Abstract
Genetic modification is a special set of gene technology that alters the genetic machinery of such living organisms as animals, plants or microorganisms. Combining genes from different organisms is known as recombinant DNA technology and the resulting organism is said to be ‘Genetically modified (GM)’, ‘Genetically engineered’ or ‘Transgenic’. The principal transgenic crops grown commercially in field are herbicide and insecticide resistant soybeans, corn, cotton and canola. Other crops grown commercially and/or field-tested are sweet potato resistant to a virus that could destroy most of the African harvest, rice with increased iron and vitamins that may alleviate chronic malnutrition in Asian countries and a variety of plants that are able to survive weather extremes. There are bananas that produce human vaccines against infectious diseases such as hepatitis B, fish that mature more quickly, fruit and nut trees that yield years earlier and plants that produce new plastics with unique properties. Technologies for genetically modifying foods offer dramatic promise for meeting some areas of greatest challenge for the 21st century. Like all new technologies, they also pose some risks, both known and unknown. Controversies and public concern surrounding GM foods and crops commonly focus on human and environmental safety, labelling and consumer choice, intellectual property rights, ethics, food security, poverty reduction and environmental conservation. With this new technology on gene manipulation what are the risks of “tampering with Mother Nature”?, what effects will this have on the environment?, what are the health concerns that consumers should be aware of? and is recombinant technology really beneficial? This review will also address some major concerns about the safety, environmental and ecological risks and health hazards involved with GM foods and recombinant technology.
Keywords: Genetically modified foods, Genetically engineered foods, Transgenic foods, Food safety, Allergenic foods, Public concerns
Introduction
Scientists first discovered in 1946 that DNA can be transferred between organisms (Clive 2011). It is now known that there are several mechanisms for DNA transfer and that these occur in nature on a large scale, for example, it is a major mechanism for antibiotic resistance in pathogenic bacteria. The first genetically modified (GM) plant was produced in 1983, using an antibiotic-resistant tobacco plant. China was the first country to commercialize a transgenic crop in the early 1990s with the introduction of virus resistant tobacco. In 1994, the transgenic ‘Flavour Saver tomato’ was approved by the Food and Drug Administration (FDA) for marketing in the USA. The modification allowed the tomato to delay ripening after picking. In 1995, few transgenic crops received marketing approval. This include canola with modified oil composition (Calgene), Bacillus thuringiensis (Bt) corn/maize (Ciba-Geigy), cotton resistant to the herbicide bromoxynil (Calgene), Bt cotton (Monsanto), Bt potatoes (Monsanto), soybeans resistant to the herbicide glyphosate (Monsanto), virus-resistant squash (Asgrow) and additional delayed ripening tomatoes (DNAP, Zeneca/Peto, and Monsanto) (Clive 2011). A total of 35 approvals had been granted to commercially grow 8 transgenic crops and one flower crop of carnations with 8 different traits in 6 countries plus the EU till 1996 (Clive 1996). As of 2011, the USA leads a list of multiple countries in the production of GM crops. Currently, there are a number of food species in which a genetically modified version exists (Johnson 2008). Some of the foods that are available in the market include cotton, soybean, canola, potatoes, eggplant, strawberries, corn, tomatoes, lettuce, cantaloupe, carrots etc. GM products which are currently in the pipeline include medicines and vaccines, foods and food ingredients, feeds and fibres. Locating genes for important traits, such as those conferring insect resistance or desired nutrients-is one of the most limiting steps in the process.
Foods derived from GM crops
At present there are several GM crops used as food sources. As of now there are no GM animals approved for use as food, but a GM salmon has been proposed for FDA approval. In instances, the product is directly consumed as food, but in most of the cases, crops that have been genetically modified are sold as commodities, which are further processed into food ingredients.
Fruits and vegetables
Papaya has been developed by genetic engineering which is ring spot virus resistant and thus enhancing the productivity. This was very much in need as in the early 1990s the Hawaii’s papaya industry was facing disaster because of the deadly papaya ring spot virus. Its single-handed savior was a breed engineered to be resistant to the virus. Without it, the state’s papaya industry would have collapsed. Today 80 % of Hawaiian papaya is genetically engineered, and till now no conventional or organic method is available to control ring spot virus.
The NewLeaf™ potato, a GM food developed using naturally-occurring bacteria found in the soil known as Bacillus thuringiensis (Bt), was made to provide in-plant protection from the yield-robbing Colorado potato beetle. This was brought to market by Monsanto in the late 1990s, developed for the fast food market. This was forced to withdraw from the market in 2001as the fast food retailers did not pick it up and thereby the food processors ran into export problems. Reports say that currently no transgenic potatoes are marketed for the purpose of human consumption. However, BASF, one of the leading suppliers of plant biotechnology solutions for agriculture requested for the approval for cultivation and marketing as a food and feed for its ‘Fortuna potato’. This GM potato was made resistant to late blight by adding two resistance genes, blb1 and blb2, which was originated from the Mexican wild potato Solanum bulbocastanum. As of 2005, about 13 % of the zucchini grown in the USA is genetically modified to resist three viruses; the zucchini is also grown in Canada (Johnson 2008).
Vegetable oil
It is reported that there is no or a significantly small amount of protein or DNA remaining in vegetable oil extracted from the original GM crops in USA. Vegetable oil is sold to consumers as cooking oil, margarine and shortening, and is used in prepared foods. Vegetable oil is made of triglycerides extracted from plants or seeds and then refined, and may be further processed via hydrogenation to turn liquid oils into solids. The refining process removes nearly all non-triglyceride ingredients (Crevel et al. 2000). Cooking oil, margarine and shortening may also be made from several crops. A large percentage of Canola produced in USA is GM and is mainly used to produce vegetable oil. Canola oil is the third most widely consumed vegetable oil in the world. The genetic modifications are made for providing resistance to herbicides viz. glyphosate or glufosinate and also for improving the oil composition. After removing oil from canola seed, which is ∼43 %, the meal has been used as high quality animal feed. Canola oil is a key ingredient in many foods and is sold directly to consumers as margarine or cooking oil. The oil has many non-food uses, which includes making lipsticks.
Maize, also called corn in the USA and cornmeal, which is ground and dried maize constitute a staple food in many regions of the world. Grown since 1997 in the USA and Canada, 86 % of the USA maize crop was genetically modified in 2010 (Hamer and Scuse 2010) and 32 % of the worldwide maize crop was GM in 2011 (Clive 2011). A good amount of the total maize harvested go for livestock feed including the distillers grains. The remaining has been used for ethanol and high fructose corn syrup production, export, and also used for other sweeteners, cornstarch, alcohol, human food or drink. Corn oil is sold directly as cooking oil and to make shortening and margarine, in addition to make vitamin carriers, as a source of lecithin, as an ingredient in prepared foods like mayonnaise, sauces and soups, and also to fry potato chips and French fries. Cottonseed oil is used as a salad and cooking oil, both domestically and industrially. Nearly 93 % of the cotton crop in USA is GM.
Sugar
The USA imports 10 % of its sugar from other countries, while the remaining 90 % is extracted from domestically grown sugar beet and sugarcane. Out of the domestically grown sugar crops, half of the extracted sugar is derived from sugar beet, and the other half is from sugarcane. After deregulation in 2005, glyphosate-resistant sugar beet was extensively adopted in the USA. In USA 95 % of sugar beet acres were planted with glyphosate-resistant seed (Clive 2011). Sugar beets that are herbicide-tolerant have been approved in Australia, Canada, Colombia, EU, Japan, Korea, Mexico, New Zealand, Philippines, Russian Federation, Singapore and USA. The food products of sugar beets are refined sugar and molasses. Pulp remaining from the refining process is used as animal feed. The sugar produced from GM sugar beets is highly refined and contains no DNA or protein—it is just sucrose, the same as sugar produced from non-GM sugar beets (Joana et al. 2010).
Quantification of genetically modified organisms (GMOs) in foods
Testing on GMOs in food and feed is routinely done using molecular techniques like DNA microarrays or qPCR. These tests are based on screening genetic elements like p35S, tNos, pat, or bar or event specific markers for the official GMOs like Mon810, Bt11, or GT73. The array based method combines multiplex PCR and array technology to screen samples for different potential GMO combining different approaches viz. screening elements, plant-specific markers, and event-specific markers. The qPCR is used to detect specific GMO events by usage of specific primers for screening elements or event specific markers. Controls are necessary to avoid false positive or false negative results. For example, a test for CaMV is used to avoid a false positive in the event of a virus contaminated sample.
Joana et al. (2010) reported the extraction and detection of DNA along with a complete industrial soybean oil processing chain to monitor the presence of Roundup Ready (RR) soybean. The amplification of soybean lectin gene by end-point polymerase chain reaction (PCR) was achieved in all the steps of extraction and refining processes. The amplification of RR soybean by PCR assays using event specific primers was also achieved for all the extraction and refining steps. This excluded the intermediate steps of refining viz. neutralization, washing and bleaching possibly due to sample instability. The real-time PCR assays using specific probes confirmed all the results and proved that it is possible to detect and quantify GMOs in the fully refined soybean oil.
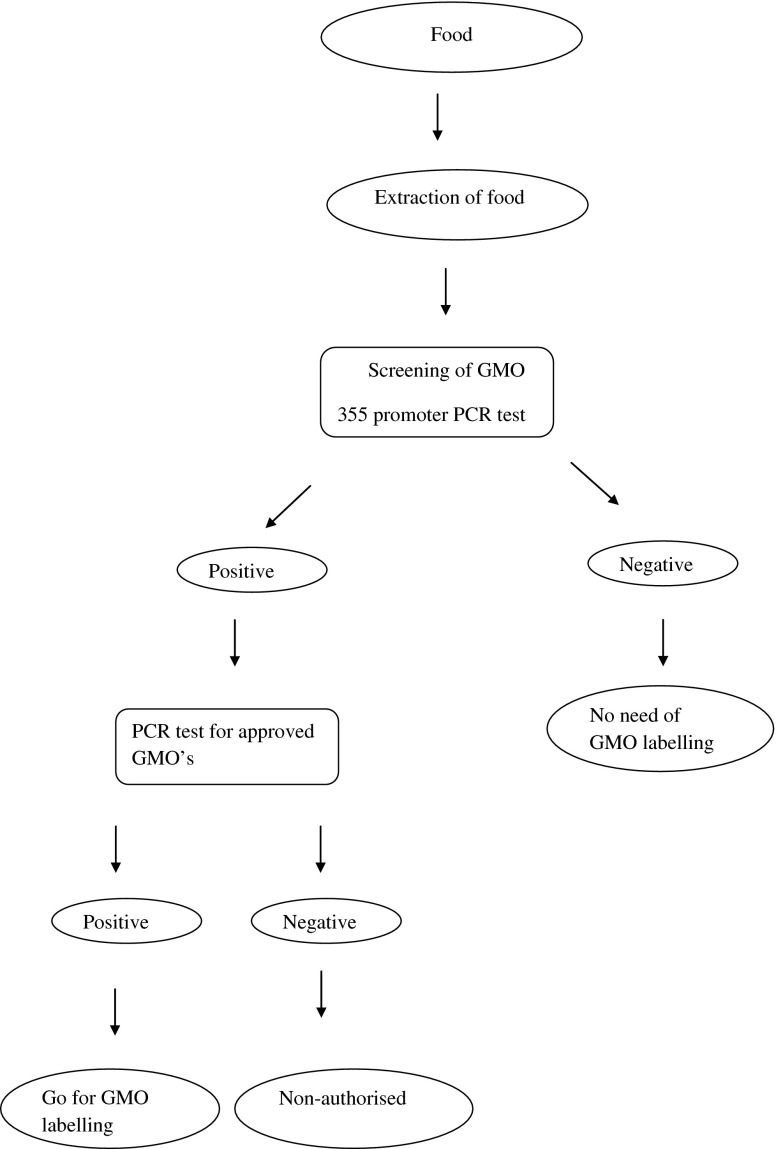
Figure 1 gives the overall protocol for the testing of GMOs. This is based on a PCR detection system specific for 35S promoter region originating from cauliflower mosaic virus (Deisingh and Badrie 2005). The 35S-PCR technique permits detection of GMO contents of foods and raw materials in the range of 0.01–0.1 %. The development of quantitative detection systems such as quantitative competitive PCR (QC-PCR), real-time PCR and ELISA systems resulted in the advantage of survival of DNA in most manufacturing processes. Otherwise with ELISA, there can be protein denaturing during food processing. Inter-laboratory differences were found to be less with the QC-PCR than with quantitative PCR probably due to insufficient homogenisation of the sample. However, there are disadvantages, the major one being the amount of DNA, which could be amplified, is affected by food processing techniques and can vary up to 5-fold. Thus, results need to be normalised by using plant-specific QC-PCR system. Further, DNA, which cannot be amplified, will affect all quantitative PCR detection systems.
Fig. 1.
Protocol for the testing of genetically modified foods
In a recent work La Mura et al. (2011) applied QUIZ (quantization using informative zeros) to estimate the contents of RoundUp Ready™ soya and MON810 in processed food containing one or both GMs. They reported that the quantification of GM in samples can be performed without the need for certified reference materials using QUIZ. Results showed good agreement between derived values and known input of GM material and compare favourably with quantitative real-time PCR. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick has been reported recently (Xiumin et al. 2012).
GM foods-merits and demerits
Before we think of having GM foods it is very important to know about is advantages and disadvantages especially with respect to its safety. These foods are made by inserting genes of other species into their DNA. Though this kind of genetic modification is used both in plants and animals, it is found more commonly in the former than in the latter. Experts are working on developing foods that have the ability to alleviate certain disorders and diseases. Though researchers and the manufacturers make sure that there are various advantages of consuming these foods, a fair bit of the population is entirely against them.
GM foods are useful in controlling the occurrence of certain diseases. By modifying the DNA system of these foods, the properties causing allergies are eliminated successfully. These foods grow faster than the foods that are grown traditionally. Probably because of this, the increased productivity provides the population with more food. Moreover these foods are a boon in places which experience frequent droughts, or where the soil is incompetent for agriculture. At times, genetically engineered food crops can be grown at places with unfavourable climatic conditions too. A normal crop can grow only in specific season or under some favourable climatic conditions. Though the seeds for such foods are quite expensive, their cost of production is reported to be less than that of the traditional crops due to the natural resistance towards pests and insects. This reduces the necessity of exposing GM crops to harmful pesticides and insecticides, making these foods free from chemicals and environment friendly as well. Genetically engineered foods are reported to be high in nutrients and contain more minerals and vitamins than those found in traditionally grown foods. Other than this, these foods are known to taste better. Another reason for people opting for genetically engineered foods is that they have an increased shelf life and hence there is less fear of foods getting spoiled quickly.
The biggest threat caused by GM foods is that they can have harmful effects on the human body. It is believed that consumption of these genetically engineered foods can cause the development of diseases which are immune to antibiotics. Besides, as these foods are new inventions, not much is known about their long term effects on human beings. As the health effects are unknown, many people prefer to stay away from these foods. Manufacturers do not mention on the label that foods are developed by genetic manipulation because they think that this would affect their business, which is not a good practice. Many religious and cultural communities are against such foods because they see it as an unnatural way of producing foods. Many people are also not comfortable with the idea of transferring animal genes into plants and vice versa. Also, this cross-pollination method can cause damage to other organisms that thrive in the environment. Experts are also of the opinion that with the increase of such foods, developing countries would start depending more on industrial countries because it is likely that the food production would be controlled by them in the time to come.
Safety tests on commercial GM crops
The GM tomatoes were produced by inserting kanr genes into a tomato by an ‘antisense’ GM method (IRDC 1998). The results show that there were no significant alterations in total protein, vitamins and mineral contents and in toxic glycoalkaloids (Redenbaugh et al. 1992). Therefore, the GM and parent tomatoes were deemed to be “substantially equivalent”. In acute toxicity studies with male/female rats, which were tube-fed with homogenized GM tomatoes, toxic effects were reported to be absent. A study with a GM tomato expressing B. thuringiensis toxin CRYIA (b) was underlined by the immunocytochemical demonstration of in vitro binding of Bt toxin to the caecum/colon from humans and rhesus monkeys (Noteborn et al. 1995).
GM maize
Two lines of Chardon LL herbicide-resistant GM maize expressing the gene of phosphinothricin acetyltransferase before and after ensiling showed significant differences in fat and carbohydrate contents compared with non-GM maize and were therefore substantially different come. Toxicity tests were only performed with the maize even though with this the unpredictable effects of the gene transfer or the vector or gene insertion could not be demonstrated or excluded. The design of these experiments was also flawed because of poor digestibility and reduction in feed conversion efficiency of GM corn. One broiler chicken feeding study with rations containing transgenic Event 176 derived Bt corn (Novartis) has been published (Brake and Vlachos 1998). However, the results of this trial are more relevant to commercial than academic scientific studies.
GM soybeans
To make soybeans herbicide resistant, the gene of 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium was used. Safety tests claim the GM variety to be “substantially equivalent” to conventional soybeans (Padgette et al. 1996). The same was claimed for GTS (glyphosate-resistant soybeans) sprayed with this herbicide (Taylor et al. 1999). However, several significant differences between the GM and control lines were recorded (Padgette et al. 1996) and the study showed statistically significant changes in the contents of genistein (isoflavone) with significant importance for health (Lappe et al. 1999) and increased content in trypsin inhibitor.
Studies have been conducted on the feeding value (Hammond et al. 1996) and possible toxicity (Harrison et al. 1996) for rats, broiler chickens, catfish and dairy cows of two GM lines of glyphosate-resistant soybean (GTS). The growth, feed conversion efficiency, catfish fillet composition, broiler breast muscle and fat pad weights and milk production, rumen fermentation and digestibilities in cows were found to be similar for GTS and non-GTS. These studies had the following lacunae: (a) No individual feed intakes, body or organ weights were given and histology studies were qualitative microscopy on the pancreas, (b) The feeding value of the two GTS lines was not substantially equivalent either because the rats/catfish grew significantly better on one of the GTS lines than on the other, (c) The design of study with broiler chicken was not much convincing, (d) Milk production and performance of lactating cows also showed significant differences between cows fed GM and non-GM feeds and (e) Testing of the safety of 5-enolpyruvylshikimate-3-phosphate synthase, which renders soybeans glyphosate-resistant (Harrison et al. 1996), was irrelevant because in the gavage studies an E. coli recombinant and not the GTS product were used. In a separate study (Teshima et al. 2000), it was claimed that rats and mice which were fed 30 % toasted GTS or non-GTS in their diet had no significant differences in nutritional performance, organ weights, histopathology and production of IgE and IgG antibodies.
GM potatoes
There were no improvements in the protein content or amino acid profile of GM potatoes (Hashimoto et al. 1999a). In a short feeding study to establish the safety of GM potatoes expressing the soybean glycinin gene, rats were daily force-fed with 2 g of GM or control potatoes/kg body weight (Hashimoto et al 1999b). No differences in growth, feed intake, blood cell count and composition and organ weights between the groups were found. In this study, the intake of potato by animals was reported to be too low (Pusztai 2001).
Feeding mice with potatoes transformed with a Bacillus thuringiensis var.kurstaki Cry1 toxin gene or the toxin itself was shown to have caused villus epithelial cell hypertrophy and multinucleation, disrupted microvilli, mitochondrial degeneration, increased numbers of lysosomes and autophagic vacuoles and activation of crypt Paneth cells (Fares and El-Sayed 1998). The results showed CryI toxin which was stable in the mouse gut. Growing rats pair-fed on iso-proteinic and iso-caloric balanced diets containing raw or boiled non-GM potatoes and GM potatoes with the snowdrop (Galanthus nivalis) bulb lectin (GNA) gene (Ewen and Pusztai 1999) showed significant increase in the mucosal thickness of the stomach and the crypt length of the intestines of rats fed GM potatoes. Most of these effects were due to the insertion of the construct used for the transformation or the genetic transformation itself and not to GNA which had been pre-selected as a non-mitotic lectin unable to induce hyperplastic intestinal growth (Pusztai et al. 1990) and epithelial T lymphocyte infiltration.
GM rice
The kind that expresses soybean glycinin gene (40–50 mg glycinin/g protein) was developed (Momma et al. 1999) and was claimed to contain 20 % more protein. However, the increased protein content was found probably due to a decrease in moisture rather than true increase in protein.
GM cotton
Several lines of GM cotton plants have been developed using a gene from Bacillus thuringiensis subsp. kurstaki providing increased protection against major lepidopteran pests. The lines were claimed to be “substantially equivalent” to parent lines (Berberich et al. 1996) in levels of macronutrients and gossypol. Cyclopropenoid fatty acids and aflatoxin levels were less than those in conventional seeds. However, because of the use of inappropriate statistics it was questionable whether the GM and non-GM lines were equivalent, particularly as environmental stresses could have unpredictable effects on anti-nutrient/toxin levels (Novak and Haslberger 2000).
GM peas
The nutritional value of diets containing GM peas expressing bean alpha-amylase inhibitor when fed to rats for 10 days at two different doses viz. 30 % and 65 % was shown to be similar to that of parent-line peas (Pusztai et al. 1999). At the same time in order to establish its safety for humans a more rigorous specific risk assessment will have to be carried out with several GM lines. Nutritional/toxicological testing on laboratory animals should follow the clinical, double-blind, placebo-type tests with human volunteers.
Allergenicity studies
When the gene is from a crop of known allergenicity, it is easy to establish whether the GM food is allergenic using in vitro tests, such as RAST or immunoblotting, with sera from individuals sensitised to the original crop. This was demonstrated in GM soybeans expressing the brasil nut 2S proteins (Nordlee et al. 1996) or in GM potatoes expressing cod protein genes (Noteborn et al. 1995). It is also relatively easy to assess whether genetic engineering affected the potency of endogenous allergens (Burks and Fuchs 1995). Farm workers exposed to B. thuringiensis pesticide were shown to have developed skin sensitization and IgE antibodies to the Bt spore extract. With their sera it may now therefore be possible to test for the allergenic potential of GM crops expressing Bt toxin (Bernstein et al. 1999). It is all the more important because Bt toxin Cry1Ac has been shown to be a potent oral/nasal antigen and adjuvant (Vazquez-Padron et al. 2000).
The decision-tree type of indirect approach based on factors such as size and stability of the transgenically expressed protein (O’Neil et al. 1998) is even more unsound, particularly as its stability to gut proteolysis is assessed by an in vitro (simulated) testing (Metcalf et al. 1996) instead of in vivo (human/animal) testing and this is fundamentally wrong. The concept that most allergens are abundant proteins may be misleading because, for example, Gad c 1, the major allergen in codfish, is not a predominant protein (Vazquez-Padron et al. 2000). However, when the gene responsible for the allergenicity is known, such as the gene of the alpha-amylase/trypsin inhibitors/allergens in rice, cloning and sequencing opens the way for reducing their level by antisense RNA strategy (Nakamura and Matsuda 1996).
It is known that the main concerns about adverse effects of GM foods on health are the transfer of antibiotic resistance, toxicity and allergenicity. There are two issues from an allergic standpoint. These are the transfer of a known allergen that may occur from a crop into a non-allergenic target crop and the creation of a neo-allergen where de novo sensitisation occurs in the population. Patients allergic to Brazil nuts and not to soy bean then showed an IgE mediated response towards GM soy bean. Lack (2002) argued that it is possible to prevent such occurrences by doing IgE-binding studies and taking into account physico-chemical characteristics of proteins and referring to known allergen databases. The second possible scenario of de novo sensitisation does not easily lend itself to risk assessment. He reports that evidence that the technology used for the production of GM foods poses an allergic threat per se is lacking very much compared to other methodologies widely accepted in the food industry.
Risks and controversy
There are controversies around GM food on several levels, including whether food produced with it is safe, whether it should be labelled and if so how, whether agricultural biotechnology and it is needed to address world hunger now or in the future, and more specifically with respect to intellectual property and market dynamics, environmental effects of GM crops and GM crops’ role in industrial agricultural more generally.
Many problems, viz. the risks of “tampering with Mother Nature”, the health concerns that consumers should be aware of and the benefits of recombinant technology, also arise with pest-resistant and herbicide-resistant plants. The evolution of resistant pests and weeds termed superbugs and super weeds is another problem. Resistance can evolve whenever selective pressure is strong enough. If these cultivars are planted on a commercial scale, there will be strong selective pressure in that habitat, which could cause the evolution of resistant insects in a few years and nullify the effects of the transgenic. Likewise, if spraying of herbicides becomes more regular due to new cultivars, surrounding weeds could develop a resistance to the herbicide tolerant by the crop. This would cause an increase in herbicide dose or change in herbicide, as well as an increase in the amount and types of herbicides on crop plants. Ironically, chemical companies that sell weed killers are a driving force behind this research (Steinbrecher 1996).
Another issue is the uncertainty in whether the pest-resistant characteristic of these crops can escape to their weedy relatives causing resistant and increased weeds (Louda 1999). It is also possible that if insect-resistant plants cause increased death in one particular pest, it may decrease competition and invite minor pests to become a major problem. In addition, it could cause the pest population to shift to another plant population that was once unthreatened. These effects can branch out much further. A study of Bt crops showed that “beneficial insects, so named because they prey on crop pests, were also exposed to harmful quantities of Bt.” It was stated that it is possible for the effects to reach further up the food web to effect plants and animals consumed by humans (Brian 1999). Also, from a toxicological standpoint, further investigation is required to determine if residues from herbicide or pest resistant plants could harm key groups of organisms found in surrounding soil, such as bacteria, fungi, nematodes, and other microorganisms (Allison and Palma 1997).
The potential risks accompanied by disease resistant plants deal mostly with viral resistance. It is possible that viral resistance can lead to the formation of new viruses and therefore new diseases. It has been reported that naturally occurring viruses can recombine with viral fragments that are introduced to create transgenic plants, forming new viruses. Additionally, there can be many variations of this newly formed virus (Steinbrecher 1996).
Health risks associated with GM foods are concerned with toxins, allergens, or genetic hazards. The mechanisms of food hazards fall into three main categories (Conner and Jacobs 1999). They are inserted genes and their expression products, secondary and pleiotropic effects of gene expression and the insertional mutagenesis resulting from gene integration. With regards to the first category, it is not the transferred gene itself that would pose a health risk. It should be the expression of the gene and the affects of the gene product that are considered. New proteins can be synthesized that can produce unpredictable allergenic effects. For example, bean plants that were genetically modified to increase cysteine and methionine content were discarded after the discovery that the expressed protein of the transgene was highly allergenic (Butler and Reichhardt 1999). Due attention should be taken for foods engineered with genes from foods that commonly cause allergies, such as milk, eggs, nuts, wheat, legumes, fish, molluscs and crustacean (Maryanski 1997). However, since the products of the transgenic are usually previously identified, the amount and effects of the product can be assessed before public consumption. Also, any potential risk, immunological, allergenic, toxic or genetically hazardous, could be recognized and evaluated if health concerns arise. The available allergen data bases with details are shown in Table 1.
Table 1.
Allergen databases (Kleter and Peijnenburg 2002)
| Name | Website | Type of allergen | Details |
|---|---|---|---|
| AgMoBiol | http://ambl.lsc.pku.edu.cn | Food, Pollen | The Agricultural Molecular Biology Laboratory of the Peking University Protein Engg. & Plant Genetic Engg. |
| Central Science Lab | http://www.csl.gov.uk/ | Proteins | Food and Drug Administration Centre for Food Safety and Applied Nutrition, Sand Hutton, York, UK |
| FARRP | http://www.farrp.org | Proteins | 658 allergens, The Food Allergy Research & Resource Program, University of Nebraska-Lincoln |
| NCFST | http://www.iit.edu/∼sgendel/fa.htm | Gluten | National Centre for Safety & Technology, Illinois Institute of Technology |
| PROTALL | http://www.ifr.bbsrc.ac.uk/protall | Plant | Biochemical and clinical data- The PROTALL project, FAIR- CT98-4356, The Institute of Food Research, UK |
| SDAP | http://129.109.73.75/SDAP/ | Proteins | Allergenic Proteins (Ivanciuc et al. 2003) |
| SwissPort | http://us.expasy.org/cgi-bin/lists?llergen.txt | Proteins | SIB Swiss Institute of Bioinformatics, Geneva) |
| WHO/International Union of Immunological Societies | http://www.allergen.org | Proteins | Nomenclature (Chapman 2008) |
| Allergome | http://www.allergome.org | Proteins | Mari and Riccioli (2004) |
| Internet Symposium on Food Allergens-2002 | http://www.food-allergens.de | – | Food Allergen data collections |
More concern comes with secondary and pleiotropic effects. For example, many transgenes encode an enzyme that alters biochemical pathways. This could cause an increase or decrease in certain biochemicals. Also, the presence of a new enzyme could cause depletion in the enzymatic substrate and subsequent build up of the enzymatic product. In addition, newly expressed enzymes may cause metabolites to diverge from one secondary metabolic pathway to another (Conner and Jacobs 1999). These changes in metabolism can lead to an increase in toxin concentrations. Assessing toxins is a more difficult task due to limitations of animal models. Animals have high variation between experimental groups and it is challenging to attain relevant doses of transgenic foods in animals that would provide results comparable to humans (Butler and Reichhardt 1999). Consequently, biochemical and regulatory pathways in plants are poorly understood.
Insertional mutagenesis can disrupt or change the expression of existing genes in a host plant. Random insertion can cause inactivation of endogenous genes, producing mutant plants. Moreover, fusion proteins can be made from plant DNA and inserted DNA. Many of these genes create nonsense products or are eliminated in crop selection due to incorrect appearance. However, of most concern is the activation or up regulation of silent or low expressed genes. This is due to the fact that it is possible to activate “genes that encode enzymes in biochemical pathways toward the production of toxic secondary compounds” (Conner and Jacobs 1999). This becomes a greater issue when the new protein or toxic compound is expressed in the edible portion of the plant, so that the food is no longer substantially equal to its traditional counterpart.
There is a great deal of unknowns when it comes to the risks of GM foods. One critic declared “foreign proteins that have never been in the human food chain will soon be consumed in large amounts”. It took us many years to realize that DDT might have oestrogenic activities and affect humans, “but we are now being asked to believe that everything is OK with GM foods because we haven’t seen any dead bodies yet” (Butler and Reichhardt 1999). As a result of the growing public concerns over GM foods, national governments have been working to regulate production and trade of GM foods.
Reports say that GM crops are grown over 160 million hectares in 29 countries, and imported by countries (including European ones) that don’t grow them. Nearly 300 million Americans, 1350 million Chinese, 280 million Brazilians and millions elsewhere regularly eat GM foods, directly and indirectly. Though Europeans voice major fears about GM foods, they permit GM maize cultivation. It imports GM soy meal and maize as animal feed. Millions of Europeans visit the US and South America and eat GM food.
Around three million Indians have become US citizens, and millions more go to the US for tourism and business and they will be eating GM foods in the USA. Indian activists claim that GM foods are inherently dangerous and must not be cultivated in India. Activists strongly opposed Bt cotton in India, and published reports claiming that the crop had failed in the field. At the same time farmers soon learned from experience that Bt cotton was very profitable, and 30 million rushed to adopt it. In consequence, India’s cotton production doubled and exports zoomed, even while using much less pesticide. Punjab farmers lease land at Rs 30,000 per acre to grow Bt cotton.
Public concerns-global scenario
In the late 1980s, there was a major controversy associated with GM foods even when the GMOs were not in the market. But the industrial applications of gene technology were developed to the production and marketing status. After words, the European Commission harmonized the national regulations across Europe. Concerns from the community side on GMOs in particular about its authorization have taken place since 1990s and the regulatory frame work on the marketing aspects underwent refining. Issues specifically on the use of GMOs for human consumption were introduced in 1997, in the Regulation on Novel Foods Ingredients (258/97/EC of 27 January 1997). This Regulations deals with rules for authorization and labelling of novel foods including food products made from GMOs, recognizing for the first time the consumer’s right to information and labelling as a tool for making an informed choice. The labelling of GM maize varieties and GM soy varieties that did not fall under this Regulation are covered by Regulation (EC 1139/98). Further legislative initiatives concern the traceability and labelling of GMOs and the authorization of GMOs in food and feed.
The initial outcome of the implementation of the first European directive seemed to be a settlement of the conflicts over technologies related to gene applications. By 1996, the second international level controversy over gene technology came up and triggered the arrival of GM soybeans at European harbours (Lassen et al. 2002). The GM soy beans by Monsanto to resist the herbicide represented the first large scale marketing of GM foods in Europe. Events such as commercialisation of GM maize and other GM modified commodities focused the public attention on the emerging biosciences, as did other gene technology applications such as animal and human cloning. The public debate on the issues associated with the GM foods resulted in the formation of many non-governmental organizations with explicit interest. At the same time there is a great demand for public participation in the issues about regulation and scientific strategy who expresses acceptance or rejection of GM products through purchase decisions or consumer boycotts (Frewer and Salter 2002).
Most research effort has been devoted to assessing people’s attitudes towards GM foods as a technology. Numerous “opinion poll”—type surveys have been conducted on national and cross-national levels (Hamstra 1998). Ethical concerns are also important, that a particular technology is in some way “tampering with nature”, or that unintended effects are unpredictable and thus unknown to science (Miles and Frewer 2001).
Consumer’s attitude towards GM foods
Consumer acceptance is conditioned by the risk that they perceive from introducing food into their consumption habits processed through technology that they hardly understand. In a study conducted in Spain, the main conclusion was that the introduction of GM food into agro-food markets should be accompanied by adequate policies to guarantee consumer safety. These actions would allow a decrease in consumer-perceived risk by taking special care of the information provided, concretely relating to health. For, the most influential factor in consumer-perceived risk from these foods is concern about health (Martinez-Poveda et al. 2009).
Tsourgiannis et al. (2011) conducted a study aimed to identify the factors that affect consumers purchasing behaviour towards food products that are free from GMO (GM Free) in a European region and more precisely in the Prefecture of Drama-Kavala-Xanthi. Field interviews conducted in a random selected sample consisted of 337 consumers in the cities of Drama, Kavala, Xanthi in 2009. Principal components analysis (PCA) was conducted in order to identify the factors that affect people in preferring consuming products that are GM Free. The factors that influence people in the study area to buy GM Free products are: (a) products’ certification as GM Free or organic products, (b) interest about the protection of the environment and nutrition value, (c) marketing issues and (d) price and quality. Furthermore, cluster and discriminant analysis identified two groups of consumers: (a) those influenced by the product price, quality and marketing aspects and (b) those interested in product’s certification and environmental protection (Tsourgiannis et al. 2011).
Snell et al. (2012) examined 12 long-term studies (of more than 90 days, up to 2 years in duration) and 12 multigenerational studies (from 2 to 5 generations) on the effects of diets containing GM maize, potato, soybean, rice, or triticale on animal health. They referenced the 90-day studies on GM feed for which long-term or multigenerational study data were available. Many parameters have been examined using biochemical analyses, histological examination of specific organs, hematology and the detection of transgenic DNA. Results from all the 24 studies do not suggest any health hazards and, in general, there were no statistically significant differences within parameters observed. They observed some small differences, though these fell within the normal variation range of the considered parameter and thus had no biological or toxicological significance. The studies reviewed present evidence to show that GM plants are nutritionally equivalent to their non-GM counterparts and can be safely used in food and feed.
GM foods: issues with respect to India
In a major setback to the proponents of GM technology in farm crops, the Parliamentary Committee on Agriculture in 2012 asked Indian government to stop all field trials and sought a bar on GM food crops such as Bt. brinjal. Raising the “ethical dimensions” of transgenics in agricultural crops, as well as studies of a long-term environmental and chronic toxicology impact, the panel noted that there were no significant socio-economic benefits to farmers.
Countries like India have great security concerns at the same time specific problems exist for small and marginal farmers. India could use a toxin free variety of the Lathyrus sativus grown on marginal lands and consumed by the very poor. GM mustard is a variety using the barnase-barstar-bar gene complex, an unstable gene construct with possible undesirable effects, to achieve male sterile lines that are used to make hybrid mustard varieties. In India we have good non-GM alternatives for making male sterile lines for hybrid production so the Proagro variety is of little use. Being a food crop, GM mustard will have to be examined very carefully. Even if there were to be benefits, they have to be weighed against the risks posed to human health and the environment. Apart from this, mustard is a cross-pollinating crop and pollen with their foreign genes is bound to reach non-GM mustard and wild relatives. We do not know what impact this will have. If GM technology is to be used in India, it should be directed at the real needs of Indian farmers, on crops like legumes, oilseeds and fodder and traits like drought tolerance and salinity tolerance.
Basmati rice and Darjeeling tea are perhaps India’s most easily identifiable premium products in the area of food. Basmati is highly prized rice, its markets are growing and it is a high end, expensive product in the international market. Like Champagne wine and truffles from France, international consumers treat it as a special, luxury food. Since rice is nutritionally a poor cereal, it is thought that addition of iron and vitamin A by genetic modification would increase the nutritional quality. So does it make any sense at all to breed a GM Basmati, along the lines of Bt Cotton? However, premium wine makers have outright rejected the notion of GM doctored wines that were designed to cut out the hangover and were supposed to be ‘healthier’. Premium products like special wines, truffles and Basmati rice need to be handled in a special, premium way (Sahai 2003).
Traceability of GMOs in the food production chain
Traceability systems document the history of a product and may serve the purpose of both marketing and health protection. In this framework, segregation and identity preservation systems allow for the separation of GM and non-GM products from “farm to fork”. Implementation of these systems comes with specific technical requirements for each particular step of the food processing chain. In addition, the feasibility of traceability systems depends on a number of factors, including unique identifiers for each GM product, detection methods, permissible levels of contamination, and financial costs. Progress has been achieved in the field of sampling, detection, and traceability of GM products, while some issues remain to be solved. For success, much will depend on the threshold level for adventitious contamination set by legislation (Miraglia et al. 2004).
Issues related to detection and traceability of GMOs is gaining interest worldwide due to the global diffusion and the related socio-economical implications. The interest of the scientific community into traceability aspects has also been increased simultaneously. Crucial factors in sampling and detection methodologies are the number of the GMOs involved and international agreement on traceability. The availability of reliable traceability strategies is very important and this may increase public trust in transparency in GMO related issues.
Heat processing methods like autoclaving and microwave heating can damage the DNA and reduce the level to detectable DNA. The PCR based methods have been standardised to detect such DNA in GM soybean and maize (Vijayakumar et al. 2009). Molecular methods such as multiplex and real time PCR methods have been developed to detect even 20 pg of genomic DNA in genetically modified EE-1 brinjal (Ballari et al. 2012).
DNA and protein based methods have been adopted for the detection and identification of GMOs which is relatively a new area of diagnostics. New diagnostic methodologies are also being developed, viz. the microarray-based methods that allow for the simultaneous identification of the increasing number of GMOs on the global market in a single sample. Some of these techniques have also been discussed for the detection of unintended effects of genetic modification by Cellini et al. (2004). The implementation of adequate traceability systems requires more than technical tools alone and is strictly linked to labelling constraints. The more stringent the labelling requirements, the more expensive and difficult the associated traceability strategies are to meet these requirements.
Both labelling and traceability of GMOs are current issues that are considered in trade and regulation. Currently, labelling of GM foods containing detectable transgenic material is required by EU legislation. A proposed package of legislation would extend this labelling to foods without any traces of transgenics. These new legislations would also impose labelling and a traceability system based on documentation throughout the food and feed manufacture system. The regulatory issues of risk analysis and labelling are currently harmonised by Codex Alimentarius. The implementation and maintenance of the regulations necessitates sampling protocols and analytical methodologies that allow for accurate determination of the content of GM organisms within a food and feed sample. Current methodologies for the analysis of GMOs are focused on either one of two targets, the transgenic DNA inserted- or the novel protein(s) expressed- in a GM product. For most DNA-based detection methods, the polymerase chain reaction is employed. Items that need consideration in the use of DNA-based detection methods include the specificity, sensitivity, matrix effects, internal reference DNA, availability of external reference materials, hemizygosity versus homozygosity, extra chromosomal DNA and international harmonisation.
For most protein-based methods, enzyme-linked immunosorbent assays with antibodies binding the novel protein are employed. Consideration should be given to the selection of the antigen bound by the antibody, accuracy, validation and matrix effects. Currently, validation of detection methods for analysis of GMOs is taking place. New methodologies are developed, in addition to the use of microarrays, mass spectrometry and surface plasmon resonance. Challenges for GMO detection include the detection of transgenic material in materials with varying chromosome numbers. The existing and proposed regulatory EU requirements for traceability of GM products fit within a broader tendency towards traceability of foods in general and, commercially, towards products that can be distinguished from one another.
Gene transfer studies in human volunteers
As of January 2009, there has only been one human feeding study conducted on the effects of GM foods. The study involved seven human volunteers who previously had their large intestines removed for medical reasons. These volunteers were provided with GM soy to eat to see if the DNA of the GM soy transferred to the bacteria that naturally lives in the human gut. Researchers identified that three of the seven volunteers had transgenes from GM soya transferred into the bacteria living in their gut before the start of the feeding experiment. As this low-frequency transfer did not increase after the consumption of GM soy, the researchers concluded that gene transfer did not occur during the experiment. In volunteers with complete digestive tracts, the transgene did not survive passage through intact gastrointestinal tract (Netherwood 2004). Other studies have found DNA from M13 virus, GFP and even ribulose-1, 5-bisphosphate carboxylase (Rubisco) genes in the blood and tissue of ingesting animals (Guertler et al. 2009; Brigulla and Wackernagel 2010).
Two studies on the possible effects of giving GM feed to animals found that there were no significant differences in the safety and nutritional value of feedstuffs containing material derived from GM plants (Gerhard et al. 2005; Beagle et al. 2006). Specifically, the studies noted that no residues of recombinant DNA or novel proteins have been found in any organ or tissue samples obtained from animals fed with GM plants (Nordlee 1996; Streit 2001).
Future developments
The GM foods have the potential to solve many of the world’s hunger and malnutrition problems, and to help protect and preserve the environment by increasing yield and reducing reliance upon synthetic pesticides and herbicides. Challenges ahead lie in many areas viz. safety testing, regulation, policies and food labelling. Many people feel that genetic engineering is the inevitable wave of the future and that we cannot afford to ignore a technology that has such enormous potential benefits.
Future also envisages that applications of GMOs are diverse and include drugs in food, bananas that produce human vaccines against infectious diseases such as Hepatitis B (Kumar et al. 2005), metabolically engineered fish that mature more quickly, fruit and nut trees that yield years earlier, foods no longer containing properties associated with common intolerances, and plants that produce new biodegradable plastics with unique properties (van Beilen and Yves 2008). While their practicality or efficacy in commercial production has yet to be fully tested, the next decade may see exponential increases in GM product development as researchers gain increasing access to genomic resources that are applicable to organisms beyond the scope of individual projects.
One has to agree that there are many opinions (Domingo 2000) about scarce data on the potential health risks of GM food crops, even though these should have been tested for and eliminated before their introduction. Although it is argued that small differences between GM and non-GM crops have little biological meaning, it is opined that most GM and parental line crops fall short of the definition of substantial equivalence. In any case, we need novel methods and concepts to probe into the compositional, nutritional, toxicological and metabolic differences between GM and conventional crops and into the safety of the genetic techniques used in developing GM crops if we want to put this technology on a proper scientific foundation and allay the fears of the general public. Considerable effort need to be directed towards understanding people’s attitudes towards this gene technology. At the same time it is imperative to note the lack of trust in institutions and institutional activities regarding GMOs and the public perceive that institutions have failed to take account of the actual concerns of the public as part of their risk management activities.
Contributor Information
A. S. Bawa, Email: amarinderbawa@yahoo.co.in
K. R. Anilakumar, Email: anilakumarkr@gmail.com
References
- Allison S, Palma PM. Commercialization of transgenic plants: potential ecological risks. BioScience. 1997;47:86–96. doi: 10.2307/1313019. [DOI] [Google Scholar]
- Ballari VR, Martin A, Gowda LR (2012) Detection and identification of genetically modified EE-1 brinjal (Solanum melongena) by single, multiplex and SYBR® real-time PCR. J Sci Food Agric. doi:10.1002/jsfa.5764, Published online 22 June 2012 [DOI] [PubMed]
- Beagle JM, Apgar GA, Jones KL, Griswold KE, Radcliffe JS, Qiu X, Lightfoot DA, Iqbal MJ. The digestive fate of Escherichia coli glutamate dehydrogenase deoxyribonucleic acid from transgenic corn in diets fed to weanling pigs. J Anim Sci. 2006;84(3):597–607. doi: 10.2527/2006.843597x. [DOI] [PubMed] [Google Scholar]
- Berberich SA, Ream JE, Jackson TL, Wood R, Stipanovic R, Harvey P, Patzer S, Fuchs RL. The composition of insect-protected cottonseed is equivalent to that of conventional cottonseed. J Agric Food Chem. 1996;44:365–371. doi: 10.1021/jf950304i. [DOI] [Google Scholar]
- Bernstein IL, Bernstein JA, Miller M, Tierzieva S, Bernstein DI, Lummus Z, Selgrade MK, Doerfler DL, Seligy VL. Immune responses in farm workers after exposure to Bacillus thuringiensis pesticides. Environ Health Perspect. 1999;107:575–582. doi: 10.1289/ehp.99107575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake J, Vlachos D. Evaluation of transgenic Event 176 “Bt” corn in broiler chicken. Poult Sci. 1998;77:648–653. doi: 10.1093/ps/77.5.648. [DOI] [PubMed] [Google Scholar]
- Brian H. Unintended effects of Bt crops. World Watch. 1999;12:9–10. [Google Scholar]
- Brigulla M, Wackernagel W. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Appl Microbiol Biotechnol. 2010;86(4):1027–1041. doi: 10.1007/s00253-010-2489-3. [DOI] [PubMed] [Google Scholar]
- Burks AW, Fuchs RL. Assessment of the endogenous allergens in glyphosate-tolerant and commercial soybean varieties. J Allergy Clin Immunol. 1995;96:1008–1010. doi: 10.1016/S0091-6749(95)70243-1. [DOI] [PubMed] [Google Scholar]
- Butler T, Reichhardt T. Long-term effect of GM crops serves up food for thought. Nature. 1999;398(6729):651–653. doi: 10.1038/19348. [DOI] [PubMed] [Google Scholar]
- Cellini F, Chesson A, Colquhoun I, Constable A, Davies HV, Engel KH, Gatehouse AMR, Karenlampi S, Kok EJ, Leguay JJ, Lehasranta S, Noteborn HPJM, Pedersen J, Smith M. Unintended effects and their detection in genetically modified crops. Food Chem Toxicol. 2004;42:1089–1125. doi: 10.1016/j.fct.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Chapman MD. Allergen nomenclature. In: Lockey RF, Dennis Ledford K, editors. Allergens and allergen immunotherapy. 4. New York: Informa Healthcare; 2008. pp. 47–58. [Google Scholar]
- Clive J (1996) Global review of the field testing and commercialization of transgenic plants: 1986 to 1995. The International Service for the Acquisition of Agri-biotech Applications. http://www.isaaa.org/kc/Publications/pdfs/isaaabriefs/Briefs%201.pdf. Retrieved on 17 July 2010
- Clive J. Global status of commercialized Biotech/GM crops. ISAAA Briefs 43. Ithaca: International Service for the Acquisition of Agri-biotech Applications; 2011. [Google Scholar]
- Conner AJ, Jacobs JME. Genetic engineering of crops as potential source of genetic hazard in the human diet. Mutat Res Genet Toxicol Environ Mutagen. 1999;443:223–234. doi: 10.1016/S1383-5742(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Crevel RWR, Lerkhof MAT, Koning MMG. Allergenicity of refined vegetable oils. Food Chem Toxicol. 2000;38(4):385–393. doi: 10.1016/S0278-6915(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Deisingh AK, Badrie N. Detection approaches for genetically modified organisms in foods. Food Res Int. 2005;38:639–649. doi: 10.1016/j.foodres.2005.01.003. [DOI] [Google Scholar]
- Domingo JL. Health risks of genetically modified foods: many opinions but few data. Science. 2000;288:1748–1749. doi: 10.1126/science.288.5472.1748. [DOI] [PubMed] [Google Scholar]
- Ewen SWB, Pusztai A. Effects of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet. 1999;354:1353–1354. doi: 10.1016/S0140-6736(98)05860-7. [DOI] [PubMed] [Google Scholar]
- Fares NH, El-Sayed AK. Fine structural changes in the ileum of mice fed on delta-endotoxin-treated potatoes and transgenic potatoes. Nat Toxins. 1998;6:219–233. doi: 10.1002/(SICI)1522-7189(199811/12)6:6<219::AID-NT30>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Frewer LI, Salter B. Public attitudes, scientific advice and the politics of regulatory policy the case of BSE. Sci Public Policy. 2002;29:137–145. doi: 10.3152/147154302781781092. [DOI] [Google Scholar]
- Gerhard F, Andrew C, Karen A. Animal nutrition with feeds from genetically modified plants. Arch Anim Nutr. 2005;59:1–40. doi: 10.1080/17450390512331342368. [DOI] [PubMed] [Google Scholar]
- Guertler P, Paul V, Albrech C, Meyer HH. Sensitive and highly specific quantitative real-time PCR and ELISA for recording a potential transfer of novel DNA and Cry1Ab protein from feed into bovine milk. Anal Bioanal Chem. 2009;393:1629–1638. doi: 10.1007/s00216-009-2667-2. [DOI] [PubMed] [Google Scholar]
- Hamer H, Scuse T (2010) National Agricultural Statistics Service (NASS), Agricultural Statistics Board, US Department of Agriculture. Acreage report, NY
- Hammond BG, Vicini JL, Hartnell GF, Naylor MW, Knight CD, Robinson EH, Fuchs RL, Padgette SR. The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by genetic incorporation of glyphosate tolerance. J Nutr. 1996;126:717–727. doi: 10.1093/jn/126.3.717. [DOI] [PubMed] [Google Scholar]
- Hamstra A (1998) Public opinion about Biotechnology. A survey of surveys. European Federation of Biotechnology, The Hague
- Harrison LA, Bailey MR, Naylor MW, Ream JE, Hammond BG, Nida DL, Burnette BL, Nickson TE, Mitsky TA, Taylor ML, Fuchs RL, Padgette SR. The expressed protein in glyphosate-tolerant soybean, 5-enolpyruvylshikimate-3-phosphate synthase from Agrobacterium sp. strain CP4, is rapidly digested in vitro and is not toxic to acutely gavaged mice. J Nutr. 1996;126:728–740. doi: 10.1093/jn/126.3.728. [DOI] [PubMed] [Google Scholar]
- Hashimoto W, Momma K, Katsube T, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of genetically engineered potatoes with designed soybean glycinin: compositional analyses of the potato tubers and digestibility of the newly expressed protein in transgenic potatoes. J Sci Food Agric. 1999;79:1607–1612. doi: 10.1002/(SICI)1097-0010(199909)79:12<1607::AID-JSFA408>3.0.CO;2-T. [DOI] [Google Scholar]
- Hashimoto W, Momma K, Yoon HJ, Ozawa S, Ohkawa Y, Ishige T, Kito M, Utsumi S, Murata K. Safety assessment of transgenic potatoes with soybean glycinin by feeding studies in rats. Biosci Biotechnol Biochem. 1999;63:1942–1946. doi: 10.1271/bbb.63.1942. [DOI] [PubMed] [Google Scholar]
- IRDC (1998) Alliance for biointegrity. http://www.biointegrity.org including Calgene FLAVR SAVR™ tomato report, pp 1–604; International Research and Development Corp. first test report, pp 1736–1738; Conclusions of the expert panel regarding the safety of the FLAVR SAVR™ tomato, ENVIRON, Arlington VA, USA pp 2355–2382; Four week oral (intubation) toxicity study in rats by IRDC, pp 2895–3000
- Ivanciuc O, Schein CH, Braun W. SDAP: database and computational tools for allergenic proteins. Nucleic Acids Res. 2003;31:359–362. doi: 10.1093/nar/gkg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joana C, Isabel M, Joana SA, Oliveira MBPP. Monitoring genetically modified soybean along the industrial soybean oil extraction and refining processes by polymerase chain reaction techniques. Food Res Int. 2010;43:301–306. doi: 10.1016/j.foodres.2009.10.003. [DOI] [Google Scholar]
- Johnson SR. Quantification of the impacts on US Agriculture of Biotechnology-Derived Crops Planted in 2006. Washington DC: National Centre for Food and Agricultural Policy; 2008. [Google Scholar]
- Kleter GA, Peijnenburg AACM. Screening of transgenic proteins expressed in transgenic food crops for the presence of short amino acid sequences identical to potential, IgE-binding linear epitopes of allergens. BMC Struct Biol. 2002;2:8–19. doi: 10.1186/1472-6807-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GBS, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA. Expression of hepatitis B surface antigen in transgenic banana plants. Planta. 2005;222:484–493. doi: 10.1007/s00425-005-1556-y. [DOI] [PubMed] [Google Scholar]
- Lack G. Clinical risk assessment of GM foods. Toxicol Lett. 2002;127:337–340. doi: 10.1016/S0378-4274(01)00517-3. [DOI] [PubMed] [Google Scholar]
- La Mura M, Allnutt TR, Greenland A, Mackay LD. Application of QUIZ for GM quantification in food. Food Chem. 2011;125:1340–1344. doi: 10.1016/j.foodchem.2010.10.002. [DOI] [Google Scholar]
- Lappe MA, Bailey EB, Childress C, Setchell KDR. Alterations in clinically important phytoestrogens in genetically modified, herbicide-tolerant soybeans. J Med Food. 1999;1:241–245. doi: 10.1089/jmf.1998.1.241. [DOI] [Google Scholar]
- Lassen J, Allansdottir A, Liakoupulos M, Olsson A, Mortensen AT. Testing times: the reception of round-up ready soya in Europe. In: Bauer M, Gaskell G, editors. Biotechnology—the making of a global controversy. Cambridge: Cambridge University Press; 2002. pp. 279–312. [Google Scholar]
- Louda SM (1999) Insect Limitation of weedy plants and its ecological implications. In: Traynor PL, Westwood J H (eds) Proceedings of a workshop on: ecological effects of pest resistance genes in managed ecosystems. Information Systems for Biotechnology. Blacksburg, Virginia, pp 43–48, http://www.isb.vt.edu
- Mari A, Riccioli D. The allergome web site—a database of allergenic molecules. Aim, structure, and data of a web-based resource. J Allergy Clin Immunol. 2004;113:S301. [Google Scholar]
- Martinez-Poveda A, Molla-Bauza MB, Gomis FJC, Martinez LMC. Consumer-perceived risk model for the introduction of genetically modified food in Spain. Food Policy. 2009;34:519–528. doi: 10.1016/j.foodpol.2009.08.001. [DOI] [Google Scholar]
- Maryanski JH. Bioengineered foods: will they cause allergic reactions? NY: U.S. Food and Drug Administration (FDA)/Centre for Food Safety and Applied Nutrition (CFSAN); 1997. [Google Scholar]
- Metcalf DD, Astwood JD, Townsend R, Sampson HA, Taylor SL, Fuchs RL (1996) Assessment of the allergenic potential of foods derived from genetically engineered crop plants. In: Crit Rev Food Sci Nutr 36(S):S165–S186. CRC, Boca Raton [DOI] [PubMed]
- Miles S, Frewer LI. Investigating specific concerns about different food hazards—higher and lower order attributes. Food Qual Prefer. 2001;12:47–61. doi: 10.1016/S0950-3293(00)00029-X. [DOI] [Google Scholar]
- Miraglia M, Berdal K, Brera C, Corbisier P, Holst-jensen A, Kok E, Marvin H, Schimmel H, Rentsch J, van Rie J, Zagon J. Detection and traceability of genetically modified organisms in the food production chain. Food Chem Toxicol. 2004;42:1157–1180. doi: 10.1016/j.fct.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Momma K, Hashimoto W, Ozawa S, Kawai S, Katsube T, Takaiwa F, Kito M, Utsumi S, Murata K. Quality and safety evaluation of genetically engineered rice with soybean glycinin: analyses of the grain composition and digestibility of glycinin in transgenic rice. Biosci Biotechnol Biochem. 1999;63:314–318. doi: 10.1271/bbb.63.314. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Matsuda T. Rice allergenic protein and molecular-genetic approach for hypoallergenic rice. Biosci Biotechnol Biochem. 1996;60:1215–1221. doi: 10.1271/bbb.60.1215. [DOI] [PubMed] [Google Scholar]
- Netherwood T. Assessing the survival of transgenic plant DNA in the human gastrointestinal tract. Nat Biotechnol. 2004;22:204–209. doi: 10.1038/nbt934. [DOI] [PubMed] [Google Scholar]
- Nordlee JA. Identification of Brazil-Nut allergen in transgenic soybeans. New Engl J Med. 1996;334:688–692. doi: 10.1056/NEJM199603143341103. [DOI] [PubMed] [Google Scholar]
- Nordlee JA, Taylor SL, Townsend JA, Thomas LA. Identification of a Brazil nut allergen in transgenic soybean. New Engl J Med. 1996;334:688–692. doi: 10.1056/NEJM199603143341103. [DOI] [PubMed] [Google Scholar]
- Noteborn HPJM, Bienenmann-Ploum ME, van den Berg JHJ, Alink GM, Zolla L, Raynaerts A, Pensa M, Kuiper HA. Safety assessment of the Bacillus thuringiensis insecticidal crystal protein CRYIA(b) expressed in transgenic tomatoes. In: Engel KH, Takeoka GR, Teranishi R, editors. ACS Symp series 605 Genetically modified foods—safety issues. Washington, D.C: American Chemical Society; 1995. pp. 135–147. [Google Scholar]
- Novak WK, Haslberger AG. Substantial equivalence of antinutrients and inherent plant toxins in genetically modified novel foods. Food Chem Toxicol. 2000;38:473–483. doi: 10.1016/S0278-6915(00)00040-5. [DOI] [PubMed] [Google Scholar]
- O’Neil C, Reese G, Lehrer SB. Allergenic potential of recombinant food proteins. Allergy Clin Immunol Int. 1998;10:5–9. [Google Scholar]
- Padgette SR, Taylor NB, Nida DL, Bailey MR, MacDonald J, Holden LR, Fuchs RL. The composition of glyphosate-tolerant soybean seeds is equivalent to that of conventional soybeans. J Nutr. 1996;126:702–716. doi: 10.1093/jn/126.3.702. [DOI] [PubMed] [Google Scholar]
- Pusztai A (2001) Safety tests on commercial crops. American Institute of Biological Sciences. actionbioscience.org, http://www.actionbioscience.org/biotech/pusztai.html viewed 2 March 2010
- Pusztai A, Ewen SWB, Grant G, Peumans WJ, van Damme EJM, Rubio L, Bardocz S. Relationship between survival and binding of plant lectins during small intestinal passage and their effectiveness as growth factors. Digestion. 1990;46(suppl 2):308–316. doi: 10.1159/000200402. [DOI] [PubMed] [Google Scholar]
- Pusztai A, Grant G, Bardocz S, Alonso R, Chrispeels MJ, Schroeder HE, Tabe LM, Higgins TJV. Expression of the insecticidal bean alpha-amylase inhibitor transgene has minimal detrimental effect on the nutritional value of peas fed to rats at 30 % of the diet. J Nutr. 1999;129:1597–1603. doi: 10.1093/jn/129.8.1597. [DOI] [PubMed] [Google Scholar]
- Redenbaugh K, Hiatt W, Martineau B, Kramer M, Sheehy R, Sanders R, Houck C, Emlay D (1992) Safety assessment of genetically engineered fruits and vegetables: a case study of the Flavr Savr Tomato. CRC Press, Boca Raton
- Sahai S. Genetically modified crops: issues for India. Fin Agric. 2003;35:7–11. [Google Scholar]
- Snell C, Bernheim A, Bergé J-B, Kuntz M, Pascal G, Paris A, Agnès ER. Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol. 2012;50:1134–1148. doi: 10.1016/j.fct.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Steinbrecher RA. From green to gene evolution: the environmental risks of genetically engineered crops. Ecologist. 1996;26:273–281. [Google Scholar]
- Streit L. Association of the Brazil nut protein gene and Kunitz trypsin inhibitor alleles with soybean protease inhibitor activity and agronomic traits. Crop Sci. 2001;41:1757–1760. doi: 10.2135/cropsci2001.1757. [DOI] [Google Scholar]
- Taylor NB, Fuchs RL, MacDonald J, Shariff AB, Padgette SR. Compositional analysis of glyphosate-tolerant soybeans treated with glyphosate. J Agric Food Chem. 1999;47:4469–4473. doi: 10.1021/jf990056g. [DOI] [PubMed] [Google Scholar]
- Teshima R, Akiyama H, Okunuki H, Sakushima J-i, Goda Y, Onodera H, Sawada J-i, Toyoda M. Effect of GM and non-GM soybeans on the immune system of BN rats and B10A mice. J Food Hyg Soc Jpn. 2000;41:188–193. doi: 10.3358/shokueishi.41.188. [DOI] [Google Scholar]
- Tsourgiannis L, Karasavvoglou A, Florou G. Consumers’ attitudes towards GM free products in a European region. The case of the Prefecture of Drama-Kavala-Xanthi in Greece. Appetite. 2011;57:448–458. doi: 10.1016/j.appet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- van Beilen JB, Yves P. Harnessing plant biomass for biofuels and biomaterials: production of renewable polymers from crop plants. Plant J. 2008;54(4):684–701. doi: 10.1111/j.1365-313X.2008.03431.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Padron RI, Moreno-Fierros L, Neri-Bazan L, Martinez-Gil AF, de la Riva GA, Lopez-Revilla R. Characterization of the mucosal and sytemic immune response induced by Cry1Ac protein from Bacillus thuringiensis HD 73 in mice. Braz J Med Biol Res. 2000;33:147–155. doi: 10.1590/S0100-879X2000000200002. [DOI] [PubMed] [Google Scholar]
- Vijayakumar KR, Martin A, Gowda LR, Prakash V. Detection of genetically modified soya and maize: impact of heat processing. Food Chem. 2009;117:514–521. doi: 10.1016/j.foodchem.2009.04.028. [DOI] [Google Scholar]
- Xiumin W, Da T, Qingfeng G, Fang T, Jianhua W. Detection of Roundup Ready soybean by loop-mediated isothermal amplification combined with a lateral-flow dipstick. Food Control. 2012;29:213–220. [Google Scholar]