Abstract
Methanolic extracts obtained from the fruiting bodies of Cantharellus cibarius (the Chanterelle) and from the mycelium of this species cultured in vitro were analyzed for the qualitative and quantitative composition of non-hallucinogenic indole compounds. The extracts were found to contain eight indole compounds: L-tryptophan, 5-hydroxytryptophan, serotonin, melatonin, indole, kynurenine sulfate, 5-methyltryptophan, and indoleacetonitrile. The extract from the fruiting bodies also contained tryptamine. The amounts of individual compounds varied widely, ranging from 0.01 to 17.61 mg/100 g DW in the fruiting bodies, and from 0.01 to 35.34 mg/100 g DW in the biomass from in vitro cultures. The quantitatively dominating compounds included: serotonin (17.61 and 20.49 mg/100 g DW, respectively) and kynurenine sulfate (3.62 and 35.34 mg/100 g DW). In addition, the material from in vitro cultures contained a considerable amount of 5-hydroxytryptophan (12.52 mg/100 g DW). The levels of the remaining indole compounds under analysis: L-tryptophan, melatonin, indole, 5-methyltryptophan, and indoleacetonitrile in the material under study were low, below 1 mg/100 g DW.
Keywords: Cantharellus cibarius, Chanterelle, Indole compounds, In vitro culture, Serotonin
Introduction
Cantharellus cibarius Fr. (Basidiomycota)—commonly known as the Chanterelle, is one of the most highly valued and most commonly harvested edible mushrooms in Europe. It is also a popular edible mushroom in Asia, Africa and in the northern part of the USA. Unfortunately, due to the fact that it is easily recognizable and harvested en masse from natural sites, it belongs to endangered species. Since the demand for this species rises, because of its culinary value, there are attempts to develop its culture under controllable conditions for commercial use. However, these attempts have not yet been successful. In natural habitats, C. cibarius occurs in deciduous and coniferous forests living in a mycorrhizal association with the pine and fir, and also with the oak, beech and hornbeam. Nevertheless, it is not a typical mycorrhizal species, since it becomes a saprophyte under certain conditions (Rangel-Castro et al. 2002). This species is rich in numerous groups of metabolites which are decisive for its dietetic and therapeutic values. They include nitrogen compounds, like assimilable proteins containing all proteinogenic amino acids (3–9 % dry weight), many free exo- and endogenous amino acids, and biogenic amines (Svrcek and Vancura 1987). The fruiting bodies of this species contain an important enzyme, homodimeric lactase, possessing lignolytic properties used in biotechnology (Ng and Wang 2004; Nakamura and Go 2005). Polysaccharides, belonging to the most important components, comprise: chitin, chitosans, and, most of all, β-glucan possessing immunostimulating properties (Kalaĉ 2009). Lipids comprise saturated and mono- and polyunsaturated fatty acids (Barros et al. 2008). Oxidation of linoleic acid in C. cibarius fruiting bodies yields 1-octen-3-ol responsible for the characteristic flavour of this mushroom (de Pinho et al. 2008). This reaction is particularly intensified during the process of drying the mushrooms (Kalaĉ 2009). Mention has also been made of the presence of six phenolic compounds: 3-, 4-, and 5-O-caffeoylquinicacid, caffeic acid, p-coumaric acid, and rutin, and five organic acids: citric, ascorbic, malic, shikimic, and fumaric (Valentao et al. 2005). A non-phenolic compound: cinnamic acid, a precursor of numerous phenolic acids and alkaloids, was found in C. cibarius (14.97 mg/kg dry weight) (Barros et al. 2009). Many of these compounds are decisive for the antioxidant properties, so they play a protective role against civilization diseases (da Silva and Jorge 2012). C. cibarius is a species distinguished from other mushroom species by the highest, comparable with those of baking yeasts, levels of vitamins of the B complex: B1, B2, B6, H; and vitamins A, D2, E, C. The presence of α, β, γ-tocopherol and carotenoids has also been investigated in this species (Barros et al. 2008). Due to a high level of carotenoids, this species is used as a natural source of those pigments for the food industry. C. cibarius is also a good source of trace elements, especially selenium, enhancing the antioxidant activity of this species (Widzicka et al. 2008). In continuation of the studies on non-hallucinogenic indole compounds in Basidiomycota species that had been carried out in our laboratory for several years, we also examined several typical edible species for the levels of compounds belonging to this group, which play the role of neurotransmitters or their precursors, exhibit antioxidant, anticancer, anti-inflammatory, analgesic and anti-ageing actions (Isbister et al. 2004).
C. cibarius had already been included in the analysis of edible mushrooms (Muszyńska et al. 2011). The aim of the present study was to initiate in vitro cultures of C. cibarius to determine optimal conditions for mycelial growth and analyze the indole compounds in the cultured biomass. The fruiting bodies from which the cultures were derived were analyzed for comparison. This article is the first description of an attempt to culture C. cibarius in vitro and is the first report on the presence of indole compounds in this material.
Materials and methods
Materials for analysis
The studies were conducted on young fruiting bodies of Cantharellus cibarius Fr. harvested from natural sites in mixed and coniferous forests in southern Poland (Brodła near Kraków) in August 2010 (deposited in the Department of Pharmaceutical Botany, Jagiellonian University, Collegium Medicum, Kraków, Poland). After taxonomic identification according to Knudsen (Knudsen and Vesterholt 2008), the sporocarps were used to set up in vitro cultures from which the obtained mycelium was also material for analysis.
In vitro culture
Mycelial cultures were derived from explants originating from the parts of young fruiting bodies of Cantharellus cibarius. These pieces of fruiting bodies were sterilized with 70 % ethyl alcohol for 15 s and then in a 15 % Domestos solution for 5 min. (hypochlorite content below 15 %, manufactured by Unilever, Hungry). After being rinsed several times with sterile redistilled water, the mycelium fragments were transferred to Petri dishes containing an agar-solidified medium with a composition according to Oddoux (1957). After growing on the solid medium, the pieces of mycelium were placed in an Erlenmeyer flask (500 mL) containing 250 mL of a liquid medium with modified Oddoux medium, and the initial biomass amounted to 0.1 g. The culture was shaken at a rate of 140 rpm (shaker ALTEL, Łódź). Cultures were incubated at a temperature of 25 ± 2 °C under 16-h light (900 lx/8 dark). The agitated liquid cultures of C. cibarius were maintained for 2 weeks and after that time subcultured.
After 2 weeks, the biomass was separated from the liquid medium using a filter paper on a Büchner funnel, rinsed with redistilled water. The obtained fresh biomass and fruiting bodies of C. cibarius were frozen and immediately dried by lyophilization (lyophilizer Freezone 4.5, Labconco; temperature: −40 °C).
Chemicals
Standards of the indole compounds: L-tryptophan, 5-hydroxytryptophan, 5-metyltryptophan, serotonin, melatonin, tryptamine, kynurenic acid, kynurenine sulfate, indoleacetic acid, indoleacetonitrile and indole were from Sigma-Aldrich (St Louis, Mo, USA). Standard solutions were prepared in methanol. Methanol, ammonium acetate, both of HPLC- grade, were from Merck (Darmstad, Germany), n-propanol, ethyl acetate, petroleum ether, all of analytical grade purity, were from Polish Chemicals Company (Gliwice, Poland). Water was purified by redistillation and filtered through a Millipore filter (Millex, Millipore Corporation, USA) under reduced pressure.
Sample preparation
Lyophilized fruiting bodies of C. cibarius and mycelium from the in vitro culture were powdered and then extracted in a percolator with petroleum ether in order to remove the lipid fraction according to the procedure developed in our laboratory (Muszyńska et al. 2007). After the extraction with ether, the material was dried and extracted with methanol in a percolator for 24 h. The extracts were combined and evaporated to dryness. To remove the remaining lipids, the concentrated extracts were frozen.
Indole compounds were isolated by using preparative TLC on aluminium-backed silica gel 60 (Merck, Art. 1.055540001) plates, onto which the methanol extracts were loaded. Chromatograms were developed in a mobile phase: n-propanol/ethyl acetate/water (7:1:2 v/v/v) and identification was performed at 280 nm. The obtained fractions were extracted with methanol, then filtered through a syringe-driven filter unit (Millex, Millipore Corporation, USA) and concentrated by distillation in a vacuum evaporator under reduced pressure at 40 °C. Dry extracts quantitatively dissolved in 1.5 mL of methanol were subjected to HPLC analysis.
HPLC analysis
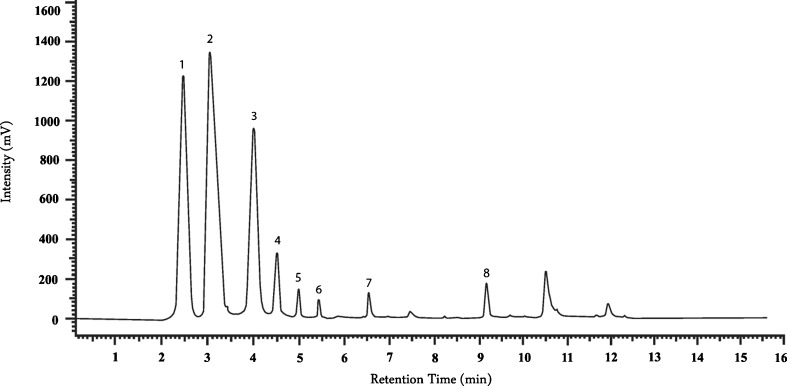
The amounts of indole compounds: L-tryptophan, 5-hydroxytryptophan, 5-metyltryptophan, serotonin, melatonin, tryptamine, kynurenic acid, kynurenine sulfate, indoleacetic acid, indoleacetonitrile, indole and indoleacetamide were determined according to the procedure developed by Kysilka (Kysilka et al. 1985) with our modifications (Muszyńska et al. 2009, 2012). Briefly, the analytical conditions were as follows: HPLC apparatus: Hitachi; pump: L-7100; column: Purospher® RP-18 (4 × 200 mm, 5 μm); solvent isocratic system: methanol/water/ammonium acetate (15:14:1 v/v/v); flow rate : 1 ml/min; detector UV: λ = 280 nm. The identification of the indole compounds was made by comparing the retention times of sample peaks with those of the standards. In order to confirm the presence of indole compounds in the tested extracts, the analysis was performed with standard solutions of indole compounds as internal standards. The presence of the tested metabolites in the sample showed up as an increase in peak height for the appropriate retention time. The quantitative analysis was carried out using the calibration curve method. The results are expressed in mg/100 g of dry weight, calculated by internal normalization of the chromatographic peak area (an example chromatogram of the extract from the mycelium of C. cibarius is presented in Fig. 1).
Fig. 1.
HPLC chromatogram of the extract from the mycelium of Cantharellus cibarius: (1) - serotonin, (2) - kynurenine sulfate, (3) - 5-hydroxytryptophan, (4) - L-tryptophan, (5) - 5-metyltryptophan, (6) - melatonin, (7) - indoleacetonitrile, (8) - indole
Statistical analysis
For each mushroom, three samples were used for the determination of every quality attribute and all the analyses were carried out in triplicate. The results were expressed as mean values with standard deviation (SD). Statistical significance was defined at p ≤ 0.05.
Results and discussion
After several attempts to establish an optimal sterilization method, we were successful in the initiation of C. cibarius mycelia in vitro culture from the hymenial part of fresh young fruiting bodies. The best biomass growth was obtained during 2-week growth cycles in shaking liquid cultures on a modified Oddoux medium. The biomass growth in the initiated cultures averaged 7.2 g DW/L of the medium. The maximum growth of C. cibarius mycelium biomass was observed at the initial medium pH value of 6.0 and at a temperature of 25 °C. In vitro cultures maintained under laboratory conditions optimized for maximum growth can provide a uniform mycelium which may be a repeatable and efficient source of metabolites. The obtained increases in biomass and the dynamics of mycelium growth did not differ from the results that we had obtained for Xerocomus badius (Fr.) Kuhn. ex Gilb—Bay Bolete and Tricholoma equestre (L.: Fr.) Kumm.—Man on Horseback, and for Calocera viscosa (Pers.: Fr.) Fr.—Yellow false coral cultures studied earlier (Muszyńska et al. 2009, 2012).
The HPLC procedure applied in the determination of the qualitative and quantitative composition of non-hallucinogenic indole compounds offered optimum conditions for the most effective separation of the metabolites analysed. The results of the analyses of the methanolic extracts of Cantharellus cibarius fruiting bodies and mycelia cultured in vitro revealed slight differences in the qualitative composition of the indole compounds under study and significant quantitative differences. Both extracts were found to contain eight different indole compounds: L-tryptophan, 5-hydroxytryptophan, serotonin, melatonin, indole, kynurenine sulfate, 5-methyltryptophan, and indoleacetonitrile. In addition, the fruiting body extracts contained tryptamine. The amounts of individual compounds varied widely, ranging from 0.01 to 17.61 mg/100 g DW for the fruiting bodies and from 0.01 to 35.34 mg/100 g DW for the mycelium cultured in vitro. The amounts of indole compounds in the methanolic extracts of the fruiting bodies and mycelium of C. cibarius are presented in Table 1. Serotonin and kynurenine sulfate (the last product of serotonin metabolism) were the quantitatively dominant compounds in both extracts. However, the mycelium from in vitro cultures contained greater amounts of these compounds. The levels of serotonin were of the same order of magnitude, but were slightly higher in the mycelium extracts (17.61 and 20.49 mg/100 g DW, respectively). On the other hand, kynurenine sulfate levels were almost 10 times higher in the material from in vitro cultures compared with the fruiting bodies (3.62 and 35.34 mg/100 g DW, respectively). The extracts from in vitro cultures were characterized by a much higher amount of 5-hydroxytryptophan (12.52 mg/100 g DW). This was also the highest level of 5-hydroxytryptophan obtained from all the species of Basidiomycota investigated in our laboratory so far. The amounts of the remaining indole compounds under analysis in the fruiting bodies and mycelium from in vitro cultures: L-tryptophan, melatonin, indole, 5-methyltryptophan, indoleacetonitrile were low, below 1 mg/100 g DW. The levels of indole compounds in the extracts of C. cibarius fruiting bodies presently under study do not diverge from the results obtained earlier for the fruiting bodies harvested from a natural site in Poland in 2007 (Muszyńska et al. 2011). The extracts studied earlier also contained a higher amount of serotonin (29.61 mg/100 g DW) and kynurenine sulfate (4.81 mg/100 g DW), and low (below 1 mg/100 g DW) amounts of other indole compounds: L-tryptophan, 5-hydroxytryptophan, melatonin, tryptamine and indoleacetonitrile. A comparison of the present data with the results obtained with methanolic extracts of other popular edible mushrooms (Agaricus bisporus (J.E. Lange)—White bottom mushroom, Boletus edulis Bull. ex Fr.—King Bolete, Lactarius deliciosus (L. Fr.) S.F. Gray—Saffron milk-cap, Leccinum rufum (Schaef.) Kreisel—Roudh-stalked boleti, Pleurotus ostreatus (Jacq.: Fr.) Kummer—Oyster mushroom, Suilus luteus L. ex Fr.—Slipery Jack, Xerocomus badius) and conditionally edible mushrooms (Armillaria mellea (Vahl.) Karst s. l.—Honey mushroom, Lactarius deterrimus Groger—False saffron milkcap, T. equestre), previously studied in our laboratory showed that the amounts of indole compounds were also very diverse and had a very wide variability range from 0.01 to 39.20 mg/100 g DW (Muszyńska et al. 2009, 2011). Only one of all the indole compounds under study, serotonin, was present in all the species investigated either previously or at present (except for L. deterrimus). The lowest serotonin content was determined in the fruiting bodies of T. equestre (0.18 mg/100 g DW), and the highest in S. luteus (34.11 mg/100 g DW). The amounts of serotonin in L. rufum, L. deliciosus and B. edulis were of the same order of magnitude as in S. luteus (31.71, 18.42, 10.14 mg/100 g DW, respectively) and these results are similar to the amount of this compound in the fruiting bodies and mycelium of C. cibarius. The levels of this compound in the commercial species of A. bisporus and P. ostreatus were slightly lower (6.52, 5.21 mg/100 g DW). The amounts of serotonin in the conditionally edible species were low and were estimated at a maximum of 2.20 mg/100 g DW in A. mellea and 0.18 mg/100 g DW in T. equestre. However, the amounts of tryptamine and tryptophan were much higher (from 2.01 to 4.46 mg/100 g DW) than in the above mentioned typical edible species. High levels of indole compounds degradation products, kynurenic acid and kynurenine sulfate, in the fruiting bodies of S. luteus, L. deliciosus and A. bisporus, ranging from 2.63 to 39.20 mg/100 g DW, appear to be of interest from a practical perspective. The amounts of the remaining indole compounds: melatonin, 5-hydroxytryptophan, indoleacetic acid and indoleacetonitrile in the fruiting bodies of the tested species were of the same order of magnitude as in the species analyzed earlier, i.e. below 1 mg/100 g DW.
Table 1.
Amounts of indole compounds under study (mg/100 g d. w.) in extracts from the fruiting bodies and mycelium of Cantharellus cibarius
| Indole compounds | Cantharellus cibarius fruiting bodies | Cantharellus cibarius mycelium from cultures |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| L-Tryptophan | 0.02 ± 0.003 | 0.64 ± 0.013 |
| 5-Hydroxytryptophan | 0.01 ± 0.001 | 12.52 ± 0.671 |
| Serotonin | 17.61 ± 0.455 | 20.49 ± 0.670 |
| Melatonin | 0.11 ± 0.006 | 0.01 ± 0.006 |
| Tryptamine | 0.02 ± 0.002 | _a |
| Indole | 0.02 ± 0.001 | 0.19 ± 0.017 |
| Kynurenine sulfate | 3.62 ± 0.032 | 35.34 ± 1.332 |
| 5-Metyltryptophan | 0.68 ± 0.006 | 0.05 ± 0.007 |
| Indoleacetonitrile | 0.02 ± 0.003 | 0.02 ± 0.002 |
Each observation mean = SD of three replicate experiments (n = 3)
aContent lower than 0.001 mg/100 g d. w.
Conclusions
The obtained results indicate that in vitro cultures of Cantharellus cibarius can be a good model for studies on the accumulation and metabolism of indole compounds in mushrooms. High levels of serotonin and its precursor, 5-hydroxytryptophan, in the fruiting bodies of C. cibarius and in its mycelium cultured in vitro also indicate a potential for the use of this material as a source of this physiologically important compound for humans. Among the indole compounds, the high level of kynurenine sulfate, which is the final product of kynurenic acid metabolism, also improves the dietetic potential of this species. Kynurenic acid is an NMDA receptor antagonist, and is responsible for adaptive processes in the human organism. Further optimization of the conditions for in vitro cultures may allow them to become an alternative for commercial cultivation of this species. This is needed, since it may be expected that the mycelium cultured in vitro is also a source of other important metabolites, possessing both culinary and medicinal values, characteristic of the fruiting bodies.
References
- Barros L, Cruz T, Baptista P, Estevinho LM, Ferreira IC. Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food Chem Toxicol. 2008;46(8):2742–2747. doi: 10.1016/j.fct.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Barros L, Duenas M, Ferreira IC, Baptista P, Santos-Buelga C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol. 2009;47(6):1076–1079. doi: 10.1016/j.fct.2009.01.039. [DOI] [PubMed] [Google Scholar]
- da Silva AC, Jorge N. Influence of Lentinus edodes and Agaricus blazei extracts on the prevention of oxidation and retention of tocopherols in soybean oil in an accelerated storage test. J Food Sci Technol. 2012 doi: 10.1007/s13197-012-0623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinho PG, Ribeiro B, Goncalves RF, Baptista P, Valentao P, Seabra RM, Andrade PB. Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms. J Agric Food Chem. 2008;56(5):1704–1712. doi: 10.1021/jf073181y. [DOI] [PubMed] [Google Scholar]
- Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol. 2004;42(3):277–285. doi: 10.1081/CLT-120037428. [DOI] [PubMed] [Google Scholar]
- Kalaĉ P. Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem. 2009;113:9–16. doi: 10.1016/j.foodchem.2008.07.077. [DOI] [Google Scholar]
- Knudsen H, Vesterholt J. Funga Nordica: agaricoid, boletoid and cyphelloid genera. Copenhagen: Nordsvamp; 2008. [Google Scholar]
- Kysilka R, Wurst M, Pacakova V, Stulik K, Haskovec L. High-performance liquid chromatographic determination of hallucinogenic indoleamines with simultaneous UV photometric and voltammetric detection. J Chromatogr. 1985;320(2):414–420. doi: 10.1016/S0021-9673(01)90521-7. [DOI] [PubMed] [Google Scholar]
- Muszyńska B, Maślanka A, Sułkowska-Ziaja K, Krzek J. TLC-UV analysis of indole compounds and other nitrogen-containing bases in fruiting bodies of Lactarius deterrimus. J Planar Chromatogr Mod TLC. 2007;20(1):55–58. [Google Scholar]
- Muszyńska B, Sułkowska-Ziaja K, Ekiert H. Indole compounds in fruiting bodies of some selected Macromycetes species and in their mycelia cultured in vitro. Pharmazie. 2009;64(7):479–480. [PubMed] [Google Scholar]
- Muszyńska B, Sułkowska-Ziaja K, Ekiert H. Indole compounds in fruiting bodies of some edible Basidiomycota species. Food Chem. 2011;125:1306–1308. doi: 10.1016/j.foodchem.2010.10.056. [DOI] [Google Scholar]
- Muszyńska B, Sułkowska-Ziaja K, Ekiert H. An antioxidant in fruiting bodies and in mycelia from in vitro cultures of Calocera viscosa (Basidiomycota)–preliminary results. Acta Pol Pharm. 2012;69(1):135–138. [PubMed] [Google Scholar]
- Nakamura K, Go N. Function and molecular evolution of multicopper blue proteins. Cell Mol Life Sci. 2005;62:2050–2066. doi: 10.1007/s00018-004-5076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TB, Wang HX. A homodimeric laccase with unique characteristics from the yellow mushroom Cantharellus cibarius. Biochem Biophys Res Commun. 2004;313(1):37–41. doi: 10.1016/j.bbrc.2003.11.087. [DOI] [PubMed] [Google Scholar]
- Oddoux L, editor. Recherches sur les mycéliums secondaires des Homobasidiés en culture pure. Lyon: Imprimerie de Trevoux; 1957. [Google Scholar]
- Rangel-Castro JI, Levenfors JJ, Danell E. Physiological and genetic characterization of fluorescent Pseudomonas associated with Cantharellus cibarius. Can J Microbiol. 2002;48(8):739–748. doi: 10.1139/w02-062. [DOI] [PubMed] [Google Scholar]
- Svrcek M, Vancura B (1987) Mushrooms of Central Europe. Państwowe Wydawnictwo Rolnicze i Leśne Warszawa
- Valentao P, Andrade PB, Rangel J, Ribeiro B, Silva BM, Baptista P, Seabra RM. Effect of the conservation procedure on the contents of phenolic compounds and organic acids in chanterelle (Cantharellus cibarius) mushroom. J Agric Food Chem. 2005;53(12):4925–4931. doi: 10.1021/jf0580263. [DOI] [PubMed] [Google Scholar]
- Widzicka E, Bielawski L, Mazur A, Falandysz J. Elemental contents in Cantharellus cibarius (Fr.) fruiting bodies and in soil from beneath the fruiting bodies in the Darżlubska Forest. Bromatol Chem Toksykol. 2008;2:121–128. [Google Scholar]