Abstract
The chemical, functional and pasting properties of cassava starch and soy protein concentrate blends intended for biofilm processing were studied. Cassava starch and soy protein concentrates were prepared and mixed at different proportions (100: 0%; 90 : 10%; 80 : 20%; 70 : 30%; 60;40% and 50: 50%). Addition of varying levels of soy protein concentrates to cassava starch led to increases in moisture (from 7.10 to 9.17%), protein ( from 0.32 to 79.03%), ash (from 0.45 to 2.67%) and fat (from 0.17 to 0.98%) contents while crude fiber, carbohydrate and amylose contents decreased from ( 1.19 to 0.38%, 90.77 to 57.01% and 29.45 to 23.04%) respectively . Water absorption capacity and swelling power of cassava starch were improved as a result of soy protein concentrate addition while syneresis and solubility value of composite blends were lower than 100% cassava starch. In general, cassava–soy protein concentrate blends formed firmer gels than cassava starch alone. There were significant (p ≤ 0.05) increases in peak viscosity (from 160.12 to 268.32RVU), final viscosity (from 140.41 to 211.08RVU) and pasting temperature (from 71.00 to 72.32 °C ) of cassava starch due to addition of soy protein concentrate. These results suggest that the addition of soy protein concentrate to cassava starch affected the studied functional properties of cassava starch as evidenced by changes such as reduced syneresis, and solubility that are desirable when considering this biopolymer as an edible biofilm.
Keywords: Cassava starch, Soy protein concentrate, Functional, Syneresis, Pasting properties
Introduction
Nigeria is the largest producer of cassava in the World producing a third more than Brazil and almost double the production capacity of Thailand and Indonesia; with the current production level being about 45 million metric tonnes per annum (NIFST 2010; Anon 2006). Starch is one of the major components of the dry matter in cassava, with a mean proximate composition of 58.90% (IITA 1990). Cassava starch has many remarkable characteristics including high paste viscosity and clarity as well as high freeze–thaw stability which are advantageous for industrial applications (Nwokocha et al. 2009).
Starch contributes greatly to the textural properties of many foods and is widely used in food and industrial applications as a thickener, colloidal stabilizer, gelling agent, bulking agent, water retention agent and adhesive (Goel et al. 1999). Starches from different sources vary in their functionality as a result of differences in their granular structure, amylose content and branch chain length distribution. Native cassava starches generally have limited functional properties which reduces their application in food systems (Huerta-Abrego et al. 2010). Their use in industrial applications is also limited by low shear stress resistance, susceptibility to thermal decomposition, high retrogradation and syneresis.
These shortcomings may be improved through physical, chemical and enzyme modification of starches or blending starches with protein isolates (Huerta-Abrego et al. 2010; Betancur-Ancona et al. 2001). In addition, proteins and polysaccharides are widely used in foods to modify food texture or functional properties (Zhang et al. 2010). The interaction of starch and protein is important in determining macroscopic properties of food products such as functional, flow, structure, stability, texture and mouth feel of food products (Li et al. 2007). In biopolymer food packages such as edible films, biopolymer interactions play an important role in determining their functional and mechanical properties (Phan et al. 2009).
Soybean is largely cultivated in Nigeria especially in the middle belt regions with Benue State accounting for about 45% of the total production in the country; with its production capacity of about 500,000 metric tonnes annually, making Nigeria the largest producer on the African continent (Anaekwe 2011). Soy protein concentrate is a soy product after soy whey (soluble carbohydrate) is removed from defatted soy flour (Jong 2006). Soy protein concentrate contain high nutritional value, essential amino acid content and excellent functional properties that makes it a valuable ingredient in various types of prepared foods, meat analogues, dairy and bakery products (Alibhai et al. 2006).
To the best of our knowledge, there is scanty of information on chemical, functional and pasting properties of cassava starch and soy protein concentrate blends. Such data could be used in food formulations as well as improving the functionality of cassava starch when used as biopolymer components in edible film preparations.
Materials and method
Source of raw material
Fresh sweet cassava (Cultivar TMS 30470) tubers and soybean (Cultivar TGX 1448-2E) seeds were procured from Crop Production department, Federal University of Technology, Minna, Nigeria.
Cassava starch extraction
Wet method as described by Ihekoronye and Ngoddy (1985) was used for cassava starch isolation. Fresh cassava tuber was manually peeled, washed with clean tap water and milled into slurries. The slurries were suspended in cold deionized water and sieved to remove the fibrous materials leaving the starch in solution. The starch layer was suspended in deionized water and centrifuged 6 to 7 times, until the settled starch gave a firm, dense deposit at the bottom. The final sediment was suspended in cold deionized water and screened through 150 μm screen to keep the cell wall off the starch slurry. Then the residue was amassed and deposited quietly for 6 h. The starch suspension obtained was dried in a convection oven at 50 °C until constant weight was achieved. The dried material was milled and sieved with a 75 μm screen to obtain the starch.
Preparation of soy protein concentrates
The isoelectric precipitation method described by Nasri and El Tinay (2007) was used in the preparation of soy protein concentrates. Defatted soybean samples were extracted by blending with 1M NaCl using flour to solvent ratio of 1:10. The peptized liquor was centrifuged at 12,000 g for 30 min. The extract was precipitated isoelectrically at pH 4.5 by addition of 0.1N HCl. The protein was allowed to dry in open air at room temperature for 24 h and then ground in an electric blender (Moulinex) to pass through a 75 μm screen and stored at 4 °C until used.
Blend formulation
Cassava starch and soy protein concentrate were mixed at different proportions (100: 0%; 90 : 10%; 80 : 20%; 70 : 30%; 60;40% and 50: 50%). A Kenwood mixer was used to achieve uniform blending.
Chemical analyses
Moisture content, protein, fat and ash contents of cassava starch, soy protein concentrate and their blends were determined using the AOAC (1995) methods. The amylose content of cassava starch and their blends was determined using the method of Williams et al. (1970) involving the preparation of stock iodine solution and iodine reagent. A 0.1 g of starch was weighed into a 100 mL volumetric flask, then 1 mL of 99.7–100% (v/v) ethanol and 9 mL 1N sodium hydroxide were carefully added. The mouth of the flask was covered with parafilm and the contents were properly mixed. The samples were heated for 10 min in a boiling water bath to gelatinize the starch (the timing was started when boiling began). The samples were removed from the water bath and allowed to cool, then made up to the mark with distilled water and shaken thoroughly. Then, 5 mL was pipetted into another 100 mL volumetric flask and 1.0 mL of 1 N acetic acid and 2.0 mL of iodine solution were added. The flask was topped up to the mark with distilled water. Absorbance (A) was read using a spectrophotometer at 620 nm wavelength. The blank contained 1 mL of ethanol and 9 mL of sodium hydroxide, boiled and topped up to the mark with distilled water. Finally, 5 mL was pipetted into a 100 mL volumetric flask; 1 mL of 1 N acetic acid and 2 mL of iodine solution were added and then topped up to the mark. This was used to standardize the spectrophotometer at 620 nm. The amylose content was calculated as:
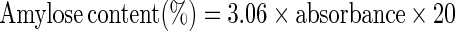 |
Determination of functional properties
Water absorption capacity, swelling power and solubility
Water absorption and swelling power patterns at 60, 70, 80 and 90 °C were determined using a modified version of Sathe and Salunkhe (1981) method. Forty milliliter of 1% starch–soy protein concentrate suspension (w/v) was prepared in a previously tarred, 50 ml centrifuge tube. The tube was placed in a water bath for 30 min at constant temperatures of 60, 70, 80 and 90 °C. Each suspension was centrifuged at 2,120 g for 15 min, the supernatant decanted and the swollen granules weighed. A 10 ml sample was taken from the supernatant, placed in a crucible and dried in an air convection oven (Imperial V, Delhi India) at 120 °C for 4 h to constant weight. Percentage solubility and swelling power were calculated using the following formulae
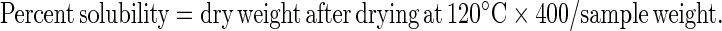 |
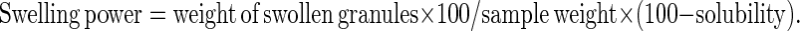 |
Water absorption capacity was measured using the same conditions as above, but expressed as weight of the gel formed per sample, divided by treated sample weight.
Gelation capacity
Gelation capacity was determined according to the method of Coffman and Garcia (1977). Suspensions of 2 to 18 g sample/100 mL in distilled water were prepared. Ten milliliter each of dispersion was transferred into a test tube. The tube was heated in a boiling water bath for 1 h, followed by rapid cooling in a cold water bath. The tubes were further cooled at 4 °C for 2 h. The least gel concentration (LGC) was determined as the concentration when the sample from the inverted test tube will not slip or fall.
Syneresis
Syneresis was determined according to the method described by Ribotta et al. (2007). Syneresis was measured using a centrifuge. A 15 g of starch and starch–soy protein dispersions were placed into 50 mL centrifuge tubes while they were hot and stored at 4 °C for 4 weeks. After storage, the gels were tempered at 20 °C for 2 h and were centrifuged at 1,500×g for 15 min at 20 °C. After centrifugation the free water was separated, weighed and expressed as percentage of the total present in the gel.
Pasting properties
Pasting parameters were determined using a rapid visco analyzer (Newport Scientific Pty Ltd., Warrie-wood NSW 2102, Australia). A 2.5 g of cassava starch and soy protein concentrate blend was weighed into a previously dried empty canister; then 25 ml of distilled water was dispensed into the canister containing the sample. The suspension was thoroughly mixed and the canister was fitted into the rapid visco analyzer. Each suspension was kept at 50 °C for 1 min and then heated up to 95 °C at 12.2 °C /min and held for 2.5 min at 95 °C. It was then cooled to 50 °C at 11.8 °C /min and kept for 2 min at 50 °C. All determinations were done in triplicate.
Statistical analysis
Data were analyzed by analysis of variance (Steel and Torrie 1980). The difference between mean values was determined by the least significant difference (LSD) test. Significance was accepted at 5% probability level (Ihekoronye and Ngoddy 1985). All the data reported in the tables are average values of triplicate determinations.
Results and discussion
Chemical composition of starch and protein concentrate blends
The chemical compositions of cassava starch and soy protein concentrate blends are presented in Table 1. The moisture, protein, ash, fat, crude fiber, carbohydrate and amylose contents ranged from 7.10 to 7.61%, 0.32 to 31.02%, 0.45 to 2.67%, 0.17 to 1.19%, 0.07 to 1.69%, 8.13 to 90.77% and 23.04 to 29.45% respectively. The moisture, protein, ash and fat contents increase with increasing level of soy protein concentrates in the blends while crude fiber, carbohydrate and amylose contents decreased. The low moisture content of the blends fell within the standard range of 0 to 10% as suitable for storage and further processing without the risk of microorganism degradation of the triglycerides as recommended by Standard Organization of Nigeria (SON 2007). The increase in protein value in the blends could be attributed to addition effect of soy protein concentrate with a high protein value (79.03%). High ash value reflects high levels of different kinds of minerals and some vitamins present in the original samples. The observed range of ash values in cassava starch and soy protein blends seem to suggest that in addition to being suitable edible biofilms, the cassava and soy protein blends may be a good source of mineral elements. The decrease in crude fiber, carbohydrate and amylose contents in the blends may be attributed to decrease in substituition level of cassava starch in the blends. As expected, cassava starch contains higher starch, fiber and amylose than soy protein concentrate. According to De La Guerivier (1976), amylose provides surface and textural regularity, elasticity and sticky characteristics to starch based products. Consequently, starch with high amylose content form harder gels (Novelo-Cen and Betancur-Ancona 2005). Therefore, 100% cassava starch may be more useful in food systems requiring high texture as they form firmer gels than the blends.
Table 1.
Chemical compositions of cassava starch and soy protein concentrate blends
| Cassava starch: soy protein concentrate | Moisture (%) | Protein (%) | Ash (%) | Fat (%) | Crude fiber (%) | Carbohydrate (%) | Amylose (%) |
|---|---|---|---|---|---|---|---|
| 100:0 | 7.1d ± 0.10 | 0.32g ± 0.01 | 0.45c ± 0.22 | 0.17b ± 0.00 | 1.2a ± 0.00 | 90.8a ± 1.22 | 29.5a ± 0.67 |
| 0:100 | 9.2a ± 0.23 | 79.0a ± 0.19 | 2.6a ± 0.17 | 0.98a ± 0.16 | 0.07c ± 0.00 | 8.1g ± 0.78 | ND |
| 90.10 | 7.4c ± 0.05 | 6.9f ± 0.43 | 1.1b ± 0.09 | 0.25b ± 0.04 | 0.98b ± 0.05 | 83.5b ± 1.01 | 28.2b ± 0.42 |
| 80:20 | 7.2c ± 0.12 | 11.4e ± 0.29 | 1.5b ± 0.15 | 0.33b ± 0.10 | 0.75b ± 0.01 | 78.3c ± 0.91 | 27.9c ± 0.70 |
| 70:30 | 7.4c ± 0.03 | 17.5d ± 0.77 | 2.2a ± 0.08 | 0.41b ± 0.03 | 0.63b ± 0.07 | 71.9d ± 0.75 | 25.6d ± 0.54 |
| 60: 40 | 7.6c ± 0.00 | 25.2c ± 0.32 | 2.7a ± 0.05 | 0.49ab ± 0.01 | 0.55bc ± 0.03 | 63.5e ± 1.30 | 24.2e ± 0.31 |
| 50:50 | 8.1b ± 0.25 | 31.0b ± 0.54 | 2.9a ± 0.11 | 0.57a ± 0.12 | 0.38b ± 0.05 | 57.0f ± 0.91 | 23.0f ± 0.27 |
Mean values and standard deviation of triplicate determinations.
Mean values with different superscript in a column are significantly (p ≤ 0.05) different from each other.
ND not determined
Water absorption capacity, swelling power, solubility and least gelation capacity
The water absorption capacity, swelling power and solubility of cassava starch and soy protein concentrate and their blends are shown in Table 2, 3 and 4 respectively. Water absorption capacity, swelling power and solubility of the blends were temperature dependent. These parameters increased with increase in temperature with the highest value at 90 °C. Water absorption capacity value ranged from 3.27 to 26.00 g/g water. The water absorption capacity of the blends increased with increasing level of soy protein concentrate. The major chemical compositions that enhance water absorption capacity in food systems are proteins and carbohydrates owing to their hydrophilic constituents such as the polar or charged side chains (Hodge and Osman 1976). The increase in water absorption capacity as a result of increased proportion of soy protein concentrate substitution of cassava starch in the blend may be partly due to higher availability of polar amino acids in soy protein concentrate since polar amino acids in protein have been reported to be primary sites for water interaction (Li et al. 2010). Therefore, the higher water absorption capacity of composite blends than 100% cassava starch was not surprising.
Table 2.
Water absorption capacity (g/ g water) of cassava starch and soy protein concentrate blends
| Blend ratio | Temperature (°C) | |||
|---|---|---|---|---|
| Cassava starch: soy protein | 60 | 70 | 80 | 90 |
| 100 : 0 | 3.3a ± 0.21 | 7.2f ± 0.05 | 10.8g ± 0.46 | 18.0f ± 0.23 |
| 90 : 10 | 3.4a ± 0.12 | 8.4e ± 0.14 | 11.6f ± 0.70 | 19.2e ± 0.10 |
| 80 : 20 | 3.4a ± 0.09 | 10.0d ± 0.37 | 14.1e ± 0.13 | 21.4d ± 0.56 |
| 70 : 30 | 3.5a ± 0.05 | 14.1c ± 0.15 | 17.3c ± 0.90 | 22.5c ± 0.75 |
| 60 : 40 | 3.6a ± 0.01 | 15.2b ± 0.01 | 19.9b ± 0.68 | 24.1b ± 0.23 |
| 50 : 50 | 3.8a ± 0.00 | 17.1a ± 0.46 | 22.9a ± 0.19 | 26.0a ± 0.55 |
Mean values and standard deviation of triplicate determinations
Mean values with different superscript in a column are significantly (p ≤ 0.05) different from each other
Table 3.
Swelling power (g sample/g water) of cassava starch and soy protein concentrate blends
| Blend ratio | Temperature (°C) | |||
|---|---|---|---|---|
| Cassava starch: soy protein | 60 | 70 | 80 | 90 |
| 100 : 0 | 3.3b ± 0.07 | 11.7f ± 0.14 | 19.0f ± 0.25 | 25.0f ± 0.14 |
| 90 : 10 | 3.3b ± 0.05 | 13.0e ± 0.56 | 21.6e ± 0.10 | 29.5e ± 0.29 |
| 80 : 20 | 3.6ab ± 0.01 | 14.0d ± 0.08 | 25.1d ± 0.19 | 33.1d ± 0.11 |
| 70 : 30 | 3.8a ± 0.05 | 15.3c ± 0.32 | 28.4c ± 0.50 | 37.4c ± 0.42 |
| 60 : 40 | 4.1a ± 0.21 | 18.0b ± 0.45 | 29.6b ± 0.13 | 38.9b ± 0.07 |
| 50 : 50 | 4.3a ± 0.13 | 19.5a ± 0.56 | 36.8a ± 0.20 | 50.2a ± 0.42 |
Mean values and standard deviation of triplicate determinations
Mean values with different superscript in a column are significantly (p ≤ 0.05) different from each other
Table 4.
Solubility (%) of cassava starch and soy protein concentrate blends
| Blend ratio | Temperature (°C) | |||
|---|---|---|---|---|
| Cassava starch: Soy protein | 60 | 70 | 80 | 90 |
| 100 : 0 | 5.6a ± 0.03 | 15.0a ± 0.01 | 21.6a ± 0.73 | 32.2a ± 0.81 |
| 90 : 10 | 4.8b ± 0.00 | 11.5b ± 0.02 | 19.3b ± 0.39 | 28.0e ± 0.57 |
| 80 : 20 | 4.8b ± 0.10 | 9.4c ± 0.06 | 18.5c ± 0.25 | 26.6d ± 0.20 |
| 70 : 30 | 4.5b ± 0.02 | 8.8cd ± 0.11 | 17.2d ± 0.10 | 23.1c ± 0.41 |
| 60 : 40 | 4.2bc ± 0.11 | 9.9c ± 0.05 | 16.1e ± 0.77 | 23.8c ± 0.85 |
| 50 : 50 | 3.9c ± 0.07 | 9.1c ± 0.01 | 17.5f ± 0.73 | 22.5a ± 0.81 |
Mean values and standard deviation of triplicate determinations
Mean values with different superscript in a column are significantly (p ≤ 0.05) different from each other
Swelling power is an indication of the water absorption index of the granules during heating (Loos et al. 1981). The swelling power of cassava starch and soy protein concentrate blends varied between 3.29 and 50.20 g water/g sample. The swelling power of the composite blends increased with increase in soy protein concentrate level and may be attributed to decrease in amylose contents in the blends due to decrease in starch content, since amylose acts as a dilutor and a swelling inhibitor (Huerta-Abrego et al. 2010). Also, the high swelling power of cassava starch and soy protein concentrate blends than cassava starch could be attributed to their high water absorption capacity. High water absorption capacity and swelling power are essential for texture (Novelo-Cen and Betancur-Ancona 2005). This may imply that such composite blends could find applications in edible films where biopolymer structure and mechanical properties of films are desirable.
Solubility values (Table 4) ranged from 3.95 to 32.19% with cassava starch having the highest solubility value. Solubility values in composite blends decreased with increase in the proportion of soy protein concentrate possibly due to decreases in amylose contents in the blends. The solubility values obtained in this study is in line with the results of Novelo-Cen and Betancur-Ancona (2005). The swelling power of cassava starch and soy protein concentrate blends varied between 3.29 and 50.20 g water/g sample.
It was observed that cassava starch had a least gelation capacity at 10% (w/v) concentration while composite blends from cassava starch and soy protein concentrates showed their least gelation capacity at 8% (w/v) concentration. The variation observed in the gelling properties of the samples might be associated to the relative ratios of different constituents such as protein, carbohydrate and lipids that make up the blends; thus suggesting that the interaction between such components have a significant role in there functional properties. Sathe and Salunkhe (1981) reported that gelation is not only a function of quantity of protein but the type of protein as well as its non-protein components. The gelation properties of cassava starch and soy protein blends will enhance their utilization in food systems that require gel formation.
Pasting properties of starch and soy protein blends
The pasting properties of cassava starch and soy protein concentrate blends are presented in Table 5. The pasting properties of cassava starch and soy protein concentrate blends showed that there were significant (p ≤ 0.05) increases in peak viscosity (from 160.12 to 268.32RVU), trough (from 85.37 to 126.67RVU), breakdown (from 65.21 to 166.83RVU), final viscosity (from 140.41 to 211.08RVU), setback (from 55.04 to 92.67RVU), peak time (from 3.69 to 4.77 min) and pasting temperature (from 71.00 to 72.32 °C) with up to 50% level of soy protein concentrate. The higher peak viscosity observed in the blends compared to 100% cassava starch could be attributed to the dilution of amylose contents in the composite blends. Zaidul et al. (2007) and Blennow et al. (2001) reported that high peak viscosity was associated with low amylose contents in flour and starch samples. Peak viscosity is an indication of the thickening power of the starch, the higher the peak viscosity the higher the thickening power. The high peak viscosity values of the composite blends may be suitable for products requiring high gel strength and elasticity as reported by Adebowale et al. (2005). Also, the high peak viscosity, trough, break down and setback values of composite blends may be attributed to their high water absorption capacity that caused increased starch swelling as observed in this study. Proteins contain many hydrophilic groups (such as –COOH, –NH2, –OH, and –SH) all of which are capable of forming cross links with starch and these cross links may be responsible for their higher paste viscosity as compared to cassava starch paste (Goel et al. 1999). High values of breakdown are associated with high peak viscosities, which in turn, are related to the degree of swelling of the starch granules during heating (Ribotta et al. 2007). Pasting temperature has been reported to relate to water binding capacity (Adebowale et al. 2005). The higher pasting temperature of composite blends may be attributed to their high water absorption capacity. The results of the pasting properties of cassava starch and soy protein blends obtained in this study was in line with those of Ribotta et al. (2007) for wheat starch and soy protein isolate mixtures.
Table 5.
Pasting properties of cassava starch and soy protein concentrate blends
| Cassava: soy protein | Peak viscosity (RVU) | Trough (RVU) | Breakdown (RVU) | Final viscosity (RVU) | Set back (RVU) | Peak time (Minutes) | Pasting temperature (°C) |
|---|---|---|---|---|---|---|---|
| 100:0 | 160.1f ± 1.23 | 85.4f ± 1.12 | 74.8e ± 0.76 | 140.4f ± 1.10 | 55.0f ± 0.65 | 3.7b ± 0.00 | 71.0b ± 0.45 |
| 90:10 | 198.5e ± 0.98 | 133.3a ± 0.90 | 65.2f ± 0.53 | 201.9b ± 0.92 | 68.7e ± 0.23 | 3.8b ± 0.01 | 71.5b ± 0.76 |
| 80:20 | 214.5d ± 0.81 | 117.1c ± 0.34 | 97.5d ± 0.90 | 188.9d ± 1.34 | 71.8d ± 0.59 | 3.9b ± 0.01 | 71.7ab ± 0.53 |
| 70:30 | 227.3c ± 1.17 | 110.3d ± 0.91 | 116.9c ± 0.47 | 186.7e ± 0.56 | 76.4c ± 0.47 | 4.4a ± 0.03 | 71.9a ± 0.81 |
| 60:40 | 252.0b ± 0.73 | 126.7b ± 0.59 | 125.4b ± 0.64 | 211.1a ± 0.90 | 84.4b ± 0.33 | 4.8a ± 0.05 | 72.1a ± 0.45 |
| 50:50 | 268.3a ± 0.86 | 101.4e ± 0.95 | 166.8a ± 0.83 | 194.2c ± 1.21 | 92.7a ± 0.32 | 4.6a ± 0.03 | 72.3a ± 0.90 |
Mean values and standard deviation of triplicate determinations
Mean values with different superscript in a column are significantly (p ≤ 0.05) different from each other
Effect of storage on syneresis of cassava starch and soy protein concentrates
Syneresis is the separation of liquid from a gel or starch-containing products and is usually viewed unfavorable because it is associated with product deterioration (Ribotta et al. 2007). Syneresis values in the blends ranged from 5.13 to 24.21% with cassava starch having higher values than cassava starch–soy protein concentrate blends (Table 6). Syneresis values were dependent on storage time. Addition of soy protein concentrate decreased syneresis of the samples during storage. This could be partly due to high water retention capacity of soy protein concentrate. This decrease in syneresis of cassava starch and soy protein concentrate mixtures during storage is not in line with the results of Ribotta et al. (2007). They reported an increase in syneresis values for wheat starch–soy protein isolate blends during storage and pointed out that such decrease in syneresis against the expected increase in value obtained in their study may be attributed to protein–starch interaction. Considering the syneresis data generated in this study, its shows that blending of cassava starch with soy protein concentration will improve its functionality compared to native cassava starch.
Table 6.
Effect of storage time on the syneresis (% w/w) of cassava starch and soy protein concentrate blends
| Blend ratio | Storage period (days) | |||
|---|---|---|---|---|
| Cassava starch: soy protein | 7 | 14 | 21 | 28 |
| 100 : 0 | 6.5a ± 0.19 | 7.4a ± 0.05 | 15.8a ± 0.27 | 24.2a ± 0.34 |
| 90 : 10 | 5.9b ± 0.50 | 6.2b ± 0.26 | 13.6b ± 0.19 | 19.9b ± 0.02 |
| 80 : 20 | 5.8b ± 0.01 | 6.5b ± 0.03 | 13.1b ± 0.12 | 18.3c ± 0.09 |
| 70 : 30 | 5.3c ± 0.00 | 6.3b ± 0.01 | 13.0b ± 0.50 | 20.2b ± 0.33 |
| 60 : 40 | 5.6b ± 0.03 | 7.7ab ± 0.03 | 12.8c ± 0.03 | 17.7d ± 0.17 |
| 50 : 50 | 5.1c ± 0.11 | 6.9a ± 0.19 | 11.9d ± 0.10 | 17.8d ± 0.40 |
Mean values and standard deviation of triplicate determinations
Mean values with different superscript in a column are significantly (p ≤ 0.05) different from each other
Conclusions
Blending of cassava starch with soy protein concentrate improved its functional such as water absorption capacity, swelling power, gelation capacity—with reduced solubility and syneresis and pasting properties. The results indicate that such blends may be useful in food systems requiring high gel strength and elasticity such as edible films considering their gelling ability, high peak viscosity with reduced starch solubility and syneresis.
Acknowledgement
Chinma, C.E. is grateful to Federal University of Technology, Minna, Nigeria for the award of postgraduate fellowship.
References
- Adebowale AA, Sanni LO, Awonorin SO. Effect of texture modifiers on the physicochemical and sensory properties of dried fufu. Food Sci Technol Int. 2005;11:373–382. doi: 10.1177/1082013205058531. [DOI] [Google Scholar]
- Alibhai Z, Mondor M, Christine Moresoli C, Lamarche F. Production of soy protein concentrates/isolates: traditional and membrane technologies. Desalination. 2006;191:351–358. doi: 10.1016/j.desal.2005.05.026. [DOI] [Google Scholar]
- Anaekwe EN (2011) Investment opportunity in Nigeria: Soybean export in Nigeria; the Opportunities. http://farriconsultingng.blogspot.com (Accessed 12th March, 2011)
- Anon (2006) Cassava master plan: a strategic action for the development of the Nigeria cassava Industry. http://www.eucord.org (accessed 26th June 2011)
- AOAC (1995) ‘Official methods of Analysis’. Association of official analytical chemist. 16th edition, Washington DC
- Betancur-Ancona DA, Chel-Guerrero LA, Camelo-Matos RI, Dávila-Ortiz G. Physicochemical and functional characterization of baby lima bean (Phaseolus lunatus) starch. Starch. 2001;53:219–226. doi: 10.1002/1521-379X(200105)53:5<219::AID-STAR219>3.0.CO;2-R. [DOI] [Google Scholar]
- Blennow A, Bay-Smidt AM, Bauer R. Amylopecin aggregation as a function of starch phosphate content studied by size exclusion chromatography and on-line refractive index and light scattering. Int J Biol Macromol. 2001;28:409–420. doi: 10.1016/S0141-8130(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Coffman CW, Garcia VV. Functional properties and amino acid content of protein isolate from mung bean flour. J Food Technol. 1977;12:473–484. doi: 10.1111/j.1365-2621.1977.tb00132.x. [DOI] [Google Scholar]
- De La Guerivier JF. Principles of the extrusion cooking process application to starch foods. Bul des Anc Ele de Ecol Fran De Meu. 1976;276:305. [Google Scholar]
- Goel PK, Singhal RS, Kulkarni PR. Studies on interaction of corn starch with casein and casein hydrolysates. Food Chem. 1999;64:383–389. doi: 10.1016/S0308-8146(98)00134-4. [DOI] [Google Scholar]
- Hodge JC, Osman EM. Carbohydrates. In: Fennema RO, editor. Principles of food science. Part 1. Food chemistry. New York: Marcel Dekker; 1976. pp. 97–200. [Google Scholar]
- Huerta-Abrego A, Segura-Campos M, Chel-Guerrero N, Betancur-Ancona D. Functional properties of starch with lima bean proteins. Food Technol Biotechnol. 2010;48:36–41. [Google Scholar]
- Ihekoronye AL, Ngoddy PO. Integrated food science and technology for the tropics. London: Macmillian; 1985. pp. 252–253. [Google Scholar]
- IITA (1990) Cassava in Tropical Africa. A reference manual. IITA, Ibadan. 15–16
- Jong L. Effect of soy protein concentrate in elastomer composites. Composites. 2006;37:438–446. doi: 10.1016/j.compositesa.2005.05.042. [DOI] [Google Scholar]
- Li JY, Yeh AI, Fan KL. Gelation characteristics and morphology of corn starch/soy protein concentrate composites during heating. J Food Eng. 2007;78:1240–1247. doi: 10.1016/j.jfoodeng.2005.12.043. [DOI] [Google Scholar]
- Li W, Shu C, Yan S, Shen Q. Characteristics of sixteen mung bean cultivars and their protein isolates. Int J Food Sci Technol. 2010;45:1205–1211. doi: 10.1111/j.1365-2621.2010.02259.x. [DOI] [Google Scholar]
- Loos PJ, Hood LF, Graham HD. Isolation and characterization of starch from Bread fruit. Cereal Chem. 1981;58:282–286. [Google Scholar]
- Nasri N, El Tinay NA. Functional properties of fenugreek (Trigonella foenum graecum) protein concentrate. Food Chem. 2007;103:582–589. doi: 10.1016/j.foodchem.2006.09.003. [DOI] [Google Scholar]
- Nigerian Institute of Food Science and Technology (NIFST) (2010) Stakeholders symposium on “cassava inclusion policy in Nigeria : experience, challenges and the way forward” organized by Cassva value addition—NIFST in collaboration with FMAN at Nicon Luxury Hotel, Abuja, Nigeria on 30th November, 2010
- Novelo-Cen L, Betancur-Ancona D. Chemical and functional properties of Phaseolus lunatus and Manihot esculenta starch blend. Starch. 2005;57:431–441. doi: 10.1002/star.200500398. [DOI] [Google Scholar]
- Nwokocha LM, Aviara NA, Senan C, Williams PA. A comparative study of some properties of cassava and cocoyam starches. Carbohydr Polym. 2009;76:362–367. doi: 10.1016/j.carbpol.2008.10.034. [DOI] [Google Scholar]
- Phan TD, Debeaufort F, Voilley A, Luu D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J Food Eng. 2009;90:548–558. doi: 10.1016/j.jfoodeng.2008.07.023. [DOI] [Google Scholar]
- Ribotta PD, Colombo A, León AE, Añón MC. Effects of soy protein on physical and rheological properties of wheat starch. Starch. 2007;59:614–623. doi: 10.1002/star.200700650. [DOI] [Google Scholar]
- Sathe SK, Salunkhe DK. Functional properties of Great Northern bean (Phaseolus vulgaris) J Food Sci. 1981;46:71–75. doi: 10.1111/j.1365-2621.1981.tb14533.x. [DOI] [Google Scholar]
- Standard Organization of Nigeria (SON) (2007). Nigerian Industrial standard for cassava starch. pp 1–8
- Steel RDG, Torrie JH. Principles and procedures of statistics: a biometrics approach. 2. New York: McGraw Hill; 1980. p. 623. [Google Scholar]
- Williams FD, Kuzina FD, Hlynka I. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–420. [Google Scholar]
- Zaidul ISM, Yamauchi H, Kim SJ, Hashimoto N, Noda T. RVA study of mixtures of wheat flour and potato starches with different phosphorus content. Food Chem. 2007;102:1105–1111. doi: 10.1016/j.foodchem.2006.06.056. [DOI] [Google Scholar]
- Zhang Q, Ismail N, Cheng LH. Flow behaviour and temperature stability of chicken muscle proteins—modified waxy corn starch blends. Int Fd Res J. 2010;17:137–145. [Google Scholar]


