Abstract
Acid soluble (ASC) and pepsin soluble (PSC) collagens were extracted from the skin, bone and muscle of a trash fish, leather jacket (Odonus niger) by three different extraction methods. Method I gave 46–50% yield for ASC, Method II gave 49–58% yield for both ASC and PSC and Method III gave 64–71% yield for PSC. The addition of pepsin had increased the yield by 30–45%. The yields of collagen from skin and bone were higher than muscle. SDS-PAGE pattern revealed that skin and bone collagen as Type I collagen with a typical (α1)2α2 chains and muscle collagen as Type V collagen with a typical α1α3α2 chains. Td values of bone and muscle collagen were high (30–32 °C) compared to skin collagen (27–28 °C). The higher imino acids (190 residues/1,000 residues) were found responsible for the higher Td values. The trash fish, leather jacket can therefore be exploited effectively for collagen as it has got good thermal properties for pharmaceutical and biomedical applications.
Keywords: Muscle, Fish Skin, Bone, Collagen, Leather
Introduction
Collagen constitutes 25% of total proteins in vertebrates. The word “collagen” is derived from the Greek words ‘kolla’ and ‘genos’ meaning glue and formation, respectively. It has got a variety of biomedical and pharmaceutical applications. Their applications include treatment of pain associated with osteoarthritis, hypertension, use in tissue engineering, implants in human, inhibition of angiogenic diseases, etc. (Rehn et al. 2001). It is also used as dermal filler, as hemostat, for drug delivery, skin substitutes, expandable intra-arterial stents and cell attachment substrate (Senaratne et al. 2006). Gelatin is a partially hydrolyzed form of collagen and it finds application in food and packaging industry for microencapsulation and light sensitive coatings (Senaratne et al. 2006).
Most of the collagen is derived from cow and pig skins. The outbreaks of certain animal diseases such as bovine spongiform encephalopathy (BSE); and foot and mouth diseases (FMD) have caused restrictions on the use of animal collagen as there is a possibility of these diseases getting transmitted to human beings (Trevitt and Singh 2003). In addition, Muslims and Jews do not accept any pig related food products while Hindus does not consume cow-based products (Pranoto et al. 2007). In such circumstances, fish collagen is considered as the best alternative because of its high availability, no risk of disease transmission and no religious barriers. The main differences of fish collagen from that of animal collagen are its high biological value, high essential amino acid content and low content of hydroxyproline.
Fish processing discards, by-catch of unutilized as well as underutilized fish species, are the promising sources for the extraction of fish collagen. Fish processing discards generally include skin, bones, scales and fins. India generates more than 2 million metric tonnes of waste every year from fish processing activities (Sudeepa et al. 2007). Processing discards from fisheries accounts for as much as 70–85% of the total weight of catch (Shahidi 1994). They are generally dumped in-land or hauled into the ocean. Disposal of these wastes also pose environmental problems for seafood processors.
Leather jacket is a trash fish belonging to the family, Balistidae that has got a huge landing in Tuticorin region of South India during the months of September to November. About 40 tonnes of this species are caught by a single trawler (53 ft) and sold at a very cheap price. These trash fishes are mainly utilized for poultry feed in whole form after drying. Besides this, there are very few companies that purchase these fishes for surimi processing discarding their frames. These trash fishes, at times, pose environmental problems in fishing harbour due to unhygienic handling (without proper icing) and less demand.
Taking into consideration the environmental problems caused by the landing of this species and growing knowledge on the utilization of processing wastes for the preparation of collagen, this study was undertaken to extract the acid and pepsin soluble collagen from skin, bone and muscle and examine their characteristics for further applications.
Materials and methods
Raw material
Trash fish, leather jacket (Odonus niger) were procured from the fishing harbour centre of Tuticorin, located in South India. The average weight and length of the fish were 140 g and 13 cm, respectively. Fishes were brought to the laboratory immediately, washed with potable water to clean the dust, dirt, sand and other extraneous matter. Then, they are fed into a mechanical deboner/mincer (Baader/601, Germany), which separated the frames and muscle. The frames were collected, from which bone and skin were segregated manually and their respective yields were calculated. The muscle along with entrails was also collected. They were held frozen at −20 °C in a deep freezer until used for the extraction.
Proximate composition and collagen content
Proximate composition of the bones, skins and muscles was determined by standard methods (AOAC 1995). Moisture content was determined by the hot air oven method. For protein analysis, Kelplus digestion system (Pelican equipments, Chennai, India) was used for the digestion of samples and Kelplus Elite Ex distillation system (Pelican equipments, Chennai, India) for the determination of nitrogen content. The crude protein was then calculated by multiplying nitrogen content with a factor 6.25. The crude fat was determined by Soxhlet method using petroleum ether (60–80 °C) as solvent in a SOCS PLUS- SCS 4 system (Pelican equipments, Chennai, India). The ash content was determined by a Muffle furnace (Servo, Salem, India) set at 500–550 °C for 15 h. Total collagen content was determined from the hydroxyproline content multiplied by the factor of 8.0. Hydroxyproline content was estimated following the colorimetric method (AOAC 1995) at 558 nm using a UV–vis Spectrophotometer (Jasco, V-530, Japan).
Extraction of collagen
Skins, bones and muscles of the leather jacket were chopped separately into small pieces and treated with 0.8 mol/L sodium chloride (NaCl) at a ratio of 1:6 (w/v) for 10 min, to remove the impurities (Montero et al. 1995). This process was repeated 3 times and then washed with cold distilled water. They were then treated with 0.1 mol/L sodium hydroxide (NaOH) at a ratio of 1:10 (w/v) for 3 days to remove the non-collagenous proteins and to prevent the effect of endogenous proteases on collagen, as per the procedure of Sato et al. (1986a). The NaOH solution was changed every day and finally washed with cold distilled water. Collagen was then extracted using acetic acid following three different methods. In the Method I, acid soluble collagen (ASC) was extracted twice using 10 volumes of 0.5 mol/L of the acetic acid for 3 days. In the Method II, acid soluble collagen was first extracted and then pepsin soluble collagen (PSC) was extracted by the addition of 0.1% (w/v) pepsin (Hi-Media Laboratories, Mumbai, India) to 0.5 mol/L of acetic acid. Pepsin was added in order to cleave the non-helical region, telopeptide. In the Method III, pepsin soluble collagen was extracted twice. After each extraction, the solution was centrifuged at 9,000 g for 20 min at 4 °C. The supernatant was salted out using 2 mol/L NaCl for 24 h at 4 °C. The precipitated collagen was centrifuged again at 9,000 g for 20 min at 4 °C. The residue was placed in the dialysis membrane-110 bags (Hi-Media Laboratories, Mumbai, India), and dialyzed against 0.02 mol/L phosphate buffer (pH 7.2) for 24 h at 4 °C. The dialyzed samples were then lyophilized (Martin Christ Lyophilizer, Alpha 1–2 LD Plus, Osterode am Harz, Germany) and held frozen at −20 °C for further analysis. The yield of collagen was calculated based on the hydroxyproline content in extracted collagen to that of the raw material.
Molecular weight determination
SDS-PAGE was performed according to the method of Laemmli (1970) using 10 g/100 ml separating gel and 1 g/100 ml stacking gel to determine the molecular weight of collagen chains. For which, collagen (10 mg) was dissolved in 1.0 ml of the sample buffer (Tris–HCl, pH 6.8 containing 2-mercaptoethanol, sucrose, bromophenol blue, 5 g/100 ml SDS) and heated at 50 °C for 10 min. Then, 20 μl was loaded along with high molecular weight protein markers (Fermentas Lifesciences, Germany). Electrophoresis was carried out at 50 mA initially and then at 100 mA. Protein bands were stained with Coomassie Brilliant Blue R250. The molecular weights were determined by comparison with standard protein markers.
Amino acid composition
Collagen samples were first hydrolyzed in vacuo using 6 mol/L HCl at 110 °C for 24 h in evacuated tubes and the hydrolysate was analyzed for amino acids using Shimadzu amino acid analyzer. Tryptophan was estimated after alkali hydrolysis by colorimetry.
Viscosity
The viscosity of collagen was determined following the procedure of Sivakumar et al. (2000) and Kimura et al. (1988) using the Ostwald’s viscometer. The viscometer was filled with 1 g/100 ml collagen dissolved in 0.1 mol/L acetic acid and immersed in a water bath set at 15 °C and allowed to stand for 10 min for equilibration. Simultaneously, 0.1 mol/L acetic acid without collagen was also immersed as control. The flow times of collagen solution and that of the control were determined using a stopwatch. Viscosities were measured at temperature intervals of about 5 °C starting from 15 °C up to 40 °C. Six determinations were made at each temperature and the average was calculated. The flow rates were used as an index for the calculation of relative viscosities. Relative viscosity (ηrel) = flow time of sample/flow time of control. Specific viscosities were computed for each temperature using the formula, specific viscosity (ηsp) = ηrel−1.
Denaturation temperature
The denaturation temperatures (Td values) of collagen were taken as the mid-point of the linear portion of the sigmoidal curve obtained by plotting ηsp/C at t °C/ηsp/C at 15 °C against temperatures, where C denotes concentration of collagen solution in mg/ml (Sivakumar et al. 2000).
Statistical analysis
The whole experiment was repeated thrice. The average mean values were calculated from three determinations and expressed with standard deviation. The results were also statistically interpreted using SPSS 13.0 to find out the least significant differences (LSD) in the proximate composition of different parts of the fish as well as in the yield and viscosity of collagen obtained by different extraction methods.
Results and discussion
Proximate composition
The leather jacket yielded 53% as frames after mechanical deboning process while muscle and offal constituted 47%. The frames included skin (25%), bone (13%), scales (9%) and fin (7%). Shahidi (1994) reported that 25% of minced meat could be obtained by mechanical deboning of processing discards. Mechanical deboning process normally removes more wastes than manual separation and the wastes generated differ in amount and composition depending on the species and process employed. Another report stated that the processing discards accounted up to 75% of the total weight of fish in filleting operations (Pigott 1986). Similar to it, the leather jacket used in this study comprised high proportions of skin and bones besides scales and fin, as processing discards. These discards were found suitable for collagen extraction. As the muscle of this fish was not utilized for consumption, it was also used for collagen extraction.
The proximate composition of skin, bone and muscle of leather jacket are given in Table 1. Skin of leather jacket contained low moisture (51.82%), fairly good amount of protein (21.65%) and very high ash content (25.03%), while the bone contained slightly high moisture, low protein and low ash contents; and thus differ statistically (P < 0.05). The skin of brown backed toadfish was reported to contain a high moisture content of 73.40% (Senaratne et al. 2006) but leather jacket skin did not contain such higher content. Muyonga et al. (2004) reported that the skin of Nile perch contained 20–22% of protein, which is in coincidence with our study. They also observed that higher ash content in the skin of adult fish was because of increased mineralization with age. But, the higher ash content noticed in the skin and bone of leather jacket could not be attributed to the increased mineralization; instead it was due to their thicker skin and inherent biochemical composition. Presence of relatively high protein in the skin and bone made them good sources for collagen extraction. The moisture content in the muscle of leather jacket was very high (85.69%) with very low protein (6.83%) and significantly different from skin and bone (P < 0.05). The moisture and crude protein contents in the muscle of fish species generally ranged between 54% and 80.3% and 16.1–27.9%, respectively (Sato et al. 1986b). Such high concentration of protein was not noticed in this species so termed as trash fish of low economic value.
Table 1.
Proximate composition of processing wastes of leather jacket (%)
| Parameters | Moisture | Protein | Fat | Ash |
|---|---|---|---|---|
| Skin | 51.82a ± 0.65 | 21.65a ± 0.50 | 0.61a ± 0.26 | 25.03a ± 0.45 |
| Bone | 68.73b ± 0.31 | 11.86b ± 0.35 | 0.91a ± 0.02 | 18.67b ± 0.10 |
| Muscle | 85.69c ± 0.05 | 6.83c ± 0.10 | 1.93b ± 0.35 | 4.97c ± 0.22 |
All values are mean ± standard deviation of triplicate analysis
Different superscripts in the same column indicate significant differences (P < 0.05)
Yield of collagen
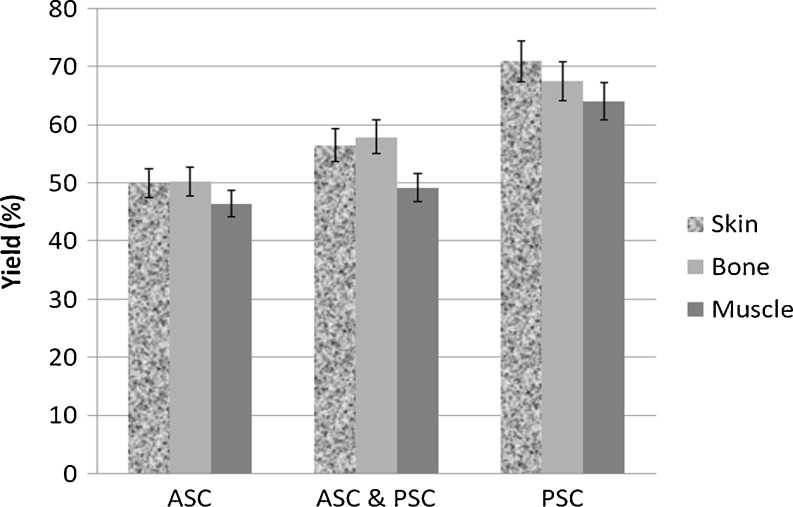
Yields of collagen obtained from the skin, bone and muscle of leather jacket are given in Fig. 1. The yield of collagen obtained from leather jacket varied from 46.48% to 70.94%. An earlier report indicated that the yield of collagen obtained from marine fish skin, bones, fins and scales generally ranged from 30% to 51% (Nagai and Suzuki 2000b). The total yield of collagen was known to vary with the type of extraction. The yield of the ASC was in general lower than the PSC. Hence, the yields obtained by Method II and III were 3–16% and 30–45% more than the Method I. Montero et al. (1990) have obtained 70% ASC yield from the muscle of hake and trout while Yamaguchi et al. (1976) could extract only 55%. In our study, only 46.48 to 50.24% of ASC alone could be obtained from the muscle of leather jacket.
Fig. 1.
Yield (%) of collagen from skin, bone and muscle of leather jacket (ASC acid soluble collagen; PSC pepsin soluble collagen)
Significant differences existed among the yields of collagen obtained by Method I, II and III (P < 0.05), except between the yields obtained by Method I and II from muscle (P > 0.05). An increase in the collagen yield was because of the addition of pepsin with acid which had completely solubilized the collagen, which was not entirely soluble in 0.5 mol/L acetic acid. Such increase in the solubility of collagen after pepsin treatment was also reported to increase by many authors (Sivakumar et al. 2000; Ogawa et al. 2004; Senaratne et al. 2006; Skierka and Sadowska 2007). Senaratne et al. (2006) have reported that the yield of collagen was increased to 54.3% with the addition of 10% (w/v) pepsin in the brown backed toadfish skin.
The relative distribution of acid and pepsin soluble collagen was understood from the yields obtained by the Method II. The yields of ASC and PSC from muscle of leather jacket were 21% and 28%, respectively. The relative distribution of the collagen fractions in Atlantic salmon muscle was reported to be 23.7% for ASC, 70.5% for PSC and 5.8% for in-soluble collagen (ISC) (Aidos et al. 1999) and such higher yield was not obtained in our study. The yields of collagen obtained from the different parts of leather jacket also showed variation. There were no significant variations in the yields of collagen obtained from skin and bone by Method I and II (P > 0.05), while that obtained from muscle was lower than the other two parts (P < 0.05). Similarly, the yields of PSC obtained by Method III showed significant differences among skin, bone and muscle (P < 0.05). The bone and the skin yielded more collagen than the muscle. Nagai and Suzuki (2000a) obtained 40.1 to 53.6% yield from the bones of skipjack tuna, Japanese seabass, ayu, yellow seabream and horse mackerel, which was quite lower than that obtained with leather jacket. The variations in the yields of collagen obtained by several authors could be due to the variations in the extraction conditions such as homogenization, shaking, mixing, initial acid solubilization and duration.
Electrophoretic characterization of collagen
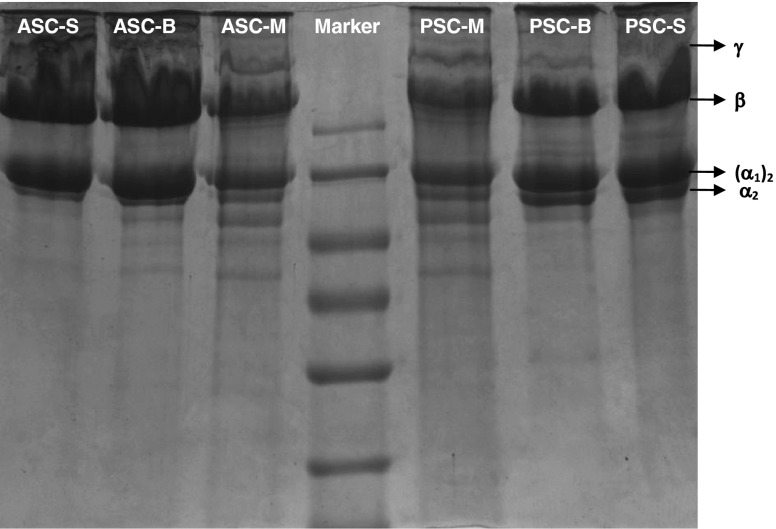
The electrophoretic pattern of ASC and PSC of skin, bone and muscle are given in Fig. 2. Inter- and intra muscular cross-linked components of collagen are β dimmers and γ trimmers. The ASC and PSC of leather jacket contained small quantity of γ trimmers and high proportions of α monomers and β dimmers similar to that reported by Yamaguchi et al. (1976). The γ chain had high molecular weight of approximately 200 kDa; while β and α chain had molecular weights below 200 kDa. The presence of β-component confirmed that the collagen contain more inter- molecular cross-links. The γ component had the ability to renature the native collagen and their presence indicated that the three chains of collagen are intra-molecularly cross-linked (Lewis and Piez 1964).
Fig. 2.
SDS-PAGE separation of skin, bone & muscle of ASC and PSC of the leather jacket (ASC acid soluble collagen; PSC pepsin soluble collagen; S skin; B bone; M muscle)
Besides the cross-linked chains, skin and bone collagens consisted two distinct α bands corresponding to α1 and α2 components, which is typical of Type I collagen. Electrophoretic pattern of bone collagen was similar to that of skin collagen. Muyonga et al. (2004) characterized the Type I skin collagen of Nile perch which consisted of two α1 and one α2 chains. The molecular weights of α subunits were 120 KDa for α1 and 115 KDa for α2. Skin and bone collagens extracted from the marine fish such as black drum and sheepshead also had similar electrophoretic patterns with molecular weights of α1 and α2 fractions as 130 and 110 kDa, respectively (Ogawa et al. 2004). Collagen extracted from skin of Baltic cod also had α chains with molecular weight below 116 kDa (Skierka and Sadowska 2007). It was apparent from the molecular weights obtained for α1 and α2 subunits that the skin and bone collagens of leather jacket were typical of Type I collagen.
Presence of another type of collagen was also documented by Kimura et al. (1991) from the scale and bone of carp which consisted of (α1)2α2 as a main component and α1α2α3 as a minor component. The electrophoretic pattern of skin and bone collagen of leather jacket also showed a slight presence of α3 subunit, as a minor component. As the α3 subunit migrates as similar as the way of migration of α1 (Kimura 1992), it is very difficult to distinguish by the normal electrophoretic procedures.
The muscle collagen showed a distinct pattern with three α chains viz. α1, α2 and α3 and their molecular weights were 125, 120 and 105 KDa, respectively. The molecular weight of α1 chain of muscle collagen corresponded with α1 chain of skin and bone collagens. But, the molecular weights of α2 and α3 chains were distinct. Sato et al. (1989) also observed a similar pattern with three α chains in fish muscle and identified it as Type V collagen. They have also reported the existence of two molecular forms of (α1)2α3 and α1α2α3 typical of Type V collagen in the ordinary muscle (Sato et al., 1994). Although Type V collagen was the major component in the muscle of fishes, Yata et al. (2001) identified it as a minor component in the skin of horse mackerel, yellow seabream and tiger puffer. Leather jacket muscle therefore contained Type V collagen as major component with α1α2α3 chains and Type I collagen as a minor component with (α1)2α2 chains and further characterization with column chromatography could help to identify the presence of minor proportion of other types of collagen.
Amino acid composition of collagen
The amino acid composition of skin, bone and muscle collagen was given in Table 2. Glycine was the major amino acid with 352.0–356.8 residues/1,000 residues. The other major amino acids were proline (90.3–103.1 residues/1,000 residues), alanine (95.1–95.9 residues/1,000 residues) and hydroxyproline (71.1–88.2 residues/1,000 residues). Fish collagen with similar amino acid compositions were also observed by many authors (Muyonga et al. 2004; Ogawa et al. 2004; Kittiphattanabawon et al. 2009). Amino acids, tryptophan and cysteine were totally absent in leather jacket collagen. Absence of cysteine was also reported by Sivakumar et al. (2000) in the skin collagen of catfish.
Table 2.
Amino acid composition of the skin, bone and muscle collagen of leather jacket
| Amino acids | Residues/1,000 total residues | ||
|---|---|---|---|
| Skin collagen | Bone collagen | Muscle collagen | |
| Hydroxyproline | 71.1 | 87.2 | 88.2 |
| Aspartic acid | 55.2 | 51.5 | 56.8 |
| Threonine | 29.4 | 24.7 | 24.2 |
| Serine | 34.3 | 29.6 | 28.6 |
| Glutamic acid | 45.3 | 35.8 | 36.3 |
| Proline | 90.3 | 103.1 | 102.2 |
| Glycine | 354.9 | 356.8 | 352.0 |
| Alanine | 95.9 | 95.1 | 94.9 |
| Valine | 12.3 | 12.1 | 12.3 |
| Methionine | 7.0 | 11.2 | 10.9 |
| Isoleucine | 12.8 | 11.5 | 11.6 |
| Leucine | 20.8 | 17.2 | 18.1 |
| Tyrosine | 26.5 | 24.1 | 24.7 |
| Phenylalanine | 28.7 | 25.8 | 25.2 |
| Histidine | 7.5 | 10.5 | 10.6 |
| Lysine | 56.0 | 52.8 | 51.4 |
| Arginine | 52.0 | 51.0 | 52.0 |
| Total | 1,000 | 1,000 | 1,000 |
| Imino acids | 161.4 | 190.3 | 190.4 |
The hydroxyproline content is in general lower in fish collagen than animal collagen, which was around 40–80 residues/1,000 amino acid residues in fish as compared to 100–130 residues/1,000 amino acid residues in meat (Sikorski et al. 1984). The hydroproline content of leather jacket was slightly higher 71–88 residues/1,000 residues than the reported values. Proline content was in general higher than hydroxyproline content in the fish collagen (Kimura et al. 1988; Yata et al. 2001) and the same was also observed in our study. The degree of hydroxylation is very useful, as it maximizes-cross-linking capacity which in turn improves the functional capacity (Montero et al. 1990).
The total imino acid content (proline and hydroxyproline) of the collagen is another important index to grade the collagen. The imino acid contents in the skin, bone and muscle collagens were 161 residues/1,000 residues, 190 residues/1,000 residues and 191 residues/1,000 residues, respectively. These contents were lower than that reported for other marine collagen obtained from shark skin having 204–207 residues/1,000 residues; sheepshead and blackdrum bone and scale having 194–200 residues/1,000 residues and Nile perch skin having 192–200 residues/1,000 residues (Kittiphattanabawon et al. 2009; Ogawa et al. 2004; Muyonga et al. 2004). These differences amongst the species were associated with the difference in the living environments, particularly habitat temperature. However, lower imino acid contents were also reported in Indian catfish skin with 173–182 residues/1,000 residues and muscle collagen with 146–151 residues/1,000 residues by Sivakumar et al. (2000). Similarly, toad fish skin collagen also contained lower imino acid content of 170 residues/1,000 residues (Senaratne et al. 2006). These fishes are obtained from Asian waters and hence have lower imino acid content which could be well correlated with the living environments. The imino acid content is also related to the denaturation temperature of the collagen. Higher imino acids contributed for higher Td values (Muyonga et al. 2004) and hence, bone and muscle collagens of leather jacket were expected to have higher Td values.
Denaturation temperatures
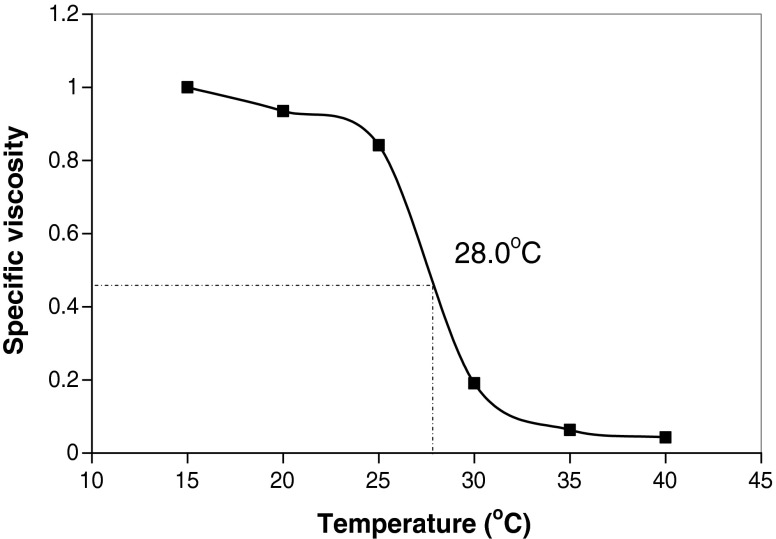
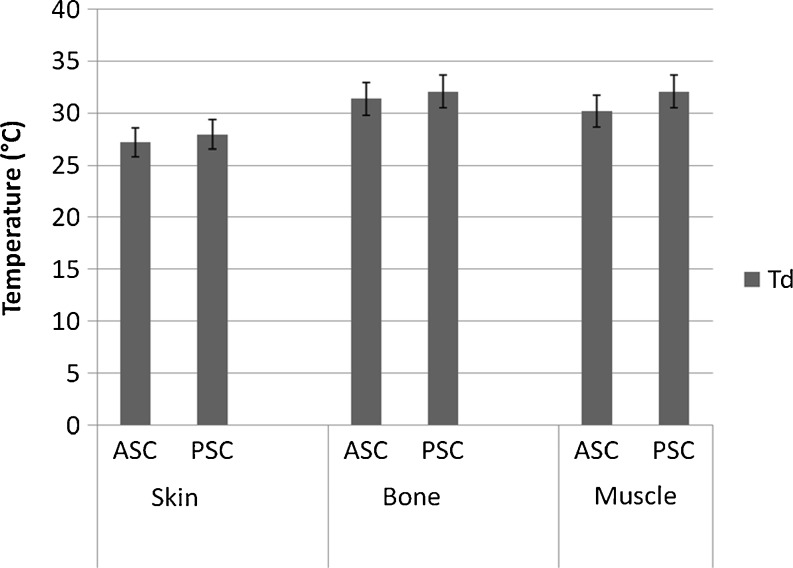
Denaturation temperature (Td) values were constructed from the specific viscosities (Fig. 3). Collagen molecule becomes ruptured at the Td and gets converted into elements of lower molecular weights. The specific viscosities started decreasing between 25 and 30 °C in skin collagen and between 30 and 35 °C in bone and muscle collagens. The Td values of skin collagen ranged from 27 to 28 °C, bone collagen from 31 to 32 °C and muscle collagen from 30 to 32 °C. Figure 4 gives the Td values of ASC and PSC extracted from different parts of fish. The Td values of the ASC were in general 1–2 °C lower than the PSC.
Fig. 3.
Denaturation temperatures of PSC of skin of leather jacket (PSC pepsin soluble collagen)
Fig 4.
Denaturation temperatures of ASC and PSC of the skin, bone and muscle of leather jacket (ASC acid soluble collagen; PSC pepsin soluble collagen)
The thermal stability of collagen was known to vary with the fish species (Kimura et al. 1988). Average Td values for the collagen obtained from common mackerel skin (26.1 °C), skipjack tuna skin (29.7 °C), ayu (29.7 °C) and brown backed toad skin (28 °C) were reported (Nagai and Suzuki 2000b; Senaratne et al. 2006). Very high Td values of 36 °C were also reported for the collagen obtained from the Nile perch skin and 34 °C from the black drum and sheepshead bones (Muyonga et al. 2004; Ogawa et al. 2004).
The Td values of collagen extracted from different parts of fish also showed variation. The Td values of bone and muscle collagen were higher (P < 0.05) than skin collagen by 3 or 4 °C. Nagai and Suzuki (2000b) also observed that the Td values of bone collagen were higher than those of skin collagen of skipjack tuna and yellow seabream. Bone and muscle being internal parts of the fish do have higher body temperatures than the skin and had contributed for higher Td values. Rigby (1968) had also demonstrated that the Td value of collagen was more correlated with the animal body temperature and environmental temperature of their habitat.
Earlier, Piez and Gross (1960) have first reported that the Td value of fish collagen was proportional to the hydroxyproline content The decrease in the thermal shrinkage with the hydroxyproline content had led to the suggestion that the hydroxy group of hydroxyproline had an important and unique role in the maintenance of the native structure. The hydroxy group of hydroxyproline was found to get involved in intermolecular rather than intramolecular cross linking (Piez and Gross 1960). The pyrrolidine rings of imino acids play an important role in the intramolecular stability of collagen by virtue of their ability to stabilize the secondary structure, whereas intermolecular strength may be in part the result of hydrogen bonding by the hydroxyl groups of hydroxyproline, serine, threonine and hydroxylysine. Therefore, the molecular structure of collagen was maintained in large part by restrictions in changes in the secondary structures of the polypeptide chain imposed by the pyrrolidine rings of proline and hydroxyproline rather than by hydrogen bonding through the hydroxyl groups of hydroxyproline (Piez and Gross 1960).
It was later described that the higher Td value for collagen was attributed to higher imino acid content rather than hydroxyproline and environmental temperature (Muyonga et al. 2004). It would be therefore more appropriate to relate the Td values of collagen with the imino acid content. Wong (1989) stated that the stable helical structure of collagen molecule was due to higher imino acid content. Piez and Gross (1960) also noticed that the cold water fish had lower Td values since the imino acid contents were very low. In this study also, the lower Td values of skin collagen was due to the lower imino acid contents compared to bone and muscle collagens. It was inferred that the stability of collagen could be a function of the total imino acids content and not necessarily related to hydroxyproline, environmental or animal body temperatures.
Conclusions
It was concluded that high collagen yield could be obtained following the extraction using acetic acid with pepsin. The collagen obtained from the leather jacket was predominantly Type I collagen, which has got applications in various fields. The higher heat stability of collagen helps them to be used as an alternative source for animal collagen that has restrictions for usage in developed countries. Suitability of tropical fish collagen in the preparation of biomedical and pharmaceutical products still needs exploration.
Acknowledgements
The authors thank the Dean, Fisheries College and Research Institute, Tuticorin for his encouragement and for providing the necessary facilities to undertake this work. This study was a part of M.F.Sc thesis submitted by the first author to the Tamil Nadu Veterinary and Animal Sciences University, Chennai, India. We are grateful to the Director, Central Institute of Fisheries Technology, Kochi, India for carrying out the amino acid analysis of the sample.
References
- Aidos I, Lie O, Espe M. Collagen content in farmed Atlantic salmon (Salmo salar L.) J Agric Food Chem. 1999;47:1440–1444. doi: 10.1021/jf980761+. [DOI] [PubMed] [Google Scholar]
- Official methods of analysis. 16. Washington: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Kimura S. Wide distribution of the skin type I collagen α3 chain in bony fish. Comp Biochem Physiol. 1992;102B:780–786. doi: 10.1016/0305-0491(92)90119-c. [DOI] [PubMed] [Google Scholar]
- Kimura S, Zhu X, Matsui R, Shijoh M, Takamizawa S. Characterization of fish muscle Type I collagen. J Food Sci. 1988;53(5):1315–1318. doi: 10.1111/j.1365-2621.1988.tb09266.x. [DOI] [Google Scholar]
- Kimura S, Mayauchi Y, Uchida N. Scale and bone Type I collagens of carp (Cyprinus carpio) Comp Biochem Physiol. 1991;99B:473–476. doi: 10.1016/0305-0491(91)90073-m. [DOI] [PubMed] [Google Scholar]
- Kittiphattanabawon P, Benjakul S, Visessanguan W, Kishimura H, Shahidi F. Isolation and characterization of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum) Food Chem. 2009;119(4):1519–1526. doi: 10.1016/j.foodchem.2009.09.037. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis MS, Piez KA. The characterization of collagen from the skin of the dogfish shark, Squalus acanthias. J Biol Chem. 1964;239(10):3336–3340. [PubMed] [Google Scholar]
- Montero P, Borderias J, Turnay J, Leyzarbe MA. Characterization of hake (Merluccius merluccius L.) and trout (Salmo irideus Gibb) collagen. J Agric Food Chem. 1990;38(3):604–609. doi: 10.1021/jf00093a004. [DOI] [Google Scholar]
- Montero P, Alvarez C, Marti MA, Borderias AJ. Plaice skin collagen extraction and functional properties. J Food Sci. 1995;60(1):1–3. doi: 10.1111/j.1365-2621.1995.tb05593.x. [DOI] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Characterization of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus) Food Chem. 2004;85(1):81–89. doi: 10.1016/j.foodchem.2003.06.006. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Preparation and characterization of several fish bone collagens. J Food Biochem. 2000;24(5):427–436. doi: 10.1111/j.1745-4514.2000.tb00711.x. [DOI] [Google Scholar]
- Nagai T, Suzuki N. Isolation of collagen from fish waste material—skin, bone and fins. Food Chem. 2000;68(3):277–281. doi: 10.1016/S0308-8146(99)00188-0. [DOI] [Google Scholar]
- Ogawa M, Portier RJ, Moody MW, Bell J, Schexnayder MA, Losso JN. Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocepahlus) Food Chem. 2004;88(4):495–501. doi: 10.1016/j.foodchem.2004.02.006. [DOI] [Google Scholar]
- Piez KA, Gross J. The amino acid composition of some fish collagens: the relation between composition and structure. J Biol Chem. 1960;235(4):995–998. [PubMed] [Google Scholar]
- Pigott GM. Surimi: the high tech raw material for fish flesh. Food Rev Int. 1986;2:213–246. doi: 10.1080/87559128609540796. [DOI] [Google Scholar]
- Pranoto Y, Lee CM, Park HJ. Characterization of fish gelatin films added with gellan and κ-carrageenan processing of fish. Int J Food Sci Technol. 2007;40(5):301–343. [Google Scholar]
- Rehn M, Veikkola T, Kukk-valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostain with integrins implicated in angiogenesis. Proc Natl Acad Sci USA. 2001;98:1024–1029. doi: 10.1073/pnas.98.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby BJ. Amino-acid composition and thermal stability of the skin collagen of the Antarctic ice-fish. Nature. 1968;219:166–167. doi: 10.1038/219166a0. [DOI] [PubMed] [Google Scholar]
- Sato K, Yoshinaka R, Sato M, Ikeda S. A simplified method for determining collagen in fish muscle. Bull Jpn Soc Sci Fisheries. 1986;52(5):889–893. doi: 10.2331/suisan.52.889. [DOI] [Google Scholar]
- Sato K, Yoshinaka R, Sato M, Shimizu Y. Collagen content in the muscle of fishes in association with their swimming movement and meat texture. Bull Jpn Soc Sci Fisheries. 1986;52(9):1595–1600. doi: 10.2331/suisan.52.1595. [DOI] [Google Scholar]
- Sato K, Yoshinaka R, Itoh Y, Sato M. Molecular species of collagen in the intramuscular connective tissue of fish. Comp Biochem Physiol. 1989;92B:87–91. [Google Scholar]
- Sato K, Sakuma K, Ohtsuki K, Kawabata M. Subunit composition of eel (Anguilla japonica) type V collagen: evidence for existence of a novel fourth α4 (V) chain. J Agric Food Chem. 1994;42:675–678. doi: 10.1021/jf00039a014. [DOI] [Google Scholar]
- Senaratne LS, Park P, Kim S. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresource Technol. 2006;97(2):191–197. doi: 10.1016/j.biortech.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Seafood processing by-products. In: Shahidi F, Botta JR, editors. Seafoods chemistry, processing, technology and quality. Glasgow: Blackie Academic Professional; 1994. pp. 11–26. [Google Scholar]
- Sikorski ZE, Scott DN, Bussion DH. The role of collagen in the quality and processing of fish. CRC Crit Rev Food Sci Nutr. 1984;20(4):301–338. doi: 10.1080/10408398409527393. [DOI] [PubMed] [Google Scholar]
- Sivakumar P, Arichandran R, Suguna L, Mariappan M, Chandrakasan G. The composition and characteristics of skin and muscle collagens from a freshwater catfish grown in biologically treated tannery effluent water. J Fish Biol. 2000;56:999–1012. [Google Scholar]
- Skierka E, Sadowska M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua) Food Chem. 2007;105(3):1302–1306. doi: 10.1016/j.foodchem.2007.04.030. [DOI] [Google Scholar]
- Sudeepa ES, Rashmi HN, Selvi AT, Bhaskar N. Proteolytic bacteria associated with fish processing waste: isolation and characterization. Int J Food Sci Technol. 2007;44(3):281–284. [Google Scholar]
- Trevitt CR, Singh PN. Variant Creutzfeldt–Jakob disease: pathology, epidemiology and public health implications. Am J Clin Nutr. 2003;78:651S–656S. doi: 10.1093/ajcn/78.3.651S. [DOI] [PubMed] [Google Scholar]
- Wong DWS. Mechanism and theory in food chemistry. New York: Van Nostrand Rehinhold; 1989. [Google Scholar]
- Yamaguchi K, Lavety J, Love RM. The connective tissues of fish VIII. Comparative studies on hake, cod and cat fish collagens. Int J Food Sci Technol. 1976;11(4):389–399. doi: 10.1111/j.1365-2621.1976.tb00737.x. [DOI] [Google Scholar]
- Yata M, Yoshida C, Fujisawa S, Mizuta S, Yoshinaka R. Identification and characterization of molecular species of collagen in fish skin. J Food Sci. 2001;66(2):247–251. doi: 10.1111/j.1365-2621.2001.tb11325.x. [DOI] [Google Scholar]