Abstract
Dunaliella bardawil, a green alga accumulates high amount of β-carotene under stress conditions. This organism has been exploited for β-carotene at industrial scale. In the present work, various metabolic inhibitors like diphenylamine (DPA), nicotine, basta, glyphosate, DCMU [3–(3′,4′-dichlophenyl)-1,1-dimethylurea] and caffeine were used in autotrophic medium, to understand their influence on carotenoid biosynthesis. The results indicated that these metabolic inhibitors influenced the production of carotenoids like wise, DPA and basta increased the contents of β-carotene (1.7 fold), glyphosate and DCMU for lutein (2.4 and 2 fold) caffeine for biomass yields (1.1 fold), while nicotine decreased the biomass yield (3.6 fold), β-carotene (2 fold) and lutein (10.5 fold).
Keywords: Dunaliella bardawil, Diphenylamine, Nicotine, Glufosinate ammonium, Glyphosate, Lutein, DCMU
Introduction
Algae could be exploited for their multifunctional properties. They are sources of useful bioactive components like phycocolloids, proteins, vitamins, minerals, carotenoids like fucoxanthin and n3 fatty acids, which are known to possess nutritional value apart from their potential biomedical applications (Bhaskar et al. 2008; Kumar et al. 2008). Carotenoids are pigments, which are mainly found in plants and algae among which β-carotene, lycopene, zeaxanthin and lutein are the most prominent and commercially important ones. Carotenoids are known to be the most efficient singlet oxygen quencher in the biological systems without the production of any oxidizing products (Sachindra et al. 2010). Among the plants carrot is known for its β-carotene (Sagar 2009; Usha and Monika 2007; Singh and Kulshrestha 2008) content and tomato for its lycopene (Gunjan et al. 2009). In case of microalgae Dunaliella, a marine halotolerant unicellular eukaryotic alga is known as a source of β-carotene. Lycopene was found to be accumulated in D. salina due to the inhibition by nicotine (Fazeli et al. 2009) a lycopene cyclase inhibitor. The β-carotene rich Dunaliella can be used in food supplements to enhance nutrition quality for health benefits. β-carotene is accumulated in oil containing globules in the chloroplasts. This cellular location of the β-carotene containing globules is consistent with widely accepted hypothesis that D. bardawil accumulates β-carotene to absorb a substantial part of the excess light (in the range of 330 to 500 nm) before this light would be able to damage the photosynthetic machinery of the cells (Lamers et al. 2008). In further support of this hypothesis, it was observed that D. bardawil cells containing high levels of β-carotene were particularly protected against photoinhibition mediated by high intensity blue light or by UV-A radiation (White and Jahnke 2002).
Carotenoid biosynthesis takes place in the plastid, but all known enzymes in the pathway are nuclear encoded and post-translationally imported into the organelle (Lichtenthaler 1999). In order to understand the biosynthetic pathway of carotenogenesis, information on carotenoid intermediates is extremely important. Different stress conditions including high light and chemical stress through the addition of carotenoid inhibitors can lead to the formation of various carotenoid intermediates which are of commercial importance for application as a pigmentation source, nutraceutical, pharmaceuticals (Raja et al. 2008) and cosmetic industries (Jin et al. 2003). β-carotene in Dunaliella mainly consists of the two steroisomers 9-cis and all-trans β-carotene (Ben-amotz et al. 1982). Irradiance and salt stress (Ben-Amotz and Avron 1983) affect the β-carotene content and the ratio of the 9-cis to all-trans isomers. These compounds have antioxidant properties and have attracted attention as potential agent in prevention of cancers (Nishino et al. 2002). Carotenoid biosynthesis is governed by the level and activity of carotenoid biosynthesis enzymes. Dunaliella when exposed to stress conditions such as salinity (Fazeli et al. 2006), high light (Hejazi and Wijffels 2003), or nutrient limitation (Raja et al. 2007), two stereoisomers of β-carotene, all-trans and 9-cis may be accumulated reaching up to 10% of the dry cell weight (Ben-amotz et al. 1982). Metabolic inhibitors like glyphosate (amino acid biosynthesis inhibitor), glufosinate ammonium which is also called as basta (glutamine synthase inhibitor in nitrogen metabolism pathway), DCMU (photosynthetic inhibitor), DPA (inhibitor of β-carotene C-4 oxygenase), nicotine (lycopene cyclase inhibitor) and caffeine (cytokinesis inhibitor) were evaluated to study their effect on growth, carotenoid profile and fatty acid profile of the marine micro alga D. bardawil under two different light intensities.
Materials and methods
Culture conditions
Dunaliella bardawil strain V-101, was obtained from CAS (Centre for Advanced Studies) in Botany, University of Madras, Chennai. Liquid cultures of D. bardawil were maintained on modified AS100 medium (Vonshak 1986) with tris buffer being replaced by NaHCO3 (2 g/L). The liquid cultures were incubated under light intensity of 35.0 ± 2.5 μmol∙ m-2∙ s-1at 25 ± 1°C for 16 h. Cultures of 15 days old were harvested by centrifugation at 3500 g for 5 min, resuspended in fresh medium and used as inoculum so as to maintain initial cell count at 15 × 104 cells ml−1 in 150 ml flasks containing 40 ml of medium. The cultures were shaken manually once a day. Diphenylamine (Sigma) and DCMU (Sigma) stocks were prepared in absolute ethanol and added to culture flasks at concentrations of 14.77 mM and 4.2 mM, respectively. Aqueous stocks of (−)-nicotine (Fluka), glufosinate ammonium (basta 15 SL, 13.5% w/w Agro Evo India Limited), glyphosate (glycel 41% excel crop care limited) and caffeine (Sigma) were added separately to the cultures at a final concentration of 3.54 mM, 1.21 mM, 29.57 mM, and 3.60 mM, respectively and incubated under two light intensities of 35.0 ± 2.5 μmol m−2 s-1 and 75.0 ± 2.5 μmol m−2 s-1 at 25±1°C for 16:8 light dark cycle. A quick screening for all the metabolic inhibitors was carried out using a range of concentrations to know the inhibitory concentration for each metabolic inhibitor and further studies for each metabolic inhibitor was carried out at its IC- 50 value.
Growth measurement
Growth was measured by counting cell numbers using a haemocytometer (Thoma, Germany). The cultures were harvested by centrifugation at 5,000 rpm for 5 min. The cells were washed with distilled water and freeze dried. The dry weight of algal biomass was determined gravimetrically and growth was expressed in terms of dry weight (Vidhyavathi et al. 2008).
Chlorophyll and total carotenoid extraction and analysis
Known quantity of freeze dried biomass was extracted with 90% acetone repeatedly until the pellet becomes colourless (Vidhyavathi et al. 2009). The pooled extracts absorbance was read at 470, 450, 645 and 661.5 nm using spectrophotometer (Model UV-160 A, Shimadzu Corporation, Kyoto, Japan). Chlorophyll and total carotenoid contents were estimated by the method of Lichtenthaler 1987.
Analysis of carotenoid by HPLC
Analyses of carotenoids were performed using a reversed phase 250 × 4.6 mm C18 (Supelco) column with an isocratic solvent system consisting of acetonitrile/methanol/dichloromethane (70: 10: 20) at a flow rate of 1.0 ml/min at 450 nm as described by (Shaish et al. 1992). β-carotene and lutein were identified using authentic standards (Sigma Co, USA).
TLC separation and analysis of carotenoids
TLC plate is activated at 100°C for 45 min. The concentrated carotenoid sample dissolved in acetone solution was spotted on silica gel TLC sheet and developed with mobile phase of Acetone: Hexane (30:70) at room temperature in the dark.
Total lipids
Cells were harvested, lyophilized, weighed and biomass was extracted using a chloroform: methanol (2: l) mixture. Solvent was then evaporated under reduced pressure and the residue was dried under slight nitrogen current and total lipid was determined.
Fatty acid analysis by GC
Methyl esters were prepared by treatment of the lipid fraction with acetyl chloride and methanol (Dayananda et al. 2007). The fatty acid methyl esters (FAME) of the mixture were analyzed by gas chromatography, the FAME were identified by comparing their retention times with those for standards (Supelco, Fame mix C8- C24), in a GC Fison 8000 series (Italy) chromatograph provided with a flame ionization detector. A capillary column of high polarity fused silica was used (Supelco SPB 1; length: 30 m; internal diameter: 0.22 mm; thickness of the film 0.25 μm). The flow of carrier gas (N2) was 1.5 ml/min, and the split ratio of the injector was 20:1. The Injector temperature was 250°C and the detector was 280°C.The starting temperature of the oven was 120°C (2 min hold) and it was increased at a rate of 5°C/min until 250°C (10 min hold). The injection volume was 1 μl.
Statistical analysis
All the analyses were carried out in triplicate. Two-way ANOVA with replication was carried out for data in Tables 1 and 2. Statistical analysis of data was carried out using Duncan’s Multiple Range Test (DMRT) (Duncan 1955) using Statistica’99. Significance was set at P ≤ 0.05.
Table 1.
HPLC analysis of carotenoid profile (expressed in relative %)a of D. bardawil in different treatments under light intensity of 35.0 ± 2.5 μmol m−2 s−1(LL) and 75.0 ± 2.5 μmol m−2(HL)
| Treatment | lutein | β carotein | Unknown | ||
|---|---|---|---|---|---|
| (HL) | (RT- 4) | (RT- 22) | (RT- 2.3) | (RT- 3.2) | (RT- 7.7) |
| Control | 20.8 cd | 39.3 cd | 5.1 d | 22.9 a | 11.9*a |
| DPA | 22.4 cd | 70.0*a | 5.4 d | 1.5 c | 0.5 cd |
| Caffeine | 24.8 cd | 41.0 b | 29.3 cd | 0.9 c | 3.8 c |
| Nicotine | 1.9 d | 19.1 c | 67.4*a | 0.9 c | 10.5 bc |
| Glyphosate | 51.3*a | 22.7 c | 12.9 c | 12.4 b | 0.5 cd |
| DCMU | 42.0 bc | 18.3 c | 11.8 d | 24.4*a | 3.3 c |
| Basta | 15.4 c | 69.0 bc | 13.3 c | 1.6 c | 0.5 cd |
| (LL) | |||||
| Control | 19.5 c | 35.8 b | 24.2*c | 5.3 c | 15.1*a |
| DPA | 22.3 b | 61.0*a | 5.4 d | 6.4 c | 5.1 c |
| Caffeine | 14.4 c | 51.0 bc | 17.3 c | 10.9 b | 6.3 c |
| Nicotine | 1.9 d | 17.1 c | 60.4a | 20.1 bc | 0.4 dc |
| Glyphosate | 42.7*a | 31.3 cd | 13.0 c | 0.0 dc | 12.9 bc |
| DCMU | 38.3 bc | 11.0 d | 18.0 d | 24.46*a | 8.3 b |
| Basta | 15.0 c | 59.0 bc | 11.0 d | 1.6 dc | 13.3 bc |
a Values are mean relative area (%) of HPLC chromatograms for three consecutive analyses.
Different alphabets in the same column represent *significant difference between the means
P ≤ 0.05. RT (retention time) at 4th and 22 nd min represent lutein, β carotein respectively, RT at 2.3, 3.2, 7.7 min represent unknown peaks.
Table 2.
Comparative fat content and fatty acid profile of D. bardawil in different treatments under light intensity of 35.0 ± 2.5 μmol m−2 s−1(LL) and 75.0 ± 2.5 μmol m−2(HL)
| Sample | FAT(%) | Fatty acid profile (expressed relative %)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| c14:0 | c 16:0 | c 18:0 | c 18:1 | c 18:2 | c 18:3 | c 20:0 | c 20:1 | ||
| Control (HL) | 33.3 c | 11.0cd | 43.0 bc | 3.0d | 29.0* a | 6.8 b | 2.0cd | 2.1 c | 3.1 c |
| DPA | 54.6*a | 12.0 b | 42.0 bc | 3.9 d | 21.4 b | 6.5cd | 2.0cd | 2.1 c | 10.1 bc |
| Caffeine | 36.7 c | 13.0 b | 40.0 bc | 8.9 c | 18.4 c | 5.0 c | 2.5 b | 2.0 c | 10.2 bc |
| Nicotine | 18.4d | 20.0* a | 10.2 ab | 37.5* a | 22.9 b | 9.4* a | ND | ND | ND |
| Glyphosate | 25.4d | 15.3 b | 49.7* a | 10.3cd | 14.4d | 2.3d | 3.0 b | 2.9cd | 2.1 c |
| DCMU | 23.7d | 11.8cd | 45.9 bc | 4.5d | 25.8cd | 4.0 c | 4.5*a | 2.5 b | 1.0cd |
| Basta | 30.3 c | 1.1d | 44.1 bc | 16.6cd | 14.2d | 5.6dc | 1.4 c | 6.0*a | 12.1*a |
| Control(LL) | 25.9d | 13.0 b | 41.0cd | 13.7 c | 19.1cd | 6.0 b | 2.0 c | 2.0 c | 3.2 c |
| DPA | 50.4*a | 14.6 b | 43.1cd | 10.6d | 13.8d | 3.4d | 4.0*bc | 5.0 bc | 5.5 c |
| Caffeine | 30.8 c | 12.0 b | 41cd | 5.5d | 18.1 c | 6.3cd | 3.5cd | 3.5cd | 10.1 a |
| Nicotine | 26.5d | 19.6*a | 14.4d | 33.9*a | 25.6*a | 6.5cd | ND | ND | ND |
| Glyphosate | 27.0d | 14.3 b | 48.7*a | 12.3 c | 12.9d | 2.3d | 4.7 a | 2.8 c | 2.0 c |
| DCMU | 23.2d | 11.4cd | 44.9cd | 9.9d | 12.8d | 8.5*a | 2.5 b | 6.0*a | 4.0 c |
| Basta | 27.2d | 1.1d | 40.1cd | 9.6d | 20.2cd | 6.6cd | 2.4cd | 5.0 bc | 15.0*a |
a Values are mean relative area (%) of GLC chromatograms for three consecutive analyses. Fatty acids with concentration less than 0.5% have not been represented in the table. ND-not detected.
C 14:0-myristic, C 16:0-palmitic, C 18:0-stearic, C 18:1-oleic, C 18:2-linoleic, C 18:3-linolenic, C 20:0-arochidonic acid and C 20:1-eicosenoic fatty acids.
Different alphabets in the same column represent * significant difference between the means
P ≤ 0.05.
Results and discussion
Biomass yield
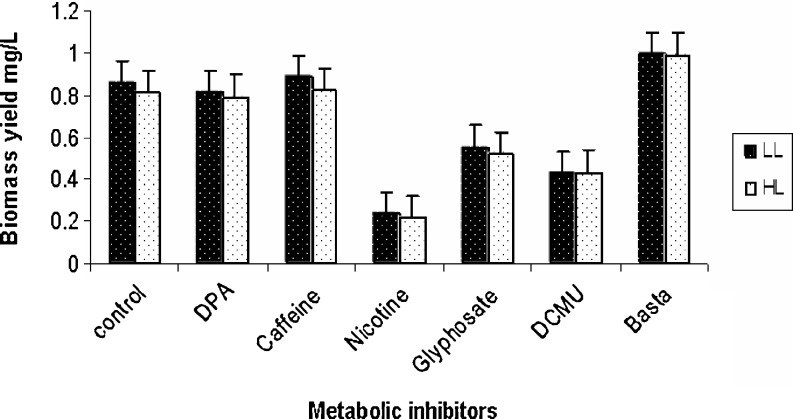
Dunaliella culture treated with nicotine showed growth inhibition, the cells became static and subsequently cells were bleached. Similar results were reported by Ishikawa and Abe (2004), who showed a decrease in the growth rate of Chlorella upon nicotine treatment. DPA treated culture showed no significant difference in biomass yield compared to control indicating that DPA did not show any toxic effect on growth of Dunaliella as it was also observed in H. pluvialis (Wang and Chen 2008). Inhibition of growth was observed in DCMU treated cultures similar to that reported for H. pluvialis (Brinda et al. 2004). Information on influence of glyphosate, glufosinate ammonium, and caffeine (cytokinesis inhibitor) on growth and carotenoid production in microalgae is scanty. Glyphosate and glufosinate ammonium treated cultures have shown reduction in biomass yield irrespective of light intensity while caffeine and DPA showed biomass yield similar to that of control (Fig. 1). It was also observed that for Dunaliella, the co3ncentration of DPA, DCMU, nicotine were required at higher concentration to study their effect compared to Haematococcus pluvialis (Brinda et al. 2004, Vidhyavathi et al. 2009). This may be due to high salt concentration in modified AS100 medium of Dunaliella (halophilic) which can significantly reduce the efficacy of the compounds as it was reported by Allnutt et al. (2000).
Fig. 1.
Biomass yield in different metabolic inhibitor treated cultures under light intensity of 35.0 ± 2.5 μmol m-2 s-1 (LL) and 75.0 ± 2.5 μmol m-2 s-1 (HL). Data represent an average of 3 replicates. Bars indicates ± Standard error
Pigment profile
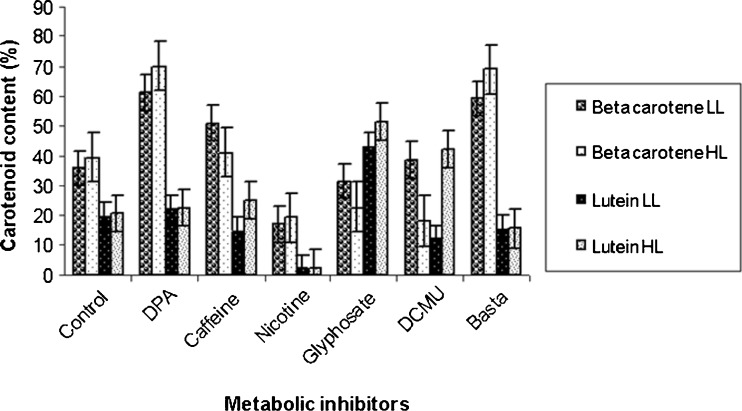
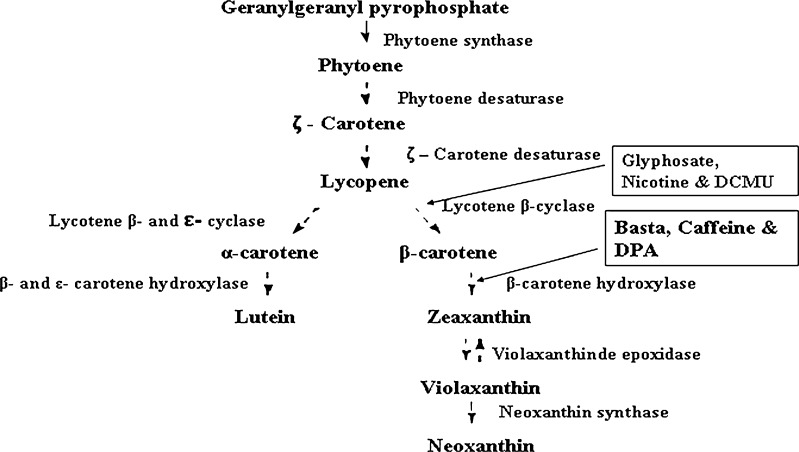
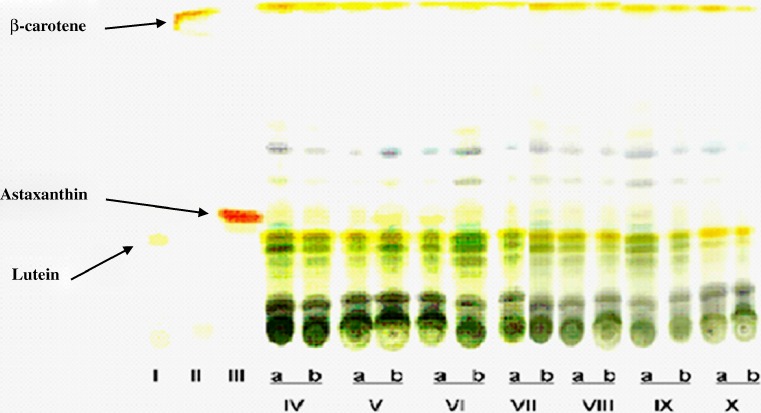
The pigments extracted with 90% acetone contained both chlorophyll and carotenoid components. The HPLC profile of the extract indicated that in D. bardawil, β-carotene constituted 35.82 ± 1.20 to 39.3 ± 1.12%, lutein 19.58 ± 1.12% of the total pigments at both the light intensities studied. The variation in HPLC profile of β-carotene and lutein in different metabolic inhibitor treated cultures at the two light intensities are shown in Fig. 2. β-carotene content increased significantly (P ≤ 0.05) in DPA treated cultures at both the light intensities while it decreased in nicotine treated cultures. In H. pluvialis also high β-carotene accumulation was reported in DPA treated culture (Fan et al. 1995). Basta treated cultures also showed significant (P ≤ 0.05) increase in β-carotene content (Table 1). Lutein content increased in cultures treated with glyphosate in both the light intensities and decreased in nicotine treated culture under 35.0 ± 2.5 μmol m−2 s-1 light intensity. There was significant (P ≤ 0.05) difference in lutein and β-carotene contents among different treatments (Table 1). Results also indicated increase in unknown carotenoids with significant (P ≤ 0.05) inhibition of both β-carotene and lutein in nicotine treated culture (Table 1). DCMU treated culture showed significant (P ≤ 0.05) decrease in chlorophyll and β-carotene contents and increase in lutein content (Table 1) under both the light intensities. In carotenoid biosynthesis pathway, α-carotene is produced by the action of the enzyme lycopene ε-cyclase (Crtl-e) from lycopene and β-carotene due to lycopene β-cyclase activity. Alpha-carotene is further converted to lutein by the action of β-and ε-carotene hydroxylase. Therefore, the increase in lutein content and decrease in β-carotene content in DCMU treated cultures may be due to inhibition of lycopene β-cyclase activity resulting in increase in lutein content through lycopene ε-cyclase (Crtl-e) and β-and ε-carotene hydroxylase activities. Cultures treated with DCMU and DPA showed yellowish physical appearance due to decreased chlorophyll content. DCMU treated cultures showed chlorophyll related product at both the light intensities indicating their possible role at various steps in the carotenoid pathway. D. bardawil under 35.0 ± 2.5 μmol m−2 s-1 light intensity, exhibited carotenoid/chlorophyll ratio as (0.23 ± 0.10) and in 35.0 ± 2.5 μmol m−2 s-1 light intensity as (0.98 ± 0.12) respectively. The postulated pathway of carotenogenesis in microalgae is shown in (Fig. 3). The first step in the carotenoid biosynthetic pathway is the formation of phytoene catalysed by phytoene synthase (Psy). Phytoene hires four double bonds before it is converted to lycopene. This process is carried out by the enzymes, phytoene desaturase (Pds) and ζ-carotene desaturase (Zds), which catalyse two symmetric dehydrogenation steps to yield ζ -carotene and lycopene respectively. Cyclization of lycopene is mediated by the enzymes, lycopene β-cyclase (Crtl-b) and lycopene ε-cyclase (Crtl-e) branching to β-carotene and α-carotene. Finally, α-carotene produce lutein catalyzed by β-and ε-carotene hydroxylase (Berg et al. 2000). Several chemical agents affect carotenogenesis in a number of microbial systems. For example, previous studies using the cyclization inhibitors, 2-(4-chlorophenylthio) triethylamine (CPTA), 2-(4-methylphenoxy) triethylamine (MPTA) and nicotine, stimulated lycopene accumulation with a concomitant increase in γ-carotene (Shaish et al. 1992). Similarly, chemical stimulators such as pyridine, imidazole, and methyl heptenone also influence lycopene formation in Blakeslea trispora and Phycomyces blakesleeanus (Nakayama et al. 1957). We also noted the parallel accumulation of β-carotene and unknown intermediates as evident from TLC separation of carotenoids from different inhibitor treated cultures (Fig. 4).
Fig. 2.
Comparison of β-carotene and lutein content in different metabolic inhibitor treated cultures under light intensity of 35.0 ± 2.5 μmol m-2 s-1 (LL) and 75.0 ± 2.5 μmol m-2 s-1 (HL). Data represent an average of 3 replicates. Bars indicate ± Standard error
Fig. 3.
Proposed pathway of carotenoid biosynthesis in Dunaliella bardawil showing specific sites of action by inhibitors
Fig. 4.
TLC separation of carotenoid extracts from different inhibitor treated cultures of microalgae and standards. (lane I)Lutein standard, (lane II) β-carotene standard, (lane III) Astaxanthin standard, (lane IV) DPA, (lane V) Nicotene, (lane VI) Control, (lane VII) Basta, (lane VIII) DCMU, (lane IX) Caffeine and (lane X) Glyphosate respectively, [(a) represent light intensity of 35.0 ± 2.5 μmol m-2 s-1, (b) represent light intensity of 75.0 ± 2.5 μmol m-2 s-1]
Fat
Fat content was found to be significantly (P ≤ 0.05) more in higher light intensity exposed culture especially in control, DPA and caffeine treated cultures compared to those exposed to lower light intensity. The fat content in nicotine treated culture was significantly less especially under light intensity of 75.0 ± 2.5 μmol m−2 s-1. Basta and glycel showed marginal difference in fat contents between two light intensities while DCMU treated culture did not show any significant (P ≤ 0.05) difference. Variation in the fatty acid profile under different culture condition is summarized in Table 2. Major fatty acids observed were palmitic acid (16:0), followed by oleic acid (18:1), myristic acid (14:0), stearic acid (18:0), linoleic acid (18:2n6c), linolenic acid(18:3n3), arachidic acid (20:0) and eicosenoic acids (20:1). Fat content was significantly (P ≤ 0.05) high in DPA treated cultures while slight (P ≤ 0.05) increase in fat content was observed in caffeine treated ones when compared to control maintained at both the light intensities.
Nicotine treated culture showed inhibition of (higer chain fatty acids) linolenic acid (18:3n3), arachidic acid (20:0) and eicosenoic acids (20:1) under both the light intensities. In all the inhibitor treated cultures palmitic acid was the major fatty acid (except nicotine) under both the light intensities including control culture. The oleic content significantly (P ≤ 0.05) increased under light intensity of 75.0 ± 2.5 μmol m−2 s-1 in all inhibitors studied except in glyphosate. In basta, DPA and caffeine treated cultures, long chain fatty acids were found in considerable proportion compared to control especially under light intensity of 75.0 ± 2.5 μmol m−2 s-1. An increase in fatty acid content under stress (high light or nitrogen starvation) conditions has been described in D. salina (Cho and Thompson 1986). Increase in fatty acids content might play an important role in the structural stability of carotene globules. The relationship between TAG biosynthesis and β-carotene accumulation has been studied (Nakayama et al. 1957) and it was suggested that both pathways were interdependent (i.e. the overproduction of β–carotene was inhibited upon chemical inhibition of TAG biosynthesis). The decrease in fat content and carotenoid content as observed in the present results with nicotine inhibitor is in accordance with the above statement. Similarly enhanced β-carotenoid content in DPA treated cultures correlated with enhanced lipid content (Tables 1 and 2). Although one to one correlation with carotenoid profile and fatty acid profile under the influence of different inhibitors could not be drawn clearly the differences in carotenoid profile and fatty acid profile observed in the present study substantiates the inhibitors role in carotenogenesis and fatty acid biosynthesis pathways.
Conclusion
From this study it may be concluded that light intensity of 35.0 ± 2.5 to 75.0 ± 2.5 μmol m−2 s-1can be used for the production of biomass, carotenoid and fatty acids. The metabolic inhibitors can enhance production of certain metabolite: DPA increased β-carotene; nicotine increased lycopene; caffeine increased biomass yield; glyphosate and DCMU increased lutein; basta increased β-carotene. Finally, a better understanding of the regulation of carotenogenesis and lipid formation enable us to further improve the overproduction of specialized carotenoids and other lipophilic products.
Acknowledgement
The authors acknowledge Department of Science and Technology, New Delhi, India for the financial support.
References
- Allnutt FCT, Kyle DJ, Grossman AR, Apt KE (2000) Methods and tools for transformation of eukaryotic algae. United State of America Patent 6027900.
- Ben-Amotz A, Avron M. Effect on the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983;72:593–597. doi: 10.1104/pp.72.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-amotz A, Katz A, Avron M. Accumulation of β-carotene in halotolerant algae: purification and characterization of β-carotene rich globules from Dunaliella bardawil. J Phycol. 1982;18:529–537. doi: 10.1111/j.1529-8817.1982.tb03219.x. [DOI] [Google Scholar]
- Berg H, Faulks R, Granado HF, Hirschberg J, Olmedilla B, Sandmann G, Southon S, Stahl W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J Sci Food Agric. 2000;80:880–912. doi: 10.1002/(SICI)1097-0010(20000515)80:7<880::AID-JSFA646>3.0.CO;2-1. [DOI] [Google Scholar]
- Bhaskar N, Chandini SK, Ganesan P, Suresh PV. Seaweeds as a source of nutritionally beneficial compounds – A review. J Food Sci Technol. 2008;45:1–13. [Google Scholar]
- Brinda BR, Sarada R, Sandesh KB, Ravishankar GA. Accumulation of astaxanthin in flagellated cell of Haematococcus pluvialis cultural and regulatory aspects. Curr Sci. 2004;87:1290–1294. [Google Scholar]
- Cho SH, Thompson GA. Properties of a fatty acid hydrolase preferentially attacking monogalactosyldiacylglycerols in Dunaliella salina chloroplasts. Biochim Biophys Acta. 1986;878:353–359. doi: 10.1016/0005-2760(86)90243-2. [DOI] [Google Scholar]
- Dayananda C, Sarada R, Kumar V, Ravishankar GA. Isolation and characterization of hydrocarbon producing green alga Botryococcus braunii from Indian freshwater bodies. Electron J Biotechn. 2007;1:78–91. [Google Scholar]
- Duncan DB. Multiple range test and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- Fan L, Vonshak A, Gabbay R, Hirshberg J, Cohen Z, Boussiba S. The biosynthetic pathway of astaxanthin in a green alga H. pluvialis as indicated by inhibition wth diphenylamine. Plant Cell Physiol. 1995;36:1519–1524. [Google Scholar]
- Fazeli MR, Tofighi H, Samadi N, Jamalifar H. Effects of salinity on β-carotene production by Dunaliella tertiolecta DCCBC26 isolated from the Urmia salt lake, North of Iran. Bioresource Technol. 2006;97:2453–2456. doi: 10.1016/j.biortech.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Fazeli MR, Tofighi H, Sobhani AM, Shahverdi AR, Sattari TN, Mirzaie S, Jamalifar H. Nicotine inhibition of lycopene cyclase enhances accumulation of carotenoid intermediates by Dunaliella salina CCAP 19/18. Eur J Phycol. 2009;44:215–220. doi: 10.1080/09670260802578526. [DOI] [Google Scholar]
- Gunjan N, Devinder K, Oberoi DPS, Dalbir SS. Thermal degradation kinetics of lycopene in oleoresin extracted from tomato paste. J Food Sci Technol. 2009;46:75–76. [Google Scholar]
- Hejazi MA, Wijffels RH. Effect of light intensity on β-carotene production and extraction by Dunaliella salina in two-phase bioreactors. Biomol Eng. 2003;20:171–175. doi: 10.1016/S1389-0344(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa E, Abe H. Lycopene accumulation and cyclic carotenoid deficiency in heterotrophic Chlorella treated with nicotine. J Ind Microbiol Biotech. 2004;31:1367–5435. doi: 10.1007/s10295-004-0179-9. [DOI] [PubMed] [Google Scholar]
- Jin ES, Feth B, Melis A. A mutant of the green algae Dunaliella salina constitutively accumulates zeaxanthin under all growth conditions. Biotechnol Bioeng. 2003;81:115–124. doi: 10.1002/bit.10459. [DOI] [PubMed] [Google Scholar]
- Kumar CS, Ganesan P, Suresh PV, Bhaskar N. Sea weeds as a source of nutritionally beneficial compounds - A review. J Food Sci Technol. 2008;45:1–13. [Google Scholar]
- Lamers PP, Janssen M, De Vos RCH, Bino RJ, Wijffels RH. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotech. 2008;26:631–638. doi: 10.1016/j.tibtech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol, Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Chichester CO, Luktone A, Mackinney G. Phytoene production in Phycomyces. Arch Biochem Biophys. 1957;66:310–315. doi: 10.1016/S0003-9861(57)80006-X. [DOI] [PubMed] [Google Scholar]
- Nishino H, Murakosh MIT, Takemura M, Kuchide M, Kanazawa M, Mou XY, Wada S. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:257–264. doi: 10.1023/A:1021206826750. [DOI] [PubMed] [Google Scholar]
- Raja R, Hemaiswarya S, Rengasamy R. Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biotechnol. 2007;74:517–523. doi: 10.1007/s00253-006-0777-8. [DOI] [PubMed] [Google Scholar]
- Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R. A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol. 2008;34:77–88. doi: 10.1080/10408410802086783. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. Changes in quality of ready-to-eat dehydrated carrot shreds during storage. J Food Sci Technol. 2010;47:94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar VR. Changes in quality of ready-to-eat dehydrated carrot shreds during storage. J Food Sci Technol. 2009;46:177–178. [Google Scholar]
- Shaish A, Ben-amotz A, Avron M. Biosynthesis of β-carotene in Dunaliella. Method Enzymol. 1992;213:439–444. doi: 10.1016/0076-6879(92)13145-N. [DOI] [Google Scholar]
- Singh P, Kulshrestha K. Nutritional quality of food supplements based on carrot powder and grits. J Food Sci Technol. 2008;45:99–101. [Google Scholar]
- Usha B, Monika G. Quality evaluation of carrot-milk cake marketed in Ludhiana. J Food Sci Technol. 2007;44:70–73. [Google Scholar]
- Vidhyavathi R, Venkatachalam L, Sarada R, Ravishankar GA. Regulation of Carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J Exp Bot. 2008;59:1409–1418. doi: 10.1093/jxb/ern048. [DOI] [PubMed] [Google Scholar]
- Vidhyavathi R, Sarada R, Ravishankar GA. Expression of carotenogenic genes and carotenoid production in Haematococcus pluvialis under under the influence of carotenoid and fatty acid synthesis. Enzyme Microb Technol. 2009;59:1409–1418. [Google Scholar]
- Vonshak A. Laboratory techniques for cultivation of Micro algae. In: Richmaond A, editor. Handbook of micro algal mass culture. Boca Raton, Florida: CRC Press; 1986. pp. 345–349. [Google Scholar]
- Wang Y, Chen T. The biosynthetic pathway of carotenoids in the astaxanthin producing green alga Chlorella zofengiensis. J Microbiol Biotechnol. 2008;24:2927–2932. doi: 10.1007/s11274-008-9834-z. [DOI] [Google Scholar]
- White AL, Jahnke LS. Contrasting effects of UV-A and UV-B on photosynthesis and photoprotection of b-carotene in two Dunaliella spp. Plant Cell Physiol. 2002;43:877–884. doi: 10.1093/pcp/pcf105. [DOI] [PubMed] [Google Scholar]