Abstract
Aims
Beta adrenergic receptor (β-AR) subtypes act through diverse signaling cascades to modulate cardiac function and remodeling. Previous in vitro studies suggest that β1-AR signaling is cardiotoxic whereas β2-AR signaling is cardioprotective, and may be the case during ischemia/reperfusion in vivo. The objective of this study was to assess whether β2-ARs also played a cardioprotective role in the pathogenesis of non-ischemic forms of cardiomyopathy.
Methods and Results
To dissect the role of β1 vs β2-ARs in modulating MLP (Muscle LIM Protein) cardiomyopathy, we crossbred MLP−/− with β1−/− or β2−/− mice. Deletion of the β2-AR improved survival, cardiac function, exercise capacity and myocyte shortening; in contrast haploinsufficency of the β1-AR reduced survival. Pathologic changes in Ca2+ handling were reversed in the absence of β2-ARs: peak Ca2+ and SR Ca2+ were decreased in MLP−/− and β1+/− /MLP−/− but restored in β2−/−MLP−/−. These changes were associated with reversal of alterations in troponin I and phospholamban phosphorylation. Gi inhibition increased peak and baseline Ca2+, recapitulating changes observed in the β2−/−/MLP−/−. The L-type Ca2+ blocker verapamil significantly decreased cardiac function in β2−/−MLP−/− vs WT. We next tested if the protective effects of β2-AR ablation were unique to the MLP model using TAC-induced heart failure. Similar to MLP, β2−/− mice demonstrated delayed progression of heart failure with restoration of myocyte shortening and peak Ca2+ and Ca2+ release.
Conclusion
Deletion of β2-ARs prevents the development of MLP−/− cardiomyopathy via positive modulation of Ca2+ due to removal of inhibitory Gi signaling and increased phosphorylation of troponin I and phospholamban. Similar effects were seen after TAC. Unlike previous models where β2-ARs were found to be cardioprotective, in these two models, β2-AR signaling appears to be deleterious, potentially through negative regulation of Ca2+ dynamics.
Keywords: Adrenergic receptors, cardiomyopathy, excitation-contraction coupling, signal transduction
1. Introduction
Beta adrenergic receptors (β-ARs) play a major role in the regulation of cardiac function. Their activation provides positive inotropic, chronotropic and lusitropic effects, however, β-ARs also play an important role in cardiac remodeling, and thus in the pathogenesis of dilated cardiomyopathy and heart failure. The continuous interaction between the underlying myocardial contractile dysfunction and the compensatory neurohumoral mechanisms activated by that dysfunction results in activation of β-AR signaling pathways that contribute to the progression of disease [1]. Of the two main β-AR subtypes in the heart (β1 and β2), β1-AR signaling is coupled to the stimulatory guanylyl nucleotide binding protein, Gs, leading to activation of adenylyl cyclase, increases in cAMP, activation of PKA and subsequent phosphorylation of key regulators of excitation-contraction coupling. β1-AR signaling has also been linked to cardiotoxic and pro-apoptotic signaling [2, 3]. In contrast, β2-ARs not only signal through Gs but also through the inhibitory G protein, Gi, which attenuates the positive inotropic and chronotropic effects of β1-stimulation and activates additional signaling pathways involved in cardioprotection [4]. Thus, some have proposed that the β1-AR is the “cardiotoxic subtype” whereas the β2-AR is the “cardioprotective subtype.” However, much of this data has been derived from in vitro studies in isolated cardiomyocytes, often with non-physiologic overexpression of the specific β-AR subtype being studied. Whether these in vitro studies will translate into in vivo models of heart failure is still unclear, although there is some in vivo data suggesting that the β2-AR is cardioprotective [5–8]. Still, the precise role of each β-AR subtype in the pathogenesis of cardiomyopathy and heart failure remains to be determined. These studies are crucial to designing the best therapeutic approach to β-AR modulation, as some have suggested that a combination of a β1-AR antagonist and a β2-AR agonist would result in a more favorable modulation of the β-AR system than the use of a non-subtype specific β-blocker alone [5].
One of the best described in vivo models of a genetic, non-ischemic cardiomyopathy is the Muscle LIM Protein (MLP) knockout mouse. MLP or cysteine-rich protein 3, contains two zinc finger LIM domains each followed by a glycine rich domain and it is known to interact with the titin-binding proteins α-actinin and T-cap at the Z-disc and β1-spectrin and the nebulin-related protein N-RAP at costameres and intercalated discs, respectively [9]. Mice deficient in MLP exhibit chamber dilation and contractile dysfunction, characteristic of dilated cardiomyopathy and transition to failure. This model is clinically relevant, as downregulation of MLP has been observed in patients with chronic heart failure [10] and mutations in the MLP gene have been identified in patients with dilated cardiomyopathy [11, 12].
Previous studies have shown that MLP cardiomyopathy can be altered by changing components of the β-AR signaling system or its downstream effectors, although the exact mechanisms have yet to be worked out. Overexpression of the β2-AR did not rescue MLP cardiomyopathy, whereas overexpression of the GRK2 inhibitor, βARKct, did [13]. Ablation of phospholamban (PLB), an inhibitor of the sarcoplasmic reticulum Ca2+ ATPase (SERCA), also rescued MLP mice, suggesting that defects in SR Ca2+ cycling play a pivotal role in progression towards heart failure in this model [14]. Although alterations in Ca2+ transients were described associated with this rescue, the mechanisms were largely undefined. In the present study, we assessed the role of β1 vs β2-AR signaling in modulating MLP cardiomyopathy and heart failure. Contrary to expectations based on other models, we found that deletion of the β2-AR rescued and deletion of the β1-AR worsened MLP cardiomyopathy, suggesting that β2-AR signaling was playing a deleterious role and β1-AR signaling a cardioprotective role. We further determined a mechanism by which β2-AR deletion restores myocyte shortening in MLP mice, through improving Ca2+ availability. To further assess if the cardioprotection provided by ablation of the β2-AR was unique to the MLP model we assessed the effects of β2-AR deletion in a model of transverse aortic constriction (TAC)-induced heart failure and confirmed that absence of β2-ARs also attenuated the progression of heart failure and restored Ca2+ dynamics.
2. Materials and Methods
A more detailed version of materials and methods is included in Supplementary Methods.
2.1 Generation of β-AR/MLP knockouts
Crosses were carried out between homozygous β1−/− and β2−/− mice (FVB background) generated by our lab [15, 16] and homozygous MLP−/− mice (FVB/Sv129), kindly provided by Dr. Ken Chien. WT littermate controls were used to ensure comparability between the different lines. β2−/−MLP−/− were generated by crossing MLP−/− with β2−/− mice which produced F1 heterozygous MLP+/− and β2+/−; these were then crossed to generate F2 double knockouts. The same approach was used to generate β1 −/−MLP−/−, however due to the near total in utero mortality of the homozygous double knockouts, only β1+/−/MLP−/− were used for further studies. Mice were genotyped by PCR to confirm β1-AR, β2-AR and MLP disruptions. All procedures were approved by the Stanford Administrative Panel on Laboratory Animal Care.
2.2 Transverse aortic constriction-induced heart failure
Heart failure was induced by TAC as previously reported [17]. TAC was performed in C57BL/6J and β2−/− in C57BL/6J background as we have previously described [18]. Echocardiography was performed before surgery and 1, 2 and 4 weeks after TAC. Sham-operated controls consisted of age-matched mice that underwent an identical surgical procedure including isolation of the aortic arch, but without banding.
2.3 Echocardiography
Images were acquired with a GE Vivid 7 ultrasound system (GE health care, Milwaukee, WI) equipped with a 10 MHz transducer. Baseline measurements included left ventricular internal dimension at end-diastole (LVIDd) and left ventricular internal dimension in systole (LVIDs). Left ventricular fractional shortening (%FS) was calculated.
2.4 Incremental treadmill exercise
Baseline metabolic measurements during exercise were performed utilizing a Simplex II metabolic rodent treadmill (Columbus Instruments, Columbus, OH) as previously described [19].
2.5 Isolation of left ventricular myocytes
Adult ventricular myocytes were isolated from 6 mo old mice based on previously published protocols [20, 21] with modifications. Experiments were performed with freshly isolated myocytes resuspended in a HEPES-buffered solution (in mM 1 CaCl2, 137 NaCl, 5.4 KCl, 15 dextrose, 1.3 MgSO4, 1.2 NaH2PO4, 20 HEPES, pH 7.4).
2.6 Myocyte shortening and relengthening
Cell contraction properties of myocytes were evaluated with a video-based sarcomere spacing acquisition system (SarcLen, IonOptix, Milton, MA) as previously described [22, 23]. Changes in sarcomere length were recorded and analyzed using IonWizard software (IonOptix, Milton, MA).
2.7 Ca2+ transient measurements
A separate set of myocytes was loaded with 0.5 µM fura 2-acetoxymethyl ester (Molecular Probes, Eugene, Oregon) for 15 min. Cells were excited at 340 and 380 nm, continuously alternated, at rates as high as 250 pairs/sec using a HyperSwitch system (IonOptix, Milton, MA). Background-corrected fura 2 ratios were collected at 510 nm. This ratio is independent of cell geometry and excitation light intensity, and reflects the intracellular Ca2+ concentration [24, 25].
2.8 Sarcoplasmic reticulum Ca2+ measurements
Caffeine 10 mM was used to induce Ca2+ release from the SR; maximum fluorescence was used as a measure of SR Ca2+, as previously described [26]
2.9 Gi protein inhibition
1.5 µg/ml Pertussis Toxin (PTX) (Enzo Life Sciences, Plymouth Meeting, PA) was administered to freshly isolated WT myocytes for 3 h to inhibit Gi as previously described [27]. Ca2+ transient measurements were then performed after PTX treatment.
2.10 Immunoblotting
Mouse hearts were homogenized; proteins were quantified and probed against SERCA2 ATPase, PLB (PLB), phospho-CaM Kinase II Thr286, calsequestrin (CSQ) (Affinity BioReagents, Rockford, IL), phospho-PLB Ser16 (Millipore, Billerica, MA), phospho-PLB Thr17 (Badrilla, Leeds, United Kingdom), Na+/ Ca2+ exchanger-1 (NCX) (Abcam) CaMKII, troponin (TnI), phosho-TnI Ser23–24 (Cell Signaling Technology, Danvers, MA) and ryanodine receptor (RyR), phospho RyR Ser2809 and phospho RyR Ser2815 (a kind gift of Dr. Andrew Marks, Columbia University).
2.11 Statistical analysis
Data are expressed as mean ± SEM. Unpaired t tests were used for comparisons between 2 groups, and ANOVA with Fisher’s test was used for differences among >2 groups. A single average value of multiple cells for each heart was used to compare the data between groups; 3–4 mice were used in each cell experiment. Significance was attained with a p<0.05.
3. Results
3.1 β-AR subtypes have opposing effects on survival and in vivo cardiac function in MLP cardiomyopathy
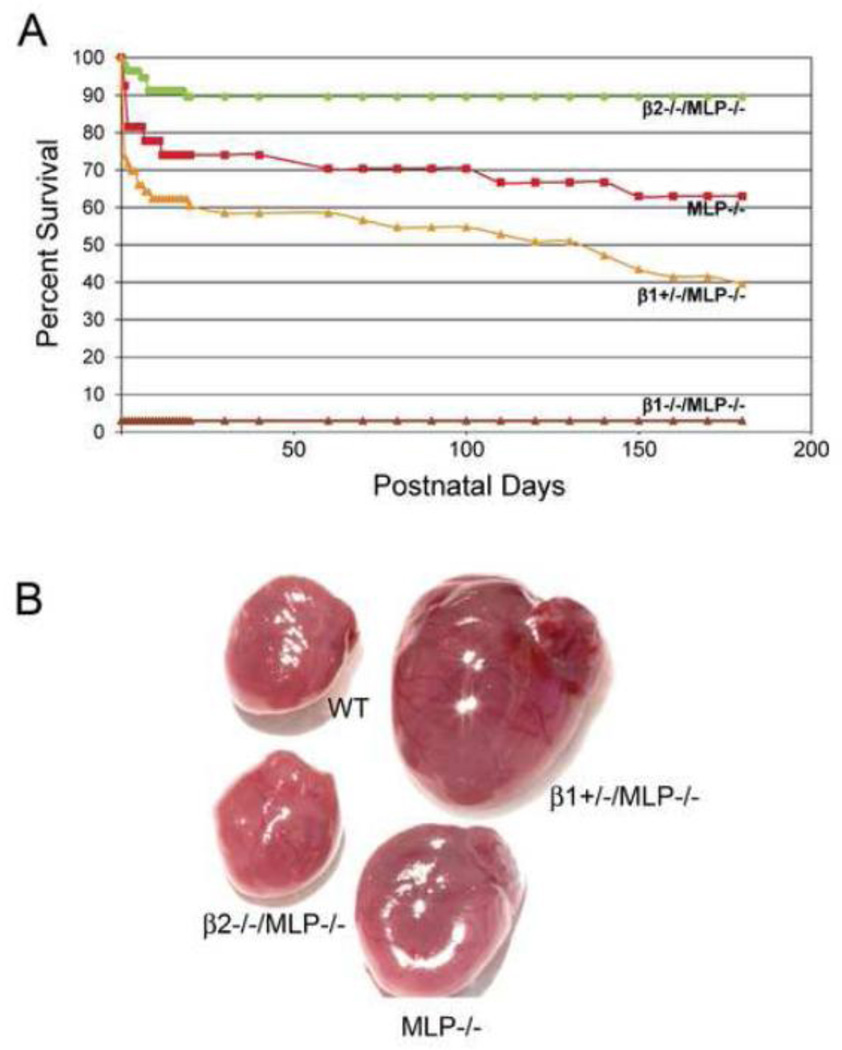
Ablation of the β1 vs. the β2-AR had dramatically opposite effects on both early and late survival of MLP−/− mice. At 2 weeks, β2−/−/MLP−/− have a 91% survival vs 74% in MLP−/− (Figure 1A). The deletion of one β1-AR allele (β1+/−/MLP−/−) reduced survival to 62%, whereas deletion of both alleles (β1−/−/MLP−/−) resulted in near total embryonic lethality (3% survival). Analysis of mice at serial embryonic stages showed that this lethality occurred between embryonic day 10 and embryonic day 15. This effect of β-AR deletion on MLP myopathy persisted as the mice aged. At 6 mos, survival of β2−/−/MLP−/− (90%) was still better than MLP−/− (63%) and survival of β1+/−/MLP−/− was worse (40%). Thus, the well-described late phase of MLP cardiomyopathy was totally reversed by deletion of the β2-AR.
Figure 1.
Opposite effects of β1-AR vs β2-AR ablation on survival in MLP−/− cardiomyopathy. (A) Kaplan-Meier survival curve, β2−/−/MLP−/− (n=57), MLP−/− ( n=27), β1+/−/MLP−/− (n=53), β1−/−/MLP−/− ( n=31). Nearly all β1−/−/ MLP−/− mice died in utero between embryonic days 10–15. (B) Reversal of marked MLP−/− cardiomegaly in β2−/−/MLP−/− but not in β1+/−/MLP−/−.
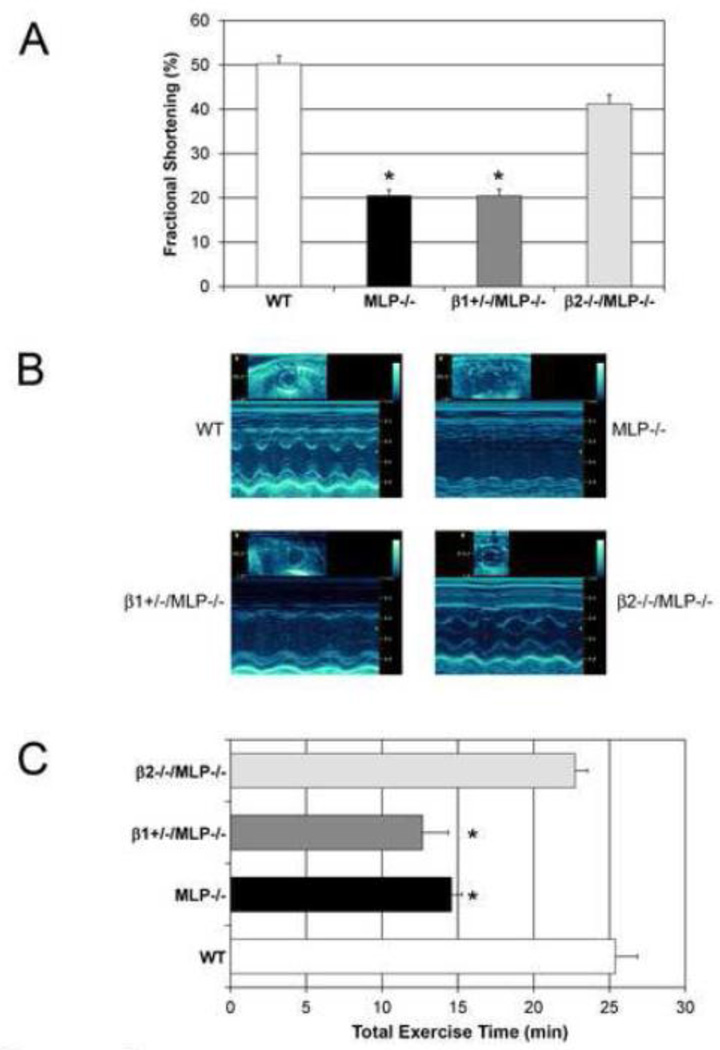
At 6 mos, MLP−/− and β1+/−/MLP−/− mice developed marked cardiomegaly, whereas β2−/−/MLP−/− did not (Figure 1B). Both absolute heart weight and HW/BW ratio was increased in MLP−/− and β1+/−/MLP−/− mice, but not in β2−/−/MLP−/− mice. Although there was an increase in body weight in β2−/−/MLP−/−, these mice were not in heart failure, therefore the increase in body weight was not related to edema (Table 1). LV function, assessed by echocardiography, was preserved in β2−/−/MLP−/− mice (FS 41.2±2%) compared to WT (50.2±1.8%, p=n.s.) and was significantly higher than MLP−/− (20.5±1.4%, p<0.05) and β1+/−/MLP−/− (FS 20.5±1.5%, p<0.05) (Figure 2 A, B). LVEDD and LVESD were increased in MLP−/− and β1+/−/MLP−/− compared to WT, but not in β2−/−/MLP−/−/ mice (Table 1). In addition, MLP−/− and β1+/−/MLP−/− had decreased exercise capacity whereas β2−/−/MLP−/− mice had normal exercise capacity (Figure 2 C).
Table 1.
Morphometry and echocardiography of 6 month old βAR-/MLP−/− mice.
| Morphometry | WT | MLP−/− | β1+/−MLP−/− | β2−/−/MLP−/− |
|---|---|---|---|---|
| BW (g) | 31.5±1.5 | 33.5±1 | 29.8±1.3 | 37.2±1.2* |
| HW (mg) | 132.6±18 | 243.3±62* | 236.1±98* | 168.5±42 |
| HW/BW (mg/g) | 4.2±0.1 | 6.9±0.7* | 7.2±0.5* | 4.9±0.3 |
| LW/BW (mg/g) | 4.8±0.2 | 5.7±0.2 | 6.2±0.4* | 4.9±0.2 |
| n | 8 | 6 | 11 | 8 |
| Echocardiography | ||||
| LVEDD (mm) | 3.4±0.1 | 5.2±0.2* | 5.9±0.4* | 3.9±0.2 |
| LVESD (mm) | 1.8±0.1 | 4.2±0.3* | 4.7±0.4* | 2.4±0.2 |
| LVPWD | 0.96±0.05 | 0.8±0.04* | 0.8±0.02* | 0.96±0.04 |
| LVPWS | 1.24±0.05 | 0.99±0.06* | 1.00±0.03* | 1.19±0.04 |
| n | 8 | 14 | 10 | 23 |
BW: body weight; HW: heart weight; LW: lung weigh; LVEDD: left ventricular end-diastolic dimension; LVESD: left ventricular end-systolic dimension; LVPWD: left ventricular posterior wall in diastole; LVPWS: left ventricular posterior wall in systole; n: number of mice.
p<0.05 vs WT.
Figure 2.
Deletion of the β2-AR restores cardiac function in MLP−/− cardiomyopathy. (A) Left ventricular function was preserved in β2−/−/MLP−/− vs MLP−/− and β1+/−/MLP−/− mice, n=14 in each group; FS in WT and β2−/− mice were not significantly different (B) Representative M-mode echocardiograms of each genotype. (C) Exercise capacity is restored in β2−/−/MLP−/− but not in β1+/−/MLP−/−, n=8 mice, *p<0.05.
It has been previously reported that β-AR density is reduced by 54% in the MLP−/− compared with WT hearts and is associated with marked attenuation of isoproterenol-stimulated adenylyl cyclase activity, indicating severe impairment of β-AR coupling [13]. The expression level of β1-AR was decreased in MLP and β2−/−MLP−/− mice compared to WT, and was actually similar to the level of expression in β1+/−/MLP−/− mice. Importantly, there was no difference in expression of the β1-AR in β2−/−/MLP−/− compared to MLP−/− mice (Supplementary Figure 1). These data suggest that the improvement in ventricular function in the β2−/− MLP−/− mice was not simply due to increasing β1-AR expression. The lack of further decrease in β1-AR expression in the β1+/−/MLP−/− compared with the MLP−/− suggests that heart failure-induced downregulation may have a lower limit beyond which further downregulation may not occur, a process which requires future evaluation.
3.2 Deletion of β2-ARs restores in vitro myocyte shortening in MLP−/− cardiomyopathy
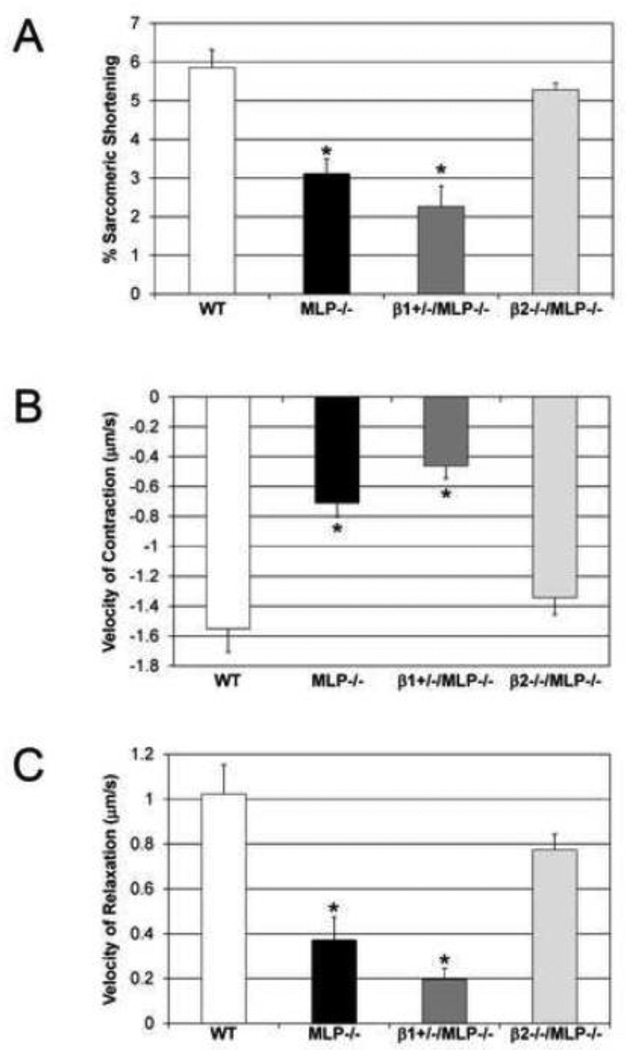
To determine if these opposing roles observed in vivo were myocyte-specific, shortening parameters were characterized in isolated adult myocytes from each genotype. Intrasarcomeric shortening was decreased in MLP−/− (3.1±0.4%) and β1+/−/MLP−/− (2.3±0.5%) vs WT (5.9±0.5%) but was preserved in β2−/−/MLP−/− (5.3±0.2%), n=4, p<0.05 (Figure 3A). These changes were observed both in the contraction and relaxation phases. MLP−/− and β1+/−/MLP−/− showed marked decreases in velocity of contraction (−54.3% and −70.1% respectively) and velocity of relaxation (−63.7% and −80.8% respectively) vs WT, whereas β2−/−/MLP−/− (Figure 3B, C) showed only a slight non-significant decrease in these velocities (−13.2% and −24.2%).
Figure 3.
Deletion of β2-ARs restores myocyte shortening in MLP−/− cardiomyopathy. (A) Sarcomeric shortening; (B) velocity of contraction and (C) relaxation were all decreased in MLP and β1+/−/MLP−/− but restored to near WT levels in β2−/−MLP−/−; WT and β2−/− myocytes were not significantly different, n=4 mice, *p<0.05 vs WT.
3.3 Deletion of β2-ARs improves Ca2+ transients in MLP cardiomyopathy
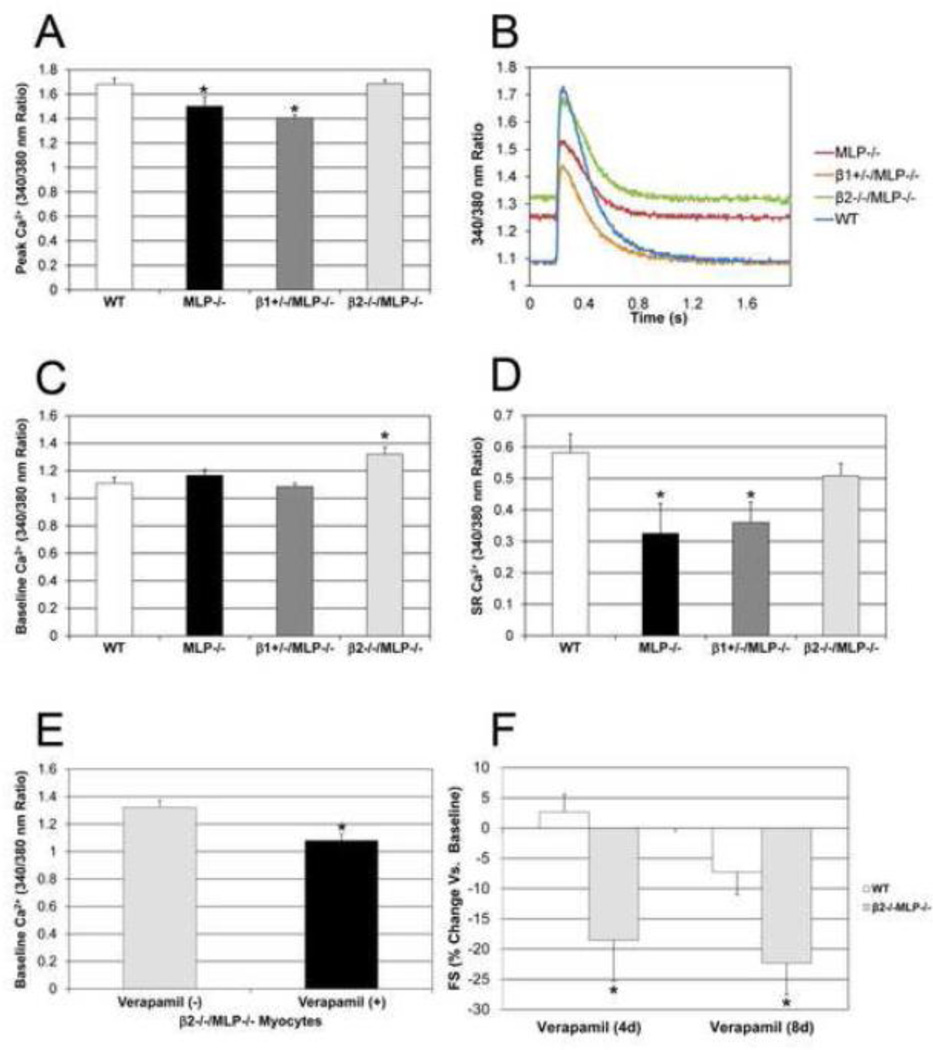
To assess the hypothesis that alterations in Ca2+ regulation could be one mechanism for the preserved function associated with deletion of the β2-AR, Ca2+ transients were measured in isolated myocytes from each genotype. Peak Ca2+ was significantly decreased in MLP−/− (1.5±0.08) and β1+/−/MLP−/− (1.40±0.03) compared to WT (1.68±0.05), whereas peak Ca2+ was restored to normal in β2−/−MLP−/− (1.69±0.03) (Figure 4 A, B). There was no difference in Ca2+ release rates between any of the genotypes. However, baseline Ca2+ was significantly increased in β2−/−/MLP−/− (1.32±0.05) compared to MLP−/− (1.17±0.04), β1+/−/MLP−/− (1.08±0.03) and WT (1.10±0.05). n=4, p<0.05 (Figure 4C). These beneficial Ca2+ effects were not limited to the cytosol: when caffeine was used to induce Ca2+ release, sarcoplasmic reticulum Ca2+ was found to be decreased in MLP−/− (0.33±0.01) and βl+/− /MLP−/− (0.36±0.07) but again restored to normal (0.58±0.06) in β2−/−/MLP−/− (0.51±0.04), n=3, p<0.05 (Figure 4D).
Figure 4.
Improved Ca2+ handling in MLP cardiomyopathy in the absence of β2-ARs. (A) Peak Ca2+ was decreased in MLP−/− and β1+/−/MLP−/− compared to WT but restored to normal in β2−/−MLP−/−. (B) Representative Ca2+ transients. (C) Baseline Ca2+ was increased in β2−/−/MLP−/− vs MLP−/−, β1+/−/MLP−/− and WT. n=4 mice, *p<0.05 vs WT. (D) Sarcoplasmic reticulum (SR) Ca2+ was restored to normal in the absence of β2-ARs. SR Ca2+ was decreased in MLP−/− and β1+/−/MLP−/− but restored in β2−/−/MLP−/−. n=3 mice, *p<0.05 vs WT. (E) Verapamil reversed the higher baseline Ca2+ observed in the β2−/−/MLP−/−, n=3 mice, *p<0.05 vs untreated. (F) FS was significantly decreased in β2−/−/MLP−/− after verapamil but not in WT, n=5, *p<0.05 vs baseline and vs WT.
To further assess the role of Ca2+ in the restoration of cardiac function observed in the β2−/− /MLP−/− mice, the L-type Ca2+ channel blocker verapamil (5 mg/kg/d ip) was administered chronically. Eight days after verapamil there was a significant decreased in baseline Ca2+ in β2−/− /MLP−/− compared to non-treated myocytes (1.08±0.05 vs 1.32±0.05 respectively) (Figure 4E) as well as peak Ca2+ (1.41±0.06 vs 1.69±0.03). This decrease in Ca2+ was associated with a significant reduction in cardiac function only in β2−/−/MLP−/− mice. After 4 days, verapamil reduced FS in β2−/−/MLP−/− by 19% vs 3% increase in WT, and after 8 days, verapamil reduced FS in β2−/−/MLP−/− by 22% vs 7% in WT (Figure 4F).
3.4 β2-ARs regulate proteins involved in excitation-contraction coupling in MLP−/− cardiomyopathy
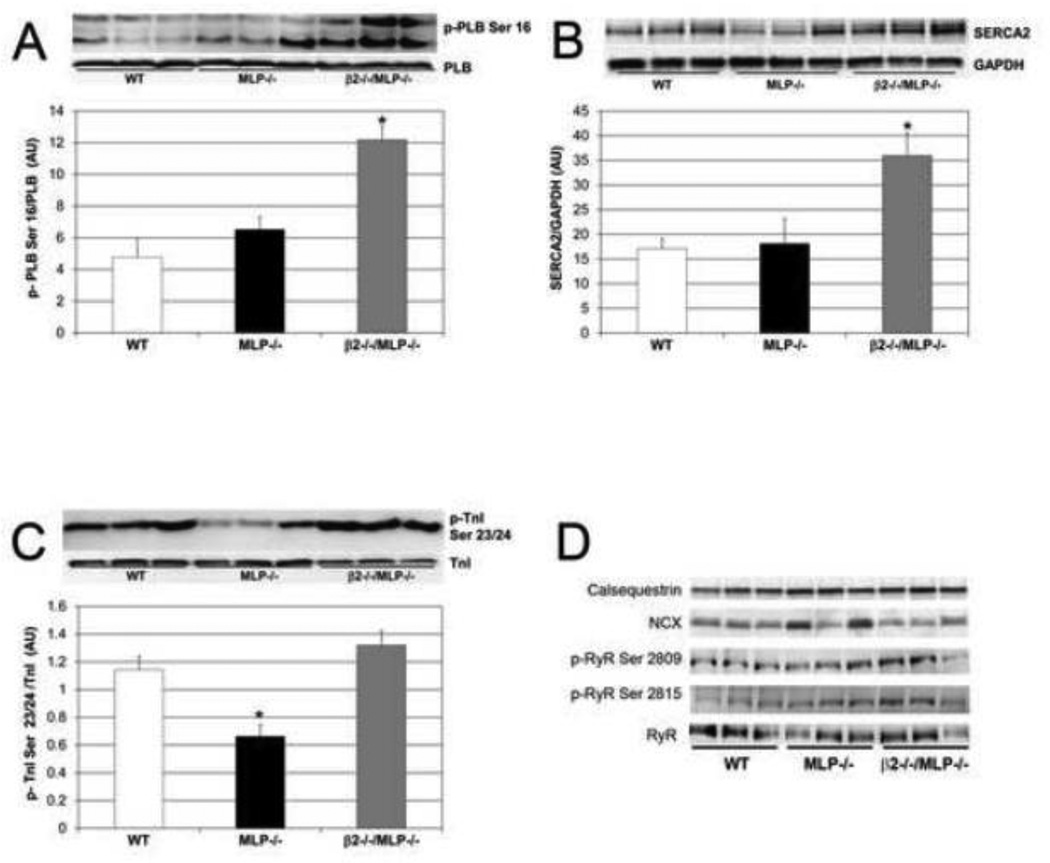
To further understand the mechanism for altered Ca2+ regulation in the cardioprotection mediated by the absence of the β2-AR, the expression and phosphorylation of key proteins involved in the modulation of excitation-contraction coupling were studied. PLB phosphorylation at Ser 16 was increased 1.9 fold in β2−/−/MLP−/− compared to MLP−/− or WT mice (Figure 5A). In addition SERCA expression was increased 2 fold in β2−/−/MLP−/− compared to MLP−/− or WT mice (Figure 5B). Also MLP−/− mice exhibit a significant decrease in TnI phosphorylation (42% less than WT), however absence of the β2-AR restores TnI phosphorylation to WT levels (Figure 5C). RyR phosphorylation at Ser 2809 and Ser 2815 (Figure 5D) was also increased in β2−/−/MLP−/− and also in MLP−/− though the latter was not statistically significant compared to WT. In contrast to the above changes, there was no difference between genotypes in the expression of, PLB, troponin, CSQ, NCX, or CaMKII, or in phosphorylation of CaMKII at Thr286 or PLB at Thr17.
Figure 5.
β2-ARs modulate proteins involved in excitation-contraction coupling in MLP−/− cardiomyopathy. (A) Phospholamban phosphorylation at Ser 16 was increased in β2−/−/MLP−/− compared to MLP−/− or WT mice, (B) SERCA expression levels were increased in β2−/−/MLP−/− compared to MLP−/− or WT mice, (C) Troponin I phosphorylation was decreased in MLP−/−, however absence of β2-ARs restores TnI to WT levels, n=3 mice, *p <0.05 vs WT. (D) Western Blots of additional proteins involved in excitation-contraction coupling.
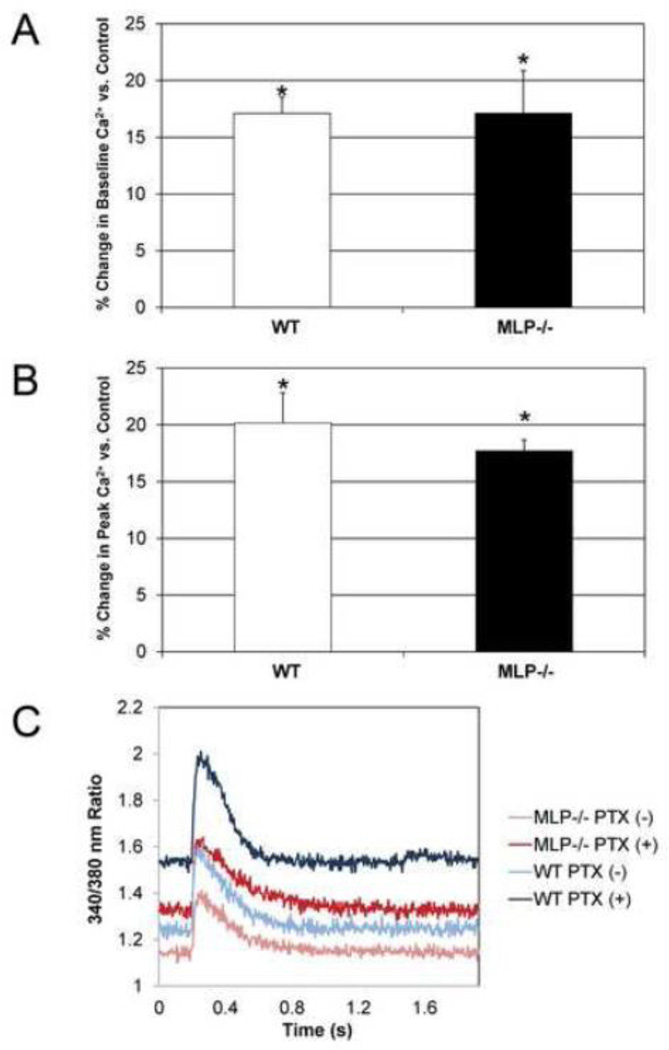
3.5 Gi inhibition recapitulates alterations in Ca2+ transients observed in β2−/− mice
To determine whether absence of β2-AR-Gi signaling could be one of the mechanisms by which ablation of the β2-AR mediates alterations in Ca2+ handling that prevent MLP−/− cardiomyopathy, pertussis toxin (PTX) was administered to WT and MLP−/− myocytes. PTX inhibition significantly increased baseline Ca2+ by 17 % both in WT and MLP−/− compared to control (Figure 6A). Peak Ca2+ was increased in PTX-treated WT cells by 20 % compared to control. A similar increase (18%) was observed in MLP−/− myocytes treated with PTX (Figure 6B), n=3, p<0.05. However, there was no change in the rate of Ca2+ release or uptake in the presence of PTX in WT or MLP−/− myocytes (Figure 6C).
Figure 6.
Gi protein inhibition in WT and MLP−/− cells recapitulates Ca2+ transients observed in β2−/−/MLP−/−. A Pertussis toxin treatment increased baseline Ca2+ (A) and peakCa2+ (B) in 6 month old WT and MLP −/− myocytes. (C) Representative Ca2+ transients after Pertussis toxin treatment, n=3 mice, *p<0.05 vs control.
3.6 Ablation of β2ARs delays the progression of heart failure after TAC
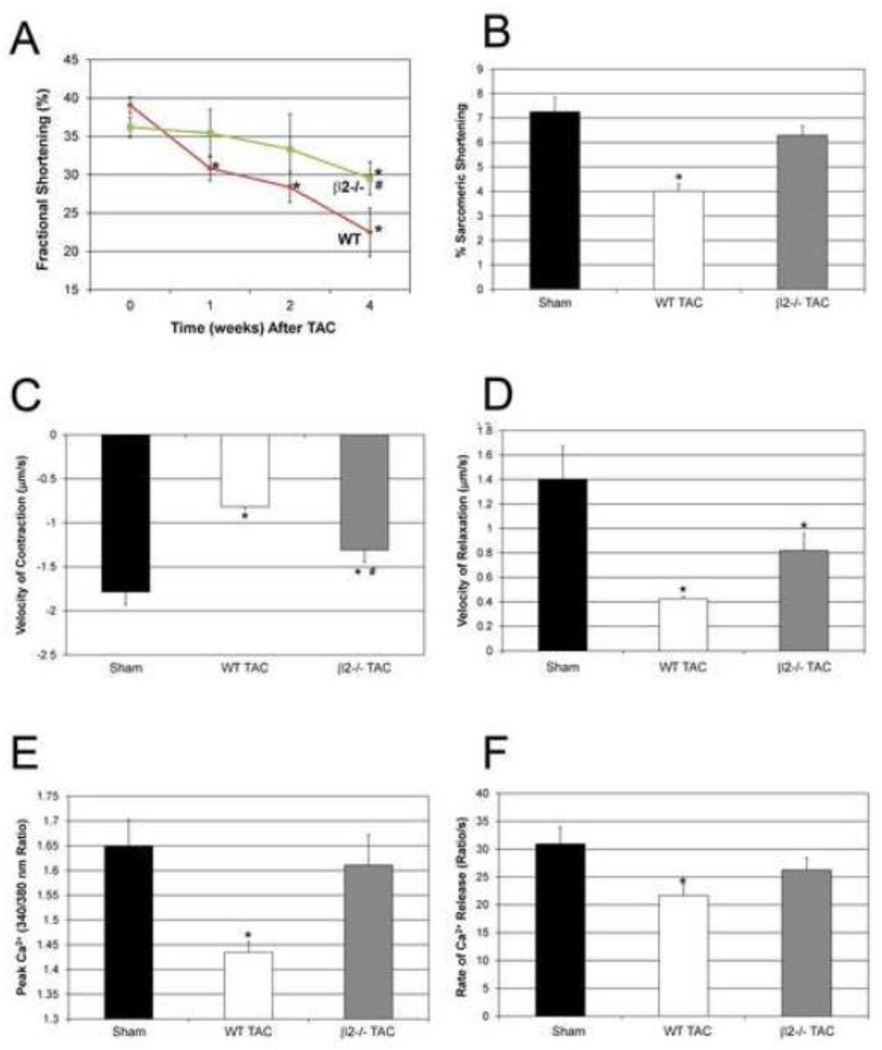
Given prior reports suggesting that β2-AR signaling is cardioprotective, we next assessed whether the cardioprotective effect of β2-AR ablation we had described was unique to the MLP model or generalizable to other models of heart failure. We thus evaluated the effects of β2-AR deletion in a model of TAC-induced heart failure. In WT mice there was a significant decrease in FS compared to baseline (39%) at 1 week (30.8%), 2 weeks (28.4%) and 4 weeks (22.5%) after TAC; in contrast, in β2−/− mice, FS was preserved at 1 and 2 weeks and only decreased at week 4 (29.5%) compared to baseline (36.2%.). Furthermore, the decrease in FS at 4 weeks in the β2−/− mice was significantly less than in WT (Figure 7A). LV/BW and HW/BW ratio were significantly increased in WT after TAC but not in β2−/− mice (Table 2). 4 weeks after TAC, there was a significant increase in myocyte cross sectional area in WT but not in the β2−/− mice (Supplementary Figure 2). Importantly, subendocardial interstitial fibrosis was significantly greater in WT compared to β2−/− mice (Supplementary Figure 3). Intrasarcomeric shortening was decreased in WT at 4 weeks after TAC (4.2±0.3%) compared to sham (7.2±0.6) but was preserved in β2−/− (6.5±0.8%) (Figure 7B).These changes were observed both in the contraction and relaxation phases. WT mice showed marked decreases in velocity of contraction (−54.3%) and velocity of relaxation (−69.8%) vs sham. Although β2−/− mice also showed a decrease in velocity of contraction (−26.8%) and relaxation (−41.6%) compared to sham, the decrease in velocity of contraction was significantly less than WT (Figure 7C, D). Similar to the MLP model, alterations in Ca2+ handling played a role in these changes. Peak Ca2+ was significantly decreased in WT after TAC (1.44±0.02) compared to sham (1.7±0.05), whereas it was restored to normal in β2−/− (1.61±0.06) (Figure 7E). Ca2+ release rate was decreased in WT vs. sham but was restored in β2−/− mice after TAC, n=4, p<0.05 (Figure 7F).
Figure 7.
Ablation of β2-ARs both delays and attenuates the progression of heart failure after transverse aortic constriction. (A) WT mice showed a significant decrease in cardiac function 1 week after TAC with further deterioration at 2 and 4 weeks. In contrast, β2−/− showed preserved function until 4 weeks after TAC and at that time point, cardiac function is significantly higher in β2−/− compared to WT. n=10 mice, *p<0.05 vs WT. (B) Sarcomeric shortening, (C) velocity of contraction, and (D) relaxation were all decreased in WT after TAC (n=4, *p<0.05 vs sham). However, in β2−/−, % shortening was preserved and the decrease in velocity of contraction was significantly less than WT (#p<0.05 vs. WT). (E) Peak Ca2+ and (F) rate of Ca2+ release were decreased in WT after TAC compared to sham but not in β2−/−, n=4 mice, *p<0.05 vs WT.
Table 2.
Morphometry and echocardiography in WT and β2−/− mice after 4 weeks of TAC.
| Morphometry | Sham | WT TAC | β2−/− TAC |
|---|---|---|---|
| BW (g) | 26.6±0.4 | 27.0±0.5 | 29.7±1.9 |
| HW (mg) | 122.2±3.8 | 236.8±28* | 169.6±14.2# |
| HW/BW (mg/g) | 4.6±0.1 | 8.8±1.1* | 5.8±0.6 # |
| LW/BW (mg/g) | 3.1±0.4 | 5.7±0.5* | 4.4±0.4# |
| n | 4 | 6 | 5 |
| Echocardiography | |||
| LVEDD (mm) | 3.5±0.1 | 4.2±0.1* | 4.0±0.1* |
| LVESD (mm) | 2.3±0.1 | 3.1±0.1* | 2.7±0.1* # |
| n | 9 | 10 | 11 |
BW: body weight; HW: heart weight; LV: left ventricle; LVEDD: left ventricular end-diastolic dimension; LVESD: left ventricular end-systolic dimension; n: number of mice.
p<0.05 vs Sham,
p<0.05 vs WT TAC.
4. Discussion
In contrast to several prior reports suggesting that β2-ARs are the cardioprotective and that β1-ARs are the cardiotoxic β-receptor subtype [2–4], the present study shows that in a well-characterized genetic cardiomyopathy, the β1-AR positively modulates survival and cardiac function whereas the β2-AR has the opposite effect. To confirm that this effect was not limited to this specific genetic myopathy, we have shown that ablation of the β2-AR also has a cardioprotective effect in TAC-induced heart failure.
A proposed cardiotoxic role for β1-ARs has been previously shown both in vitro and in vivo. Studies in isolated myocytes have shown that stimulation of β1-ARs increases apoptosis via cAMP [28, 29] and CaMKII-dependent mechanisms, since its inhibition protects against apoptosis induced by excessive β1 -AR stimulation [30]; and mice with cardiac-directed overexpression of the β1-AR develop progressive heart failure [31]. In contrast, in isolated myocytes β2-AR stimulation has antiapoptotic effects against catecholamines, hypoxia and free radicals [32]. We have previously shown that preconditioning does not protect β2−/− hearts from ischemia/reperfusion injury [33]. We have also shown, both in vivo and in vitro, that deletion of the β2−/− increases cardiotoxicity of the anticancer agent doxorubicin [6, 34]. Finally, the therapeutic effects of chronic β1-AR blockade are superior when supplemented with β2-AR stimulation in rats with myocardial infarction-induced heart failure [5, 35].
Our results regarding β2-AR signaling are consistent with several studies suggesting that in chronic heart failure, β2-AR signaling can switch from being beneficial to detrimental. Studies in canine heart failure have shown that the major component of the blunted response to nonselective β-AR stimulation in heart failure was caused by β2-AR activation, resulting in a pertussis toxin-sensitive, Gi-mediated inhibition of the β1-AR-induced increase in L-type Ca2+ current [36]. Also, low level blockade of the β2-AR restores the decreased response to β-AR stimulation associated with overexpression of NCX, again suggesting β2-AR-mediated inhibition of β1-AR signaling. [37]. Although transgenic overexpression of the β2-AR initially increases contractility [38], these mice eventually develop cardiomyopathy as they age, with the severity related to the dose of β2-AR protein [39]. Thus, overexpression of either the β1-AR or the β2-AR is capable of producing toxic effects on the heart, although this appears at a much lower level of expression of the β1-AR (5-fold) [31] compared to the β2-AR (100-fold) [39]. In addition, in hypertrophic cardiomyopathy due to a mutant myosin heavy chain, β2-AR overexpression resulted in worsening heart failure [40] and β2-AR antagonists protect against ventricular fibrillation in dogs susceptible to malignant arrhythmias [41]. Taken together, these results suggest that the differential role of β-receptor subtypes in the pathogenesis of cardiomyopathy is more complex than previously appreciated. Absence of β2-ARs may be protective or deleterious depending on the context of the underlying disease. We have previously shown that although baseline cardiovascular function is not altered in β2−/− mice, peak [Ca2+]i and the rate of Ca2+ release are increased, potentially due to the loss of inhibitory signaling through Gi [15, 42]. In this study we have shown in two separate models, MLP−/− cardiomyopathy and TAC-induced heart failure, that ablation of β2-AR signaling is cardioprotective. The mechanism for this cardioprotection is in part via enhancement of Ca2+ signaling, through removal of the normal brake imposed by β2-AR-Gi signaling. However, in heart failure models where oxidative stress plays a primary pathogenic role, such as acute doxorubicin cardiotoxicity or ischemic-reperfusion, β2-AR deletion becomes deleterious. In acute doxorubicin, we have shown that this effect is also mediated by enhancement of intracellular Ca2+, however, in the case of oxidative stress, the increased Ca2+ is deleterious, predisposing to opening of the mitochondrial permeability transition pore [42]. Thus, the type, duration and intensity of a cardiac stress dramatically influences whether the β2-AR subtype regulates cardiotoxic vs. cardioprotective signaling.
Previous investigators have altered components of adrenergic signaling and have also demonstrated the ability to modulate MLP−/− cardiomyopathy: overexpression of βARKct [13] or knockout of PLB [14] improved function and survival, whereas overexpression of the β2-AR did not [13]. However, the exact mechanisms by which these manipulations achieved this rescue have not been fully explored. The current study provides evidence that ablation of β2-AR signaling in MLP−/− restores myocyte inotropy and lusitropy to near normal via positive modulation of Ca2+ handling, increased phosphorylation of PLB and SERCA levels as well as increased phosphorylation of TnI. The functional alterations induced by deletion of the β2-AR we found in vivo were recapitulated at the myocyte level. Absence of β2-ARs restores intrasarcomeric shortening, which was decreased in MLP−/− and β1+/−MLP−/−, to normal. This restoration is associated with improvement in Ca2+ handling: β2−/−/MLP−/− myocytes achieve peak Ca2+ levels similar to WT, an effect that is in part due to higher baseline Ca2+ but also due to the restoration of SR Ca2+ levels. We hypothesize that the increase in baseline Ca2+, although not sufficient to alter resting cardiac function in the β2−/−, may rescue the MLP myopathy due to a similar inotropic effect as seen with the use of cardiac glycosides [43]. This level of increased diastolic Ca2+ is not enough to cause diastolic dysfunction, as would occur when Ca2+ reuptake or removal is more severely impaired.
To further test this hypothesis, we administered the L-type Ca2+ blocker verapamil chronically at 5 mg/kg i.p. for one week, as previously reported [44]. This protocol was sufficient to decrease the high baseline Ca2+ in β2−/−MLP−/− to levels similar to non-treated WT myocytes. Importantly, this decrease in Ca2+ was detrimental for the β2−/−MLP−/− but not WT mice, significantly deteriorating cardiac function in the β2−/−MLP−/− and thus negating the rescue effect due to the absence of β2-ARs. Although MLP−/− have a higher baseline Ca2+, their peak Ca2+ and SR Ca2+ content were decreased and the time to maximal Ca2+ uptake was increased; these alterations are restored in the β2−/−/MLP−/−. In addition, absence of the β2-AR increases phophorylation of PLB at Ser 16, increasing SERCA expression and TnI phosphorylation. RyR phosphorylation at Ser 2809 and Ser 2815 is increased in β2−/−MLP−/−and trends higher in MLP−/−, consistent with previous findings in heart failure [45]. This alteration in RyR phosphorylation is not reversed despite the improvement in cardiac function in the double knockouts. However, no detrimental effects such as arrhythmia were seen in the β2−/−MLP−/− both at rest and with exercise stress. Although we looked for alterations in additional signaling pathways with potential crosstalk with the β2-AR (PI3K, Akt, p38, JNK and ERK), we did not find any significant differences. Additional signaling alterations could still play a role, in addition to Ca2+, in the reversal of the MLP−/− cardiomyopathy.
Previous studies have shown in non-failing myocytes that β2-AR stimulation augments L-type Ca2+ current in a PKA-dependent manner but fails to phosphorylate PLB, indicating that β2-AR-induced cAMP/PKA signaling is highly localized [46]. β2-AR Gi signaling limits β2-AR Gs signaling to subsarcolemmal domains, and prevents Gs-PKA phosphorylation of multiple proteins involved in excitation-contraction coupling. [32] In the absence of β2-AR signaling, this restriction may be lifted, thus explaining the observed increase in phosphorylation of key targets involved in positive inotropic and lusitropic responses.
The beneficial effects of β2-AR ablation are in part recapitulated by inhibiting β2-AR-Gi signaling, suggesting a mechanism by which β2-ARs mediate a negative inotropic effect in the context of a failing heart. Neumann et al. have shown, in preparations from patients with idiopathic dilated cardiomyopathy, an increase in myocardial Gi-protein, compared with preparations from non-failing hearts suggesting that an increase in myocardial Gi could be causally related to heart failure [47]. Previous studies have shown a dramatic effect of β2-AR-mediated Gi stimulation on cardiac function in isolated myocytes [48]. In addition, inhibition of Gi with pertussis toxin permits PLB phosphorylation and a de novo relaxant effect following β2-AR stimulation, converting the localized β2-AR signaling to a global signaling mode similar to that of β1-ARs [46].
Our study is limited by the inability to induce long term highly specific pharmacological inhibition of β2-AR and Gi signaling in vivo to further demonstrate the role of β2-AR signaling in this model of cardiomyopathy. In addition, it is possible that extracardiac effects of the global β-AR knockouts, such as hormonal and neural differences could partially contribute to the phenotypic response. However, if anything, it would be expected that β2-AR ablation would tend to increase afterload and thus worsen contractile function; we have not seen any differences in afterload in the β2−/− [15]. In the current study, we have demonstrated the effect of β2-AR deletion on MLP−/− cardiomyopathy by crossing homozygous β2−/− with homozygous MLP−/− mice, however there could be additional modifications associated with this type of genetic long-term ablation of β-AR function that could contribute to the restoration of cardiac function in this model. These alterations would not be too dissimilar from humans with genetic forms of dilated cardiomyopathy.
In conclusion, we have demonstrated that deletion of the β2-AR prevents MLP cardiomyopathy, restoring myocyte shortening and improving Ca2+ availability. This deleterious role of the β2-AR is not limited to this one genetic model, as ablation of the β2-AR has a similar cardioprotective effect in TAC-induced heart failure, also restoring Ca2+ handling. Our results suggest that β2-ARs can play a deleterious role in some forms of cardiomyopathy and add to the complexity of our understanding of β2-AR signaling. Depending on the context, β2-ARs may play a cardioprotective or a cardiotoxic role. The implications of our data for the clinical use of β2-AR antagonists or agonists in human heart failure suggest a much more complex interaction between these cell signaling pathways and underlying pathologic processes.
Supplementary Material
Highlights.
Unexpectedly, deletion of the β2-AR prevents the development of MLP cardiomyopathy.
Deletion of β2-ARs restores abnormal Ca2+ signaling in MLP cardiomyopathy.
Pathologic changes in Ca2+ are reversed in the absence of β2-Gi signaling.
Deletion of β2-ARs is also cardioprotective in TAC-induced heart failure.
Therefore, β2-AR signaling can play a deleterious role in some forms of cardiomyopathy.
Acknowledgements
We are grateful to Dr. Donald Bers at UC Davis for his advice regarding calcium measurements.
Funding
This work was supported by the National Institutes of Health Grant HL061535 to D.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- 1.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 3.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, et al. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3'-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 5.Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, et al. Cardioprotective and survival benefits of long-term combined therapy with beta2 adrenoreceptor (AR) agonist and beta1 AR blocker in dilated cardiomyopathy postmyocardial infarction. J Pharmacol Exp Ther. 2008;325:491–499. doi: 10.1124/jpet.107.135335. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, et al. Differential cardioprotective/cardiotoxic effects mediated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005;289:H2441–H2449. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- 7.Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, et al. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med. 2004;32:1041–1048. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- 8.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 9.Miller MK, Granzier H, Ehler E, Gregorio CC. The sensitive giant: the role of titin-based stretch sensing complexes in the heart. Trends Cell Biol. 2004;14:119–126. doi: 10.1016/j.tcb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Zolk O, Caroni P, Bohm M. Decreased expression of the cardiac LIM domain protein MLP in chronic human heart failure. Circulation. 2000;101:2674–2677. doi: 10.1161/01.cir.101.23.2674. [DOI] [PubMed] [Google Scholar]
- 11.Mohapatra B, Jimenez S, Lin JH, Bowles KR, Coveler KJ, Marx JG, et al. Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol Genet Metab. 2003;80:207–215. doi: 10.1016/s1096-7192(03)00142-2. [DOI] [PubMed] [Google Scholar]
- 12.Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111:943–955. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]
- 13.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr., et al. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 15.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 16.Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP, Jr., Barsh GS, et al. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci U S A. 1996;93:7375–7380. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 18.Zhao M, Chow A, Powers J, Fajardo G, Bernstein D. Microarray analysis of gene expression after transverse aortic constriction in mice. Physiol Genomics. 2004;19:93–105. doi: 10.1152/physiolgenomics.00040.2004. [DOI] [PubMed] [Google Scholar]
- 19.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 21.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 22.Duan J, McFadden GE, Borgerding AJ, Norby FL, Ren BH, Ye G, et al. Overexpression of alcohol dehydrogenase exacerbates ethanol-induced contractile defect in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1216–H1222. doi: 10.1152/ajpheart.00780.2001. [DOI] [PubMed] [Google Scholar]
- 23.Olsson MC, Palmer BM, Stauffer BL, Leinwand LA, Moore RL. Morphological and functional alterations in ventricular myocytes from male transgenic mice with hypertrophic cardiomyopathy. Circ Res. 2004;94:201–207. doi: 10.1161/01.RES.0000111521.40760.18. [DOI] [PubMed] [Google Scholar]
- 24.Nagata K, Liao R, Eberli FR, Satoh N, Chevalier B, Apstein CS, et al. Early changes in excitation-contraction coupling: transition from compensated hypertrophy to failure in Dahl salt-sensitive rat myocytes. Cardiovasc Res. 1998;37:467–477. doi: 10.1016/s0008-6363(97)00278-2. [DOI] [PubMed] [Google Scholar]
- 25.Silver RB. Ratio imaging: measuring intracellular Ca++ and pH in living cells. Methods Cell Biol. 2003;72:369–387. doi: 10.1016/s0091-679x(03)72018-4. [DOI] [PubMed] [Google Scholar]
- 26.Semsarian C, Ahmad I, Giewat M, Georgakopoulos D, Schmitt JP, McConnell BK, et al. The L-type calcium channel inhibitor diltiazem prevents cardiomyopathy in a mouse model. J Clin Invest. 2002;109:1013–1020. doi: 10.1172/JCI14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, Herzig S. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci U S A. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 29.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 30.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci U S A. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of beta2-adrenergic receptor Gi signaling in the heart: the good, the bad, and the ugly. Circ Res. 2005;97:507–509. doi: 10.1161/01.RES.0000184615.56822.bd. [DOI] [PubMed] [Google Scholar]
- 33.Tong H, Bernstein D, Murphy E, Steenbergen C. The role of beta-adrenergic receptor signaling in cardioprotection. Faseb J. 2005;19:983–985. doi: 10.1096/fj.04-3067fje. [DOI] [PubMed] [Google Scholar]
- 34.Fajardo G, Zhao M, Powers J, Bernstein D. Differential cardiotoxic/cardioprotective effects of beta-adrenergic receptor subtypes in myocytes and fibroblasts in doxorubicin cardiomyopathy. J Mol Cell Cardiol. 2006;40:375–383. doi: 10.1016/j.yjmcc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmet I, Lakatta EG, Talan MI. Pharmacological stimulation of beta2-adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev. 2005;10:289–296. doi: 10.1007/s10741-005-7543-3. [DOI] [PubMed] [Google Scholar]
- 36.He JQ, Balijepalli RC, Haworth RA, Kamp TJ. Crosstalk of beta-adrenergic receptor subtypes through Gi blunts beta-adrenergic stimulation of L-type Ca2+ channels in canine heart failure. Circ Res. 2005;97:566–573. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- 37.Sato M, Gong H, Terracciano CM, Ranu H, Harding SE. Loss of beta-adrenoceptor response in myocytes overexpressing the Na+/Ca(2+)-exchanger. J Mol Cell Cardiol. 2004;36:43–48. doi: 10.1016/j.yjmcc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, et al. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 39.Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 40.Freeman K, Lerman I, Kranias EG, Bohlmeyer T, Bristow MR, Lefkowitz RJ, et al. Alterations in cardiac adrenergic signaling and calcium cycling differentially affect the progression of cardiomyopathy. J Clin Invest. 2001;107:967–974. doi: 10.1172/JCI12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billman GE, Castillo LC, Hensley J, Hohl CM, Altschuld RA. Beta2-adrenergic receptor antagonists protect against ventricular fibrillation: in vivo and in vitro evidence for enhanced sensitivity to beta2-adrenergic stimulation in animals susceptible to sudden death. Circulation. 1997;96:1914–1922. doi: 10.1161/01.cir.96.6.1914. [DOI] [PubMed] [Google Scholar]
- 42.Fajardo G, Zhao M, Berry G, Wong LJ, Mochly-Rosen D, Bernstein D. beta2-adrenergic receptors mediate cardioprotection through crosstalk with mitochondrial cell death pathways. J Mol Cell Cardiol. 2011;51:781–789. doi: 10.1016/j.yjmcc.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bers DM. Cardiac Inotropy and Ca Mismanagement. In: Bers DM, editor. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kluwer Academic Publishers; 2001. pp. 273–331. [Google Scholar]
- 44.Dong R, Liu P, Wee L, Butany J, Sole MJ. Verapamil ameliorates the clinical and pathological course of murine myocarditis. J Clin Invest. 1992;90:2022–2030. doi: 10.1172/JCI116082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 46.Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, et al. G(i) protein-mediated functional compartmentalization of cardiac beta(2)-adrenergic signaling. J Biol Chem. 1999;274:22048–22052. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- 47.Neumann J, Schmitz W, Scholz H, von Meyerinck L, Doring V, Kalmar P. Increase in myocardial Gi-proteins in heart failure. Lancet. 1988;2:936–937. doi: 10.1016/s0140-6736(88)92601-3. [DOI] [PubMed] [Google Scholar]
- 48.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.