Abstract
Biomedical researchers often assume that sponsors, subjects, families, and disease-associated advocacy groups contribute to research solely because of altruism. This view fails to capture the diverse interests of many participants in the emerging research enterprise. In the past two decades, patient groups have become increasingly active in the promotion and facilitation of genetics research. Simultaneously, a significant shift of academic biomedical science toward commercialization has occurred, spurred by U.S. federal policy changes. The concurrent rise in both the roles that subjects play and the commercial interests they have presents numerous ethical challenges. We examine the interests of different research participants, finding that these interests are not addressed by current policies and practices. We conclude that all participants should be given a voice in decisions affecting ownership, access to, and use of commercialized products and services, and that researchers and institutions should negotiate issues relating to control of research results and the sharing of benefits before the research is performed.
The paragraph above is from a prototypical consent form for DNA banking in genetics research. The statement reflects the prevalent view among researchers, institutions, and sponsors in regard to the allocation of intellectual property rights and associated monies that result from such research (Beskow et al. 2001). As reflected in standardized consent forms, current institutional policies and practices that govern biomedical research assume that subjects act solely because of altruism and that the sole duty of researchers is to disclose their intentions, rather than to recognize any interest of subjects in commercial products that may result from the research. This view was fostered by the California Supreme Court, when, more than a decade ago, it held that John Moore did not have a property interest in his tissues, but only had a right to be informed about both the intent to develop a cell line and the potential commercial interests of the clinician researchers with whom he interacted (Moore v. Regents of the University of California 1990).
This view fails to capture the myriad interests and motives of the different actors involved in the research enterprise, including academic scientists, public and private sponsors, commercial institutions, patient groups, and research subjects themselves. A consideration of these interests reveals many areas of potential conflict. The resolution of these issues early in the research process may facilitate research and better protect the rights and interests of patients and research subjects.
The Emerging Collaborative-Research Enterprise
Patient groups are becoming more active in the promotion and facilitation of preclinical and clinical research. Although patient groups have long played a key role through participation in research (e.g., by helping coordinate subject identification and recruitment and fund-raising to support research), a new type of relationship is emerging as groups become key players in the promotion of studies of the causal role of genetics in diseases (Lindee 2000). This developing role has occurred concurrently with two other significant changes in biomedical research: the creation and rapid evolution of technology transfer and the explosion in biotechnology science and investment. The concurrent rise in research subjects’ roles and commercial interests raises new ethical and policy challenges.
Various groups have started or are starting foundations for the funding of research, compiling disease-specific registries of patients and genealogical and medical databases, establishing tissue and DNA banks to provide resources crucial for genetics research, and developing scientific expertise that can make substantive contributions to the direction and performance of research. Demonstrating the number and breadth of interest groups, the Genetic Alliance lists more than 500 members from family and patient support groups, advocacy groups, health professionals, universities, companies, and the government. Individuals involved in these initiatives and their families often participate directly in research. The resultant close collaborative relationships with scientists greatly facilitate targeted research by providing access to affected communities, giving highly motivated assistance in identifying and soliciting potential participants from these communities, giving credibility to the researchers that can increase trust and participation, and sharing the costs of recruitment.
Several recent examples illustrate the range of subject and group involvement. These examples suggest that the nature of the relationships that exist between patients, their families and the larger community of families at risk, and the genetics research community is changing. These cases reveal that the participants can have different interests in the outcomes of research and that these interests may conflict with those of other actors. By identifying the interests in the products and benefits of research that are at stake, we hope to begin a conversation and negotiation among those involved to develop policies for protecting the interests of subjects that will allocate the costs, burdens, and benefits in an equitable manner.
Canavan Disease
Dan and Debbie Greenberg and their children and more than 150 other families from around the world participated in an extensive collaborative relationship with Dr. Reuben Matalon that led fruitfully to the discovery of the aspartoacylase gene in 1993 (Kaul et al. 1993). These families participated in the studies; helped identify, solicit, and collect blood samples from other afflicted families; and secured research support from the Canavan Foundation, the National Tay Sachs and Allied Diseases Association, the United Leukodystrophy Foundation, and various local groups. Rabbi Josef Ekstein, Executive Director of Dor Yeshorim–Committee for Prevention of Jewish Genetic Diseases, in Brooklyn, provided access to approximately six thousand stored blood samples. These were used by the researchers to rapidly identify several mutations in Ashkenazi Jewish families and to estimate population frequencies (Kaul et al. 1994).
Unbeknownst to the families, the researchers and Miami Children’s Hospital (MCH) secured U.S. patent 5,679,635, which covered all diagnostic and therapeutic uses of the gene in October 1997. MCH embarked on what the families believed to be a restrictive and unduly expensive licensing program. On October 30, 2000, three involved families, the National Tay Sachs and Allied Diseases Association, and Dor Yeshorim filed suit against MCH, in an attempt to prevent continued use of the patent in a manner that the plaintiffs believe is immoral, unfair to those who made the research possible, and likely to restrict access to the test (Greenberg v. Miami Children’s Hospital 2000; Marshall 2000).
Pseudoxanthoma Elasticum (PXE)
In the mid-1990s, Sharon Terry created PXE International (Kolata 2000b). The foundation helped identify and solicit participation from affected families, established a registry and a repository, and raised money to support studies through use of these resources. PXE International negotiated with researchers to whom they provided support and access to biomaterials for research, and through use of Material Transfer Agreements, retained authorship in any papers and ownership rights in any patents, to ensure broad and affordable availability of the test and to retain influence over downstream development (Pennisi 2000; Smaglik 2000). The gene implicated in the disorder was identified in early 2000 (Bergen et al. 2000; Le Saux et al. 2000), and the foundation’s ownership increases the likelihood that the interests of those most affected by PXE will be represented in decisions about licensing and treatment research and development.
α-1-Antitrypsin Deficiency
The Alpha-1 Foundation was created to expedite research into therapies and a cure for α-1-antitrypsin deficiency. The Foundation has provided monetary research support, it has created a confidential research registry as well as a DNA bank, it sponsors annual research symposia, and it helped secure funding for a program and faculty chair at the University of Florida. The Foundation is developing proactive policies that will best protect the interests of the α-1-antitrypsin deficiency community in new discoveries resulting from collaborative and sponsored research, while directly promoting research for treatments and a cure.
Recognizing Interests in Genetics Research
The current “market” in genetics research includes patients and families, disease-associated advocacy groups, foundations, governmental agencies such as the National Institutes of Health (NIH), researchers, universities, biotechnology firms, and pharmaceutical companies. All have common goals: the discovery of the genetic causes of disease, the broad availability of testing to patients, and, ultimately, the development of treatments or cures. However, individuals and groups have more diverse interests, motivations, and incentives for performing, funding, participating in, and promoting research. It is important to understand those interests to develop strategies that best satisfy all of the parties. Of course, not all interests and motives can be captured in an analysis such as this, but our generalizations may help the understanding of what the limits of our knowledge are in this area, by spurring conversation among interested parties, and may provide a foundation for basic policies that can be adapted to different needs and situations.
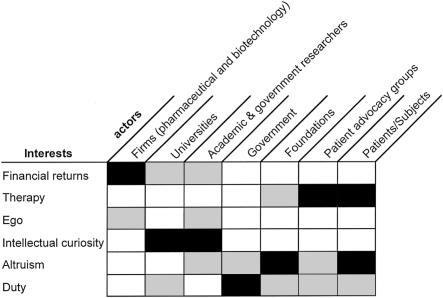
The key interests that typify the contributions of firms, foundations, government, researchers, advocacy groups, and patient participants are presented in figure 1 and include the following:
-
1. Financial return.
Contributions to research are treated as investments on which a monetary reward and reasonable rate of return is expected and, to the extent feasible, negotiated. The type of investments may be financial or otherwise, but here the payoffs are expected to be monetary.
-
2. Therapy.
Various actors invest money, time, expertise, and other resources for fundamental knowledge about the disease, development and provision of carrier, prenatal, and population-screening tests, and ultimately development of preventative measures, treatments, and cures. The motive here is self-interest in treatment or cure for a disease.
-
3. Ego (self-esteem).
Motivation comes from the intangible rewards of reputation, notoriety, prizes, and awards.
-
4. Intellectual curiosity.
Reflecting the very basic scientific motives of seeking knowledge, making discoveries, and understanding the world around us, intellectual curiosity is distinguished from therapy, in that knowledge is valued in itself, independently of its instrumental value as a relief from disease.
-
5. Altruism.
Contributions of money, time, and active research promotion and participation are treated as an unalloyed gift to help those who will most benefit in the future from the potential fruits of the research. Under a pure altruism model, there is no expectation of return or personal benefit.
-
6. Duty.
Individuals, professionals, and organizations are obliged to act for the good of others. Patients and those who act as their advocates may feel obligated to promote and participate in research for themselves, for their families, and for those in the affected or at-risk disease community; government may be compelled to support research on behalf of citizens who are or may be afflicted, to promote the public health, or even for reasons of foreign policy or national security.
Figure 1 graphically depicts the contrasting interests of different research participants, providing a heuristic method that permits the express consideration of interests. From our consideration of these interests, we have drawn various inferences.
Figure 1.
A heuristic method for consideration of interests of various actors involved in genetics research. All actors may be influenced in part by any of these factors, but we posit that some interests will dominate, as suggested by deeper shading of the intersecting boxes.
Biotechnology and pharmaceutical firms will invest risk capital in downstream research and development, such as taking a promising drug through animal and human testing, only if they are assured of some measure of market exclusivity. Any retained interests of research collaborators or of those who made upstream discoveries increases the risk and depresses the motivation for the firm to undertake a development effort (Heller and Eisenberg 1998). The firm rightfully operates for the purpose of earning a return on its investment, and the motivations for performing research are largely—if not entirely—financial. We believe, however, that the firm should be willing to compensate those who contribute to its research and development efforts, but demands must be reasonable in relation to the contributions, the likely payoffs, and the risks of the research. Simply, any economic actor who draws on and profits from resources such as public funds or participation by collaborating disease-associated advocacy groups should not be freely permitted to use and profit from those public and private resources without paying for them.
Understanding the motives and interests of universities is more problematic. Arguably, the primary purpose of a research university is to contribute to learning and development of new knowledge. These institutions are typically nonprofit, reflecting that they have duties to society to contribute to the public welfare. But the institutions have also become increasingly capitalist ventures, centered around technology transfer. Technology-transfer departments are permitted by federal law to cover patenting and licensing expenses with royalty income and typically will return portions of excess revenue to faculty and their departments, as well as to the university itself for educational and research purposes (U.S. Code 2001). Universities, then, may be viewed as schizophrenic, defending the open and free sharing of ideas traditionally valued in the academy while simultaneously pursuing the protection of intellectual property and profits.
Technology-transfer programs have, in turn, created a monetary incentive for researchers themselves, who can earn quite substantial sums from inventions licensed to industry as well as from more-direct commercial ties, such as equity ownership in commercial ventures. Despite growing concerns about financial conflicts of interest (Cho et al. 2000; DeAngelis 2000), the incidence of financial incentives and relationships has become more prevalent within the biomedical research community (Krimsky et al. 1996; Angell 2000). We posit that government and university researchers remain primarily motivated by intellectual curiosity and academic freedom, for they likely could earn higher salaries in industry (Kreeger 2001).
One of the most difficult issues that NIH, other federal and state funding agencies, and nonprofit foundations face is the assurance that their financial sponsorship of research promotes the greatest public good. On the one hand, patenting and technology transfer are viewed as effective tools for promoting disclosure of inventions, spurring investment, rewarding success, and moving basic research off the bench and into the marketplace. Intellectual property rights can ensure firms the exclusivity they need to justify expenditures of risk capital for development of marketable products and services. On the other hand, these public institutions—along with patients and advocacy organizations—should be wary of having their expenditures totally appropriated by private firms or universities. Although citizens, patients, and patients' advocates want treatments and cures, they also want these to be available at reasonable prices so that they will be broadly accessible by the community. They also do not want to be made to pay twice for an invention resulting from research to which they materially contributed, with financial, scientific, participatory, or other kinds of assistance—once through their direct support and “again through higher monopoly prices and restricted supply” (Eisenberg 1996). Justice concerns arise if the costs of products are set too high and result in material inequities in access by participants.
Of course, it is problematic to assume that government, private foundations, or patient advocacy groups established by affected families will necessarily reflect the interests and values of those afflicted with a particular disease. These groups often reflect the interests and perspectives of a relatively small number in the community who happen to be particularly active. Sometimes, competing goals and priorities emerge that lead to the creation of separate institutions. In several cases, separate foundations have evolved, with one focusing on issues such as political advocacy, prevention, and education while another seeks the development of therapies and a cure.
Although we cannot say that the interests of patients and advocacy groups are identical, we believe that these organizations are the best situated to represent, protect, and serve the interests of those most affected. First, for genetic diseases, there is a sense in which the affected families have “voted.” As in the case of PXE, families have joined the group, put their names in a registry, and perhaps even deposited their genetic material for research, clearly indicating support for the institution and its representation of their interests. Second, other institutions and individuals (e.g., companies, researchers, and universities) do not have the interests of the affected families as their primary interests. As we noted above, companies have financial obligations to their stockholders and to the bottom line. Researchers have obligations to develop knowledge and to meet the needs of their universities. Only the patient advocacy and support groups have the interests of the affected families as their primary interests, even if they have other interests as well.
The difficult balancing suggested above has led to policies—particularly within the federal government—that favor nonexclusive over exclusive licensing, except in cases in which substantial investment farther downstream is required to bring an invention to market (Katz and Merz 2001). In the 1980s, there was also a regulatory attempt to require the “reasonable pricing” of products developed under Cooperative Research and Development Agreements between industry and the federal government (Brody 1996). The financial risk posed by such conditions were viewed as an impediment to downstream investment, and these requirements were dropped. Notwithstanding, the federal government does not give away its inventions; the NIH collected royalties of more than $50 million in fiscal year 2000, which funds are used to reward inventors, pay licensing expenses in the Institutes and Centers, and sponsor educational and research activities (Office of Technology Transfer, PHS/NIH).
To the extent that royalties—including those paid to the government—raise prices to consumers, there is a fundamental justice issue in how such royalties are distributed. If the royalties are used to fund downstream development—say, of therapies, the costs of these efforts will effectively be internalized to the community most likely to benefit from them. This should be acceptable to the community, as long as the costs do not lead to material inequities in access. If the money is, instead, used to reward prior discovery or treated as profit or other return on the contribution of the community, then it may be less acceptable. In our view, fairness demands that profits be distributed among those who contributed to the research in an equitable manner. The patient community may not want a financial return, instead preferring to have an influence on access, pricing, and the terms guiding ownership and control of downstream developments.
Current policies and practices in research largely reflect none of these interests, and the default stipulation noted in the beginning of this essay that there will be no sharing of financial returns with subjects fails to respect the contributions, or protect the interests, of subjects and the disease communities they seek to help (Weijer and Emanuel 2000). Strategies and policies that respect the contributions of the many involved parties need to be developed. For example, for products resulting from population-based studies, the Human Genome Organisation (HUGO) Ethics Committee recommended that ∼1%–3% of net profits be returned to the populations, to support humanitarian aid or health care infrastructure (HUGO Ethics Committee 2000; Weijer 2000; Berg 2001). This rate was arbitrarily chosen and does not reflect any economic analysis of relative contribution or fairness. Others have recommended that even greater amounts be shared with subjects (Weir and Horton 1995). As a very-well-publicized example, deCODE Genetics of Iceland executed an agreement with the Icelandic government in 2000. This license granted, to deCODE, the exclusive use of a centrally compiled population health database, which deCODE will combine with proprietary genealogic and genetic databases. In return, deCODE will pay all expenses incurred by the government that are related to deCODE’s effort to build and maintain the central database—as well as an annual inflation-adjusted payment of 70 million KR (currently slightly less than $700,000) and 6% of its annual pretax profit, up to an additional 70 million KR (Icelandic Ministry of Health and Social Security 2000). To put these figures in context, we compare them to Iceland’s total public expenditure on health, which was slightly more than 40 billion KR in 1998 (National Economics Institute, Iceland). Similar policies for benefit sharing were under discussion prior to the collapse, in December 2000, of the Framingham Genomics initiative (Kolata 2000a; Rosenberg 2001) and have been used to recognize the contributions of individuals and agrarian communities in the developing world for their “traditional knowledge” and technology relating to rare and useful agricultural stock (Gupta 1998).
We believe it is unacceptable to presume that patients, subjects, disease-associated advocacy groups, foundations, and government (and, in turn, taxpayers) are all pure altruists, as policies and practices now do presume, especially when these stakeholders have contributed in a meaningful way to the research enterprise. It is unfair to these groups for their “investments” to be wholly appropriated by firms or universities with no commitment to return to the community something of value that they can both access and afford. We believe that there has been a market failure with respect to the value added to the research enterprise by patient and subject groups, and ways should be found to recognize and reward their contributions. What that return should be will vary depending upon the relative contributions made.
Of course, there are economic concerns and practical problems with the general claim that subjects should share in the financial rewards of research. Subjects often stand to benefit indirectly as consumers from any new knowledge and therapies that result from research in which they participate. This is a reasonable claim as long as prices neither extract exorbitant profits from the subjects nor create unfair limitations on access. In addition, the contribution of individual subjects may be quite minimal, given that they bear little physical, psychosocial, or financial risk from the research. Subjects could be compensated by up-front payments for their time, pain, and effort, and, although this may make research more expensive because it is not contingent on the success of the studies, such compensation is a reasonable way to acknowledge individuals’ roles and the expected value of their contributions. Alternatively, providing individuals with any share of potential profits (e.g., royalties) may simply be unmanageable, and the burden of such royalties might provide a disincentive to downstream investment unless they are reasonable and manageable. A middle ground would be to distribute, for example, a single share of stock in any biotechnology business venture associated with the research, because it might be worth very little unless the research is successful. Perhaps a new class of preferred subject-class stock could be created for this purpose.
Thus, unless there is some group that represents participants, there may be no good way to recognize and reward individuals’ contributions. Of course, representative organizations are not always present or involved. Although there are many disease-associated advocacy groups, there remain many diseases that have either no collective representation or limited collective representation, and many individuals may not be affiliated with the groups that do exist. Nonetheless, when an advocacy group is present, it is likely that researchers will work with the group if they see that the group increases the ability to perform their research. If the group contributes too little or demands too much, then researchers may look for individual participants separate from the group. Advocacy groups thus serve two functions: adding value by facilitating research and providing a collective voice to individual participants, backed by the power to negotiate and frame the ways in which research and commercialization take place.
Entities involved in the commercial aspects of research (including companies and universities that develop intellectual property portfolios from which royalty revenues can be earned) should be expected, as a matter of public policy and research ethics practice, to openly negotiate with foundations and disease-associated advocacy group and to resolve issues regarding ownership, control of downstream use, limits on financial profit-taking from inventions, equitable profit sharing, and other acknowledgments of all contributions before the research is done. When individuals participate, researchers and institutional review boards should consider the value that their contribution has toward the performance of the research and should provide just compensation. The claims made herein about benefits sharing are based purely in equity and not property or other rights; to put it simply, we believe it is the right thing to do. For their part, subjects and disease foundations must also be reasonable about what their contributions are worth. Much more work needs to be done to evaluate the relative value of contributions, perhaps with the goal of the development of standards to be used in these negotiations.
Acknowledgments
Supported in part by NIH grant HG02034, to Stanford University, and by a gift from deCODE Genetics and grants from the Alpha-1 Foundation and the Rockefeller Foundation, to the University of Pennsylvania Center for Bioethics. J.F.M. received a consulting fee from the Alpha-1 Foundation for legal and ethical advice relating to intellectual property matters, received an honorarium from the Canavan Disease Screening Consortium for participation in a meeting between Canavan Disease Screening Consortium members and Miami Children’s Hospital, and has agreed to provide pro bono expert testimony in the Canavan litigation. The opinions expressed are solely those of the authors. We thank Marcie Merz and several anonymous reviewers for comments about an earlier draft of the manuscript for this article.
Electronic-Database Information
URLs for data in this article are as follows:
- Genetic Alliance, http://www.geneticalliance.org/members.html (for member list)
- National Economic Institute, Iceland, http://www.ths.is/rit/buskop/t0401.xls (for general government total expenditures from 1980 to 1998)
- Office of Technology Transfer, PHS/NIH, http://ott.od.nih.gov/NewPages/TTstats00.pdf (for NIH technology-transfer activities from fiscal year 1993 to fiscal year 2000)
References
- Angell M (2000) Is academic medicine for sale? New Engl J Med 342:1516–1518 [DOI] [PubMed] [Google Scholar]
- Berg K (2001) The ethics of benefit sharing. Clin Genet 59:240–243 [DOI] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, ten Brink JB, de Jong PT (2000) Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 25:228–231 [DOI] [PubMed] [Google Scholar]
- Beskow LM, Burke W, Merz JF, Barr PA, Terry S, Penchaszadeh VB, Gostin LO, Gwinn M, Khoury MJ (2001) Informed consent for population-based research involving genetics. JAMA 286:2315–2321 [DOI] [PubMed] [Google Scholar]
- Brody B (1996) Public goods and fair prices: balancing technological innovation with social well-being. Hastings Cent Rep 26:5–11 [PubMed] [Google Scholar]
- Cho MK, Shohara R, Schissel A, Rennie D (2000) Policies on faculty conflicts of interest at US universities. JAMA 284:2203–2208 [DOI] [PubMed] [Google Scholar]
- DeAngelis CD (2000) Conflict of interest and the public trust. JAMA 284:2237–2238 [DOI] [PubMed] [Google Scholar]
- Eisenberg RS (1996) Public research and private development: patents and technology transfer in government-sponsored research. Virginia Law Rev 82:1663–1727 [Google Scholar]
- Greenberg v Miami Children’s Hospital, case 00C-6779 (ND Illinois 2000) [Google Scholar]
- Gupta AK (1998) Rewarding local communities for conserving biodiversity: the case of the honey bee in protection of global biodiversity: converging strategies. In: Guruswamy LD, McNeely JA (eds) Protection of global biodiversity: converging strategies. Durham: Duke University Press, pp 180–189 [Google Scholar]
- Heller M, Eisenberg R (1998) Can patents deter innovation? the anticommons in biomedical research. Science 280:698–701 [DOI] [PubMed] [Google Scholar]
- HUGO Ethics Committee (2000) Statement on benefit-sharing. Clin Genet 58:364–366 [DOI] [PubMed] [Google Scholar]
- Icelandic Ministry of Health and Social Security (2000) Agreement between the Minister for Health and Social Security and Islensk erf-agreining egf. relating to the issue of an operating license for the creation and operation of a health sector database, January 21. Ministry of Health and Social Security, Reykjavik [Google Scholar]
- Katz D, Merz JF (2000) Patents and licensing, policy, patenting of inventions developed with public funds. In: Mehlman MJ, Murray T (eds) Encyclopedia of ethical, legal, and policy issues in biotechnology. New York: John Wiley & Sons, pp 854–866 [Google Scholar]
- Kaul R, Gao GP, Aloya M, Balamurugan K, Petrosky A, Michals K, Matalon R (1994) Canavan disease: mutations among Jewish and non-Jewish patients. Am J Hum Genet 55:34–41 [PMC free article] [PubMed] [Google Scholar]
- Kaul R, Gao GP, Balamurugan K, Matalon R (1993) Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat Genet 5:118–123 [DOI] [PubMed] [Google Scholar]
- Kolata G (2000a) Use of research is reconsidered. New York Times, December 29:A19 [Google Scholar]
- ——— (2000b) Who owns your genes? New York Times, May 15:A1 [Google Scholar]
- Kreeger KY (2001) Science salaries. The Scientist 14:35 [Google Scholar]
- Krimsky S, Rothenberg LS, Stott P, Kyle G (1996) Financial interests of authors in scientific journals: a pilot study of 14 publications. Sci Eng Ethics 2:395–410 [DOI] [PubMed] [Google Scholar]
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B, Quaglino D, Pasquali-Ronchetti I, Pope FM, Richards A, Terry S, Bercovitch L, de Paepe A, Boyd CD (2000) Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet 25:223–227 [DOI] [PubMed] [Google Scholar]
- Lindee MS (2000) Genetic disease since 1945. Nat Rev Genet 1:236–241 [DOI] [PubMed] [Google Scholar]
- Marshall E (2000) Genetic testing: families sue hospital, scientist for control of Canavan gene. Science 290:1062 [DOI] [PubMed] [Google Scholar]
- Moore v Regents of the University of California, 51 Cal 3d 120, 271 Cal Rptr 146, 793 P 2d 479 (1990) [Google Scholar]
- Pennisi E (2000) Biomedical research: patients help track down disease gene. Science 288:1565–1567 [DOI] [PubMed] [Google Scholar]
- Rosenberg R (2001) Questions still linger on Heart Study access: private industry’s right to use publicly funded data for profit remains at issue. Boston Globe, February 21:D4 [Google Scholar]
- Smaglik P (2000) Tissue donors use their influence in deal over gene patent terms. Nature 407:821. [DOI] [PubMed] [Google Scholar]
- US Code, title 35, sec 202(c) (2001) [Google Scholar]
- Weijer C (2000) Benefit-sharing and other protections for communities in genetic research. Clin Genet 58:367–368 [DOI] [PubMed] [Google Scholar]
- Weijer C, Emanuel EJ (2000) Ethics: protecting communities in biomedical research. Science 289:1142–1143 [DOI] [PubMed] [Google Scholar]
- Weir RF, Horton JR (1995) DNA banking and informed consent—part 2. IRB 17:1–8 [PubMed] [Google Scholar]