Abstract
Diffusion tensor imaging (DTI) studies have demonstrated white matter (WM) abnormalities in tracts involved in emotion processing in autism spectrum disorder (ASD), but little is known regarding the nature and distribution of WM anomalies in relation to ASD trait severity in adults. Increasing evidence suggests that ASD occurs at the extreme of a distribution of social abilities. We aimed to examine WM microstructure as a potential marker for ASD symptom severity in a combined clinical–neurotypical population. SIENAX was used to estimate whole brain volume. Tract-based spatial statistics (TBSS) was used to provide a voxel-wise comparison of WM microstructure in 50 high-functioning young adults: 25 ASD and 25 neurotypical. The severity of ASD traits was measured by autism quotient (AQ); we examined regressions between DTI markers of WM microstructure and ASD trait severity. Cognitive abilities, measured by intelligence quotient, were well-matched between the groups and were controlled in all analyses. There were no significant group differences in whole brain volume. TBSS showed widespread regions of significantly reduced fractional anisotropy (FA) and increased mean diffusivity (MD) and radial diffusivity (RD) in ASD compared with controls. Linear regression analyses in the combined sample showed that average whole WM skeleton FA was negatively influenced by AQ (p = 0.004), whilst MD and RD were positively related to AQ (p = 0.002; p = 0.001). Regression slopes were similar within both groups and strongest for AQ social, communication and attention switching scores. In conclusion, similar regression characteristics were found between WM microstructure and ASD trait severity in a combined sample of ASD and neurotypical adults. WM anomalies were relatively more severe in the clinically diagnosed sample. Both findings suggest that there is a dimensional relationship between WM microstructure and severity of ASD traits from neurotypical subjects through to clinical ASD, with reduced coherence of WM associated with greater ASD symptoms. General cognitive abilities were independent of the relationship between WM indices and ASD traits.
Keywords: Autism spectrum disorder, Autism quotient, Diffusion tensor imaging, Tract-based spatial statistics, White matter
Highlights
-
•
Novel comparison of white matter microstructure in neurotypical and autistic adults
-
•
White matter coherence related to autistic trait severity in combined sample
-
•
The relationship between social intelligence and white matter is independent of IQ.
-
•
White matter anomalies are significantly more pronounced in the autistic subjects.
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterised by impaired communication skills and poor social reciprocity in combination with repetitive and stereotypic behaviours (WHO, 1994). Recent estimates show that autism affects approximately 1 in 88 (Centers for Disease Control and Prevention, 2012). Symptoms are thought to arise from aberrant neurodevelopment in childhood. A recent report by Raznahan et al. (2013) has questioned the theory that this altered development is underpinned by early brain overgrowth, as previously found using magnetic resonance imaging (MRI) (Courchesne et al., 2001), suggesting that brain volume in ASD remains more similar to neurotypical controls than previously thought.
Consequently, ASD may be associated with subtle differences in brain structure, as opposed to abnormalities in overall volume. Social and communication functions, which are impaired in ASD, are mediated by specialised brain regions that are connected in complex networks, such as the limbic (Dalgleish, 2004) and mirror neuron systems (Rizzolatti et al., 2001). The limbic network is supported structurally by well-defined white matter (WM) tracts, including the cingulum, inferior longitudinal fasciculus (ILF) and arcuate fasciculus (Catani et al., 2002; Catani and Thiebaut de Schotten, 2008). The white matter connections underlying the mirror neuron system, which include the frontal motor areas, posterior parietal cortex and superior temporal sulcus (Rizzolatti and Craighero, 2004), are less-well defined. These are, however, likely to be connected by the cingulum and superior longitudinal fasciculus (SLF) (Catani et al., 2002; Catani and Thiebaut de Schotten, 2008).
Diffusion tensor imaging (DTI) provides information about WM microstructure using measures of water diffusion in tissue (Basser and Pierpaoli, 1996). DTI measures include: fractional anisotropy (FA), which represents the directional dependence of water diffusion (ranging from 0 to 1, with 0 representing no anisotropy, or isotropic diffusion, and 1 representing infinite anisotropy); mean diffusivity (MD), which measures the overall magnitude of diffusion; axial diffusivity (AD) which measures the magnitude of diffusion along the principal diffusion direction; and radial diffusivity (RD), which is the magnitude of diffusion perpendicular to the principal direction. Most previous DTI studies of ASD have been carried out in children and adolescents. The majority have reported decreases in FA and increases in MD compared to neurotypical controls (Barnea-Goraly et al., 2004; Groen et al., 2011; Pugliese et al., 2009; Shukla et al., 2011a). Such changes are indicative of aberrant WM and may represent irregular organisation of WM tracts, relatively low axon density and/or deficient axon myelination (Beaulieu, 2002).
Some DTI studies of ASD have been conducted with adults included in the cohort, but samples have often been heterogeneous in terms of age, which may confound their findings (Alexander et al., 2007; Keller et al., 2007; Kleinhans et al., 2012). Evidence shows that age and developmental stage contribute to DTI findings in ASD children and adolescents (Kleinhans et al., 2012). Further, DTI studies of WM maturation show that the majority of FA and MD changes occur prior to 30 years of age, though maturation trajectories differ in each white matter tract (Hasan et al., 2010; Lebel et al., 2012). Thus, heterogeneity in age may explain some apparently paradoxical reports that children with ASD have increased FA (Ben Bashat et al., 2007; Weinstein et al., 2011). The typical WM developmental process is non-linear (Yap et al., 2013) and is therefore difficult to control for by simply covarying age in a mixed adult–child sample. We considered a preferable approach to studying WM anomalies in ASD would be to select a sample of adults, rather than children, for this investigation.
Previous DTI investigations of samples containing ASD adults have usually employed voxel-based techniques, including statistical parametric mapping (SPM), or region of interest (ROI) techniques, such as deterministic tractography which follows pathways of maximal diffusion in order to trace WM tracts. One problem with techniques such as ROI-based deterministic tractography and other ROI methodologies is that their interpretation requires a prior hypothesis. Only those WM tracts pre-selected as regions of interest can be subjected to analysis, thus potential WM alterations in other tracts will not be measured. Previous voxel-based methodologies are limited by the partial volume effects of registration error and smoothing techniques (Jones et al., 2005; Smith et al., 2006). Findings in ASD adults using these techniques include reduced FA in the corpus callosum, which mediates cross-talk between the two cerebral hemispheres (Thakkar et al., 2008). Reduced corpus callosum FA was also reported in a combined sample of children and adults with ASD (Alexander et al., 2007). Reduced FA in adults with ASD has been further reported in the inferior fronto-occipital fasciculus (IFOF), uncinate fasciculus (UF) (Pugliese et al., 2009) and cerebellar WM tracts (Catani et al., 2008). Increased MD, another marker of altered WM microstructure, has been measured in the ILF and cingulum (Pugliese et al., 2009). Co-localised reductions in FA and increases in RD have been reported in frontal, medial, temporal and parietal brain regions (Bloemen et al., 2010). Alterations in the number of streamlines obtained using tractography have been reported in the ILF, IFOF, UF and corpus callosum (Thomas et al., 2010).
In contrast to the limitations of ROI and previous voxel-based techniques, there are advantages to employing tract-based spatial statistics (TBSS) (Smith et al., 2006) which is an automated method that enables voxel-wise comparison of WM parameters, including FA and MD. Like other voxel-based techniques, TBSS removes the requirement for prior determination of tracts of interest, but TBSS additionally moderates registration errors and reduces partial volume effects. It achieves this by aligning the FA data from each subject to a common space before extracting a WM skeleton comprising the core of the WM voxels, thus excluding the more variable voxels at the extremities of the WM (Smith et al., 2006; Smith et al., 2007). TBSS has been applied to DTI scans of ASD children and adolescents, and WM tract alterations have been reported compared to controls (Ameis et al., 2011; Barnea-Goraly et al., 2010; Bode et al., 2011; Cheng et al., 2010; Jou et al., 2011; Kumar et al., 2010; Noriuchi et al., 2010; Sahyoun et al., 2010; Shukla et al., 2011a; Shukla et al., 2011b; Weinstein et al., 2011). Recently, Kleinhans et al. (2012) applied TBSS to a combined adolescent–adult cohort, with findings showing widespread reductions in FA and increases in MD and RD. TBSS has not, to the best of our knowledge, yet been used to study brain structure in a purely adult cohort of ASD.
ASD clinical characteristics are often considered to be on a continuum with social and communication skills found in the general population (Baron-Cohen et al., 2001; Constantino and Todd, 2003; Robinson et al., 2011). Some evidence suggests that the severity of ASD traits correlates with WM microstructure in clinical populations. For example, a negative correlation has been shown between FA in the cingulum and restricted and repetitive behaviours (Autism Diagnostic Interview — Revised (ADI-R)) in adults with ASD (Thakkar et al., 2008). Catani et al. (2008) reported a negative correlation between ADI-R social score and FA of the left cerebellar peduncle in adults with Asperger syndrome. On the other hand, some studies have not found significant correlations between WM characteristics and measures of ASD severity in clinically defined groups (Barnea-Goraly et al., 2010; Kleinhans et al., 2012; Shukla et al., 2010). Others have investigated whether the presence of ASD traits in non-clinical populations is correlated with WM microstructure characteristics. Kumar et al. (2010) reported a positive correlation between ASD traits and tract volume of the left UF in neurotypical subjects. Iidaka et al. (2012) identified a positive correlation between ASD traits and the volume of WM tracts connecting the superior temporal sulcus and the amygdala in healthy individuals. Both studies suggest that larger WM tract volume is associated with more severe ASD-like symptoms in neurotypical subjects. Alexander et al. (2007) reported a negative correlation between abnormalities in corpus callosum FA and social responsiveness scale (SRS) scores in a heterogeneous sample of neurotypical and ASD children and adults. These findings indicate that the relationship between WM microstructure and ASD symptoms could extend beyond the clinical syndrome into the neurotypical population, but to date studies have either only investigated ASD and neurotypical subjects separately or have been confounded by the inclusion of both child and adult subjects.
Intelligence quotient (IQ) has been shown to correlate with WM microstructure (Alexander et al., 2007) and is likely to influence, or be influenced by, DTI parameters. We therefore selected high-functioning individuals in order to minimise this confound. This enabled us to investigate any ASD-specific effects whilst minimising any potential confound of low general cognitive ability.
To our knowledge no previous investigation has examined a high functioning adult cohort of clinical and non-clinical subjects in the type of analyses we present.
2. Material and methods
2.1. Participants
Twenty-six high-functioning young adults with ASD and 25 age-matched neurotypical controls were recruited locally. None of the subjects had a history of neuropsychiatric disorders including anxiety, attention deficit hyperactivity disorder, depression and epilepsy. All MRI scans were visually inspected for neurological abnormalities, with no irregularities observed. The study was approved by the local Ethics committee and each participant gave written informed consent. Full-scale, verbal and performance IQ were measured using the four-scale Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). All subjects had an IQ > 80. The autism quotient (AQ) self-report questionnaire was administered to all subjects. The Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989) semi-structured interview was conducted with the ASD subjects to confirm their diagnosis. One subject was subsequently excluded from the study as their diagnosis could not be re-confirmed, leaving a total of 25 in the ASD group. Two-tailed t-tests were used to compare demographic measures between groups.
2.2. Data acquisition and pre-processing
Whole-brain MRI was carried out on a 1.5 T Siemens Magnetom Avanto scanner (Siemens, Erlangen, Germany) with 40 mT/m gradients and a 12-channel receive head coil. A T1-weighted three-dimensional fast low angle shot sequence was acquired with flip angle = 15°; TR = 11 ms; TE = 4.94 ms; voxel size = 1 mm isotropic; and slices = 176. The DTI protocol consisted of a twice-refocused spin echo diffusion-weighted echo planar imaging sequence with 60 unique gradient directions (b = 1000 s/mm2). Three images without diffusion weighting (b = 0) were interleaved. The parameters were: TR = 7300 ms; TE = 81 ms; voxel size = 2.5 mm isotropic; and 60 axial slices. The protocol also included resting-state and task-based functional MRI (fMRI), and the results of which will be reported elsewhere. The total imaging protocol took 42 min.
All scans were visually inspected for abnormalities, motion and other artefacts. Plots of estimated head motion during the diffusion-weighted scan were visually inspected and rotation was found to be minimal. There was no appreciable evidence that motion or other artefacts varied between groups. The DTI data were pre-processed using TractoR version 2.1 (Clayden et al., 2011) and FMRIB Software Library (FSL) version 4.1 (Smith et al., 2004). Briefly, within a given subject, a reference b = 0 volume was brain-extracted (Smith, 2002) and the diffusion-weighted volumes were registered to this reference to correct for eddy current distortions. A diffusion tensor was derived at each voxel using a standard least-squares process to provide a voxel-wise calculation of FA, MD, RD and AD.
2.3. Whole brain volume measurements
Whole brain, total grey matter (GM) and total WM volumes, both raw and normalised for intracranial volume, were calculated on the T1-weighted scans using the FSL tool SIENAX (Smith et al., 2001; Smith et al., 2002). Briefly, SIENAX brain extracts the images, affine-registers them to MNI152 space and then uses partial volume estimation to segment tissue types. The skull is used for normalisation. Group comparisons of normalised volumes were calculated using linear regression with age, gender and full-scale IQ as covariates.
2.4. Voxel-wise analysis of diffusion tensor imaging data
TBSS was carried out using FSL 4.1. All participants' FA data were projected onto a mean FA image using the non-linear registration tool FNIRT to register to the FMRIB58_FA template. The registered data was thinned to create a mean FA skeleton restricted to voxels with the highest FA at the centre of the major WM tracts. Following visual assessment of the optimal threshold value, the skeleton was thresholded at the recommended level of FA = 0.2 in order to remove confounding low-FA voxels, which may be caused by partial volume effects of GM or cerebrospinal fluid. Each participant's aligned FA data were projected onto the skeleton and voxel-wise cross-participant statistics was applied using non-parametric permutation testing within TBSS. Results were corrected for multiple comparisons using family wise error (FWE) and thresholded using threshold-free cluster enhancement (TFCE), as per the standard TBSS protocol. Only clusters surviving FWE p < 0.05 are reported. The locations of significant clusters were determined using the FSL atlas tools . This process was repeated for MD, RD and AD. TBSS fill was used for visualisation.
FSL tools were used to ascertain those WM regions that were most significantly different between the ASD and neurotypical groups. This was achieved by overlaying a skeleton of the John's Hopkins University (JHU) ICBM-DTI-81 atlas on the TBSS results. As delineated by the atlas, the number of voxels in particular WM tracts that had a significant group difference in FA and MD was recorded. To control for tract size, this was normalised as a percentage of the total number of voxels within the skeleton of the WM tract. Any WM regions in the skeleton which fell outside of the atlas were recorded as ‘unclassified WM’.
2.5. Whole white matter skeleton analysis of diffusion tensor imaging data
The values for each DTI metric were averaged across the whole WM skeleton for each participant. Group comparisons of mean WM skeleton FA, MD, RD and AD were made using linear regression.
2.6. Relationship between structural measures and clinical scores
Linear regression was used to investigate the relationship between the DTI parameters averaged across the whole WM skeleton and AQ. AQ measures the extent of autistic traits in both neurotypical controls and individuals with ASD in social, communication, attention switching, attention to detail and imagination domains. This makes the AQ an ideal tool for assessing ASD-like symptoms in the whole study population. Regressions were carried out in a combined sample of all subjects in order to investigate whether any relationship between ASD traits and WM microstructure applied across the entire study population.
We carried out further analyses in order to confirm that the ASD and control group did not differ substantially in terms of their relationships with AQ, and hence that the study of the entire population as one continuum was reasonable. This was done through a standard statistical model comparison for each diffusion measure, where one model treats the data set as a whole (as was carried out previously), whilst the other includes separate AQ slope and intercept terms for each group. The latter model was fully flexible, allowing for any combination of relationships between diffusion parameters and AQ in the two groups. In addition, we plotted the regression slopes from each fit for comparison.
Linear regression was used to investigate the relationship between the whole WM skeleton diffusion parameters and each sub-domain of the AQ score across all participants. Multiple comparisons correction was applied to control the false discovery rate (FDR), and only p values < 0.05 after correction were considered significant.
In order to visualise which WM voxels were contributing to these results, the relationships between DTI measures and AQ were estimated in each voxel across all subjects using TBSS. Only clusters surviving FWE p < 0.05 are reported.
2.7. Covariates
All DTI-based statistics included age, gender, full-scale IQ and un-normalised whole brain volume as covariates. These parameters were selected as covariates because they have previously been shown to influence DTI parameters (Alexander et al., 2007; Clayden et al., 2012; Kanaan et al., 2012; Menzler et al., 2011) and could thus confound findings.
3. Results
3.1. Demographics
Table 1 summarises participant demographics. Analysis with two-tailed t-tests showed no significant group differences in age, verbal IQ, performance IQ or full-scale IQ. AQ was significantly higher in the ASD group, with an overlap in scores between the groups.
Table 1.
Participant demographics and cognitive test scores.
| Control (n = 25)a | ASD (n = 25)a | t-statistic | p value | |
|---|---|---|---|---|
| Gender | 20M:5F | 21M:4F | – | – |
| Handedness | 25R:0L | 23R:2L | – | – |
| Age (years) | 24.50 (4.02) [18.83–33.30] |
23.22 (4.05) [18.31–31.90] |
1.12 | 0.27 |
| Full-scale IQ | 122.08 (8.02) [94–138] |
119.40 (11.59) [105–136] |
0.95 | 0.35 |
| Verbal IQ | 120.44 (8.43) [106–136] |
116.84 (12.56) [84–135] |
1.19 | 0.24 |
| Performance IQ | 118.40 (7.01) [103–129] |
117.36 (11.04) [88–135] |
0.40 | 0.69 |
| AQ | 13.60 (7.86) [4–37] |
36.60 (6.53) [22–49] |
− 11.23 | < 0.0001 |
| ADOSb | − | 9.22 (4.11) [3–20] |
− | − |
Data are expressed as mean (SD) [range].
Combined social and communication sub-scores.
Linear regression analysis controlling for age, gender and full-scale IQ showed no significant group differences in whole brain volume (t = − 0.69; p = 0.49), total GM volume (t = − 0.80; p = 0.43) or total WM volume (t = − 0.36; p = 0.72).
3.2. Whole white matter skeleton diffusion measurements
FA values averaged across the whole WM skeleton in each subject were significantly lower in the ASD group compared to controls (t = − 3.54; p = 0.001), whilst MD (t = 2.78; p = 0.008) and RD (t = 3.32; p = 0.002) were significantly elevated in the ASD group. There were no significant group differences in AD (t = 0.98; p = 0.33).
3.3. Voxel-wise diffusion measurements
The voxel-wise group comparison between ASD and controls showed widespread clusters of significantly reduced FA in the ASD group (p < 0.05; FWE-corrected) (see Fig. 1A). This included WM tracts bilaterally in the frontal, temporal, parietal and occipital lobes, in addition to the corpus callosum. The same group-wise comparison for MD showed similarly widespread increases of MD in the ASD group compared to controls (Fig. 1B). This was comparable to those seen in the FA comparison, though the fornix did not contain any significant clusters and affected voxels were more widespread in the temporal lobe and the right cingulum. Widespread significant increases in RD in the ASD group compared to controls were observed in a very similar pattern (Fig. 1C). No significant group differences in AD were detected.
Fig. 1.
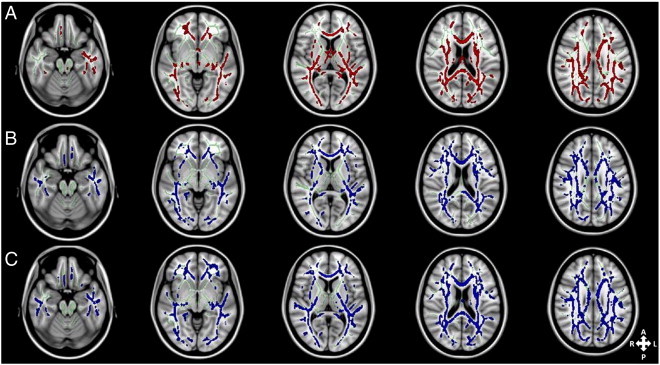
Axial slices of the cohort's mean white matter skeleton (green) overlaid with (A) red clusters depicting white matter voxels with significantly lower fractional anisotropy (FA) in subjects with autism spectrum disorder (ASD) compared to healthy controls (p < 0.05; FWE-corrected). Tracts in all major lobes of the cerebrum (frontal, parietal, occipital and temporal lobes) and the corpus callosum connecting the two brain hemispheres are affected. (B and C) Blue clusters showing regions with significantly higher mean diffusivity (MD) (B) and radial diffusivity (RD) (C) in ASD compared to controls (p < 0.05; FWE-corrected). Similarly to the FA results, the clusters are widespread throughout the cerebrum. There were no significant differences in axial diffusivity (AD) between the two groups. TBSS fill was used for visualisation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2 shows the 20 WM tracts, as outlined by the JHU-ICBM-DTI-81 atlas, which had the highest percentage of voxels showing significantly reduced FA and elevated MD in the ASD group in comparison to neurotypical controls. WM tracts key for long-range pathways in the brain, such as the corona radiata, SLF, and the corpus callosum, showed large areas with significant group differences in WM microstructure. Tracts involved in social processing, such as the fornix, ILF and IFOF, also presented with large proportions of affected WM microstructure in ASD. In WM not encompassed by the atlas, 29.69% had significantly reduced FA in ASD compared to neurotypical controls, whilst 30.27% showed significantly elevated MD.
Table 2.
The 20 WM tracts in the skeletonised JHU-ICBM-DTI-81 atlas which had the greatest proportion of voxels showing significantly reduced FA and significantly elevated MD in ASD compared to neurotypical controls.
| WM tract | Proportion of voxels with significantly (p < .05) reduced FA in ASD (%) | WM tract | Proportion of voxels with significantly (p < .05) elevated MD in ASD (%) |
|---|---|---|---|
| La Tapetum | 100.00 | CC — genu | 80.25 |
| Ra Tapetum | 100.00 | L SFOF | 79.49 |
| L SFOFa | 89.74 | L Sagittal stratumb | 77.55 |
| R Retrolenticular ICa | 80.84 | R Sagittal stratum | 75.17 |
| R Superior CRa | 79.97 | L SLF | 71.20 |
| R PTRa | 79.59 | R Posterior CR | 64.64 |
| CCa — genu | 76.99 | L PTR | 63.59 |
| Fornix — column and body | 76.61 | L Retrolenticular IC | 62.26 |
| L Fornix stria terminalis | 72.78 | R Anterior CR | 60.86 |
| L PTR | 72.39 | L Anterior CR | 60.04 |
| L UF | 68.85 | R PTR | 58.20 |
| CC — splenium | 68.40 | R Retrolenticular IC | 56.47 |
| R Posterior CR | 67.86 | R SLF | 55.59 |
| R SLFa | 67.02 | L Superior CR | 54.12 |
| L Superior CR | 62.21 | R Superior CR | 53.89 |
| CC — body | 58.62 | L Posterior CR | 51.20 |
| R SFOF | 58.57 | L External capsule | 47.19 |
| L Posterior CR | 57.28 | L Fornix — stria terminalis | 46.84 |
| L SLF | 56.90 | CC — body | 42.64 |
| L Retrolenticular IC | 56.29 | R External capsule | 40.58 |
Abbreviations: CC (corpus callosum); CR (corona radiata); IC (internal capsule); L (left); PTR (posterior thalamic radiation); R (right); SFOF (superior fronto-occipital fasciculus); SLF (superior longitudinal fasciculus); UF (uncinate fasciculus).
Sagittal stratum includes the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFOF).
3.4. Relationship between structural measures and clinical scores
In the entire study population, whilst controlling for age, gender, full-scale IQ and whole brain volume, FA averaged over the whole WM skeleton was negatively influenced by AQ (t = − 3.04; p = 0.004). Highly significant positive relationships were observed between MD and AQ (t = 3.24; p = 0.002) and RD and AQ (t = 3.42; p = 0.001). A weaker positive influence was seen by AQ on AD (t = 1.52; p = 0.14). See Fig. 2 for plots of the regressions. One control participant had an outlying high AQ score. Results found when excluding this high outlier remained very similar to those found whilst including them.
Fig. 2.
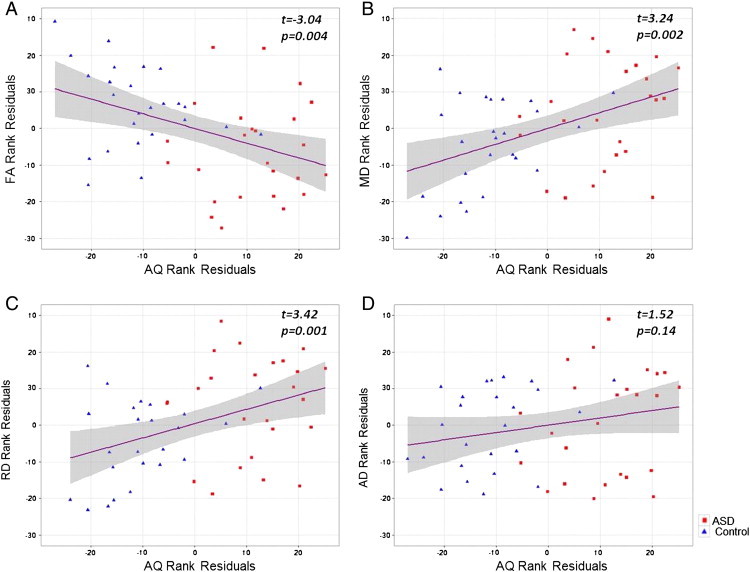
Scatter plots showing results of linear regression controlling for age, gender, full-scale intelligence quotient (IQ) and whole brain volume. Red squares denote participants diagnosed with an autism spectrum disorder (ASD); blue triangles represent neurotypical controls. Grey shading shows the standard error of the fit. (A) A significant negative relationship between autism quotient (AQ) and fractional anisotropy (FA) (t = − 3.04; p = 0.004) in addition to significant positive relationships for AQ with (B) mean diffusivity (MD) (t = 3.24; p = 0.002) and (C) radial diffusivity (RD) (t = 3.42; p = 0.001). (D) There was a weak positive influence of AQ on axial diffusivity (AD) (t = 1.52; p = 0.14). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Results from the model comparison showed no significant improvement in explained variance observed when using a regression model allowing for any combination of different relationships between diffusion parameters and AQ in the two groups, as opposed to the regression model using the entire study sample (F statistics: FA = 2.74, MD = 0.44, RD = 0.77, AD = 1.07; all p > 0.24). Thus, the added complexity of the full model is not required to describe the data, and one fit line is sufficient to encapsulate all of the data. Plots of the regression slopes from these combined sample relationships are shown alongside regression slopes from within-group regressions in Fig. 3. The slopes of the regressions look similar, which once again indicates that the AQ–WM relationships are consistent across the spectrum from neurotypical controls through to autism. Some of the confidence intervals overlap zero. This may be because variance was higher in the individual groups, particularly the ASD group, as would be expected.
Fig. 3.
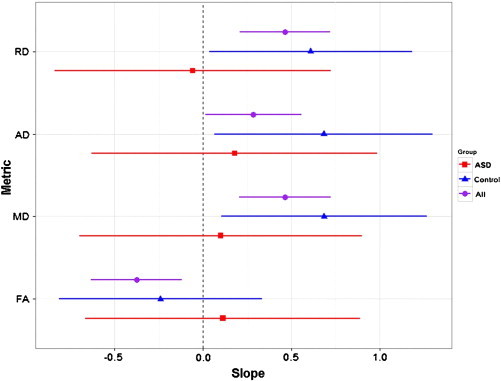
Plots of the regression slopes between the DTI metrics and AQ across all participants (purple circle), and within ASD (red square) and neurotypical control (blue triangle) groups separately. All regressions controlled for age, gender, full-scale intelligence quotient (IQ) and whole brain volume. Points represent the value of the regression slope; lines show the 95% confidence interval of the fit. The plots show greater variance in the separate groups, particularly the ASD group. Slopes were similar for regression in the combined sample in comparison to regressions in the separate groups, particularly for MD and AD. Slopes for FA and RD were more different, but model comparison showed no significant difference in the fits (all p > 0.24). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Voxel-wise analysis of relationships between the diffusion measures and AQ, controlling for age, gender, full-scale IQ and whole brain volume, indicated widespread clusters throughout the WM of the left hemisphere and bilaterally in the occipital lobe in which MD was positively influenced by AQ (p < 0.05; FWE-corrected) (Fig. 4A). There was a significant positive relationship between RD and AQ in a limited number of voxels from the SLF in the left hemisphere only (see Fig. 4B). There were no significant voxel-wise relationships between AQ and either FA or AD.
Fig. 4.
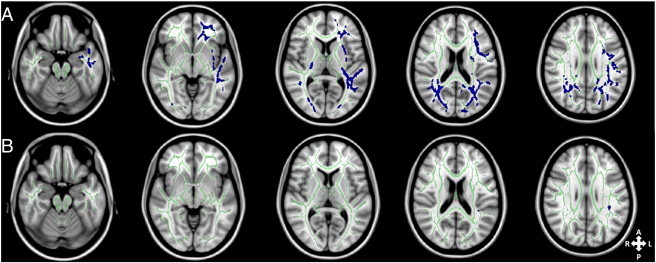
Axial slices of the group white matter skeleton (green) overlaid with blue clusters showing white matter voxels in which autism quotient (AQ) is positively related (p < 0.05; FWE-corrected) with (A) mean diffusivity (MD) widespread through the left hemisphere and bilaterally in the occipital lobe and (B) with radial diffusivity (RD) in voxels of the left hemisphere forming part of the left superior longitudinal fasciculus (SLF). There were no significant relationships between AQ and fractional anisotropy (FA) or axial diffusivity (AD). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Linear regressions between sub-divisions of the AQ score and diffusion measures averaged across the whole WM skeleton, controlling for age, gender, full-scale IQ and whole brain volume, are summarised in Table 3. There were significant negative relationships between FA and the AQ social and communication and attention switching domains. MD and RD showed highly significant positive relationships with the AQ social, communication, attention switching and imagination domains. The only significant relationship for AD was with the imagination domain.
Table 3.
Results of linear regressions across both groups between AQ sub-scores and DTI parameters averaged throughout the white matter skeleton.
| AQ domain | DTI metric | Est | p valuea |
|---|---|---|---|
| Social | FA | − 0.38 | 0.008 |
| MD | 0.38 | 0.01 | |
| RD | 0.41 | 0.005 | |
| AD | 0.13 | 0.34 | |
| Communication | FA | − 0.44 | < 0.001 |
| MD | 0.39 | 0.006 | |
| RD | 0.43 | 0.003 | |
| AD | 0.13 | 0.35 | |
| Attention switching | FA | − 0.51 | < 0.001 |
| MD | 0.40 | 0.006 | |
| RD | 0.47 | 0.001 | |
| AD | 0.06 | 0.66 | |
| Imagination | FA | − 0.28 | 0.06 |
| MD | 0.40 | 0.006 | |
| RD | 0.38 | 0.009 | |
| AD | 0.28 | 0.04 | |
| Attention to detail | FA | − 0.24 | 0.11 |
| MD | 0.31 | 0.04 | |
| RD | 0.29 | 0.06 | |
| AD | 0.22 | 0.12 |
p values were adjusted for multiple comparisons using false discovery rate correction. Those p values remaining significant (p < 0.05) after correction are displayed in bold text.
4. Discussion
Our study is the first to investigate the dimensional relationship between WM microstructure and ASD traits in an adult cohort of combined clinical and non-clinical cases. We found that WM characteristics, as evidenced by reduced FA and increased MD and RD, correlate with ASD symptom severity in the combined sample. Regressions between WM measures and ASD traits within each group were very similar in strength to the corresponding regression for all subjects. These results support the concept of a dimensional relationship between WM microstructure and ASD symptomatology in young adults. Group comparisons further support this finding, with more widespread WM disruption found in subjects diagnosed with clinical ASD in comparison to controls.
ASD, particularly in highly-functioning individuals, is often considered to be at the extreme of a continuum of social and communication skills (Baron-Cohen et al., 2001; Constantino and Todd, 2003; Robinson et al., 2011). Our findings provide support for the hypothesis that dimensionality of ASD traits, including social–communication ability and attention switching, is closely related to WM microstructure. The results we show across the whole WM skeleton are compatible with Alexander et al. (2007) who indicated that there was a dimensional relationship between corpus callosum microstructure and ASD traits in a combined clinical–control sample of adolescents and adults. Our finding is also consistent with previous studies which have reported correlations between ASD traits and WM tract volume in neurotypical young adults (Iidaka et al., 2012; Kumar et al., 2010). Further, our results are consistent with reports of correlations between ASD severity and greater WM disruption within adult ASD groups alone (Catani et al., 2008; Thakkar et al., 2008; Thomas et al., 2010). Our study investigated the relationship between WM microstructure and ASD traits more comprehensively across the entire WM skeleton in a combined clinical and non-clinical sample, and is thus able to show the extent of this relationship more clearly.
Reductions in FA and increases in MD are thought to reflect reduced organisation of the WM, reduced axonal density, and/or reduced myelination (Basser and Pierpaoli, 1996; Beaulieu, 2002), although the precise biology underpinning particular FA and MD values cannot be determined due to restrictions in the resolution of DTI. Elevated RD represents increased water diffusion perpendicular to axon bundles, which may reflect reduced axon density, increased axon diameter, increased membrane permeability (Takahashi et al., 2002) and/or reduced myelination (Song et al., 2002). Our results indicate that it is these types of WM characteristics which relate to ASD traits. Upon investigation of the specificities of each WM metric's relationship with ASD traits, we found that the relationship between FA and ASD traits was strongest for social, communication and attention switching domains and weakest for imagination and attention to detail. This supports the concept of distributed WM connectivity particularly influencing cognitive flexibility, social and communication ASD behaviours. MD and RD also showed strong associations with the core social and communication features of ASD as well as with attention switching and imagination. Interestingly, MD had a stronger relationship with imagination than with social ability. This is in contrast to FA and RD which were more strongly related to social skills. In summary, we found that MD is more strongly associated with a wider complement of ASD traits than FA, which is more closely linked to attention switching and the core social–communication ASD behaviours. A similar dissociation was also observed by Catani et al. (2008).
Group comparisons using TBSS showed that WM characteristics were significantly altered in ASD compared to neurotypical controls. Specifically, we found significantly decreased FA in the ASD group, alongside increased MD and RD. We did not detect a significant difference in AD between the two groups. These results are compatible with Kleinhans et al.'s (2012) recent report of widespread WM anomalies in a heterogeneous cohort of adolescents and adults with ASD using TBSS. The results of the group comparison indicate that clinically-significant ASD is associated with a greater degree of WM abnormalities than is found in neurotypical controls. Coupled with the findings of the relationship with AQ, a spectrum of WM changes, with ASD at one extreme of a distribution, is supported. Maintenance of AD coupled with an increase in RD could signify reduced axon myelination (Song et al., 2002) or increased membrane permeability (Takahashi et al., 2002). These observations are suggestive of involvement of processes responsible for the myelination and preservation of axons in development of ASD, though elucidation of this is outside the remit of DTI.
Our finding of widespread WM aberrations in ASD adults are also consistent with the results of previous voxel-based and tractography studies in adults showing ASD-related WM anomalies in the temporal lobe and cortico-thalamic tracts (Lee et al., 2007); limbic tracts, such as the cingulum, fornix and UF (Pugliese et al., 2009); the arcuate fasciculus (Nagae et al., 2012); tracts of the mirror neuron system, such as the arcuate fasciculus and IFOF (Pugliese et al., 2009); and in the corpus callosum which connects the two cerebral hemispheres (Alexander et al., 2007; Thakkar et al., 2008; Thomas et al., 2010). Our analysis was not limited to prior regions of interest and so we were able to demonstrate the more widespread nature of WM anomalies in a group of ASD adults. We investigated the relative contributions of particular WM tracts to the group difference in WM microstructure. The results showed that tracts which link widespread regions of the brain were affected in ASD. These included the superior fronto-occipital fasciculus, corpus callosum, internal capsule and corona radiata. Tracts associated with social processing were also majorly affected, including the fornix and uncinate fasciculus, by changes in FA, and the ILF, IFOF, and to a lesser extent, the fornix, by changes in MD. However, we did not find that limbic tracts were singularly affected to a degree which would have been expected from previous ROI-based studies. This indicates that the structural deficits associated with ASD are more wide-reaching than previously reported, and appear to be related to ASD traits, such as imagination, repetitive behaviours and flexibility, which are likely to require the recruitment of several brain regions.
Despite the widespread nature of these WM changes our participants had relatively high IQs. The group comparisons and regressions between ASD traits and WM microstructure were independent of IQ, which was well matched between groups and controlled for in all statistical tests. The relationship between WM microstructure and severity of social and communication impairments is thus independent of general cognition. This implies that we are observing a relationship between WM microstructure and cognitive faculties that are specifically linked to ASD behaviours, such as social cognition, as opposed to general cognitive ability. The finding of a close relationship between WM microstructure and social cognition independently from general cognition is consistent with evidence that corpus callosum agenesis is associated with increased incidence of ASD traits, but no significant difference in IQ scores (Lau et al., 2012). Evidence suggests that social and communication processing involve co-ordinated activation of a network of brain areas in both hemispheres (Dalgleish, 2004; Rizzolatti et al., 2001). This need for recruitment of widely dispersed brain regions during social processing means that WM abnormalities are particularly likely to exert a negative impact on effective communication between them. Kanwisher (2010) reviews evidence suggesting that several social functions, including face recognition and thinking about another person's thoughts, are localised to functionally specialised brain regions, each of which will need to communicate via WM tracts. Additional recent evidence indicates that intelligent cognition may occur via multiple networks (Hampshire et al., 2012), suggesting that some elements of duplication may provide a ‘back-up’ for functions tested by conventional measures of general intelligence.
We did not find a significant difference in whole brain volume, total GM volume or total WM volume between the groups. This is consistent with previous reports that brain volumes of adults with ASD are similar to those of neurotypical controls (Aylward et al., 2002; Courchesne et al., 2001; Raznahan et al., 2013), implying that alterations in the structural wiring of the brain underpin ASD symptomatology to a greater extent than brain volume itself.
Limitations of the study include the following issues: First, at present only post-mortem studies could confirm the exact nature of the WM changes we report, since DTI resolution is too low to allow evaluation of single axons. Second, the values of WM indices in our participants are highly variable in both groups. Some of the within-group AQ–WM relationships had an estimated slope in the opposite direction compared to the analysis within the entire study sample. Although this was minor and we found no significant difference in the slopes, this finding highlights the variability in the relationship between WM microstructure and ASD symptom severity. Further studies on larger cohorts would be helpful to confirm the findings. Further, variability does currently limit the usefulness of DTI measures as biomarkers of change at an individual level. Third, we recognise that the AQ is not a ‘gold standard’ of ASD traits: it is a self-report measure, which may influence its validity. Finally, it would be valuable to conduct a further study that combines DTI with fMRI, which would enable analysis of the relationship between WM microstructure, brain activity, and the strength of ASD traits in a mixed neurotypical and clinical sample.
5. Conclusions
In conclusion, our findings indicate that the strength of ASD traits is related to WM microstructure in both neurotypical and clinically identified participants, but that WM anomalies are more severe in those diagnosed with ASD. Our findings are consistent with recent evidence from studies of separate neurotypical and ASD groups that the dimensional characteristics of the ASD phenotype reflect a distributed variability of WM microstructure affecting tracts involved in social and communication processing in addition to attention switching. IQ was maintained in these individuals, thus our findings imply that a dissociation exists between WM features linked to IQ-related abilities and WM characteristics associated with ASD traits.
Financial disclosures
CAC wishes to acknowledge European Union Grant FP7-2009-C 238292. CRG is funded by a UCL Grand Challenge studentship. JR is funded by an Erasmus Mundus Chinese Collaborative PhD studentship. The authors have no conflicts of interest.
Acknowledgements
We thank all participants who took part in the study as well as Tina Banks and Charlotte Sanderson for assistance in acquiring the data. We would also like to thank Martin King for helpful statistical discussions.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Alexander A.L., Lee J.E., Lazar M., Boudos R., DuBray M.B., Oakes T.R., Miller J.N., Lu J., Jeong E.-K., McMahon W.M., Bigler E.D., Lainhart J.E. Diffusion tensor imaging of the corpus callosum in autism. NeuroImage. 2007;34(1):61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Ameis S.H., Fan J., Rockel C., Voineskos A.N., Lobaugh N.J., Soorya L., Wang A.T., Hollander E., Anagnostou E. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: a diffusion tensor imaging study. PLoS One. 2011;6(11):e28044. doi: 10.1371/journal.pone.0028044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward E.H., Minshew N.J., Field K., Sparks B.F., Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss A. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N., Lotspeich L., Reiss A. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Archives of General Psychiatry. 2010;67(10):1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. 1996;213(2):560–570. doi: 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D., Kronfeld-Duenias V., Zachor D.A., Ekstein P.M., Hendler T., Tarrasch R., Even A., Levy Y., Ben Sira L. Accelerated maturation of white matter in young children with autism: a high b value DWI study. NeuroImage. 2007;37(1):40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Bloemen O.J.N., Deeley Q., Sundram F., Daly E.M., Barker G.J., Jones D.K., van Amelsvoort T.A.M.J., Schmitz N., Robertson D., Murphy K.C., Murphy D.G.M. White matter integrity in Asperger syndrome: a preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Research. 2010;3(5):203–213. doi: 10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- Bode M.K., Mattila M., Kiviniemi V., Rahko J., Moilanen I., Ebeling H., Tervonen O., Nikkinen J. White matter in autism spectrum disorders — evidence of impaired fiber formation. Acta Radiologica. 2011;52:1169–1174. doi: 10.1258/ar.2011.110197. [DOI] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Daly E., Embiricos N., Deeley Q., Pugliese L., Curran S., Robertson D., Murphy D.G.M. Altered cerebellar feedback projections in Asperger syndrome. NeuroImage. 2008;41(4):1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of autism spectrum disorders — Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. Morbidity and Mortality Weekly Report. Surveillance Summaries. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Cheng Y., Chou K., Chen I., Fan Y., Decety J., Lin C. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. NeuroImage. 2010;50(3):873–882. doi: 10.1016/j.neuroimage.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Clayden J.D., Muñoz Maniega S., Storkey A.J., King M.D., Bastin M.E., Clark C.A. TractoR: magnetic resonance imaging and tractography with R. Journal of Statistical Software. 2011;44(8) [Google Scholar]
- Clayden J.D., Jentschke S., Muñoz M., Cooper J.M., Chadwick M.J., Banks T., Clark C.A., Vargha-Khadem F. Normative development of white matter tracts: similarities and differences in relation to age, gender, and intelligence. Cerebral Cortex. 2012;22(8):1738–1747. doi: 10.1093/cercor/bhr243. [DOI] [PubMed] [Google Scholar]
- Constantino J.N., Todd R.D. Autistic traits in the general population — a twin study. Archives of General Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Karns C.M., Davis H.R., Ziccardi R., Carper R.A., Tigue Z.D., Chisum H.J., Moses P., Pierce K., Lord C., Lincoln A.J., Pizzo S., Schreibman L., Haas R.H., Akshoomoff N.A., Courchesne R.Y. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. The emotional brain. Nature Reviews Neuroscience. 2004;5(7):583–589. doi: 10.1038/nrn1432. [DOI] [PubMed] [Google Scholar]
- Groen W.B., Buitelaar J.K., van der Gaag R.J., Zwiers M.P. Pervasive microstructural abnormalities in autism: a DTI study. Journal of Psychiatry & Neuroscience. 2011;36(1):32–40. doi: 10.1503/jpn.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Highfield R.R., Parkin B.L., Owen A.M. Fractionating human intelligence. Neuron. 2012;76(6):1225–1237. doi: 10.1016/j.neuron.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Hasan K.M., Kamali A., Abid H., Kramer L.A., Fletcher J.M., Ewing-Cobbs L. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Structure & Function. 2010;214(4):361–373. doi: 10.1007/s00429-009-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T., Miyakoshi M., Harada T., Nakai T. White matter connectivity between superior temporal sulcus and amygdala is associated with autistic trait in healthy humans. Neuroscience Letters. 2012;510(2):154–158. doi: 10.1016/j.neulet.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Symms M.R., Cercignani M., Howard R.J. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26(2):546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Jou R.J., Mateljevic N., Kaiser M.D., Sugrue D.R., Volkmar F.R., Pelphrey K.A. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. American Journal of Neuroradiology. 2011;32(9):1607–1613. doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R.A., Allin M., Picchioni M., Barker G.J., Daly E., Shergill S.S., Woolley J., McGuire P.K. Gender differences in white matter microstructure. PLoS One. 2012;7(6):e38272. doi: 10.1371/journal.pone.0038272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N. Functional specificity in the human brain: a window into the functional architecture of the mind. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11163–11170. doi: 10.1073/pnas.1005062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T.A., Kana R.K., Just M.A. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;18(1):23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Pauley G., Richards T., Neuhaus E., Martin N., Corrigan N.M., Shaw D.W., Estes A., Dager S.R. Age-related abnormalities in white matter microstructure in autism spectrum disorders. Brain Research. 2012;1479:1–16. doi: 10.1016/j.brainres.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Sundaram S.K., Sivaswamy L., Behen M.E., Makki M.I., Ager J., Janisse J., Chugani H.T., Chugani D.C. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral Cortex. 2010;20(9):2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Lau Y.C., Hinkley L.B.N., Bukshpun P., Strominger Z.A., Wakahiro M.L.J., Baron-Cohen S., Allison C., Auyeung B., Jeremy R.J., Nagarajan S.S., Sherr E.H., Marco E.J. Autism traits in individuals with agenesis of the corpus callosum. Journal of Autism and Developmental Disorders. 2012;43(5):1106–1118. doi: 10.1007/s10803-012-1653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Bigler E.D., Alexander A.L., Lazar M., DuBray M.B., Chung M.K., Johnson M., Morgan J., Miller J.N., McMahon W.M., Lu J., Jeong E.-K., Lainhart J.E. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neuroscience Letters. 2007;424(2):127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Goode S., Heemsbergen J., Jordan H., Mawhood L., Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19(2):185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Menzler K., Belke M., Wehrmann E., Krakow K., Lengler U., Jansen A., Hamer H.M., Oertel W.H., Rosenow F., Knake S. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. NeuroImage. 2011;54(4):2557–2562. doi: 10.1016/j.neuroimage.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Nagae L.M., Zarnow D.M., Blaskey L., Dell J., Khan S.Y., Qasmieh S., Levy S.E., Roberts T.P.L. Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. American Journal of Neuroradiology. 2012:1–6. doi: 10.3174/ajnr.A3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M., Kikuchi Y., Yoshiura T., Kira R., Shigeto H., Hara T., Tobimatsu S., Kamio Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Research. 2010;1362:141–149. doi: 10.1016/j.brainres.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Pugliese L., Catani M., Ameis S., Dell'Acqua F., Thiebaut de Schotten M., Murphy C., Robertson D., Deeley Q., Daly E., Murphy D.G.M. The anatomy of extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. NeuroImage. 2009;47(2):427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Wallace G.L., Antezana L., Greenstein D., Lenroot R., Thurm A., Gozzi M., Spence S., Martin A., Swedo S.E., Giedd J.N. Compared to what? Early brain overgrowth in autism and the perils of population norms. Biological Psychiatry. 2013:1–13. doi: 10.1016/j.biopsych.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Craighero L. The mirror–neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Fogassi L., Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Robinson E.B., Munir K., Munafò M.R., Hughes M., McCormick M.C., Koenen K.C. Stability of autistic traits in the general population: further evidence for a continuum of impairment. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(4):376–384. doi: 10.1016/j.jaac.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun C.P., Belliveau J.W., Mody M. White matter integrity and pictorial reasoning in high-functioning children with autism. Brain and Cognition. 2010;73(3):180–188. doi: 10.1016/j.bandc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Lincoln A.J., Müller R.-A. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(12):1269–1278. doi: 10.1016/j.jaac.2010.08.018. (1278.e1–2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Müller R.-A. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D.K., Keehn B., Smylie D.M., Müller R.-A. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49(5):1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., De Stefano N., Jenkinson M., Matthews P.M. Normalized accurate measurement of longitudinal brain change. Journal of Computer Assisted Tomography. 2001;25(3):466–475. doi: 10.1097/00004728-200105000-00022. [DOI] [PubMed] [Google Scholar]
- Smith S., Zhang Y., Jenkinson M., Chen J., Matthews P., Federico A., De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith S., Jenkinson M., Woolrich M., Beckmann C., Behrens T., Johansen-Berg H., Bannister P., De Luca M., Drobnjak I., Flitney D., Niazy R., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J., Matthews P. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T., Mackay C., Watkins K., Ciccarelli O., Cader M., Matthews P., Behrens T. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S., Johansen-Berg H., Jenkinson M., Rueckert D., Nichols T., Miller K., Robson M., Jones D., Klein J., Bartsch A., Behrens T. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Hackney D.B., Zhang G., Wehrli S.L., Wright A.C., O'Brien W.T., Uematsu H., Wehrli F.W., Selzer M.E. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16192–16196. doi: 10.1073/pnas.252249999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Polli F.E., Joseph R.M., Tuch D.S., Hadjikhani N., Barton J.J.S., Manoach D.S. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD) Brain. 2008;131(Pt. 9):2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Humphreys K., Jung K.-J., Minshew N., Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex. 2010;47(7):863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. [Google Scholar]
- Weinstein M., Ben-Sira L., Levy Y., Zachor D.A., Ben Itzhak E., Artzi M., Tarrasch R., Eksteine P.M., Hendler T., Ben Bashat D. Abnormal white matter integrity in young children with autism. Human Brain Mapping. 2011;32(4):534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 10th ed. World Health Organization; Geneva: 1994. International Classification of Diseases. [Google Scholar]
- Yap Q.J., Teh I., Fusar-Poli P., Sum M.Y., Kuswanto C., Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. Journal of Neural Transmission. 2013 doi: 10.1007/s00702-013-0971-7. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]


