Abstract
Dichotic listening (DL) tests are among the most frequently included in batteries for the diagnosis of auditory processing disorders (APD) in children. A finding of atypical left ear advantage (LEA) for speech-related stimuli is often taken by clinical audiologists as an indicator for APD. However, the precise etiology of ear advantage in DL tests has been a source of debate for decades. It is uncertain whether a finding of LEA is truly indicative of a sensory processing deficit such as APD, or whether attentional or other supramodal factors may also influence ear advantage. Multivariate machine learning was used on diffusion tensor imaging (DTI) and functional MRI (fMRI) data from a cohort of children ages 7–14 referred for APD testing with LEA, and typical controls with right-ear advantage (REA). LEA was predicted by: increased axial diffusivity in the left internal capsule (sublenticular region), and decreased functional activation in the left frontal eye fields (BA 8) during words presented diotically as compared to words presented dichotically, compared to children with right-ear advantage (REA). These results indicate that both sensory and attentional deficits may be predictive of LEA, and thus a finding of LEA, while possibly due to sensory factors, is not a specific indicator of APD as it may stem from a supramodal etiology.
Keywords: Diffusion Magnetic Resonance Imaging, Functional Magnetic Resonance Imaging, Attention, Dichotic listening tests, Functional laterality, Auditory processing disorder
Highlights
-
•
Left-ear advantage (LEA) in speech-related dichotic listening tests is atypical.
-
•
LEA is predicted by differences in functional activation in frontal eye fields.
-
•
LEA also predicted by differences in WM microstructure in left auditory radiation.
-
•
LEA is therefore not specific for auditory processing disorder (APD) in children.
1. Introduction
Auditory processing disorder (APD) is a deficit in the neural processing of auditory stimuli (Asha. American Speech-Language-Hearing Association, 2005), distinct from higher-order cognitive function, which is estimated to affect 2–3% of school-aged children (Chermak and Musiek, 1997). Children diagnosed with APD have normal peripheral hearing but manifest deficits in one or more areas of higher-order auditory perception. The disorder is highly heterogeneous in nature, with a long list of behavioral indications (Asha. American Speech-Language-Hearing Association, 2005), such as difficulty understanding spoken language in noisy backgrounds or competing messages; difficulty following complex auditory directions or commands; difficulty in sound localization and lateralization; and difficulty in auditory discrimination. Thus, there is a wide variety of tests used in diagnostic procedures for APD (Emanuel, 2002; Emanuel et al., 2011; Jerger and Musiek, 2000). Behavioral findings from a study of over 1000 children in the U.K. suggest that many children referred for APD testing may in fact suffer a cognitive and/or attention deficit rather than a sensory processing deficit (Moore et al., 2010). Thus, a key difficulty in APD diagnosis is differential diagnosis of APD as opposed to other supramodal influences such as attention, memory, cognition, and ability to follow directions (Cacace and Mcfarland, 2005; Katz and Tillery, 2005).
Dichotic listening (DL) tests are among the most frequently included and important tests used when diagnosing APD in children (Emanuel, 2002; Emanuel et al., 2011). In DL tasks, two different auditory stimuli are presented simultaneously to the right and left ears and the listener is required to report what was heard. Most individuals report speech-related stimuli presented to their right ear with greater accuracy compared to their left ear in the “free recall” condition (individuals report stimuli in either order), a phenomenon known as the right-ear advantage (REA). The REA has been a robust finding (Hugdahl, 2002; Hugdahl and Hammar, 1997), since it was first described in the 1960s. However, not every individual demonstrates a REA; somewhere around 15–20% of right-handed individuals exhibit either no ear advantage (NEA) or a left-ear advantage (LEA) (Bryden, 1988). Moncrieff (2011) also reports that the prevalence of LEA in typically achieving children is approximately 20%.
Many clinical audiologists consider ear advantage (EA) scores to be indicators of hemispheric dominance for language as well as neurologically-based auditory processing, language, and learning disorders (Keith, 1984). The common interpretation among clinical audiologists is that a REA for verbal stimuli indicates left hemisphere dominance for language. A LEA for verbal stimuli, on the other hand, is considered to indicate mixed or reversed dominance for language: a finding that is common among children with phonologic, reading, language and learning disorders (Hugdahl, 2005; Kimura, 1961; Newman and Sandridge, 2007). The rationale for the inclusion of DL tests in test batteries is that abnormal findings can result from the presence of APD (Asha. American Speech-Language-Hearing Association, 2005; Debonis and Moncrieff, 2008). Thus, a finding of LEA may indicate a sensory deficit which is thought to be associated with right-hemisphere language dominance. However, both the etiologies of right-hemisphere language dominance and of EA are unknown, lending doubt to this interpretation. Attentional or other supramodal influences may be responsible, or partly responsible, for EA, right-hemisphere dominance, or both.
The association between LEA and right-hemispheric dominance is also uncertain. This construct is based on evidence of loci of language function obtained from multiple studies (Kimura, 1961). However, the prevalence of NEA/LEA is significantly greater than the prevalence of right-hemisphere lateralization for language processing in right-handed individuals. The prevalence of right-hemisphere lateralization is estimated at only between 1 and 5% (Knecht et al., 2000; Loring et al., 1990). Also, validation studies in normal adults and epileptic patients of speech-related DL (e.g. Bethmann et al., 2007; Fernandes et al., 2006; Fontoura et al., 2008; Hugdahl et al., 1997; Hund-Georgiadis et al., 2002; Strauss et al., 1987; Van Ettinger-Veenstra et al., 2010; Zatorre, 1989) confirmed the REA as predictive of left-hemisphere dominance, but not LEA as predictive of right-hemisphere dominance.
Moreover, the interpretation of LEA made by clinical audiologists (e.g. that of indicating mixed or reversed language dominance and a possible sensory deficit) has never been tested using techniques of evoked potentials or imaging studies. American Academy of Audiology (Musiek et al., 2010) recognized the need for brain imaging and other electrophysiologic research to ascertain the status of the central auditory nervous system in children and adults. In this study we investigate whether functional MRI (fMRI) and diffusion tensor MRI (DTI) data can be used to predict whether a given individual will show a REA or a LEA. Toward this end, we use multivariate machine learning (ML) techniques (Haynes and Rees, 2006; Norman et al., 2006; O'toole et al., 2007; Pereira et al., 2009). These techniques represent a novel method for analyzing neuroimaging data which provide several advantages over standard analyses. While standard analyses (e.g. Worsley et al., 2002) are mass-univariate, multivariate analyses may show greater sensitivity (Pereira et al., 2009) to detect significantly different patterns of activation or structural differences between groups, when these differences are spread out over several regions. Mass-univariate analyses must implement a procedure for multiple statistical comparisons across regions, and while several regions may individually fail to meet a given threshold (corrected for multiple comparisons), the data combined over those regions may in fact meet that threshold.
2. Materials and methods
2.1. Participants
The LEA study cohort consisted of right-handed children 7–14 years old (N = 13) who were recruited from the auditory processing disorders (APD) clinic at Cincinnati Children's Hospital Medical Center (CCHMC). All children were native English speakers with no diagnosed cognitive or neurological pathologies or hearing loss. These children had been referred for APD assessment due to listening and hearing complaints despite normal peripheral hearing, and were administered the SCAN3 APD test battery (Keith, 2009). Children with a LEA were identified from results of the competing words free recall subtest of the SCAN3 battery. Parents reported complaints such as difficulty understanding speech in background noise, following oral instructions, and rapid speech. These children often had difficulty following directions and difficulty localizing the source of the signal/speech, and frequently requested speakers to repeat oral information. However, parents reported no concerns or symptoms in regard to cognitive or neurological pathology; thus, in accordance with standard clinical practice, no specific cognitive or neurological evaluation was conducted. None of the participants received a diagnosis of APD after administration of the test battery (which was a chance occurrence, as APD diagnosis was not used as an inclusion or exclusion criterion).
Typically developing children (N = 20) were recruited as controls from the Cincinnati area via flyer and word of mouth. All of these children had a typical right ear advantage (REA) on the competing words free recall subtest of the SCAN3 test battery. The Institutional Review Boards at CCHMC and the University of Cincinnati approved all experiments. Informed consent from one parent and assent from each child were obtained before testing. Demographic information is reported in Tables 1 and 2 for those children for whom usable fMRI and DTI data were successfully obtained, respectively. All children were right handed based on a questionnaire filled out by parents that included a question “is your child right/left handed/inconsistent”. They were asked to base their response according to which hand the child used for writing, throwing, striking a match, scissors, toothbrush, spoon, knife, and a computer mouse. There were 2 children identified from the chart review who were reported as being left handed by their parents so they were not invited to participate in the study.
Table 1.
Demographic and behavioral data on children classified as left-ear advantage (LEA) and right-ear advantage (REA) whose data was included in the fMRI study.
| REA | LEA | p | |
|---|---|---|---|
| #M, #F | 10M, 2F | 10M, 2F | 1.00 |
| Age (months) | 133.6 ± 24.3 | 129.9 ± 23.4 | 0.71 |
| # words identified in right ear (SCAN3 Competing words free recall) | 17.3 ± 1.7 | 12.2 ± 1.8 | < .001 |
| # words identified in left ear (SCAN3 Competing words free recall) | 14.2 ± 2.0 | 16.8 ± 2.2 | < 0.01 |
| Sqrt. # of retained frames | 10.2 ± 1.0 | 9.5 ± 1.5 | 0.20 |
Table 2.
Demographic and behavioral data on children classified as left-ear advantage (LEA) and right-ear advantage (REA) whose data was included in the DTI study.
| REA | LEA | p | |
|---|---|---|---|
| #M, #F | 12M, 2F | 8M, 2F | 0.71 |
| Age (months) | 131.1 ± 27.0 | 131.3 ± 25.0 | 0.98 |
| # words identified in right ear (SCAN3 competing words free recall) | 17.7 ± 1.1 | 12.0 ± 1.7 | < .001 |
| # words identified in left ear (SCAN3 competing words free recall) | 14.1 ± 2.0 | 16.7 ± 2.3 | < .01 |
2.2. Audiological testing
Peripheral hearing sensitivity for both ears was verified via standard pure tone audiometry and immittance testing in a sound proof booth. All children had pure-tone thresholds of 15 dB HL or better at octave frequencies ranging from 250 Hz to 8000 Hz, and Type-A tympanograms. There was no significant difference in pure tone average (PTA) for either ear (p > 0.5, unpaired T-test).
Following peripheral auditory testing, the Dichotic Competing Words (CW) subtest of the SCAN3 battery (Keith, 2009), typically used to test children for APD, was administered to all children. Two different monosyllabic words were presented to both ears simultaneously and the children were instructed to repeat both words in any order, called the “free recall” response mode. The test included 20 word pairs presented dichotically. The word pairs were aligned for onset and offset to eliminate any cue for the first word heard. The EA score was calculated as the mathematical difference between the right ear (RE) and left ear (LE) raw score. A positive value is considered a REA and a negative value a LEA. The EA scores were compared to age-matched normative data. All children in the LEA group had an atypical LEA with prevalence of 10% or less compared to the normative data.
As part of their APD assessment, additional subtests of the SCAN3 test battery including Auditory Figure Ground, Filtered Words, Competing Words-Directed Ear and Competing Sentences had been administered to the children with LEA prior to recruitment in the study. For the Auditory Figure Ground (AFG) subtest, test stimuli consist of 20 monaural words presented in multi-talker speech background noise at an SNR of + 8 dB; the stimuli are presented at intensity of 8 dB greater than the background noise. For the Filtered Words (FW) subtest, the test stimuli consist of one syllable words that have been low-pass filtered at 750 Hz with a roll-off of 30 dB per octave. Twenty words are administered to each ear. The Competing Words-Directed Ear (CW-DE) subtest includes monosyllabic word pairs presented to both ears simultaneously. The child is instructed to repeat both words in a prescribed order: repeating from the right ear first for the first 15 word pairs then repeating from the left ear first for the second 15 word pairs. The Competing Sentences subtest consists of sentence pairs presented dichotically in a focused attention mode of administration. In this mode, the child is instructed to repeat only the stimuli presented to the right ear for the first 10 sentence pairs followed by repeating only the stimuli presented to the left ear for the second 10 sentence pairs. Normative data is available for all subtests of the SCAN3 according to the child's age.
2.3. fMRI scans
All scans were acquired on a Philips 3T Achieva system. The event-related fMRI paradigm was similar to that used in a previously published study (Van Den Noort et al., 2008). The paradigm consisted of word pairs taken from the CW paradigm. 20 word pairs were presented dichotically. Silent gradient intervals were used for the word presentations (Schmithorst and Holland, 2004); this method allows the presentation of stimuli without any background scanner noise. This technique has been shown to provide similar or better activation than using continuous scanning (Vannest et al., 2009). The children responded orally by repeating back the heard words. For the control task, the two words were presented diotically, one after the other. Diotic presentation was selected as a control task in order to control for cognitive processes related to receptive language, expressive language, working memory, and sublexical auditory processing. A 6-second scanning period (in which three image volumes are acquired) followed a 5-second stimulus presentation period. fMRI–EPI scan parameters were: TR/TE = 2000/38 ms, matrix = 64 × 64, FOV = 24 cm × 24 cm, SENSE factor = 2, slice thickness = 5 mm, 25 slices acquired covering the whole brain. The stimuli were presented using Presentation software (Neurobehavioral Systems Inc., Albany, CA) and the order of presentation was randomized at runtime. One scan run was obtained with 40 trials (20 dichotic, 20 diotic) with 11 s per trial (5 second stimulus presentation, 6 second scanning period) for a total acquisition time of 7 min 20 s. In-scanner performance was monitored via an MRI-compatible microphone.
2.4. DTI scans
The 15-direction standard Philips EPI-DTI sequence was used with the following parameters: FOV = 22.4 cm × 22.4 cm, matrix = 112 × 112, slice thickness = 2 mm, 60 slices were acquired for 2 mm isotropic resolution over the whole brain, b value = 1000 s/mm2, SENSE factor = 2.
2.5. First-level analyses
The fMRI scans were motion-corrected using an affine transformation and a pyramid iterative algorithm (Thevenaz et al., 1998) (scans were not smoothed prior to analysis as a “searchlight” procedure (described below) is to be used.). The motion correction was performed separately for the sets of 1st, 2nd, and 3rd scans after the silent period. The motion correction was performed repeatedly for each set of reference images. The remainder of the 1st level analysis for fMRI was performed using in-house routines written in IDL (ENVI, Boulder, CO). The optimal motion correction results (e.g. which set of reference of images were chosen) were selected using the maximum number of frames meeting a cost function threshold selected via visual inspection (Szaflarski et al., 2006). The entire dataset was discarded if there were not at least 47 retained frames. This happened for 9 participants, leaving a total of 24 participants with usable data. A General Linear Model (GLM) was performed separately on the 1st, 2nd, and 3rd scans after the silent period with manner of presentation (diotic vs. dichotic) the variable of interest, and linear and quadratic terms to account for scanner drift as covariates of no interest. Results were combined into a single Z-score map of functional activation and transformed into stereotactic (Talairach) space (Talairach and Tournoux, 1988) at 3.75 mm × 3.75 mm by 5 mm resolution.
The DTI scans were pre-processed in a similar manner as that described in Schmithorst et al. (2005). Scans were visually inspected for gross motion artifacts and slice drop-outs due to motion during the diffusion sensitizing gradients. This resulted in datasets being discarded from 9 participants, leaving a total of 24 participants with usable data (21 participants had usable data for both DTI and fMRI). Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) maps were computed from the tensor components. These maps were transformed into standard Montreal Neurological Institute (MNI) space using the following procedure (routines written in SPM8, Wellcome Institute of Cognitive Neurology, London, UK). The T1-weighted anatomical images were segmented into gray, white, and CSF images using the segmentation procedure in SPM8. The FA maps were co-registered to the white matter maps using a 6-parameter rigid-body transformation, and this transformation was applied to the other DTI parameter maps. The white matter maps for each child were normalized to the white matter template (using the non-linear normalization routine). This transformation was then applied to the DTI parameter maps. Only voxels with FA > 0.25 and white matter probability > 0.9 were retained for further analysis.
2.6. ML analyses
ML analyses were performed using in-house code written in IDL (ENVI, Boulder, CO). The ML analyses were performed using a searchlight approach with a 5 × 5 × 5 cube and a Support Vector Machine (SVM) classifier (Vapnik, 1995). The searchlight cube was chosen to be similar in magnitude to spatial filters typically used for voxelwise fMRI and DTI analyses (e.g. Schmithorst and Holland, 2006; Schmithorst et al., 2005, 2007, 2011). The SVMLight program (Joachims, 1999) was used when there was more than one independent variable; in-house code was written in IDL for the special case of one independent variable. The ML analyses only included voxels where each participant had a usable data point (e.g. in the brain for fMRI, in white matter with FA > 0.25 and WM probability > 0.9 for DTI).
The classifier accuracy in distinguishing LEA children from REA children was estimated using leave-one-subject-out cross-validation. To avoid biasing the classification accuracy estimator (Pereira et al., 2009), the following steps were performed only on the training data for each cross-validation run. For each voxel, the mean value (Z-score map for fMRI; FA, RD, MD, or AD for DTI) was determined for the 5 × 5 × 5 cube centered on the voxel (the mean value, rather than a weighted average found via e.g. Gaussian filtering, was chosen due to its superior SNR). Feature selection: Feature selection was performed by ranking all voxels in the brain. The metric used was accuracy on an SVM classifier (using data from a single voxel only) estimated using leave-one-subject-out cross-validation (since these steps involve only the training data for each cross-validation run in which one participant is left out, each training run for estimation of classifier accuracy using leave-one-subject-out cross-validation will therefore include data from N-2 participants.). The voxels were ranked according to the performance of the classifier, and only data from the top-ranked voxels were retained for further analysis. The number of voxels to retain was chosen a priori as between 1 and 10. Classification: An SVM classifier was trained for each set (e.g. from 1 to 10) of retained voxels, and the number of voxels to retain was determined based on which classifier performed the best. The voxels to retain and the parameters for the classifier were stored and then used to classify the test subject.
One of the children was scanned twice, on different days. Both datasets were retained for analysis, since having more training data available is always optimal for ML. When the doubly-scanned participant was the test subject for the cross-validation, however, the other scan from the participant was removed from the training set to avoid bias. In order to estimate accuracy from cross-validation, the doubly-scanned participant was counted as being correctly classified if he/she was correctly classified both times, incorrectly classified if he/she was incorrectly classified both times, and assigned a value of 0.5 if one of the datasets resulted in a correct classification while the other resulted in an incorrect classification.
For those classifiers shown to be better than chance level, the classifiers were re-trained using the entire dataset using the above procedure. Classifier maps (incorporating all voxels included in the classifier and 5 × 5 × 5 cubes centered on them) were constructed to display the relevant voxels (e.g. where the searchlight was located). For each region, ROIs were drawn and the fMRI or DTI values for each class (REA vs. LEA) were obtained. Data values were averaged from both sets of the doubly-scanned participant. Post-hoc analyses were performed on the resulting data to further investigate the significance of the regions included in the classifiers (relevant regions may incorporate less than 125 voxels due to the part of the searchlight cube being outside the region of usable data. Also, we note that in this study we are classifying individual subjects and the training data is independent from the test data, unlike applications such as classification of cognitive states from neuroimaging data, where the training and test data are correlated. Thus, the null distribution is equivalent to that obtained from chance classification and it is not necessary to use permutation testing to derive the null distribution.).
2.7. Post-hoc tractography analysis
To investigate the DTI results further, the white matter parcellation map (ICBM DTI-81 Atlas) (Mazziotta et al., 1995) was used to classify the relevant white matter region. Additionally, probabilistic tractography (Behrens et al., 2003) was performed, using the voxels relevant for classification from the DTI results as the starting seed points. The probabilistic tractography was performed using routines in FSL (fMRIB, Oxford, UK). The streamlines were transformed back into MNI space and then averaged across participants.
2.8. Post-hoc fMRI analyses to investigate possible effects of participant motion
Differences in participant motion between the LEA and REA children could conceivably affect the results. Thus, an additional analysis was conducted on the average activation in the ROI. A GLM was performed with functional activation as the independent variable and side of ear advantage, participant motion (parameterized by the square root of the number of retained frames), and their interaction as dependent variables.
3. Results
Based on the classification, there were significant differences in (out-of-scanner) performance in the left and right ears of the competing words free recall test between the groups; however, there were no significant differences in age, sex or motion inside the scanner between the groups for either the fMRI or the DTI study (Tables 1 and 2). As all the control participants demonstrated a REA, it was not necessary to exclude any participant based on the dichotic testing results.
Performance on the other subtests of the SCAN3 test battery, administered to all children with LEA, is given in Table 3 as age-normed percentile scores (scores were converted into Z-scores for further statistical analysis). There was no significant difference from normal for Auditory Figure Ground (T(11) = − 1.41, ns), Competing Sentences (T(11) = − 1.44, ns) or Competing Words Directed Ear (T(11) = 1.21, ns). However, there was a significant difference from normal for the Filtered Words subtest (T(11) = 3.39, p < 0.01).
Table 3.
Performance (as age-normed percentile rank) on four subtests of the SCAN3 test for auditory processing disorders: Auditory Figure Ground, Filtered Words, Competing Words-Directed Ear and Competing Sentences, for all children with a LEA.
| Participant # | Auditory Figure Ground | Filtered Words | Competing Words-Directed Ear | Competing Sentences |
|---|---|---|---|---|
| 1 | 9 | 37 | 63 | 37 |
| 2 | 5 | 63 | 37 | 37 |
| 3 | 50 | 50 | 84 | 37 |
| 4 | 75 | 84 | 50 | 63 |
| 5 | 25 | 50 | 84 | 50 |
| 6 | 25 | 75 | 75 | 16 |
| 7 | 25 | 75 | 63 | 63 |
| 8 | 25 | 75 | 50 | 63 |
| 9 | 91 | 91 | 75 | 50 |
| 10 | 50 | 50 | 63 | 37 |
| 11 | 50 | 75 | 5 | 2 |
| 12 | 37 | 91 | 75 | 50 |
For the fMRI task, there were highly significant within-subject correlations between the in-scanner performance during the dichotic condition and the results of the DL test performed outside the scanner (the same words were used for both tests, although the in-scanner presentation order was randomized.). For the number correct in the right ear, overall correlation was R = 0.96 (p < 0.001); in children with right-ear advantage, correlation was R = 0.97 (p < 0.001); in children with left-ear advantage, correlation was R = 0.82 (p < 0.005). For the number correct in the left ear, overall correlation was R = 0.95 (p < 0.001); in children with right-ear advantage, correlation was R = 0.94 (p < 0.001); in children with left-ear advantage, correlation was R = 0.94 (p < 0.001) (due to the difference in correlation coefficients (R = 0.94 vs. R = 0.82) between in-scanner and out-of-scanner performance in the LEA group between the left and right ears, we tested for an in-scanner performance × side interaction on out-of-scanner performance; the result was not significant (T(20) = 0.23, p > 0.5).) For the ear advantage (# correct right − # correct left), overall correlation was R = 0.99 (p < 0.001); for the children with right-ear advantage, correlation was R = 0.86 (p < 0.001); for the children with left-ear advantage, correlation was R = 0.96 (p < 0.001). Root mean square (rms) differences in scores between the two test administrations were also computed: rms differences for the right ear, left ear, and difference were 0.94, 0.96, and 0.68, respectively. No child displayed a difference in the side of ear advantage between the two tests. A systematic difference between the two test administrations was reached at a trend level for the right ear (T(23) = 2.055, p = 0.051) and the left ear (T(23) = 1.74, p = 0.095), with children performing better on average in the scanner, likely due to a training effect. However, these effects canceled each other out in the computation of ear difference scores, where no significant effect was seen (T(23) = 0.29, p > 0.5). All children performed very well in the diotic condition, with each child correctly identifying at least 34 out of the 40 words presented. No difference was seen between REA and LEA children (p > 0.5, unpaired T-test).
A classifier of LEA vs. REA was successfully trained for the fMRI data. The accuracy of the classifier was 87.5% (95% confidence interval 67.6%–95.3%). The accuracy is significantly different from chance (p < 0.001). The relevant region (Fig. 1; Table 4) is the left middle/superior frontal gyrus in the region of the frontal eye fields (BA 8). Post-hoc analysis (Fig. 2) revealed greater activation for children with REA during the diotic presentation in the left frontal eye fields (p < 1e − 4, one-sample T-test) as compared to the dichotic presentation; however, no difference was found in children with LEA (p > 0.5, one-sample T-test). Analyzing possible effects of participant motion, the GLM revealed a significant main effect of EA side (T(20) = 5.24, p < 1e − 4) but no significant main effect of motion (T(20) = 0.9, p > 0.5); there was a trend towards an interaction (T(20) =− 1.7, p < 0.1). Removing the effects of motion via stepwise regression for both groups still resulted in a highly significant difference between groups (p < 1e − 4, unpaired T-test). These results allow us to conclude that our results are not unacceptably biased by participant motion.
Fig. 1.
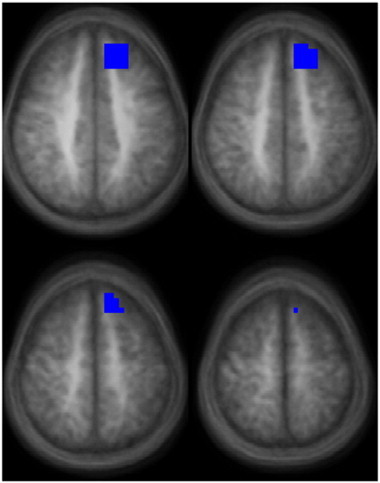
Region found to predict LEA or REA during a speech-related dichotic listening task in children for the functional contrast of listening to words presented dichotically vs. words presented diotically (images in radiologic orientation; slice locations: Z = + 41 mm to Z = + 56 mm, Talairach coordinate system.).
Table 4.
Region (from Fig. 1) found to predict left-ear advantage (LEA) or right-ear advantage (REA) for the functional contrast of listening to words presented dichotically vs. words presented diotically.
| Region | X, Y, Z (mm Talairach) | # Voxels |
|---|---|---|
| Left middle/superior frontal gyrus (BA 8) | − 16, 33, 45 | 61 |
Fig. 2.
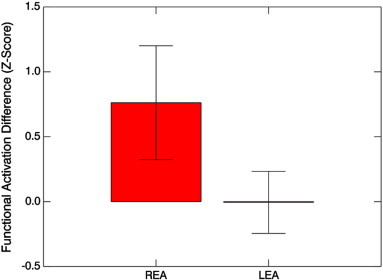
Functional activation (Z-scores, mean ± s.d.) for the contrast of diotic listening vs. dichotic listening, for the region of the left frontal eye fields (shown in Fig. 1), for children with LEA vs. children with REA.
From the DTI data, no classifier of LEA vs. REA was successfully trained for FA, MD, or RD. However, a classifier was successfully trained for the AD data. The accuracy of the classifier was 87.5% (95% confidence interval 67.6%–95.3%). The accuracy is significantly different from chance (p < 0.001). The relevant region (Fig. 3; Table 5) is in the left internal capsule. This region was classified as the retrolenticular part using the DTI atlas; however, the atlas does not distinguish between the retrolenticular and sublenticular parts. Post-hoc analysis (Fig. 4) revealed greater AD for children with LEA compared to those with REA (p < 0.005, unpaired T-test). This region also had greater MD in children with LEA (p < 0.01, unpaired T-test), but no significant difference in FA (p > 0.5, unpaired T-test) or RD (p > 0.5, unpaired T-test). AD was correlated with MD (R = 0.56, p < 0.01) and FA (R = 0.55, p < 0.01) but not RD (R = − 0.19, p > 0.35). For convenience the results of both classifiers are summarized in Table 6.
Fig. 3.
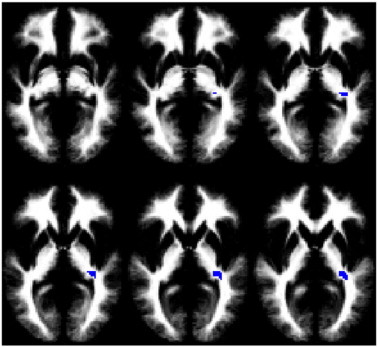
Region found to predict LEA or REA during a speech-related dichotic listening task in children for axial diffusivity (images in radiologic orientation; slice locations: Z = − 4 mm to Z = + 6 mm, MNI coordinate system.).
Table 5.
Region (from Fig. 2) found to predict left-ear advantage (LEA) or right-ear advantage (REA) from axial diffusivity (AD).
| Region | X, Y, Z (mm MNI) | # Voxels |
|---|---|---|
| Left internal capsule (sublenticular region) | − 32, − 31, 3 | 58 |
Fig. 4.
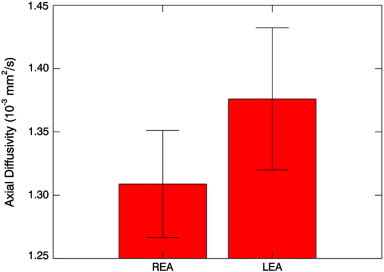
Axial diffusivity (10− 3 mm2/s; mean ± s.d.) for the region shown in Fig. 3 for children with LEA vs. children with REA.
Table 6.
Performance of the classifiers used to predict LEA or REA during a speech-related dichotic listening task in children.
| Classifier | Accuracy (%) | Accuracy (95% confidence limit) | p | REA accuracy (%) | LEA accuracy (%) |
|---|---|---|---|---|---|
| fMRI (dichotic vs. diotic) | 87.5 | 67.6–95.3 | < 0.001 | 83.3 | 91.2 |
| DTI (AD) | 87.5 | 67.6–95.3 | < 0.001 | 85.7 | 90.0 |
The tractography results revealed the most probable connections to be to the posterior part of the thalamus and the auditory cortex (Fig. 5, left), indicating the relevant white matter tract as the auditory radiations, and showing the region in Fig. 4 to be the sublenticular, and not the retrolenticular, part of the internal capsule. However, the tractography results also show connections though the corticospinal tract (Fig. 5, right).
Fig. 5.
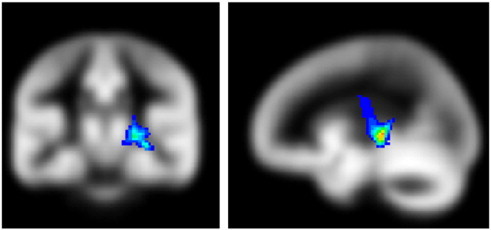
Probabilistic tractography results using the region in Fig. 3 as the starting seed, thresholded from a minimum of 1% of streamlines passing through each voxel, showing the relevant tracts to be the auditory radiations (left) and corticospinal tract (right) (slice locations: Coronal slice, Y = − 25.5 mm; sagittal slice, X = − 22.5 mm, MNI coordinate system).
4. Discussion
There are some neuroimaging and electrophysiology studies in the literature that investigate dichotic listening (Eichele et al., 2005; Jancke and Shah, 2002; Jancke et al., 2001; Thomsen et al., 2004; Westerhausen et al., 2006, 2009a). Schmithorst et al. (2011) published a DTI study that investigated the structural correlates of some tests used to diagnose APD in normal children. However, this is the first study, to our knowledge, which uses machine-learning techniques to predict results of one of the tests, i.e. the competing words free recall subtest. Machine-learning techniques are much more powerful than standard statistical analyses for ascertaining the clinical value of diagnostic tests as they relate to a given pathology. While standard statistical analyses are only capable of informing the investigator that the average result of a given test or tests (to a given degree of statistical significance) differs dependent on pathology, machine-learning techniques inform on the sensitivity and specificity of a specific test battery.
4.1. DTI classifier
LEA was found to be predicted by increased AD in the posterior limb of the internal capsule, including projections to the auditory cortex (indicating the sublenticular portion). The AD values did not significantly correlate with RD. Increased MD, but no significant difference in RD or FA, was found in children with LEA (despite this difference in MD, we were unable to successfully train a classifier using MD. A region that meets a nominal (uncorrected) threshold for significant between-group differences will not necessarily be detected when attempting to train a classifier, due to the fact that the whole brain is searched each training run. In fact, the region may not even be found with a conventional voxelwise GLM analysis, due to the necessity to correct for multiple comparisons. Additionally, the mathematics of an SVM classifier is different from a GLM.).
Physiological interpretation of DTI parameters is open to debate, and there are a number of possible explanations for differences in AD. One interpretation is that increased AD indicates a pattern of reduced tortuosity in the fiber anatomy and/or increased axonal fiber organization (Dubois et al., 2008). This result has been shown histologically in rat experiments (Takahashi et al., 2000) and increased AD (without any change in RD) has been shown developmentally in adolescent older vs. younger males (Ashtari et al., 2007). Also, many studies have shown changes in AD resulting from axonal or neuronal injury or degeneration; however, the direction of the change varies depending on the specific pathology. Increased AD was found as a result of neurodegeneration in patients with amyotrophic lateral sclerosis (Metwalli et al., 2010). However, axonal injury in a mouse model of multiple sclerosis was found to result in decreased AD (Budde et al., 2009; Kim et al., 2006). Yet another possibility is that increased AD results from decreased neurofibrils (such as microtubules and neurofilaments) and loss of glial cells (Kinoshita et al., 1999) in response to intoxication with methylmercury chloride (MMC). To make matters more complicated yet, patients with optic neuritis displayed an initial decrease of AD, followed by an increase over baseline 1 year after onset (Naismith et al., 2009). Future research needs to be performed to better understand the etiology of differences in AD. Nevertheless, we find the explanations of axonal injury, decreased neurofibrils, or neurodegeneration unlikely for our study, given that the axonal injury and decreased neurofibrils were found in mice exposed to toxins, and our study cohort consisted of normal children without obvious neuropathology.
Therefore, we consider reduced tortuosity and increased organization as the most likely explanation for the increased AD. Increased organization (as reflected by increased AD) is consistent with enhanced efferent connectivity. Cortical feedback (through the corticofugal pathway) is known to alter the representation of auditory information at the subcortical level of processing (Luo et al., 2008; Ma and Suga, 2007, 2008; Suga et al., 2002; Xiao and Suga, 2002) in animal models. This feedback is both excitatory and inhibitory (Luo et al., 2008). Thus, children with LEA may be exhibiting increased inhibition of the signal from the right ear due to increased connectivity in the efferent auditory pathway. This may be possibly the result of inefficiencies in the gray matter pruning process, which begins around the younger age range of our study (Giedd et al., 1999).
However, we cannot rule out the DTI results being due to impaired afferent connectivity, as DTI is unable to resolve the directionality of the connectivity difference (e.g. thalamo-cortical vs. cortico-thalamic). Thalamo-cortical projections appear to be related to multimodal and polysensory integration (Kriegeskorte, 2009; Kriegeskorte et al., 2008). If impaired afferent connectivity is the reason for the increase in AD, the causal direction of the relationship between LEA and deficits in multimodal integration is at present unclear.
The tractography results also indicate connections to the motor/premotor regions. This may be an artifact, due to our large effective voxel size (e.g. from the 5 × 5 × 5 searchlight region used) encompassing part of the posterior limb of the internal capsule. However, it may also be the case that LEA is associated with differences in connectivity to premotor regions, as they have been hypothesized to be implicated in speech processing via articulatory representations (Hickok and Poeppel, 2007; Kluender and Lotto, 1999; Liberman and Whalen, 2000; Wilson et al., 2004).
4.2. fMRI classifier
Children with REA activate the left frontal eye fields (BA 8) to a greater extent under the diotic than under the dichotic condition, while no difference was found in children with LEA. These results are consistent with previously published studies suggesting that attentional factors may play an important role in side of ear advantage. Frontal eye field activation has been noted in several neuroimaging studies involving auditory attention (Lipschutz et al., 2002; Tzourio et al., 1997; Zatorre et al., 1999) and dichotic listening (Thomsen et al., 2004). The frontal eye fields play an important role in attentional explanations of the REA (Kinsbourne, 1970, 1973, 1975, 1980). In this model, greater activation of the left hemisphere to language stimuli extends to the lateral-orienting frontal eye fields, biasing attention contralaterally towards the right side of space (Astafiev et al., 2003; Corbetta et al., 1998; Kodaka et al., 1997; Taylor et al., 2008; Wardak et al., 2006). This results in a right-sided advantage for the detection of sensory stimuli including visual, somatosensory, and auditory. In the children with REA, this right-sided attentional bias (as reflected by frontal eye field activation) is greater in the absence of dichotic competition, as is expected as there is no distractor on the contralateral side.
Our findings that side of ear advantage may be related to attentional differences are also consistent with previously published studies investigating laterality and dichotic listening performance in individuals with attention deficit hyperactivity disorder (ADHD). Normal controls, but not adults with ADHD, displayed a REA for word recognition (Hale et al., 2006); the interaction was statistically significant, despite the small sample size (22 controls vs. 22 adults with ADHD). ADHD participants also displayed worse performance for words presented to the right ear compared to controls. Interestingly, individuals with ADHD also displayed better performance overall during dichotic listening involving the presentation of emotional stimuli, and also better performance in the left ear, leading the authors to posit greater right hemisphere and lower left hemisphere contribution in individuals with ADHD. Children with ADHD displayed a REA during a version of dichotic listening when they were instructed to focus attention on the left ear (Oie et al., in press); similar results were also found in adults (Dramsdahl et al., 2011), again indicating a link between spatial attention and ADHD.
4.3. Implications for clinical practice
Side of ear advantage was found to be strongly predicted by differences in activation in the frontal eye fields related to the mode of presentation (diotic vs. dichotic). These results are consistent with LEA being the result of differences in directional biases of attention, as persons with an attentional deficit may focus their listening attention differently than typical listeners, resulting in a left ear advantage (LEA) on free recall dichotic listening tests. However, our results are also consistent with a neuroanatomical underpinning to LEA at the sensory level. Differences in connectivity in the area of the auditory radiations in the left hemisphere (connecting the medial geniculate to auditory cortex) were also shown to predict a finding of LEA as accurately as functional activation differences in the frontal eye fields. Whether this is due to excess efferent, inhibitory connections (as we find more likely) or due to impaired afferent, ascending connections are unknown at this time. Such a deficit is likely associated with impaired speech comprehension, as it inhibits the flow of information into the left auditory cortex and subsequently into language processing areas in the left hemisphere. Therefore, although previous investigations indicate that LEA is sensitive to sensory deficits, we find that it cannot be taken as a specific indicator for modality specific APD.
Our results also support the concern of Moore et al. (2010) that many children referred for APD testing may in fact have a cognitive/attention rather than an auditory perceptual deficit, underscoring the importance of dissociating supramodal influences in APD diagnostic test batteries. In addition to attention and cognition, other examples of supramodal influence include memory, ability to follow instructions, motivation, etc. While none of the study participants with a LEA received a diagnosis of APD, and they performed on average at near-normal or above-normal levels on the other SCAN3 subtests (not involving dichotic listening), they were referred for APD testing with complaints regarding speech perception. The between-test differences in performance are not surprising: large differences in patterns of correlations between brain connectivity and task performance on individual tests used for APD diagnosis, including degraded speech, have previously been found (Schmithorst et al., 2011).
Our results also support previous studies (e.g. Bethmann et al., 2007; Hugdahl et al., 1997) showing that a finding of LEA is not predictive for atypical right-hemispheric language dominance, as we have identified two mechanisms by which LEA could also arise in a child with normal left-hemisphere dominance.
4.4. The etiology of ear advantage
Additionally, our results can inform the ongoing debate over the etiology of ear advantage, for which there is currently no consensus. In the structural model of DL (Kimura, 1964, 1967, 1973), the (weaker) ipsilateral connections in the ascending auditory pathway are suppressed (via some sort of “occlusion” mechanism) at some point in the auditory pathway during presentation of dichotic stimuli. Each hemisphere then exclusively receives input from the contralateral ear. Input from the left ear must therefore traverse interhemispherically from the right auditory cortex across the corpus callosum before it can be processed in the language areas of the left hemisphere. Therefore stimuli presented to the right ear have a more direct connection to the language areas, without the need for a “callosal relay” (Westerhausen and Hugdahl, 2008; Zaidel, 1983) resulting in delay and/or attenuation of auditory information for stimuli presented to the left ear. In the attentional model (Kinsbourne, 1970, 1973), the processing of language stimuli in the left hemisphere biases subsequent attention towards the contralateral (right) hemispace. This model is based on a “filter” theory of selective attention (Broadbent, 1958), in which due to limited capacity for information processing in a given channel, only a proportion of sensory input is accepted for subsequent processing, the remainder being rejected. Since the right-ear message is mainly projected towards an already-activated left hemisphere, the attentional system is biased towards accepting the message presented in the right ear, and rejecting that presented in the left ear.
Evidence in support of the structural model comes from commisurotomized patients who can recognize monaural stimuli but completely fail to recognize stimuli presented to the left ear during a dichotic presentation (Sidtis, 1988; Sparks and Geschwind, 1968; Springer and Gazzaniga, 1975; Springer et al., 1978). The structural model finds additional support from magnetoencephalography (MEG) studies (Brancucci et al., 2004; Della Penna et al., 2007) that show inhibition of the ipsilateral auditory pathway for dichotic stimuli with similar fundamental frequencies and for dichotic stimuli with different intensities. However, one of the most important findings favoring the attentional model is that change in lateralization occurs when listeners are instructed to direct attention to either the left or the right ear (Foundas et al., 2006; Hugdahl, 1995; Hugdahl and Andersson, 1986). Additionally, the REA has also been shown to be a right side of space advantage, in several experiments which either used loudspeakers instead of earphones (Hublet et al., 1976; Morais, 1975; Morais and Bertelson, 1973), or simulated the position of sounds by altering amplitude and/or phase (Morais and Bertelson, 1975). Also supporting the attentional model are studies showing a REA even for monaural stimulation (Henry, 1979, 1983), undercutting the assumption in the structural model that an occlusion mechanism is necessary to elicit a REA.
These two models (structural vs. attentional/supramodal), however, are not mutually exclusive. A recent study (Westerhausen et al., 2009b) demonstrated interactions between top-down (e.g. free-report vs. focused attention) and bottom-up (interaural intensity difference) factors for ear advantage, suggesting that attentional and sensory components are not independent, but interacting. Our results, in which EA was found to be predicted both by attentional factors and by neuranatomical differences below the level of the auditory cortex, lend support to this framework. It should be pointed out, however, that our results are confounded by the fact that the LEA children were referred for APD testing whereas the REA children were not.
4.5. Relation of LEA to other measures of auditory processing
While children with LEA did not show a significant difference from normal on either Auditory Figure Ground (speech in noise) or Competing Sentences, they did perform significantly better than normal on low-pass Filtered Words. For this task, it is likely that participants are using spectral information (normally processed in the right hemisphere) to aid with lexical decision (Obleser et al., 2008; Schonwiesner et al., 2005), and children with LEA may have a more direct input into the right hemisphere, as information does not need to traverse interhemispherically across the corpus callosum if the main input is from the left ear. In normal children, structural connectivity across the corpus callosum has been associated with performance on the Filtered Words test (Schmithorst et al., 2011). However, further research will be necessary on this topic.
4.6. Limitations
The study is subject to some limitations. The study population was biased towards males, which might limit its generalizability; however, sex differences are not consistently reported in DL (Bryden, 1988), and while a recent meta-analysis (Voyer, 2011) found some evidence of greater laterality in males, the effect size was rather small (d = 0.054). Additionally, this study utilized the free-recall version of dichotic listening, in which the listener reports back the two words heard in any order. Further research will investigate whether performance on directed-ear versions of dichotic listening tests may also be predicted via neuroimaging data. In this study lateralization was taken as a dichotomous variable; however, it can also be parameterized as a continuous variable and predicted using a different type of classifier such as Support Vector Regression (Vapnik et al., 1997).
A possible limitation is the selection of the control group from a community population. A more matched control group would have consisted of children with REA referred for APD testing due to complaints regarding speech perception but not eventually diagnosed with APD. The choice of control population to use involves a tradeoff of information obtained about the precise neuroanatomical and neurofunctional correlates of EA versus information available about the clinical relevance of DL testing for APD in children referred for APD testing. If (hypothetically) attentional factors would accurately predict LEA in children referred for APD testing versus REA in children referred for APD testing, we would be able to draw the same conclusion as we have in the current study. However, a much stronger conclusion would have been available from a (hypothetical) failure to predict LEA from attentional factors: in our current study design, we would have been able to conclude that a finding of LEA is specific to a sensory processing deficit and hence of more significant clinical value for APD diagnosis. As the main focus of this study was to investigate the clinical relevance of EA for APD diagnosis, we chose to use a community-based sample for our control population.
5. Conclusion
In children referred for APD testing, LEA during a dichotic listening task involving speech-related stimuli was found to be predicted by greater axial diffusivity in the sublenticular part of the left internal capsule; and lesser functional activation in the left frontal eye fields during diotic speech-related presentations relative to dichotic presentations. Results show that LEA may be predicted by attentional or other supramodal differences as well as sensory deficits and therefore not specific to APD.
Acknowledgment
The authors thank Tom Mitchell, Ph.D., for the helpful discussion regarding the implementation of ML.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Asha. American Speech-Language-Hearing Association (Central) Auditory processing disorders [technical report] 2005. www.asha.org/policy Available from.
- Ashtari M., Cervellione K.L., Hasan K.M., Wu J., Mcilree C., Kester H., Ardekani B.A., Roofeh D., Szeszko P.R., Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. NeuroImage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Astafiev S.V., Shulman G.L., Stanley C.M., Snyder A.Z., Van Essen D.C., Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. Journal of Neuroscience. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G., Clare S., Matthews P.M., Brady J.M., Smith S.M. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bethmann A., Tempelmann C., De Bleser R., Scheich H., Brechmann A. Determining language laterality by fMRI and dichotic listening. Brain Research. 2007;1133:145–157. doi: 10.1016/j.brainres.2006.11.057. [DOI] [PubMed] [Google Scholar]
- Brancucci A., Babiloni C., Babiloni F., Galderisi S., Mucci A., Tecchio F., Zappasodi F., Pizzella V., Romani G.L., Rossini P.M. Inhibition of auditory cortical responses to ipsilateral stimuli during dichotic listening: evidence from magnetoencephalography. European Journal of Neuroscience. 2004;19:2329–2336. doi: 10.1111/j.0953-816X.2004.03302.x. [DOI] [PubMed] [Google Scholar]
- Broadbent D. Pergamon; London: 1958. Perception and Communication. [Google Scholar]
- Bryden M.P. An overview of the dichotic listening procedure and its relation to cerebral organization. In: Hugdahl K., editor. Handbook of Dichotic Listening: Theory, Methods, and Research. Wiley; Chichester, UK: 1988. pp. 1–43. [Google Scholar]
- Budde M.D., Xie M., Cross A.H., Song S.K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. Journal of Neuroscience. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace A.T., Mcfarland D.J. The importance of modality specificity in diagnosing central auditory processing disorder. American Journal of Audiology. 2005;14:112–123. doi: 10.1044/1059-0889(2005/012). [DOI] [PubMed] [Google Scholar]
- Chermak G., Musiek F.E. Singular Publishing Group; San Diego: 1997. Central Auditory Processing Disorders. New Perspectives. [Google Scholar]
- Corbetta M., Akbudak E., Conturo T.E., Snyder A.Z., Ollinger J.M., Drury H.A., Linenweber M.R., Petersen S.E., Raichle M.E., Van Essen D.C., Shulman G.L. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Debonis D.A., Moncrieff D. Auditory processing disorders: an update for speech-language pathologists. American Journal of Speech-Language Pathology. 2008;17:4–18. doi: 10.1044/1058-0360(2008/002). [DOI] [PubMed] [Google Scholar]
- Della Penna S., Brancucci A., Babiloni C., Franciotti R., Pizzella V., Rossi D., Torquati K., Rossini P.M., Romani G.L. Lateralization of dichotic speech stimuli is based on specific auditory pathway interactions: neuromagnetic evidence. Cerebral Cortex. 2007;17:2303–2311. doi: 10.1093/cercor/bhl139. [DOI] [PubMed] [Google Scholar]
- Dramsdahl M., Westerhausen R., Haavik J., Hugdahl K., Plessen K.J. Cognitive control in adults with attention-deficit/hyperactivity disorder. Psychiatry Research. 2011;188:406–410. doi: 10.1016/j.psychres.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Perrin M., Mangin J.F., Cointepas Y., Duchesnay E., Le Bihan D., Hertz-Pannier L. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele T., Nordby H., Rimol L.M., Hugdahl K. Asymmetry of evoked potential latency to speech sounds predicts the ear advantage in dichotic listening. Brain Research. Cognitive Brain Research. 2005;24:405–412. doi: 10.1016/j.cogbrainres.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Emanuel D.C. The auditory processing battery: survey of common practices. Journal of the American Academy of Audiology. 2002;13:93–117. (quiz 8–9) [PubMed] [Google Scholar]
- Emanuel D.C., Ficca K.N., Korczak P. Survey of the diagnosis and management of auditory processing disorder. American Journal of Audiology. 2011;20:48–60. doi: 10.1044/1059-0889(2011/10-0019). [DOI] [PubMed] [Google Scholar]
- Fernandes M.A., Smith M.L., Logan W., Crawley A., Mcandrews M.P. Comparing language lateralization determined by dichotic listening and fMRI activation in frontal and temporal lobes in children with epilepsy. Brain and Language. 2006;96:106–114. doi: 10.1016/j.bandl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Fontoura D.R., Branco Dde M., Anes M., Costa J.C., Portuguez M.W. Language brain dominance in patients with refractory temporal lobe epilepsy: a comparative study between functional magnetic resonance imaging and dichotic listening test. Arquivos de Neuro-Psiquiatria. 2008;66:34–39. doi: 10.1590/s0004-282x2008000100009. [DOI] [PubMed] [Google Scholar]
- Foundas A.L., Corey D.M., Hurley M.M., Heilman K.M. Verbal dichotic listening in right and left-handed adults: laterality effects of directed attention. Cortex. 2006;42:79–86. doi: 10.1016/s0010-9452(08)70324-1. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hale T.S., Zaidel E., Mcgough J.J., Phillips J.M., Mccracken J.T. Atypical brain laterality in adults with ADHD during dichotic listening for emotional intonation and words. Neuropsychologia. 2006;44:896–904. doi: 10.1016/j.neuropsychologia.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Haynes J.D., Rees G. Decoding mental states from brain activity in humans. Nature Reviews. Neuroscience. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- Henry R.G. Monaural studies eliciting an hemispheric asymmetry: a bibliography. Perceptual and Motor Skills. 1979;48:335–338. doi: 10.2466/pms.1979.48.1.335. [DOI] [PubMed] [Google Scholar]
- Henry R.G. Monaural studies eliciting an hemispheric asymmetry; a bibliography: II. Perceptual and Motor Skills. 1983;56:915–918. doi: 10.2466/pms.1983.56.3.915. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nature Reviews. Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hublet C., Morais J., Bertelson P. Spatial constraints on focused attention: beyond the right-side advantage. Perception. 1976;5:3–8. doi: 10.1068/p050003. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Dichotic listening: probing temporal lobe functional integrity. In: Davidson R.J., Hugdahl K., editors. Brain Asymmetry. The MIT Press; Cambridge, MA: 1995. [Google Scholar]
- Hugdahl K. Dichotic listening in the study of auditory laterality. In: Hugdahl K., editor. The Asymmetrical Brain. MIT Press; Cambridge, MA: 2002. pp. 441–476. [Google Scholar]
- Hugdahl K. Symmetry and asymmetry in the human brain. European Review. 2005;13(S2):119–133. [Google Scholar]
- Hugdahl K., Andersson L. The “forced-attention paradigm” in dichotic listening to CV-syllables: a comparison between adults and children. Cortex. 1986;22:417–432. doi: 10.1016/s0010-9452(86)80005-3. [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Hammar A. Test–retest reliability for the consonant-vowel syllables dichotic listening paradigm. Journal of Clinical and Experimental Neuropsychology. 1997;19:667–675. doi: 10.1080/01688639708403752. [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Carlsson G., Uvebrant P., Lundervold A.J. Dichotic-listening performance and intracarotid injections of amobarbital in children and adolescents. Preoperative and postoperative comparisons. Archives of Neurology. 1997;54:1494–1500. doi: 10.1001/archneur.1997.00550240046011. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M., Lex U., Friederici A.D., Von Cramon D.Y. Non-invasive regime for language lateralization in right- and left-handers by means of functional MRI and dichotic listening. Experimental Brain Research. 2002;145:166–176. doi: 10.1007/s00221-002-1090-0. [DOI] [PubMed] [Google Scholar]
- Jancke L., Shah N.J. Does dichotic listening probe temporal lobe functions? Neurology. 2002;58:736–743. doi: 10.1212/wnl.58.5.736. [DOI] [PubMed] [Google Scholar]
- Jancke L., Buchanan T.W., Lutz K., Shah N.J. Focused and nonfocused attention in verbal and emotional dichotic listening: an FMRI study. Brain and Language. 2001;78:349–363. doi: 10.1006/brln.2000.2476. [DOI] [PubMed] [Google Scholar]
- Jerger J., Musiek F. Report of the consensus conference on the diagnosis of auditory processing disorders in school-aged children. Journal of the American Academy of Audiology. 2000;11:467–474. [PubMed] [Google Scholar]
- Joachims T. MIT Press; Cambridge, MA: 1999. Making Large-scale SVM Learning Practical. Advances in Kernel Methods — Support Vector Learning. [Google Scholar]
- Katz J., Tillery K.L. Can central auditory processing tests resist supramodal influences? American Journal of Audiology. 2005;14:124–127. doi: 10.1044/1059-0889(2005/013). (discussion 43–50) [DOI] [PubMed] [Google Scholar]
- Keith R.W. Dichotic listening in children. In: Beasley D.S., editor. Audition in Children: Methods of Study. College-Hill Press; San Diego, CA: 1984. [Google Scholar]
- Keith R. Pearson Education; San Antonio, TX: 2009. SCAN-3 for Children: Tests for Auditory Processing Disorder. [Google Scholar]
- Kim J.H., Budde M.D., Liang H.F., Klein R.S., Russell J.H., Cross A.H., Song S.K. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiology of Disease. 2006;21:626–632. doi: 10.1016/j.nbd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kimura D. Cerebral dominance and the perception of verbal stimuli. Canadian Journal of Psychology. 1961;15:166–171. [Google Scholar]
- Kimura D. Left-right differences in the perception of melodies. Journal of Experimental Psychology. 1964;16:355–358. [Google Scholar]
- Kimura D. Functional asymmetry of the brain in dichotic listening. Cortex. 1967;3:163–168. [Google Scholar]
- Kimura D. The asymmetry of the human brain. Scientific American. 1973;228:70–78. doi: 10.1038/scientificamerican0373-70. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y., Ohnishi A., Kohshi K., Yokota A. Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environmental Research. 1999;80:348–354. doi: 10.1006/enrs.1998.3935. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The cerebral basis of lateral asymmetries in attention. Acta Psychologica. 1970;33:193–201. doi: 10.1016/0001-6918(70)90132-0. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The control of attention by interaction between the cerebral hemispheres. In: Kornblum S., editor. Attention and Performance IV. Academic Press; New York: 1973. pp. 239–255. [Google Scholar]
- Kinsbourne M. The mechanism of hemispheric control of the lateral gradient of attention. In: Rabbitt P.M.A., Dornic S., editors. Attention and Performance V. Academic Press; London: 1975. pp. 81–97. [Google Scholar]
- Kinsbourne M. Dichotic imbalance due to isolated hemisphere occlusion or directional rivalry? Brain and Language. 1980;11:221–224. doi: 10.1016/0093-934x(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Kluender K.R., Lotto A.J. Virtues and perils of an empiricist approach to speech perception. Journal of the Acoustical Society of America. 1999;105:503–511. [Google Scholar]
- Knecht S., Drager B., Deppe M., Bobe L., Lohmann H., Floel A., Ringelstein E.B., Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kodaka Y., Mikami A., Kubota K. Neuronal activity in the frontal eye field of the monkey is modulated while attention is focused on to a stimulus in the peripheral visual field, irrespective of eye movement. Neuroscience Research. 1997;28:291–298. doi: 10.1016/s0168-0102(97)00055-2. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N. Relating population-code representations between man, monkey, and computational models. Frontiers in Neuroscience. 2009;3:363–373. doi: 10.3389/neuro.01.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Ruff D.A., Kiani R., Bodurka J., Esteky H., Tanaka K., Bandettini P.A. Matching categorical object representations in inferior temporal cortex of man and monkey. Neuron. 2008;60:1126–1141. doi: 10.1016/j.neuron.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman A.M., Whalen D.H. On the relation of speech to language. Trends in Cognitive Sciences. 2000;4:187–196. doi: 10.1016/s1364-6613(00)01471-6. [DOI] [PubMed] [Google Scholar]
- Lipschutz B., Kolinsky R., Damhaut P., Wikler D., Goldman S. Attention-dependent changes of activation and connectivity in dichotic listening. NeuroImage. 2002;17:643–656. [PubMed] [Google Scholar]
- Loring D.W., Meador K.J., Lee G.P., Murro A.M., Smith J.R., Flanigin H.F., Gallagher B.B., King D.W. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28:831–838. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- Luo F., Wang Q., Kashani A., Yan J. Corticofugal modulation of initial sound processing in the brain. Journal of Neuroscience. 2008;28:11615–11621. doi: 10.1523/JNEUROSCI.3972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Suga N. Multiparametric corticofugal modulation of collicular duration-tuned neurons: modulation in the amplitude domain. Journal of Neurophysiology. 2007;97:3722–3730. doi: 10.1152/jn.01268.2006. [DOI] [PubMed] [Google Scholar]
- Ma X., Suga N. Corticofugal modulation of the paradoxical latency shifts of inferior collicular neurons. Journal of Neurophysiology. 2008;100:1127–1134. doi: 10.1152/jn.90508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J.C., Toga A.W., Evans A., Fox P., Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development: the international consortium for brain mapping (ICBM) NeuroImage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Metwalli N.S., Benatar M., Nair G., Usher S., Hu X., Carew J.D. Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Research. 2010;1348:156–164. doi: 10.1016/j.brainres.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Moncrieff D.W. Dichotic listening in children: age-related changes in direction and magnitude of ear advantage. Brain and Cognition. 2011;76:316–322. doi: 10.1016/j.bandc.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Moore D.R., Ferguson M.A., Edmondson-Jones A.M., Ratib S., Riley A. Nature of auditory processing disorder in children. Pediatrics. 2010;126:e382–e390. doi: 10.1542/peds.2009-2826. [DOI] [PubMed] [Google Scholar]
- Morais J. The effects of ventriloquism on the right-side advantage for verbal material. Cognition. 1975;3:127–139. [Google Scholar]
- Morais J., Bertelson P. Laterality effects in diotic listening. Perception. 1973;2:107–111. doi: 10.1068/p020107. [DOI] [PubMed] [Google Scholar]
- Morais J., Bertelson P. Spatial position versus ear of entry as determinant of the auditory laterality effects: a stereophonic test. Journal of Experimental Psychology. Human Perception and Performance. 1975;1:253–262. doi: 10.1037//0096-1523.1.3.253. [DOI] [PubMed] [Google Scholar]
- Musiek F.E., Baran J.A., Bellis T.J., Chermak G.D., Hall Iii J.W., Keith R.W., Medwestky L., Loftus West K., Young M., Nagle S. 2010. American Academy of Audiology Clinical Practice Guidelines: Diagnosis, Treatment and Management of Children and Adults with Central Auditory Processing Disorder. [Google Scholar]
- Naismith R.T., Xu J., Tutlam N.T., Snyder A., Benzinger T., Shimony J., Shepherd J., Trinkaus K., Cross A.H., Song S.K. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology. 2009;72:589–594. doi: 10.1212/01.wnl.0000335766.22758.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C.W., Sandridge S.A. Diagnostic audiology. In: Hughes G., Pensak M., editors. Clinical Otology. Thieme Press; New York, NY: 2007. [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Obleser J., Eisner F., Kotz S.A. Bilateral speech comprehension reflects differential sensitivity to spectral and temporal features. Journal of Neuroscience. 2008;28:8116–8123. doi: 10.1523/JNEUROSCI.1290-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie M., Skogli E.W., Andersen P.N., Hovik K.T., Hugdahl K. Differences in cognitive control in children and adolescents with combined and inattentive subtypes of ADHD. Child Neuropsychology. 2013 doi: 10.1080/09297049.2012.741224. (in press) [DOI] [PubMed] [Google Scholar]
- O'toole A.J., Jiang F., Abdi H., Penard N., Dunlop J.P., Parent M.A. Theoretical, statistical, and practical perspectives on pattern-based classification approaches to the analysis of functional neuroimaging data. Journal of Cognitive Neuroscience. 2007;19:1735–1752. doi: 10.1162/jocn.2007.19.11.1735. [DOI] [PubMed] [Google Scholar]
- Pereira F., Mitchell T., Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. NeuroImage. 2009;45:S199–S209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magnetic Resonance in Medicine. 2004;51:399–402. doi: 10.1002/mrm.10706. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K. Functional MRI evidence for disparate developmental processes underlying intelligence in boys and girls. NeuroImage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Wilke M., Dardzinski B.J., Holland S.K. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human Brain Mapping. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Plante E. Object identification and lexical/semantic access in children: a functional magnetic resonance imaging study of word-picture matching. Human Brain Mapping. 2007;28:1060–1074. doi: 10.1002/hbm.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Plante E. Diffusion tensor imaging reveals white matter microstructure correlations with auditory processing ability. Ear and Hearing. 2011;32:156–167. doi: 10.1097/AUD.0b013e3181f7a481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwiesner M., Rubsamen R., Von Cramon D.Y. Hemispheric asymmetry for spectral and temporal processing in the human antero-lateral auditory belt cortex. European Journal of Neuroscience. 2005;22:1521–1528. doi: 10.1111/j.1460-9568.2005.04315.x. [DOI] [PubMed] [Google Scholar]
- Sidtis J. Dichotic listening after commissurotomy. In: Hugdahl K., editor. Handbook of Dichotic Listening: Theory, Methods and Research. Wiley & Sons; New York: 1988. pp. 161–184. [Google Scholar]
- Sparks R., Geschwind N. Dichotic listening after section of neo-cortical commisures. Cortex. 1968;4:3–16. [Google Scholar]
- Springer S., Gazzaniga M. Dichotic testing of partial and complete split-brain subjects. Neuropsychologia. 1975;13:341–346. doi: 10.1016/0028-3932(75)90011-1. [DOI] [PubMed] [Google Scholar]
- Springer S., Sidtis J., Wilson D., Gazzaniga M. Left ear performance in dichotic listening following commissurotomy. Neuropsychologia. 1978;16:305–312. doi: 10.1016/0028-3932(78)90024-6. [DOI] [PubMed] [Google Scholar]
- Strauss E., Gaddes W.H., Wada J. Performance on a free-recall verbal dichotic listening task and cerebral dominance determined by the carotid amytal test. Neuropsychologia. 1987;25:747–753. doi: 10.1016/0028-3932(87)90112-6. [DOI] [PubMed] [Google Scholar]
- Suga N., Xiao Z., Ma X., Ji W. Plasticity and corticofugal modulation for hearing in adult animals. Neuron. 2002;36:9–18. doi: 10.1016/s0896-6273(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Schmithorst V.J., Altaye M., Byars A.W., Ret J., Plante E., Holland S.K. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Annals of Neurology. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Ono J., Harada K., Maeda M., Hackney D.B. Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology. 2000;216:881–885. doi: 10.1148/radiology.216.3.r00se41881. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers, Inc.; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Taylor P.C., Rushworth M.F., Nobre A.C. Choosing where to attend and the medial frontal cortex: an FMRI study. Journal of Neurophysiology. 2008;100:1397–1406. doi: 10.1152/jn.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P., Ruttimann U.E., Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Transactions on Image Processing. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thomsen T., Rimol L.M., Ersland L., Hugdahl K. Dichotic listening reveals functional specificity in prefrontal cortex: an fMRI study. NeuroImage. 2004;21:211–218. doi: 10.1016/j.neuroimage.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Tzourio N., Massioui F.E., Crivello F., Joliot M., Renault B., Mazoyer B. Functional anatomy of human auditory attention studied with PET. NeuroImage. 1997;5:63–77. doi: 10.1006/nimg.1996.0252. [DOI] [PubMed] [Google Scholar]
- Van Den Noort M., Specht K., Rimol L.M., Ersland L., Hugdahl K. A new verbal reports fMRI dichotic listening paradigm for studies of hemispheric asymmetry. NeuroImage. 2008;40:902–911. doi: 10.1016/j.neuroimage.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Van Ettinger-Veenstra H.M., Ragnehed M., Hallgren M., Karlsson T., Landtblom A.M., Lundberg P., Engstrom M. Right-hemispheric brain activation correlates to language performance. NeuroImage. 2010;49:3481–3488. doi: 10.1016/j.neuroimage.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Vannest J.J., Karunanayaka P.R., Altaye M., Schmithorst V.J., Plante E.M., Eaton K.J., Rasmussen J.M., Holland S.K. Comparison of fMRI data from passive listening and active-response story processing tasks in children. Journal of Magnetic Resonance Imaging. 2009;29:971–976. doi: 10.1002/jmri.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnik V. Springer; New York: 1995. The Nature of Statistical Learning Theory. [Google Scholar]
- Vapnik V., Golowich S., Smola A. Support vector method for function approximation, regression estimation, and signal processing. In: Mozer M., Jordan M., Petsche T., editors. Neural Information Processing Systems. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- Voyer D. Sex differences in dichotic listening. Brain and Cognition. 2011;76:245–255. doi: 10.1016/j.bandc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Wardak C., Ibos G., Duhamel J.R., Olivier E. Contribution of the monkey frontal eye field to covert visual attention. Journal of Neuroscience. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R., Hugdahl K. The corpus callosum in dichotic listening studies of hemispheric asymmetry: a review of clinical and experimental evidence. Neuroscience and Biobehavioral Reviews. 2008;32:1044–1054. doi: 10.1016/j.neubiorev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Westerhausen R., Woerner W., Kreuder F., Schweiger E., Hugdahl K., Wittling W. The role of the corpus callosum in dichotic listening: a combined morphological and diffusion tensor imaging study. Neuropsychology. 2006;20:272–279. doi: 10.1037/0894-4105.20.3.272. [DOI] [PubMed] [Google Scholar]
- Westerhausen R., Gruner R., Specht K., Hugdahl K. Functional relevance of interindividual differences in temporal lobe callosal pathways: a DTI tractography study. Cerebral Cortex. 2009;19:1322–1329. doi: 10.1093/cercor/bhn173. [DOI] [PubMed] [Google Scholar]
- Westerhausen R., Moosmann M., Alho K., Medvedev S., Hamalainen H., Hugdahl K. Top-down and bottom-up interaction: manipulating the dichotic listening ear advantage. Brain Research. 2009;1250:183–189. doi: 10.1016/j.brainres.2008.10.070. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Saygin A.P., Sereno M.I., Iacoboni M. Listening to speech activates motor areas involved in speech production. Nature Neuroscience. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Liao C.H., Aston J., Petre V., Duncan G.H., Morales F., Evans A.C. A general statistical analysis for fMRI data. NeuroImage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nature Neuroscience. 2002;5:57–63. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- Zaidel E. Disconnection syndrome as a model for laterality effects in the normal brain. In: Hellige J.B., editor. Cerebral Hemisphere Asymmetry: Method, Theory, and Application. Praeger; New York: 1983. pp. 95–151. [Google Scholar]
- Zatorre R.J. Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium amytal test. Neuropsychologia. 1989;27:1207–1219. doi: 10.1016/0028-3932(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Mondor T.A., Evans A.C. Auditory attention to space and frequency activates similar cerebral systems. NeuroImage. 1999;10:544–554. doi: 10.1006/nimg.1999.0491. [DOI] [PubMed] [Google Scholar]


