Abstract
Evidence suggests a relationship between dietary fat intake, obesity and colorectal cancer, implying a role for fatty acid (FA) metabolism in intestinal tumorigenesis that is incompletely understood. Liver fatty acid binding protein (L-Fabp), a dominant intestinal FA binding protein, regulates intestinal FA trafficking and metabolism and L-Fabp deletion attenuates diet-induced obesity. Here we examined whether changes in intestinal FA metabolism following L-Fabp deletion modify adenoma development in ApcMin/+ mice. Compound L-Fabp−/−ApcMin/+ mice were generated and fed a 10% fat diet balanced equally between saturated, monounsaturated and polyunsaturated fat. L-Fabp−/−ApcMin/+ mice displayed significant reductions in adenoma number and total polyp area compared to ApcMin/+controls, reflecting a significant shift in distribution toward smaller polyps. Adenomas from L-Fabp−/−ApcMin/+ mice exhibited reductions in cellular proliferation, high-grade dysplasia and nuclear β-catenin translocation. Intestinal FA content was increased in L-Fabp−/−ApcMin/+ mice and lipidomic profiling of intestinal mucosa revealed significant shifts to polyunsaturated FA species with reduced saturated FA species. L-Fabp−/−ApcMin/+mice also demonstrated corresponding changes in mRNA expression of enzymes involved in FA elongation and desaturation. Furthermore, adenomas from L-Fabp−/−ApcMin/+mice displayed significant reductions in mRNA abundance of nuclear hormone receptors involved in cellular proliferation and in enzymes involved in lipogenesis. These findings collectively implicate L-Fabp as an important genetic modifier of intestinal tumorigenesis and identify FA trafficking and metabolic compartmentalization as an important pathway linking dietary fat intake, obesity and intestinal tumor formation.
Keywords: Obesity, fatty acid trafficking, fatty acid metabolism, lipogenesis, intestinal tumorigenesis
INTRODUCTION
There is a strong foundation of epidemiologic and experimental work to support a role for environmental modifiers including high dietary fat intake and obesity in the pathogenesis of CRC, and increasing evidence suggests that the type of dietary fat is important (1-4). Several studies have found a strong association between saturated fat intake and CRC risk (5-7), while diets rich in ω-3 polyunsaturated fatty acids (PUFA) may suppress CRC development (8-10). These studies highlight the role of fatty acid (FA) metabolism in the pathogenesis of intestinal tumors and suggest that intestinal metabolism of specific dietary fats modify CRC risk. Despite the importance of these observations, however, little progress has been made towards a molecular genetic understanding of the pathways that link consumption of high-saturated fat diets to intestinal tumorigenesis.
FA metabolism is a focal point for metabolic regulation, acting to modulate gene expression, growth and survival pathways in both physiologic and pathologic settings (11). Fatty acid binding proteins (Fabps) include a large multigene family of highly abundant, cytosolic lipid binding proteins that coordinate intracellular lipid trafficking and regulate metabolic and inflammatory pathways (11, 12). A dominant Fabp in mammalian intestine, liver fatty acid binding protein (L-Fabp) plays a critical role in intestinal FA trafficking and compartmentalization (13). Beyond a role in modulating lipid flux however, the expression of L-Fabp within CRC tumor tissue has been linked to histologic (tumor) differentiation, the incidence of lymph node metastasis and overall prognosis (14-16). Those studies imply that L-Fabp expression may modulate signaling pathways involved in CRC initiation and progression, although the mechanisms involved are currently obscure.
We have previously demonstrated that mice with germline deletion of L-Fabp (L-Fabp−/−) are protected against diet-induced obesity and hepatic steatosis when fed a high saturated fat diet, but not when fed a high PUFA diet (17-19). We have also shown significant alterations in enterohepatic FA, cholesterol, and bile acid metabolism in L-Fabp−/− mice, findings which collectively demonstrate a role for L-Fabp as a metabolic sensor with a hierarchy of sensitivity to intraluminal and dietary fat species (20-22). These experimental observations, together with the findings linking dietary fat intake to CRC susceptibility, led us to ask whether alterations in intestinal FA metabolism associated with L-Fabp deletion affect intestinal tumor initiation and progression. Here, we report that L-Fabp deletion significantly reduces intestinal adenoma formation in the ApcMin/+ model of tumorigenesis and demonstrate that this protection is associated with alterations in the metabolic utilization of intestinal FA species.
MATERIALS AND METHODS
Animals and tissue processing
All studies were approved by the Animal Studies Committee at Washington University School of Medicine and conformed to criteria outlined in the National Institutes of Health “Policy on Humane Care and Use of Laboratory Animals. L-Fabp−/−ApcMin/+ mice were generated and fed a 10% fat diet containing similar proportions of saturated, monounsaturated and polyunsaturated FAs (#8626 Teklad Mouse Breeder Diet, Harlan Teklad, Madison, WI). Mice were weighed and sacrificed at 104 ± 4 days of age. Intestines were formalin-fixed and pinned for inspection with a dissecting microscope. Intestinal polyps were examined blinded to genotype and classified according to location in the small intestine (SI). Each section was photographed using a Photometrics CooLSNAPcf camera (Photometrics, Tucson, AZ). Polyp size was quantitated using Metavue software (Molecular Devices, Sunnyvale, CA). In separate groups of 104-day old mice, normal intestine and polyp tissue were snap frozen for mRNA and protein expression studies. Additional groups of mice were sacrificed at 53 ± 2 days of age (prior to gross polyp development), and intestinal mucosa obtained for lipidomic profiling and gene expression studies. Where indicated, food consumption and fecal lipid studies were performed on mice at ~10 weeks of age housed in metabolic cages (19).
Immunohistochemical studies, bromodeoxyuridine labeling, and dysplasia measurements
Four-micron sections used to assess polyp histology following staining with hematoxylin and eosin (H&E). Dysplasia was assessed by an independent blinded gastrointestinal pathologist (IN). Proliferative activity within polyps was measured by bromodeoxyuridine (BrdU) incorporation as described previously (23). Immunohistochemical studies were performed using rabbit anti-L-Fabp (a generous gift from Dr. Jeffrey Gordon), rabbit anti-Fatty Acid Synthase (Bethyl Laboratories, Montgomery, TX), or mouse anti-β-catenin (BD Biosciences, San Jose, CA). As demonstrated previously (21), the anti-L-Fabp antiserum used in this study is specific for L-Fabp with no cross reactivity to either ileal lipid binding protein or intestinal fatty acid binding protein, the other dominant intestinal fatty acid binding proteins.
Western blotting
Tissue extracts were prepared as described (21) in denaturing sample buffer, separated by SDS-PAGE, and transferred onto a polyvinylidene difluoride membrane (Millipore). The membranes were probed sequentially with rabbit anti-L-Fabp, anti-PPAR-α or anti-β actin (Sigma Aldrich, St. Louis, MO). Proteins of interest were visualized with enhanced chemiluminescence reagents (GE Healthcare, Waukesha, WI) and scanned using Image-Quant software (GE Healthcare Biosciences, Pittsburgh, PA).
Analysis of Tissue Lipid Content and fecal fat content
Frozen tissue (~100 mg) was homogenized and protein concentration determined (BioRad, Hercules, CA; DC protein assay). Lipid extractions were performed (21) and TG, free fatty acids (FFA), and cholesterol content were determined using commercially available kits (Wako, Richmond, VA) and normalized to protein content of the homogenate. Fecal fat content was determined gravimetrically as previously described (17).
Lipidomic profiling
A modified Bligh-Dyer extraction method was used to extract lipids from scraped intestinal mucosa in the presence of internal standards. The free fatty acids were further derivatized into amides to improve the sensitivity of mass spectrometry (MS). Sample analysis was performed with a Shimadzu 10A HPLC system coupled to a TSQ Quantum Ultra triple quadrupole mass spectrometer. A pooled sample from each extracted study sample was used as the quality control (QC) sample. Data processing was conducted with Xcalibur software (Thermo, Waltham, MA). The concentration of lipids were calculated as concentration of internal standard multiplied by peak area ratio of analyte to internal standard, based on an assumption that the MS responses of analyte and internal standard are the same.
Quantitative PCR
Normal intestinal tissue and tumor samples from individual animals were flash frozen and RNA extracted. Q-PCR was performed in triplicate on an ABI Step One Plus Detection System (Applied Biosystems, Carlsbad, CA) using SYBR Green PCR Master Mix (Applied Biosystems) and primer pairs (Supplementary Table S1) designed by Primer Express Software (Applied Biosystems). Relative gene expression was determined using the comparative threshold cycle method (Applied Biosystems User Bulletin I). Relative mRNA abundance in L-Fabp−/−ApcMin/+ mice was expressed as fold change compared to mRNA levels in normal mucosa from ApcMin/+ mice, normalized to GAPDH expression.
PGE2 analysis
Normal tissue and tumor samples from individual (three per genotype) animals were excised individually and flash frozen. Cleared homogenates were prepared (23) and analyzed using a PGE2 monoclonal immunoassay kit (Cayman Chemical, Ann Arbor, MI).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA). In most cases, Student’s t test was used to determine p values. For multiple group comparison, one-way ANOVA with Bonferroni post test was used to calculate differences between groups. Statistical significance was set at p ≤ 0.05. All values are reported as mean ± SE.
RESULTS
L-Fabp deletion in the ApcMin/+ background attenuates small intestinal adenoma burden
At 15 weeks, L-Fabp−/−ApcMin/+ mice manifested 35% fewer polyps (Figure 1A) than ApcMin/+ mice, with a regional decrease in polyp multiplicity in all sections of SI (Figure 1B), most evident in the proximal (Figure 1C) and distal intestine. There was a 42% reduction in total SI polyp area in L-Fabp−/−ApcMin/+ mice compared to ApcMin/+ mice (Figure 1D). This reduction was most significant in the proximal and middle sections of SI with a trend toward reduced polyp area in the distal SI (Figure 1E). Polyps were then divided into quartiles by size (area) and the distribution between genotypes compared. L-Fabp−/−ApcMin/+ mice exhibited a significantly greater proportion of polyps in the smallest size quartile compared to ApcMin/+ mice (Figure 1F). Our findings suggest that polyp number increases from proximal to distal SI in ApcMin/+ mice, an observation broadly consistent with our prior observations (23). We interpet the generally comparable reduction in polyp area (Figure 1D) and polyp number (Figure 1A) in L-Fabp−/−ApcMin/+ mice to imply that L-Fabp deletion reduces both polyp initiation and progression.
Figure 1.
Germline deletion of L-Fabp reduces small intestinal adenoma burden in ApcMin/+ mice. A, reduced mean polyp number per mouse in L-Fabp−/−ApcMin/+ mice. B, regional reduction in polyp number. C, representative photographs of reduced proximal small intestine polyposis. D, reduction in polyp area. E, regional reduction in polyp area. F, increased proportion of smaller polyps in L-Fabp−/−ApcMin/+ mice. *, p ≤ 0.05, **, p ≤ 0.01.
L-Fabp deletion in the ApcMin/+ background reduces cellular proliferation, dysplasia, and Wnt signaling in small intestinal polyps
We next examined histologic markers of adenoma progression and differentiation in polyps from both genotypes. L-Fabp−/−ApcMin/+ mice exhibited reduced proliferation in adenomas compared to ApcMin/+ mice in the proximal and middle intestine (Figure 2A-B). There was a 26% reduction in the number of polyps containing high grade dysplasia in L-Fabp−/−ApcMin/+ mice compared to ApcMin/+ controls (Figure 2C-D). Given the reduced proportion of tumors with high grade dysplasia in L-Fabp−/−ApcMin/+ mice, we evaluated nuclear translocation of β—catenin (Figure 2E-F). We found a significant reduction in staining for nuclear β—catenin in proximal SI polyps from L-Fabp−/−ApcMin/+ mice compared to ApcMin/+ mice. These findings suggest that L-Fabp deletion produces alterations in pathways related to cellular proliferation and differentiation in intestinal epithelium.
Figure 2.
Reduced proliferation, high grade dysplasia and nuclear β-catenin translocation in polyps of L-Fabp−/−ApcMin/+ mice. A, reduction in % of BrdU-positive cells/polyp. B, representative BrdU staining (brown nuclei) of proximal small intestine polyps. C, reduction in proportion of polyps with high-grade dysplasia. D, representative example of high-grade dysplasia from ApcMin/+ mice (left) versus low-grade dysplasia from L-Fabp−/−ApcMin/+ mice (right). E, reduction in nuclear β-catenin translocation. F, representative β-catenin staining (brown nuclei) of proximal small intestine polyps. ***, p ≤ 0.001.
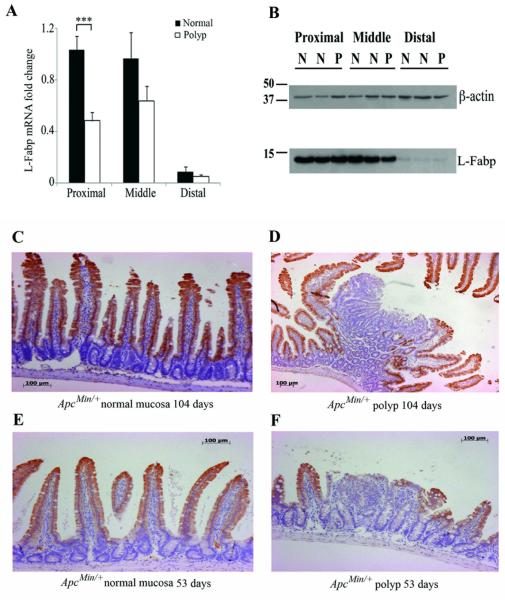
Reduced L-Fabp expression in intestinal adenomas in ApcMin/+ mice
Because germline deletion of L-Fabp results in reduced polyp burden, we examined the expression of L-Fabp in adenomas compared to normal intestinal mucosa in ApcMin/+ mice (i.e., mice with a wild-type L-Fabp allele). A gradient of L-Fabp expression was observed in normal intestinal mucosa from proximal to distal SI (Figure 3A). L-Fabp mRNA expression was reduced in proximal intestinal polyps by ~2 fold compared to normal mucosa. The proximal to distal gradient of L-Fabp expression was confirmed at the protein level using Western blot (Figure 3B), although differences in protein expression between normal mucosa and polyps were not apparent, likely due to high levels of L-Fabp expression in normal tissue surrounding polyps. Therefore, immunohistochemistry was performed to examine the expression of L-Fabp in normal mucosa and polyp tissue. Normal intestinal mucosa demonstrated robust immunohistochemical staining of L-Fabp (Figure 3C), while adenomas demonstrated either a loss or absence of L-Fabp expression (Figure 3D). 53 day old ApcMin/+ mice demonstrated a similar staining pattern in normal and early polyp tissue (Figure 3E-F) to that observed in 104 day old mice, indicating that the loss of L-Fabp expression may be an early event in intestinal tumorigenesis.
Figure 3.
Reduced L-Fabp expression in intestinal adenomas compared to normal intestinal mucosa in ApcMin/+ mice. A, Gradient of L-Fabp mRNA expression in normal intestinal mucosa and reduction in L-Fabp mRNA abundance in intestinal polyps compared to normal intestinal mucosa. B, L-Fabp protein levels in normal intestinal mucosa and intestinal polyps. C-D,Reduced L-Fabp expression in small intestinal polyp (D) compared to normal mucosa (C) at 15 weeks. E-F, Reduced L-Fabp expression in small intestine polyp (F) compared to normal mucosa (E) apparent at 53 days.
L-Fabp deletion in the ApcMin/+ background increases intestinal free fatty acid, triglyceride and cholesterol content and alters distribution of fatty acid species
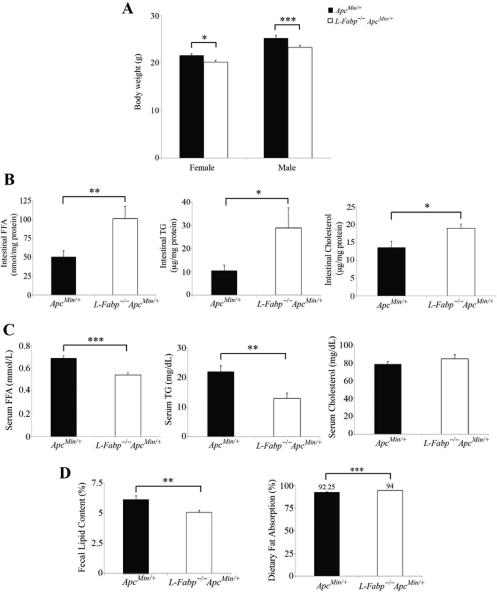
We have previously demonstrated protection against diet-induced obesity associated with alterations in the kinetics of intestinal FA transport in female L-Fabp−/− mice (17, 19). We found that L-Fabp−/−ApcMin/+ mice weighed significantly less than their ApcMin/+ counterparts at 104 ± 4 days of age (Figure 4A). L-Fabp−/−ApcMin/+ mice exhibited increased intestinal FFA, TG and cholesterol content compared to ApcMin/+ mice along with a concomitant decrease in serum FFA and TG (Figure 4B-C). Analysis of fecal lipid content in mice from both genotypes revealed reduced fecal fat and a subtle yet significant increase in dietary fat absorption in L-Fabp−/−ApcMin/+ mice (Figure 4D). These data suggest that L-Fabp deletion alters intestinal lipid trafficking in the ApcMin/+ background.
Figure 4.
Reduced body weight and altered lipid trafficking and metabolism in L-Fabp−/−ApcMin/+ mice. A, Reduced weight gain on 10% fat diet at 104 days. B, Increased intestinal FFA (left), TG (center) and cholesterol (right) in 53 day-old L-Fabp−/−ApcMin/+ mice. C, reduced serum FFA (left) and TG (center) and unchanged serum cholesterol (right) in 53 day-old L-Fabp−/−ApcMin/+ mice. D, reduced fecal lipid content (left) and increased dietary fat absorption (right) in L-Fabp−/−ApcMin/+ mice.
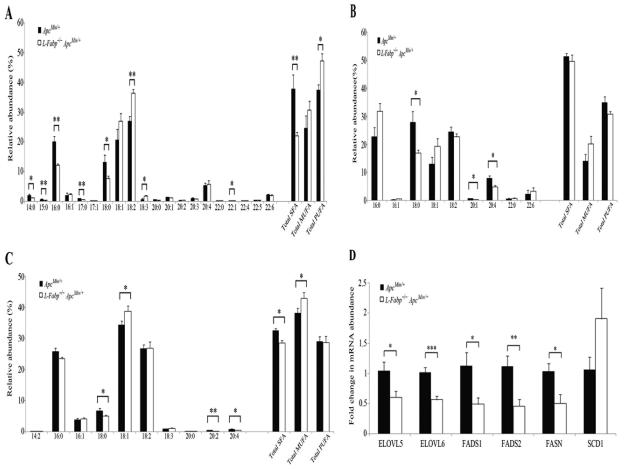
We next turned to a lipidomics approach to examine whether the changes in lipid abundance in mucosal extracts from proximal SI of 53 day old mice reflect a corresponding change in FA species within lipid classes. As shown in Figure 5A, there was a significant shift in relative abundance of intestinal FFA species between genotypes, with a decrease in total saturated FA species and an increase in total PUFA species in L-Fabp−/−ApcMin/+ mice. ApcMin/+ mice also exhibited a significant increase in relative abundance of shorter chain saturated FA species (14-18 carbons), while L-Fabp−/−ApcMin/+ mice demonstrated increased abundance of essential dietary polyunsaturated FA (18:2, 18:3). There was also a significant shift in the relative abundance of FA species in diacylglycerides (DG) between genotypes (Figure 5B and Supplementary Table S2), with broadly similar patterns to those observed above for individual FFA species. Diacylglycerides containing 18:0 FA were decreased in mucosal extracts from L-Fabp−/−ApcMin/+ mice and DGs containing arachidonic acid (20:4) were significantly increased in ApcMin/+ mice. We then examined the relative composition of triglyceride FA species in these extracts (Figure 5C and Supplementary Table S3). The abundance of TGs that contain the saturated FA species 18:0 was significantly decreased in L-Fabp−/−ApcMin/+ mice, as was the abundance of TGs containing arachidonic acid (20:4). These data collectively suggest that L-Fabp functions as a critical component of intestinal FA trafficking and compartmentalization, with a distinct hierarchy of FA sensitivity. The findings further suggest that L-Fabp deletion results in a significant shift in metabolic channeling of DGs and TGs containing 20:4.
Figure 5.
Altered composition of intestinal FFA, DG and TG in L-Fabp−/−ApcMin/+ mice by lipidomics. A, altered relative abundance of FFA species with shift to PUFA species in L-Fabp−/−ApcMin/+ mice. B, altered relative abundance of FA species in DG between genotypes. DG from ApcMin/+ mice have an increased proportion of 18:0 FAs and arachidonic acid (20:4). C, altered relative abundance of FA species in TG. TG from ApcMin/+ mice displayed increased relative abundance of saturated FAs and arachidonic acid (20:4). D, altered mRNA abundance of enzymes involved in FA biosynthesis, elongation and desaturation.
L-Fabp deletion significantly alters mRNA expression of enzymes involved in FA elongation and desaturation
The demonstration of differences in FA composition among intestinal FFA, DG and TG species between genotypes prompted us to examine the expression of enzymes involved in FA biosynthesis, elongation and desaturation. We found that mRNA abundance of fatty acid elongases 5 and 6 (ELOVL5 and ELOVL6) and fatty acid desaturases 1 and 2 (FADS1 and FADS2) were reduced by 40-60%, while fatty acid synthase (FASN) mRNA was reduced 2-fold in SI mucosa of 53 day old L-Fabp−/−ApcMin/+ mice (Figure 5D). There was also a qualitative reduction in FASN protein expression as determined by immunostaining (Supplementary Figure S1). Collectively, these findings imply that altered FA trafficking as a result of L-Fabp deletion produces significant adaptive changes in long chain FA biosynthesis, elongation and desaturation, which likely further contribute to the altered FA profiles observed.
L-Fabp deletion significantly alters mRNA expression of nuclear hormone receptors involved in FA metabolism
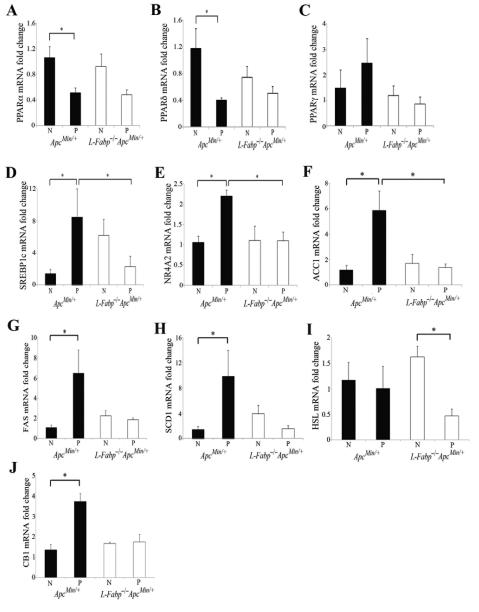
Given the pronounced differences in intestinal FA composition in intestinal mucosa at 53 days of age, we investigated gene expression changes of transcription factors known to be involved in FA metabolism and whose regulation has been implicated in intestinal tumorigenesis in normal mucosa and polyps from mice of both genotypes. The peroxisome proliferator activated receptor family of transcription factors are activated by endogenous lipid ligands (24). We found that the abundance of both PPARα and PPARδ mRNAs was significantly reduced in adenomas compared to normal intestinal mucosa in ApcMin/+ mice (Figure 6A-B). There was a trend to reduced mRNA expression of PPARα and PPARδ between polyps and normal intestine in L-Fabp−/−ApcMin/+ mice, but PPARα protein expression was significantly reduced in polyps of L-Fabp−/−ApcMin/+ mice (Figures 6A-B, Supplementary Figure S2). No significant differences were found between genotypes in the expression of PPARγ (Figure 6C). Sterol regulatory element-binding protein-1 (SREBP-1) is a master regulator of lipogenic pathways and has been shown to be upregulated in CRC (25). mRNA expression of SREBP-1c was significantly upregulated in polyps compared to normal mucosa in ApcMin/+ mice, but significantly reduced in polyps from L-Fabp−/−ApcMin/+ mice compared to expression in polyps in ApcMin/+ mice (Figure 6D). Similarly, the NR4A orphan nuclear receptors are linked to the regulation of FA metabolism and expression of NR4A2 in particular is increased in CRC (26, 27). mRNA abundance of NR4A2 was also upregulated in polyps from ApcMin/+ mice and significantly reduced in polyps from L-Fabp−/−ApcMin/+ mice (Figure 6E). Given the changes in NR4A2 and the known modulation of cyclooxgenase-mediated prostanoid signaling by NR4A2, we then measured mRNA abundance of cyclooxygenase-2 (COX-2) and levels of prostaglandin E2 (PGE2) in normal and polyp tissue from mice of both genotypes. There was a trend toward reduced COX-2 mRNA abundance (37% reduction in relative mRNA abundance, p=0.13) and reduced PGE2 levels (62 μg PGE2/mg protein vs. 83 μg PGE2/mg protein, p=0.3) in polyps from L-Fabp−/−ApcMin/+ mice compared to ApcMin/+ mice, despite similar expression levels in normal intestine (data not shown). These results collectively suggest that intestinal tumorigenesis is accompanied by altered expression of key transcription factors that govern FA metabolic pathways important in sustaining cell growth and proliferation, and that L-Fabp deletion appears to prevent these metabolic changes at the transcriptional level.
Figure 6.
Alterations of tumor lipid metabolism in polyps from L-Fabp−/−ApcMin/+ mice compared with ApcMin/+ mice. Altered mRNA expression in proximal small intestine polyps (P) compared to normal proximal small intestine (N) of the PPAR family (A-C), SREBP1C (D), NR4A2 (E), enzymes involved in de novo lipogenesis (F-H), lipolysis (I) and energy utilization (J). Gene expression is normalized to expression in normal proximal SI mucosa of ApcMin/+ mice.
L-Fabp deletion significantly alters mRNA expression of enzymes involved in de novo lipogenesis and lipolysis
Activation of FA metabolism and de novo FA biosynthesis is an early event in intestinal tumorigenesis and a number of studies have demonstrated upregulation of key enzymes involved in de novo fatty acid biosynthesis in CRC and reduced tumor growth upon their inhibition (28-31). Here we demonstrate that mRNA expression of three key lipogenic enzymes, acetyl coA carboxylase (ACC), fatty acid synthase (FASN), and stearoyl-coA desaturase (SCD-1), were all significantly upregulated in the transformation from normal intestinal mucosa to adenoma in ApcMin/+ mice (Figure 6F-H). However, there was no corresponding change in mRNA expression for these genes in L-Fabp−/−ApcMin/+ mice (Figure 6F-H, Supplementary Figure S2). Alterations in fatty acid lipolysis pathways have also been shown to be part of the adaptive changes in lipid metabolism in CRC (26). The expression of hormone sensitive lipase (HSL), an enzyme involved in lipolysis, was significantly reduced in polyps from L-Fabp−/−ApcMin/+ mice compared to normal mucosa, while expression remained unchanged in ApcMin/+ mice (Figure 6I). Finally, the expression of cannabinoid receptor 1 (CB1), an endocannabinoid receptor implicated in the control of energy and metabolism through alterations in FA balance and known to be altered in CRC (32), was significantly upregulated in the polyps of ApcMin/+ mice compared to normal mucosa, while its expression remained unchanged in L-Fabp−/−ApcMin/+ mice (Figure 6J). Taken together, these results suggest that L-Fabp deletion modulates pathways of de novo FA lipogenesis and influences the metabolic compartmentalization of FA. These metabolic shifts in turn are associated with decreased intestinal adenoma burden in L-Fabp−/−ApcMin/+ mice.
DISCUSSION
The relationship between dietary fat intake, obesity and CRC has focused attention on the pathways that integrate elements of intestinal lipid metabolism and pathways involved in tumorigenesis. We have previously demonstrated that L-Fabp plays an important role in the metabolic response to dietary fat and is a modifier of the obesity trait associated with high saturated fat feeding (18, 19). The key finding of this study is that L-Fabp deletion in ApcMin/+ mice not only protects against weight gain, but also significantly attenuates intestinal tumor initiation and progression. This protection from adenoma development is associated with alterations in intestinal FA and TG content and composition and in the expression of transcription factors and enzymes involved in lipogenesis and energy utilization. Taken together, these findings suggest that L-Fabp is an important genetic modifier of intestinal tumorigenesis and identify FA trafficking as a novel pathway linking dietary fat intake, obesity and intestinal adenoma formation.
The requirement of FA and lipid substrates for membrane synthesis, energy homeostasis and cell signaling is an increasingly recognized hallmark of neoplastic transformation (28, 33, 34). These FAs are either imported from the extracellular milieu, synthesized de novo or mobilized from intracellular stores through specific adaptations in lipid metabolism critical for tumor cell growth and proliferation. Consistent with the need for robust lipid synthesis, neoplastic cells demonstrate increased expression of critical enzymes involved in lipogenesis, including ACC, FASN and SCD1 (33). Studies have demonstrated that inhibiting each of these enzymes in vitro and/or in vivo results in diminished tumor cell proliferation, decreased cell viability or decreased tumor size, suggesting that interruption of FA synthesis may be a potential chemotherapeutic strategy in the treatment of cancer (35-41). Here we demonstrate upregulated expression of lipogenic enzymes ACC1, FASN, and SCD1 in intestinal adenomas in ApcMin/+ mice, yet find no corresponding increase in these lipogenic genes in L-Fabp−/−ApcMin/+ mice (Figure 6). This suggests that alterations in FA metabolic trafficking caused by L-Fabp deletion may reduce the ability of neoplastic cells to upregulate lipogenesis necessary to support the initiation and/or proliferation of intestinal tumors. These findings suggest that the adaptations in FA trafficking and lipogenesis observed during tumorigenesis in ApcMin/+ mice are reduced or delayed in L-Fabp−/−ApcMin/+ mice, resulting in reduced intestinal adenoma formation and proliferation. Further experimental confirmation using more direct measures of lipid substrate utilization and turnover will of course be required to be able to make firm conclusions regarding the relative importance of these pathways implicated in intestinal tumorigenesis.
The aforementioned adaptive changes in tumor lipid metabolism are proposed to reflect activation of oncogenic pathways that in turn alter the activity of key transcription factors (42). For example, the transcriptional regulation of lipogenic genes in colorectal neoplasia has been shown to be regulated by SREBP-1 (25). Here we show that L-Fabp deletion significantly reduces the expression of SREBP-1 in intestinal polyps. Our findings suggest that this decrease is associated with reduced lipogenesis and fewer and smaller intestinal polyps. We also demonstrate reduced expression of the nuclear orphan receptor NR4A2 in L-Fabp−/−ApcMin/+ mice, which is of interest in relation to recent studies demonstrating that this gene plays a key role integrating eicosanoid and FA metabolic pathways (26, 27). We demonstrated increased NR4A2 expression in polyps from ApcMin/+ mice but not in polyps from L-Fabp−/−ApcMin/+ mice (Figure 6). These data, together with the lipidomic data demonstrating decreased abundance of DG and TG species containing 20:4 FA in mucosal extracts from L-Fabp−/−ApcMin/+ mice and the trend toward reduced COX-2 mRNA and PGE2 in polyps from L-Fabp−/−ApcMin/+ mice, suggest that L-Fabp expression may modulate eicosanoid and prostanoid signaling, pathways that are important in intestinal tumorigenesis.
An unresolved question is whether the changes in proliferation and gene expression in polyps from L-Fabp−/−ApcMin/+ mice arise as a direct result of altered intracellular FA trafficking and compartmentalization within neoplastic cells, or whether these changes reflect altered lipid substrate availability and utilization in the extracellular milieu that restrict polyp growth. These questions assume additional significance since L-Fabp expression was consistently reduced in intestinal polyps compared to normal intestinal mucosa in ApcMin/+ mice and apparent even in 8 week-old mice, suggesting that loss of L-Fabp is an early event in intestinal tumorigenesis. Consistent with this, studies in human CRC have demonstrated changes in L-Fabp expression in early stage intestinal adenomas with progressive loss of L-Fabp expression during CRC development and progression, and the lowest levels of expression occurring in poorly differentiated tumors (14, 43). These findings suggest that L-Fabp expression is important in the promotion of normal intestinal differentiation, and that pathways involved in regulating L-Fabp expression may be disrupted in transformed and de-differentiated enterocytes. Another possibility is that the transformation of normal intestinal epithelium into dysplastic adenoma and adenoma progression is dependent on the transfer of lipid substrates from surrounding normal intestinal epithelium. Recently, Nieman et al reported reduced tumor burden and metastases in FABP4−/− mice compared to wild-type mice (44), along with reduced transfer of lipid substrates from adipocytes to ovarian cancer cells. Those findings raise the question of whether similar adaptations may in part explain our current observations. In this scenario, deletion of L-Fabp may result in altered trafficking of substrates along with alterations in key pathways of lipogenesis, reducing the availability of substrates necessary for tumorigenesis. Thus, it is possible that the attenuation in polyp burden in L-Fabp−/−ApcMin/+ mice reflects altered lipid trafficking required for the earliest stages of adenoma progression. Our findings bear further consideration in the context of other work showing that low-density lipoprotein receptor-related protein LRP1 modulates intracellular cholesterol storage and fatty acid synthesis through Wnt signaling pathways (45). These findings have added significance since L-Fabp regulates hepatobiliary cholesterol metabolism in-vivo (17, 22). Here we find increased intestinal cholesterol content associated with reduced lipogenesis and nuclear β-catenin translocation in L-Fabp−/−ApcMin/+ mice, raising the possibilty that L-Fabp may also play a role in the crosstalk between sterol and oxysterol metabolism and Wnt signaling pathways.
Lipidomic profiling revealed altered intestinal FFA, DG, and TG FA species between genotypes, with a shift from saturated FAs in ApcMin/+ mice to PUFAs in L-Fabp−/−ApcMin/+ mice. These findings, in conjunction with our previous studies demonstrating reduced diet-induced obesity and hepatic steatosis in L-Fabp−/− mice fed a high saturated but not PUFA diet, suggest that L-Fabp deletion alters the metabolism of specific FA species. While in vitro data demonstrates that L-Fabp has higher affinity for long-chain PUFAs than saturated FA species, the adaptations that take place in-vivo are more complicated to interpret since both L-Fabp and intestinal fatty acid binding protein (I-Fabp) are abundantly expressed within enterocytes. Studies have demonstrated different functions for murine I-Fabp and L-Fabp in intestinal FA trafficking and energy homeostasis that may explain some of the subtle differences in FA composition in different lipid classes noted in our study (46). For example, it is possible that L-Fabp plays a larger role in saturated FA metabolism and I-Fabp assumes a larger role in PUFA metabolism. Alternatively, our findings are also consistent with a role for L-Fabp in the metabolism of essential dietary PUFA. In this scenario, the shift in relative abundance from increased saturated FAs in ApcMin/+ mice to increased PUFAs in L-Fabp−/−ApcMin/+ mice may reflect reduced utilization and metabolism of essential dietary PUFA precursors and increased reliance on saturated FAs in L-Fabp−/−ApcMin/+ mice. This differential processing of dietary FAs in L-Fabp−/−ApcMin/+ mice might then lead to reduced production of longer chain ω—3 and ω—6 FAs involved in pathways of tumor initiation and progression, the result of defective trafficking caused by L-Fabp deletion. Although FA utilization was not specifically examined in the current study, the observation that DG and TG species containing arachidonic acid were more abundant in ApcMin/+ mice compared to L-Fabp−/−ApcMin/+ mice, further substantiates the possibility that there might be reduced production of important eicosanoid and prostanoid precursors in L-Fabp−/−ApcMin/+ mice. In support of this possibility, we found trends towards reduced COX-2 and PGE2 expression in polyps from L-Fabp−/−ApcMin/+ mice compared to ApcMin/+ mice. In addition, we found significant reductions in mRNA expression of enzymes involved in FA elongation and desaturation, which also suggests that alterations in FA trafficking in L-Fabp−/−ApcMin/+ mice may reduce generation of long chain FA substrates necessary for tumor initiation and progression. Confirmation of this hypothesis will require further studies in these lines of mice to examine whether changes in eicosanoid precursor and prostaglandin production play a role in reduced polyp growth in L-Fabp−/−ApcMin/+ mice.
In conclusion, the present findings suggest that an array of adaptive changes in FA metabolism, focused on maximizing lipogenesis and suppressing FA oxidation, occurs in intestinal tumors to support the lipid substrate requirements of rapidly proliferating dysplastic cells. We propose that L-Fabp modulates intestinal tumorigenesis by altering cellular FA and lipid trafficking and compartmentalization and by altering lipid substrate availability. The net result of these alterations is reduced lipid synthesis and utilization for energy homeostasis, membrane synthesis and lipid signaling required for tumor initiation and progression. Our results suggest that lipid trafficking may represent a novel pathway in understanding mechanisms involved in intestinal tumorigenesis and raise the possibility that strategies to target FA flux may eventually become a therapeutic target in the prevention and treatment of intestinal tumors.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Hui Jiang, Dave Scherrer, and Dr. Daniel S. Ory of the Metabolomics Core Facility of Washington University School of Medicine for their assistance with lipidomic profiling of intestinal mucosal extracts, Coen Klos, MD, for technical support, and Kymberli Carter and Angela Hamer of the DDRCC Morphology Core at Washington University School of Medicine for their assistance with immunohistochemistry.
GRANT SUPPORT
American Society of Colon and Rectal Surgeons Research Foundation Career Development Award (SD), National Institutes of Health grants HL-38180, DK-56260, DDRCC DK-52574 (Morphology and Murine Models Core; to NOD).
Financial Support: American Society of Colon and Rectal Surgeons Research Foundation Career Development Award (SD) and National Institutes of Health Grants DK-56264 and HL-38180 and Digestive Diseases Research Core Center Grant DK-52574 (NOD).
REFERENCES
- 1.de la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–80. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7:253–63. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Willett WC. Dietary factors and risk of colon cancer. Ann Med. 1994;26:443–52. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 5.Zaridze D, Filipchenko V, Kustov V, Serdyuk V, Duffy S. Diet and colorectal cancer: results of two case-control studies in Russia. Eur J Cancer. 1992;29A:112–5. doi: 10.1016/0959-8049(93)90586-5. [DOI] [PubMed] [Google Scholar]
- 6.Sandler RS, Lyles CM, Peipins LA, McAuliffe CA, Woosley JT, Kupper LL. Diet and risk of colorectal adenomas: macronutrients, cholesterol, and fiber. J Natl Cancer Inst. 1993;85:884–91. doi: 10.1093/jnci/85.11.884. [DOI] [PubMed] [Google Scholar]
- 7.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 8.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74:159–64. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavani A, Pelucchi C, Parpinel M, Negri E, Franceschi S, Levi F, et al. n-3 polyunsaturated fatty acid intake and cancer risk in Italy and Switzerland. Int J Cancer. 2003;105:113–6. doi: 10.1002/ijc.11018. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90. doi: 10.1093/ajcn/70.1.85. [DOI] [PubMed] [Google Scholar]
- 11.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nature reviews. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–34. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. The Journal of biological chemistry. 2010;285:32679–83. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrie LC, Dundas SR, Curran S, Murray GI. Liver fatty acid binding protein expression in colorectal neoplasia. Br J Cancer. 2004;90:1955–60. doi: 10.1038/sj.bjc.6601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyall MS, Dundas SR, Curran S, Murray GI. Profiling markers of prognosis in colorectal cancer. Clin Cancer Res. 2006;12:1184–91. doi: 10.1158/1078-0432.CCR-05-1864. [DOI] [PubMed] [Google Scholar]
- 16.Pei H, Zhu H, Zeng S, Li Y, Yang H, Shen L, et al. Proteome analysis and tissue microarray for profiling protein markers associated with lymph node metastasis in colorectal cancer. J Proteome Res. 2007;6:2495–501. doi: 10.1021/pr060644r. [DOI] [PubMed] [Google Scholar]
- 17.Newberry EP, Kennedy SM, Xie Y, Luo J, Davidson NO. Diet-induced alterations in intestinal and extrahepatic lipid metabolism in liver fatty acid binding protein knockout mice. Mol Cell Biochem. 2009;326:79–86. doi: 10.1007/s11010-008-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-Fabp / mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G307–14. doi: 10.1152/ajpgi.00377.2007. [DOI] [PubMed] [Google Scholar]
- 19.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 2006;44:1191–205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 20.Newberry EP, Kennedy SM, Xie Y, Luo J, Crooke RM, Graham MJ, et al. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-Fabp -/- mice. J Lipid Res. 2012;53:744–54. doi: 10.1194/jlr.M020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newberry EP, Xie Y, Kennedy S, Han X, Buhman KK, Luo J, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. The Journal of biological chemistry. 2003;278:51664–72. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y, Newberry EP, Kennedy SM, Luo J, Davidson NO. Increased susceptibility to diet-induced gallstones in liver fatty acid binding protein knockout mice. J Lipid Res. 2009;50:977–87. doi: 10.1194/jlr.M800645-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanc V, Henderson JO, Newberry RD, Xie Y, Cho S-J, Newberry EP, et al. Deletion of the AU-Rich RNA Binding Protein Apobec-1 Reduces Intestinal Tumor Burden in Apcmin Mice. Cancer Res. 2007;67 doi: 10.1158/0008-5472.CAN-07-1593. [DOI] [PubMed] [Google Scholar]
- 24.Peters JM, Shah YM, Gonzalez FJ. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat Rev Cancer. 2012;12:181–95. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JN, Mahmoud MA, Han WF, Ripple M, Pizer ES. Sterol regulatory element-binding protein-1 participates in the regulation of fatty acid synthase expression in colorectal neoplasia. Exp Cell Res. 2000;261:159–65. doi: 10.1006/excr.2000.5054. [DOI] [PubMed] [Google Scholar]
- 26.Holla VR, Wu H, Shi Q, Menter DG, DuBois RN. Nuclear orphan receptor NR4A2 modulates fatty acid oxidation pathways in colorectal cancer. The Journal of biological chemistry. 2011;286:30003–9. doi: 10.1074/jbc.M110.184697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holla VR, Mann JR, Shi Q, DuBois RN. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. The Journal of biological chemistry. 2006;281:2676–82. doi: 10.1074/jbc.M507752200. [DOI] [PubMed] [Google Scholar]
- 28.Notarnicola M, Messa C, Caruso MG. A significant role of lipogenic enzymes in colorectal cancer. Anticancer Res. 2012;32:2585–90. [PubMed] [Google Scholar]
- 29.Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB, et al. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71:6888–98. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Y, Ginanni N, Tota MR, Wu M, Bays NW, Richon VM, et al. Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin Cancer Res. 2008;14:5735–42. doi: 10.1158/1078-0432.CCR-07-5074. [DOI] [PubMed] [Google Scholar]
- 31.Mason P, Liang B, Li L, Fremgen T, Murphy E, Quinn A, et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS One. 2012;7:e33823. doi: 10.1371/journal.pone.0033823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafsson SB, Palmqvist R, Henriksson ML, Dahlin AM, Edin S, Jacobsson SO, et al. High tumour cannabinoid CB1 receptor immunoreactivity negatively impacts disease-specific survival in stage II microsatellite stable colorectal cancer. PLoS One. 2011;6:e23003. doi: 10.1371/journal.pone.0023003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–77. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–25. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 36.Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–94. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- 37.Kuhajda FP, Jenner K, Wood FD, Hennigar RA, Jacobs LB, Dick JD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci U S A. 1994;91:6379–83. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–93. [PubMed] [Google Scholar]
- 39.Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. 2011;9:1551–61. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- 40.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–65. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 41.Zaytseva YY, Rychahou PG, Gulhati P, Elliott VA, Mustain WC, O'Connor K, et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 2012;72:1504–17. doi: 10.1158/0008-5472.CAN-11-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 43.Davidson NO, Ifkovits CA, Skarosi SF, Hausman AM, Llor X, Sitrin MD, et al. Tissue and cell-specific patterns of expression of rat liver and intestinal fatty acid binding protein during development and in experimental colonic and small intestinal adenocarcinomas. Lab Invest. 1993;68:663–75. [PubMed] [Google Scholar]
- 44.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrand J, Bruban V, Zhou L, Gong W, El Asmar, May P, et al. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009;284:381–8. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lagakos WS, Gajda AM, Agellon L, Binas B, Choi V, Mandap B, et al. Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G803–14. doi: 10.1152/ajpgi.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.