. McGill was supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
List of Abbreviations
- APAP
Acetaminophen
- NAPQI
N-acetyl-p-benzoquinone imine
- JNK
c-jun-N-terminal kinase
- AIF
apoptosis-inducing factor
- RIP
receptor interacting protein kinase
- MLK
mixed-lineage kinase
- FADD
Fas associated death domain
- ALT
alanine aminotransferase
- GSH
glutathione
- GSSG
glutathione disulfide
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- H&E
hematoxylin & eosin
- PI
propidium iodide
- ASK1
apoptosis signaling-regulating kinase 1
- ARC
apoptosis repressor with caspase recruitment domain
- Drp1
dynamin related protein 1
REFERENCES
- 1.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 3.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 6.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 7.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 9.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 10.Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- 12.Williams CD, Farhood A, Jaeschke H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflammation and liver injury. Toxicol Appl Pharmacol . 2010;247:169–178. doi: 10.1016/j.taap.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 14.McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181:133–141. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- 18.Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Williams CD, Koerner MR, Lampe JN, Farhood A, Jaeschke H. Mouse strain-dependent caspase activation during acetaminophen hepatotoxicity does not result in apoptosis or modulation of inflammation. Toxicol Appl Pharmacol. 2011;257:449–458. doi: 10.1016/j.taap.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 21.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Moquin D, Chan FK. The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 24.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 29.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An J, Mehrhof F, Harms C, Lättig-Tünnemann G, Lee SL, Endres M, Li M, Sellge G, et al. ARC is a novel therapeutic approach against acetaminophen-induced hepatocellular necrosis. J Hepatol. 2012 Oct 6; doi: 10.1016/j.jhep.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 2000;54:509–516. doi: 10.1093/toxsci/54.2.509. [DOI] [PubMed] [Google Scholar]
- 34.Simpson KJ, Lukacs NW, McGregor AH, Harrison DJ, Strieter RM, Kunkel SL. Inhibition of tumour necrosis factor alpha does not prevent experimental paracetamol-induced hepatic necrosis. J Pathol. 2000;190:489–494. doi: 10.1002/(SICI)1096-9896(200003)190:4<489::AID-PATH534>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021–1029. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- 36.James LP, Kurten RC, Lamps LW, McCullough S, Hinson JA. Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol. 2005;97:8–14. doi: 10.1111/j.1742-7843.2005.pto_97102.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 42.Elgass K, Pakay J, Ryan MT, Palmer CS. Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta. 2012 May 10; doi: 10.1016/j.bbamcr.2012.05.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Nguyen D, Alavi MV, Kim KY, Kang T, Scott RT, Noh YH, Lindsey JD, et al. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2011 Dec 8;2:e240. doi: 10.1038/cddis.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Mice were treated with 200 mg/kg acetaminophen (APAP) with or without control (CTRLM) or RIP3 (RIP3M) morpholino pretreatment and plasma ALT was measured. (B) Wild-type (RIP3 +/+) and RIP3−/− mice were treated with 300 mg/kg and plasma ALT was measured. Data represent mean ± SE of n=3–6.