Abstract
This study was designed to optimize drying and inactivation of heat-labile inhibitors conditions of soybean by using a fluidized bed dryer, in order to shorten treatment time and to reduce losses in end-product quality such as soy flour color and soy protein solubility. The independent variables were initial moisture of soybeans, heating time and temperature of air entering the fluidization chamber. The response variables studied were final moisture of soybeans, inactivation of urease, soy flour color and soy protein solubility. Response surface methodology was able to model the response of the different studied variables. For each response group, relevant terms were included into an equation; the behavior of response was predicted within the experimental area and was presented as a response surface. The results suggested that a combination of soybean initial moisture of 0.14 g/g (wb), treatment time of 3.4 min and hot-air temperature of 136.5 °C could be a good processing combination of parameters for heating soybean using hot-air in order to reduce treatment time and quality losses in soybean flour. Thus, fluidized bed drying technology may be used as an alternative industrial method to eliminate the antinutritional factors.
Keywords: Soybean quality, Fluidized bed drying, Processing optimization, Heat-labile inhibitor inactivation
Introduction
Soybean is widely used in human and animal nutrition because of its favorable agronomic characteristics, relatively low price, high content and quality of its proteins and oil. Moreover, proteins’ functional properties are significant for the development of different types of foods for human beings. It has been reported that soy consumption has beneficial effects on nutrition and health. These effects include lowering of plasma cholesterol, prevention of cancer, diabetes, and obesity, and protection against bowel and kidney disease (Friedman and Brandon 2001).
New soy foods are continually being developed including cheese, drinks, salami and textured soy protein. Inclusion of soy products has been applied for developing protein enriched foods, as gluten free breads, wheat breads, starch and meat based products, and pasta (Nunes et al. 2003; Marco and Rosell 2008; Ribotta et al. 2010; Martínez et al. 2009). Soybean proteins, particularly, are used in many different ways in human food, including infant formulas, flours, protein isolates and concentrates, and textured fibers.
However, the presence of several antinutrients, mainly protease inhibitors and lectins, reduces the nutritional value of soybean. Protease inhibitors are protein molecules, which have the ability to inhibit the activity of proteolytic enzymes within the alimentary tract. In raw soybeans these account for about 6% of the protein content and their main constituents are the heat labile Kunitz inhibitors and the heat stable Bowman-Birk inhibitor. The first inhibits mainly trypsin and the second inhibits both trypsin and chymotrypsin (Liener 1994). Processing of soybean improves its digestibility and destroys much of the inhibitors, making soy foods edible and altering texture and flavor. The most common method for protease inhibitors destruction is heat treatment, which results in their denaturation. The degree of denaturation depends on the extent and severity of the treatment. On the other hand, such treatments cause undesirable changes. These include loss of available lysine, formation of unnatural amino acids from protein-carbohydrate browning reactions and of cross-linked amino acids (Faldet et al. 1992; Friedman and Brandon 2001; Žilic et al. 2006; Agrahar-Murugkar and Jha 2010).
Fluidized bed heating systems leads to a rigorous mixing between solids and media, enhancing heat and mass transfer rates. The relevant characteristic of fluidized bed dryers is that particles are suspended in the medium and move randomly like a fluid. Osella et al. (1997) and Soponronnarit et al. (2001) reported that the fluidized bed appears to be an adequate procedure for the fulfillment of both soybean drying and inactivation of urease and trypsin inhibitor. Wiriyaumpaiwong et al. (2004) studied the performance of four different heating techniques, regarding moisture reduction, urease inactivation, protein solubility and lysine content. They concluded that soybeans treated by spouted bed, extruders, fluidized bed and infrared radiation showed that each heating treatment technique appears to be capable of satisfactorily inactivating urease, but provides radical differences in the degree of proteins denaturation and aggregation and, consequently, in protein solubility. Later, Prachayawarakorn et al. (2006) investigated drying characteristics and inactivation of urease in soybean dried by superheated-steam and hot-air fluidized beds. They found that urease enzyme was inactivated, along with maintaining protein solubility and lysine content being in standard range, as soybean was treated at temperatures between 135 and 150 °C for the hot-air fluidized bed. They also pointed that dry soybean should be added with a certain amount of water (<200 g/kg dry matter) in order to accelerate the enzymatic inactivation before the large amount of protein insolubilization is developed.
According to the references described above, the fluidized bed hot air is an alternative method to eliminate the antinutritional factors in soybean along with maintaining protein solubility. The optimal processing conditions, as time, temperature and soybean moisture, are very important to accomplish these tasks; therefore the aim of this study was to optimize processing conditions for fluidized bed drying/inactivation of soybeans, in order to reduce losses in flour quality.
Materials and methods
Material
Raw soybean was purchased from local market. Soybean grains presented an initial moisture content of ~0.07 g/g wet matter (moisture was determined at 105 °C to constant weight).
The soybean was rewetted from its delivery moisture and then equilibrated: a measured amount of water (to reach the final moisture content from 0.073 to 0.207 g/g wb) was added to the samples, the mixtures were put in hermetic plastic containers and were left in a cool room at 6–8 °C for 5–7 days to ensure uniform moisture content through the kernels. Before processing, water content of soybean samples were checked, grains were taken out of cold storage, and left in environmental condition until grains reached room temperature.
Heat treatment
Soybean samples (300 g) were treated in a laboratory fluidized bed dryer (model FT31, Armfield Limited, England). The cylindrical chamber was 30 cm height and had an internal diameter of 20 cm. Air was drawn through a mesh filter and blown by a centrifugal fan over an electrical heater and through a stainless steel filter gauze before being delivered to the distributor gauze at the base of the chamber, which holds the fluidized bed and distributes the air uniformly. A filter bag was placed on the top of the cylindrical chamber to retain stray particles. Temperatures were measured using K type thermocouples connected to a data logger with an accuracy of ±1 °C. Air speed was measured at different points of the cylinder by a wind speed anemometer (an accuracy of 0.1 m/s).
Samples were load in the fluidization chamber after the temperature reached the steady state. Then they were removed at prefixed times and cooled at room temperature. A constant air velocity of 1.9 m/s was used. Gas bubbles were produced above the distributor and moved to the bed surface, thus soybean moved freely. Soponronnarit et al. (2001) modeled the drying rate of soybean in fluidized bed dryer and tested air speeds between 2.4 and 4.1 m/s, and concluded that the minimum superficial air speed required for fluidization was 1.9 m/s.
In order to study the effects of the processing parameters on soybean quality the following factors were selected as independent variables: soybean initial moisture (0.073–0.207 g/g wb) (Xtw), treatment time (3–15.1 min) and temperature of the air entering the fluidization chamber (113–147 °C). Levels of design variables were selected on the basis of previous experiments and cited references.
The response variables studied, related to soybean quality, were soybean final moisture, inactivation of urease, soy flour color and soy protein solubility. After each treatment, soybean samples were left to cool, milled using a blade mill and sifted through a No. 40 US standard sieve to achieve soy flour for further analysis.
Soybean quality
Moisture content
The moisture content of soybean flour after treatment (Xft) was determined by drying in an electrical oven at 105 °C to constant weight. Each sample was analyzed in duplicate.
Urease activity
It was determined by the AACC method 22–90 (AACC 1995). It measures the change in pH resulting from the action of urease converting urea to ammonia. The pH difference value was measured by the CG 837 pH meter (Schott, Germany). Urease activity was expressed as pH difference. The urease level was used to evaluate under processing conditions and, consequently, the presence of toxic factors such as trypsin inhibitors (Araba and Dale 1990). Each sample was analyzed in duplicate.
Flour color
It was determined with a Minolta 508 d spectrophotometer (D65 illuminant, and 10º angle of observer). Readings were recorded as CIELAB, L*, a*, and b* values. The coordinates of L*, a* and b* serve to define the location of any color in the uniform color space. L* (lightness) component closely matches human perception of lightness of the color (L* = 0 yields black and L* = 100 indicates white), and a* (redness) component represents the position between red/magenta and green, and b* (yellowness) component represents the position between yellow and blue. At least three readings were taken from each milled sample.
Protein solubility
Soluble protein was measured by dispersion of soybean meal in 50 mM phosphate buffer (pH 7.0) for 30 min (samples were stirred each 5 min). Then the samples were centrifuged at 14.000 g for 10 min to remove insoluble material. The protein content of the supernatants was determined using the Bradford protein assay procedure. The solubility of the protein, expressed as a percentage (DPS%) was calculated by dividing the protein content of the extracted solution (PS) by the protein content of the original soybean sample (determined by AACC 46–13 Micro Kjeldhal Method, AACC 1995). Each sample was analyzed in triplicate.
Statistical methods
A rotatable central composite with three factors and five levels was generated using response surface regression procedures (Statgraphics plus 5.0). The center point in the design was repeated four times to calculate the repeatability of the method (Montgomery 2001). The results were analyzed by multiple regression method. Quality of the models fitness was evaluated by ANOVA (Statgraphics plus 5.0). The experimental results were applied to obtain the regression models. The fit of model to the experimental data was given by the coefficient of determination, R2, which explains the extent of the variance in a modeled variable that can be explained with the model. Multiple regression equations included only significant coefficients (p < 0.05). The simplest models (linear or quadratic) with high coefficient of determination (>80) were included in this study. Three-dimensional response surface plots were generated for each quality parameter. Calculation of optimal processing parameters for fluidized bed drying of soybeans was performed using multiple response method called desirability (Ferreira et al. 2007). This optimization method incorporates desires and priorities for each of the variables.
Results and discussion
The assays were performed according to the experimental design and soybean flour quality parameters for the processing factor combination were determined at each experimental point (Table 1). Coefficient of determination (R2) is the proportion of variation in the response attributed to the model that is for which the model accounts. For each response group a linear or quadratic equation was formed with relevant terms (p < 0.05) to obtain coefficients of determination higher than 80% and the simplest possible model. Based on these equations, behavior of response may be predicted within the experimental area and presented as a response surface.
Table 1.
Central composite design arrangement and experimental result for the respo-e variables of heat treated soybean
| Independent variables | Dependent variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Xtw | Timea | Tb | Xfw | Urease | L* | a* | b* | PS | DPS% |
| 0.073 | 7.5 | 130 | 0.055 ± 0.004 | 0.28 ± 0.02 | 88.48 ± 1.68 | 0.43 ± 0.01 | 20.23 ± 0.40 | 0.020 ± 0.001 | 17.09 |
| 0.100 | 3.0 | 140 | 0.062 ± 0.006 | 0.50 ± 0.03 | 87.43 ± 0.88 | −0.57 ± 0.90 | 19.70 ± 0.90 | 0.028 ± 0.001 | 23.45 |
| 0.100 | 3.0 | 120 | 0.072 ± 0.003 | 2.06 ± 0.15 | 87.62 ± 1.14 | −0.45 ± 0.00 | 21.11 ± 0.63 | 0.061 ± 0.002 | 51.96 |
| 0.100 | 12.0 | 120 | 0.058 ± 0.001 | 1.76 ± 0.09 | 88.04 ± 0.44 | 0.00 ± 0.00 | 20.69 ± 0.10 | 0.023 ± 0.001 | 19.26 |
| 0.100 | 12.0 | 140 | 0.046 ± 0.003 | 0.07 ± 0.01 | 84.21 ± 1.68 | 2.49 ± 0.05 | 22.10 ± 0.44 | 0.005 ± 0.000 | 4.20 |
| 0.140 | 7.5 | 130 | 0.079 ± 0.005 | 0.25 ± 0.03 | 87.06 ± 1.39 | −0.06 ± 0.00 | 20.97 ± 0.34 | 0.027 ± 0.001 | 22.62 |
| 0.140 | 7.5 | 113 | 0.087 ± 0.004 | 2.01 ± 0.13 | 87.69 ± 0.88 | −0.60 ± 0.01 | 20.97 ± 0.21 | 0.044 ± 0.002 | 37.88 |
| 0.140 | 7.5 | 147 | 0.056 ± 0.001 | 0.02 ± 0.01 | 86.30 ± 1.04 | 1.85 ± 0.02 | 21.15 ± 0.25 | 0.003 ± 0.000 | 2.29 |
| 0.140 | 7.5 | 130 | 0.074 ± 0.007 | 0.24 ± 0.02 | 87.91 ± 1.76 | −0.26 ± 0.00 | 19.54 ± 0.39 | 0.024 ± 0.001 | 20.43 |
| 0.140 | 7.5 | 130 | 0.067 ± 0.006 | 0.24 ± 0.02 | 87.23 ± 0.44 | −0.08 ± 0.00 | 20.48 ± 0.10 | 0.024 ± 0.001 | 20.45 |
| 0.140 | 7.5 | 130 | 0.073 ± 0.007 | 0.21 ± 0.01 | 86.72 ± 1.47 | 0.08 ± 0.00 | 20.71 ± 0.37 | 0.025 ± 0.001 | 21.41 |
| 0.140 | 15.1 | 130 | 0.055 ± 0.006 | 0.15 ± 0.01 | 86.52 ± 1.21 | 0.96 ± 0.01 | 19.07 ± 0.27 | 0.016 ± 0.001 | 13.91 |
| 0.180 | 3.0 | 120 | 0.120 ± 0.005 | 1.99 ± 0.13 | 85.90 ± 0.94 | −0.84 ± 0.00 | 22.18 ± 0.24 | 0.048 ± 0.002 | 41.23 |
| 0.180 | 3.0 | 140 | 0.122 ± 0.005 | 0.26 ± 0.02 | 86.14 ± 1.72 | −0.72 ± 0.00 | 21.21 ± 0.45 | 0.031 ± 0.001 | 25.99 |
| 0.180 | 12.0 | 120 | 0.087 ± 0.003 | 1.17 ± 0.08 | 86.98 ± 1.74 | 0.15 ± 0.00 | 21.44 ± 0.43 | 0.023 ± 0.001 | 19.43 |
| 0.180 | 12.0 | 140 | 0.049 ± 0.001 | 0.05 ± 0.00 | 84.35 ± 0.93 | 2.29 ± 0.03 | 22.14 ± 0.24 | 0.001 ± 0.000 | 0.98 |
| 0.207 | 7.5 | 130 | 0.116 ± 0.003 | 0.24 ± 0.02 | 85.73 ± 0.77 | −0.21 ± 0.00 | 21.09 ± 0.19 | 0.020 ± 0.001 | 16.83 |
| 0.140 | Untreated | 0.113 ± 0.006 | 2.11 ± 0.20 | 85.80 ± 1.20 | −0.90 ± 0.01 | 23.42 ± 0.33 | 0.117 ± 0.005 | 100 | |
Xtw: initial soybean moisture (g/g); Xft: moisture content of soybean after treatment (g/g); Urease: Urease activity (pH difference); L*, a* and b*: color parameters; PS: Protein Solubility; DPS%: heat-treated sample PS x 100/non-treated sample PS; a treatment time (min); b temperature of the air entering the fluidization chamber (°C)
Moisture content
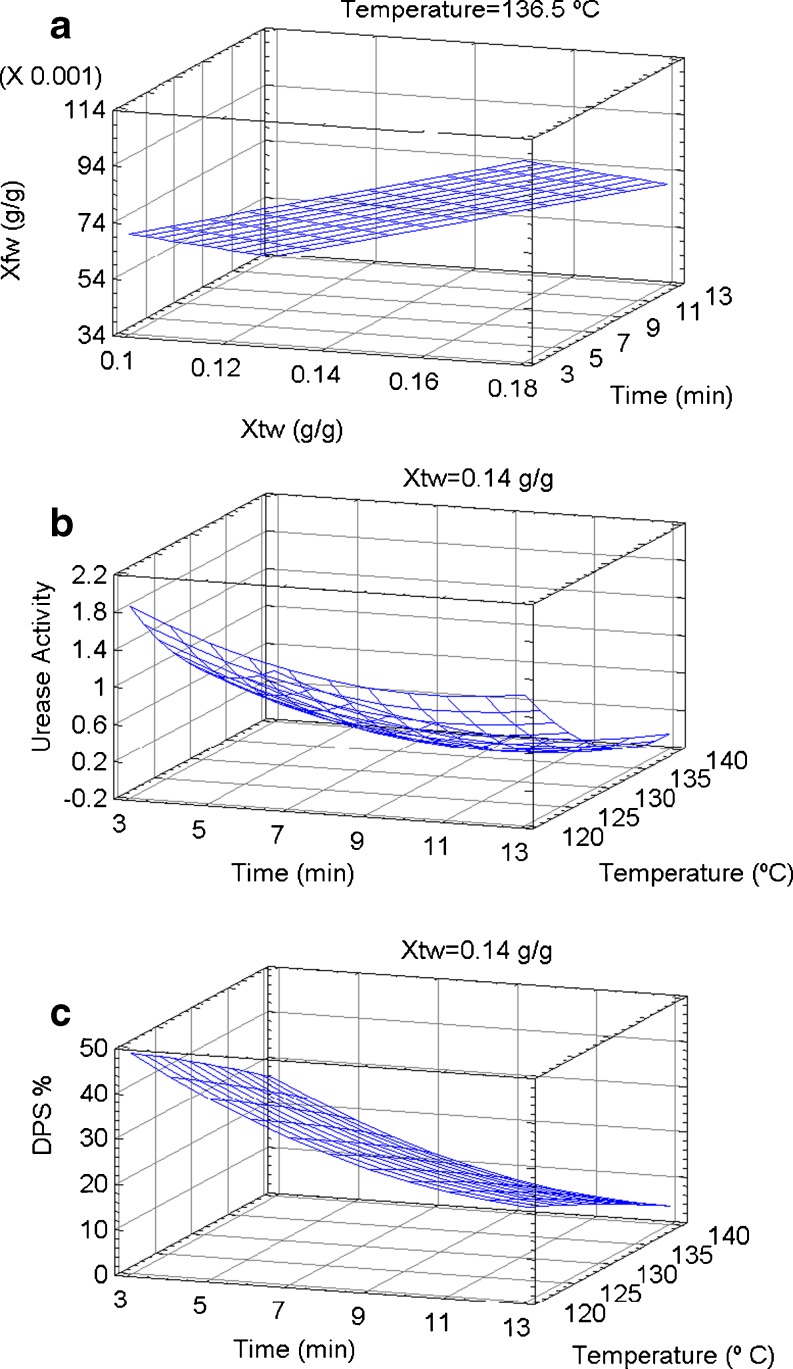
A linear model, which accounted for 83.81% of the variability in the data, could be fitted for soybean final moisture (Xfw). The significant model regression coefficients and the square coefficient of the fitting model (R2) estimated by the ANOVA analysis are shown in Table 2. As was expected, negative linear effect of treatment time and hot-air temperature and positive linear effect of soybean initial moisture were observed on Xfw (Fig. 1a).
Table 2.
Significant coefficients (95% confidence interval) of the design of the regression fitting model
| Xfw (g/g wb) | Urease (pH diff) | DPS% | L* | a* | |
|---|---|---|---|---|---|
| co-tant | 0.1460 | 71.3103 | 166.2580 | 53.1863 | 52.7471 |
| A:Xtw (g/g wb) | 0.4441** | – | – | −63.0148* | – |
| B:Time | −0.0036** | −0.3934* | −10.1995** | – | −1.4559** |
| C:Temperature | −0.0008* | −0.9658** | −0.2821** | 0.5434** | −0.7797** |
| AA | – | – | – | – | – |
| AB | – | – | – | – | – |
| AC | – | – | – | – | – |
| BB | – | 0.0124* | 0.2192** | – | – |
| BC | – | – | – | −0.0181* | 0.0129** |
| CC | – | 0.0033** | – | – | 0.0029** |
| R2 | 83.81 | 94.67 | 98.29 | 86.48 | 97.85 |
| SRE | 0.0108 | 0.2708 | 2.6167 | 0.6677 | 0.2291 |
| MAE | 0.0075 | 0.1299 | 1.4636 | 0.3495 | 0.1182 |
*, ** significant at p < 0.05 and p < 0.01 respectively; R2: square coefficient of the fitting model (indicates the percentage of variability for which the model accounts). A: Xtw, total water fraction; B: Treatment time and C: Hot-air temperature. Xfw: water fraction after drying process; DPS%: heat-treated sample PS x 100/non-treated sample PS. Urease: Urease activity. SRE: standard error of estimate, MAE: mean absolute error
Fig. 1.
Effects of treatment time and temperature on soybean final moisture (Xfw), urease activity (pH diff) and protein solubility (DPS%)
Urease activity
With no technological importance to itself, urease serves as an indicator for the adequacy of the heat treatment given to soybean meal (Parsons et al. 1991). Urease is used as an indirect indicator of the presence of antinutritional factors, such as trypsin inhibitors, that show underprocessing of soybean products. Moreover, the recommended maximum level of urease is controversial, with acceptable values varying from 0.2 or less (McNaughton et al. 1981) to 0.5 units of pH change (Waldroup et al. 1985). Acceptable values of urease activity (ΔpH) should be lower than 0.3 (Rhee 2006; CAA 2010). In the present study, non-treated soybean flour presented a ΔpH of 2.11. Regarding to the heating process, a quadratic model was fitted for urease inactivation. The coefficient of determination of urease activity accounted for 94.67% of the variability in the data, respectively (Table 2). Negative linear effect of treatment time and air temperature and positive quadratic effect of treatment time and air temperature on urease activity were provided. These results indicated that the urease activity dropped as the level of these parameters increased but the rate of inactivation was lower at higher values of treatment time and air temperature, as it is shown in surface plots presented in Fig. 1b.
The predicted values obtained from the fitted model equation (Table 2) considered as variables in Fig. 1b, showed that at 120 °C it was not possible to reach the acceptable values of urease activity (ΔpH ≤ 0.3). On the other hand, at 130 and 140 °C, it took about 6.1 and 3.8 min, respectively to reach the acceptable values of urease activity (ΔpH ≤ 0.3) and the final moisture level at these temperatures was lower to 0.11 g/g.
Color changes
Color is an important attribute of soybean flours, contributing to consumer preference. It is produced by chemical reactions including Maillard reaction and caramelisation. The Maillard reaction occurs between the free amino group of lysine and/or other amino acids and the carbonyl groups of reducing sugars such as glucose and maltose, and they are favored in systems with intermediate moisture content, temperatures >50 °C and pH 4–7. Whereas caramelisation depends on direct degradation of sugars and needs more drastic conditions (temperatures >120 °C, pH <3 or >9, and very low water activity Kroh 1994).
Non-treated soybean flour presented L*: 85.80, a*: −0.90 and b*: 23.42. Flour color changed significantly as consequence of the heating and drying process.
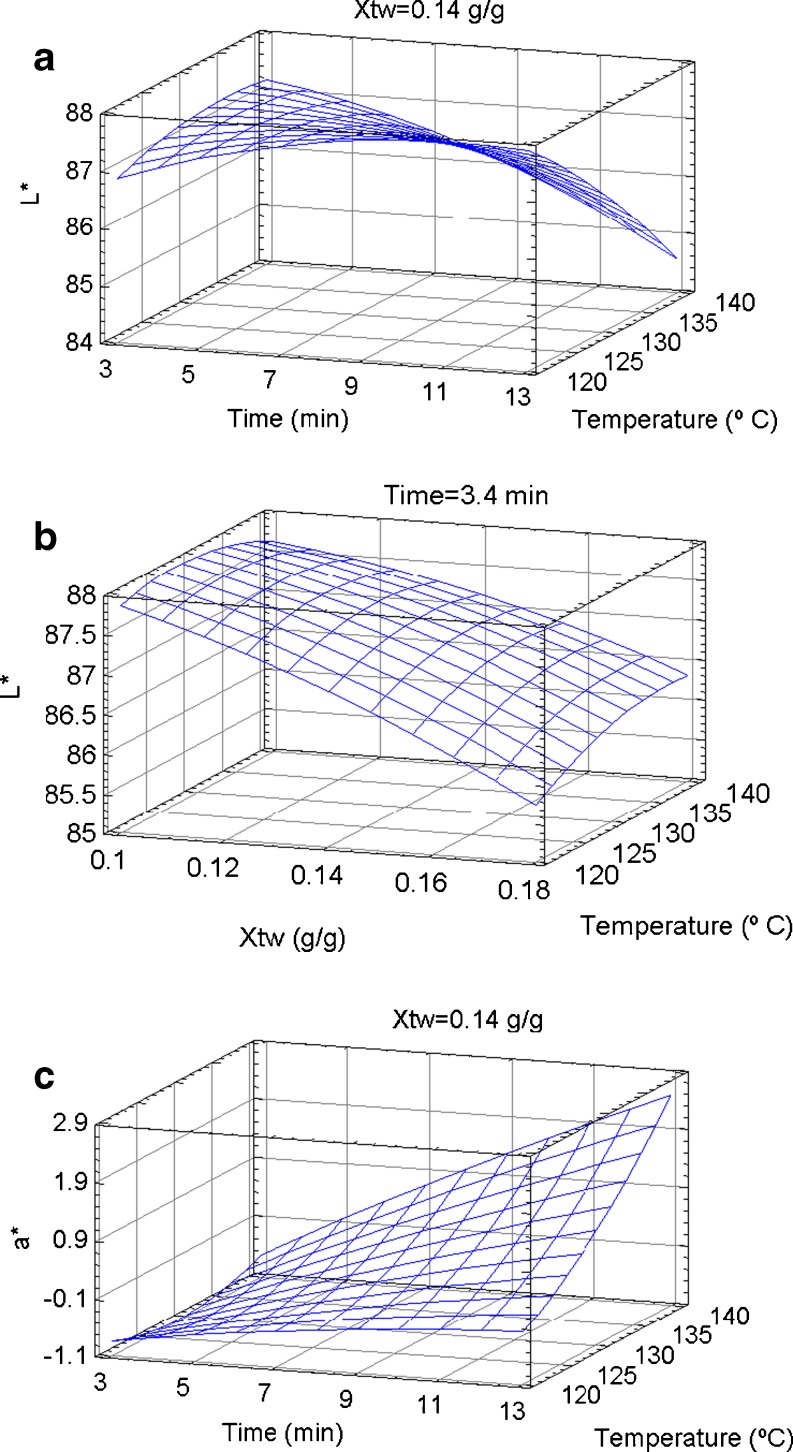
Quadratic models were fitted for L* and a* color components. Coefficients of determination L* and a* accounted for 86.47 and 97.85% of the variability in the data, respectively (Table 2). It was not possible to fit a model for b* component.
Negative linear effect of Xtw, positive linear effect of hot-air temperature and negative crossed effect of time-temperature were observed on L* component. Negative linear effect of treatment time and hot-air temperature and positive quadratic and crossed effect of hot-air temperature and time-temperature, respectively, were observed on a* component. These results indicated that lightness decreased continuously as the soybean initial moisture content increased, and the same effect had the temperature of hot-air only at higher treatment times (Fig. 2a and b). On the other hand, redness increased as consequence of the increment of treatment time and hot-air temperature, showing an increment of magenta color (Fig. 2c). Since the colors produced during Maillard reaction include from pale yellow to very dark brown, these behaviors can be attributed to soybean very high lysine content and their availability to Maillard reactions. Similar trends in L* and a* components were obtained by Rufián-Henares et al. (2009), who studied the color changes in cereal and soy flour as consequence of heating at180 °C.
Fig. 2.
Effects of processing parameters on color flour parameters. Xtw: initial soybean moisture (g/g)
Soybean protein solubility
Urease activity is useful for detecting undercooking of soybean products, but is of limited use for detecting overcooking. Both under- and over-heating result in poor quality of soybean products. Over-heating produces changes to the chemical structure of many essential amino acids resulting in a decrease of the nutritive value. The reduction in protein quality of soybean meal as the result of over processing is due to the destruction of lysine and cystine and reduced digestibility of the lysine and cystine that is not destroyed (Dudley-Cash 1999), as consequence of Maillard reactions. Protein solubility has been shown to be a good indicator of protein quality for over-processing.
Protein solubility changed significantly as consequence of heating process. A quadratic model was fitted for protein solubility loss and the coefficient of determination of the model accounted for 98.29% of the variability in the data, respectively (Table 2). Negative linear effect of hot-air temperature and treatment time, positive quadratic effect of treatment time were observed on protein solubility losses. Major negative effect on soy protein solubility was provided by hot-air temperature and treatment time (Fig. 1c). In a previous study, Ribotta et al. (2004) confirmed the decrease in protein solubility and the aggregation of protein due to the heating process by mean of size exclusion chromatography and sodium dodecyl sulphate polyacrylamide gel electrophoresis
Optimization
Extra experiments were carried out to validate the accuracy of the model, which showed to be adequately reproducible within ±10% of error. As well explaining the behavior of variables by the contour curves, the models fitted in this study could also be applied for optimization using desirability function (Ferreira et al. 2007). As was described previously, optimum processing should produce the highest level of urease inactivation, but, at the same time, to keep protein solubility and color parameters as similar to the original ones as possible. Therefore, optimization was conducted in order to maximize the urease inactivation, soy protein solubility and to minimize final soybean moisture, L* and a*; simultaneously. The results for this optimization suggested, regarding a desirability function value = 0.57, that a combination of soybean initial moisture of 0.14 g/g (wb), treatment time of 3.4 min and hot-air temperature of 136.5 °C could be a good combination of processing parameters for heating and drying of soybean using hot-air in order to reduce treatment time and end-product quality losses. The values predicted by the equations of the model for each response are: soybean final moisture 0.086 g/g (wb), urease activity (ΔpH) 0.20, L* component 87.03, a* component −0.49 and solubility of proteins, expressed as a percentage (DPS%), 24.18%.
Conclusion
The influence of soybean initial moisture and the parameters of fluidized bed drying on soybean quality characteristics were evaluated in this study. Response surface methodology was able to model efficiently the influence of soybean moisture, air temperature and heating time on soybean quality parameters. The study showed that the fluidized bed drying process was able to eliminate the antinutritional factors, minimizing losses in soy flour quality. According to the results, the fluidized bed drying process may be used before flour milling to eliminate the antinutritional factors avoiding severe flour color changes but decreasing protein solubility.
Acknowledgments
The authors would like to thank the Consejo Nacional de Ciencia y Técnica, the Secretaría de Ciencia y Tecnología, Universidad Nacional de Córdoba and the Agencia Nacional de Promoción Científica y Tecnológica for financial support.
References
- Approved methods of the AACC. St. Paul: American Association of Cereal Chemists; 1995. [Google Scholar]
- Agrahar-Murugkar D, Jha K. Effect of drying on nutritional and functional quality and electrophoretic pattern of soyflour from sprouted soybean (Glycine max) J Food Sci Technol. 2010;47(5):482–487. doi: 10.1007/s13197-010-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araba M, Dale NM. Evaluation of protein solubility as an indicator of underprocessing of soybean meal. Poultry Sci. 1990;69:1749–1752. doi: 10.3382/ps.0691749. [DOI] [Google Scholar]
- CAA (CODIGO ALIMENTARIO ARGENTINO) (2010) De la Canal y Asociados. Buenos Aires, Argentina
- Dudley-Cash WA. Methods for determining quality of soybean protein important. Feedstuffs. 1999;71:10–11. [Google Scholar]
- Faldet MA, Satter LD, Broderick GA. Determining optimal treatment of soybeans by measuring available lysine chemically and biologically with rats to maximize protein utilization by ruminants. J Nutrition. 1992;122:151–160. doi: 10.1093/jn/122.1.151. [DOI] [PubMed] [Google Scholar]
- Ferreira SLC, Bruns RE, Ferreira HS, et al. Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chem Acta. 2007;597:179–186. doi: 10.1016/j.aca.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Friedman M, Brandon DL. Nutritional and health benefits of soy proteins. J Agric Food Chem. 2001;49:1069–1086. doi: 10.1021/jf0009246. [DOI] [PubMed] [Google Scholar]
- Kroh LW. Caramelisation in food and beverages. Food Chem. 1994;51:373–379. doi: 10.1016/0308-8146(94)90188-0. [DOI] [Google Scholar]
- Liener IE. Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nut. 1994;34:31–67. doi: 10.1080/10408399409527649. [DOI] [PubMed] [Google Scholar]
- Marco C, Rosell CM. Breadmaking performance of protein enriched gluten free breads. European Food Res Tech. 2008;227:1205–1213. doi: 10.1007/s00217-008-0838-6. [DOI] [Google Scholar]
- Martínez CS, Ribotta PD, León AE, et al. Pastas elaboradas con harina de trigo pan sustituidas con harina de soja. In: Pérez GT, León AE, et al., editors. Ciencia y tecnología de los alimentos, avances en formulación y nutrición. Córdoba: Ministerio de Ciencia y Tecnología; 2009. pp. 187–197. [Google Scholar]
- McNaughton JM, Reece FN, Deaton JW. Relationships between color, trypsin inhibitor contents, urease index of soybean meal and effects on broiler performance. Poultry Sci. 1981;60:393–400. doi: 10.3382/ps.0600393. [DOI] [Google Scholar]
- Montgomery DC. Design and analysis of experiments. 5. New York: Wiley; 2001. [Google Scholar]
- Nunes MC, Batista P, Raymundo A, et al. Vegetable proteins and milk puddings. Coll Surf B: Biointerfaces. 2003;31:21–29. doi: 10.1016/S0927-7765(03)00040-7. [DOI] [Google Scholar]
- Osella CA, Gordo NA, González RJ, et al. Soybean heat-treated using a fluidized bed. Lebensm.-Wiss. u.-Technol. 1997;30:676–680. [Google Scholar]
- Parsons CM, Hashimoto K, Wedekind KJ, et al. Soybean protein solubility in potassium hydroxide: an in vitro test of in vivo protein quality. J Anim Sci. 1991;69:2918–2924. doi: 10.2527/1991.6972918x. [DOI] [PubMed] [Google Scholar]
- Prachayawarakorn S, Prachayawasina P, Soponronnarit S. Heating process of soybean using hot-air and superheated-steam fluidized-bed dryers. LWT. 2006;39:770–778. doi: 10.1016/j.lwt.2005.05.013. [DOI] [Google Scholar]
- Rhee KC (2006) Effects of processing on nutrient content of soybean meal. In Feed formulation. Technical report series. American Soybean Association. Singapore
- Ribotta PD, Ausar SF, Morcillo MH, et al. Production of gluten free bread using soybean flour. J Science Food Agric. 2004;84:1969–1974. doi: 10.1002/jsfa.1915. [DOI] [Google Scholar]
- Ribotta PD, Pérez GT, Añón MC, et al. Optimization of additive combination for improved soy-wheat bread quality. Food Biop Tech. 2010;3:395–405. doi: 10.1007/s11947-008-0080-z. [DOI] [Google Scholar]
- Rufián-Henares JA, Delgado-Andrade C, Morales FJ. Assessing the Maillard reaction development during the toasting process of common flours employed by the cereal products industry. Food Chem. 2009;114:93–99. doi: 10.1016/j.foodchem.2008.09.021. [DOI] [Google Scholar]
- Soponronnarit S, Swasdisevi T, Wetchacama S, et al. Fluidized bed drying of soybeans. J Stored Prod Res. 2001;37:133–151. doi: 10.1016/S0022-474X(00)00015-1. [DOI] [PubMed] [Google Scholar]
- Waldroup PW, Ramsey BE, Helwing HM, et al. Optimum processing for soybean meal used in broiler diets. Poultry Sci. 1985;64:2314–2320. doi: 10.3382/ps.0642314. [DOI] [Google Scholar]
- Wiriyaumpaiwong S, Soponronnarit S, Prachayawarakorn S. Comparative study of heating processes for full-fat soybeans. J Food Eng. 2004;65:371–382. doi: 10.1016/j.jfoodeng.2004.01.036. [DOI] [Google Scholar]
- Žilic S, Bozovic I, Savic S, et al. Heat processing of soybean kernel and its effect on lysine availability and protein solubility. Central Eur J Biol. 2006;1:572–582. doi: 10.2478/s11535-006-0039-x. [DOI] [Google Scholar]