Abstract
Objective
The Theory of Structural Dissociation of the Personality (TSDP) proposes that dissociative identity disorder (DID) patients are fixed in traumatic memories as “Emotional Parts” (EP), but mentally avoid these as “Apparently Normal Parts” of the personality (ANP). We tested the hypotheses that ANP and EP have different biopsychosocial reactions to subliminally presented angry and neutral faces, and that actors instructed and motivated to simulate ANP and EP react differently.
Methods
Women with DID and matched healthy female actors (CON) were as ANP and EP (DIDanp, DIDep, CONanp, CONep) consecutively exposed to masked neutral and angry faces. Their brain activation was monitored using functional magnetic resonance imaging. The black-and-white dotted masks preceding and following the faces each had a centered colored dot, but in a different color. Participants were instructed to immediately press a button after a perceived color change. State anxiety was assessed after each run using the STAI-S. Final statistical analyses were conducted on 11 DID patients and 15 controls for differences in neural activity, and 13 DID patients and 15 controls for differences in behavior and psychometric measures.
Results
Differences between ANP and EP in DID patients and between DID and CON in the two dissociative parts of the personality were generally larger for neutral than for angry faces. The longest reaction times (RTs) existed for DIDep when exposed to neutral faces. Compared to DIDanp, DIDep was associated with more activation of the parahippocampal gyrus. Following neutral faces and compared to CONep, DIDep had more activation in the brainstem, face-sensitive regions, and motor-related areas. DIDanp showed a decreased activity all over the brain in the neutral and angry face condition. There were neither significant within differences nor significant between group differences in state anxiety. CON was not able to simulate genuine ANP and EP biopsychosocially.
Conclusions
DID patients have dissociative part-dependent biopsychosocial reactions to masked neutral and angry faces. As EP, they are overactivated, and as ANP underactivated. The findings support TSDP. Major clinical implications are discussed.
Keywords: Dissociative identity disorder, Neuroimaging, Backward masking, Face perception, Emotional ambiguity, Hypervigilance
Highlights
-
•
Neural/behavioral differences between EP and ANP exist at a preconscious level.
-
•
EP but not ANP showed a positive attentional bias to masked facial stimuli.
-
•
Masked neutral faces elicited dorsal brainstem and occipitotemporal activity in EP.
-
•
EP’s reaction pattern suggests preconscious fixation particularly on neutral faces.
-
•
Actors were unable to mimic the neural/behavioral reactions of DID patients.
1. Introduction
Dissociative Identity Disorder (DID) is the most complex of dissociative disorders (American Psychiatric Association, 1994). According to the Theory of Structural Dissociation of the Personality (TSDP) (Nijenhuis et al., 2002; Van der Hart et al., 2006), DID is a severe form of posttraumatic stress disorder (PTSD) encompassing different types of dissociative parts of the personality. In TSDP, personality is understood as a whole biopsychosocial system, and dissociative parts as subsystems of this whole system. Van der Hart et al. (2006) propose a distinction between “Emotional Parts” (EP) and “Apparently Normal Parts” (ANP) of the personality. DID involves more than one EP and more than one ANP. Switching between these dissociative parts is a major characteristic of DID. EP is fixed in traumatic memories. As ANP, DID patients may claim a degree of amnesia for these memories, do not or not sufficiently personify traumatic experiences and memories, and attempt to mentally avoid trauma-related stimuli. TSDP distinguishes different prototypical subtypes of EP (Nijenhuis and Den Boer, 2009). Some subtypes show strong emotional reactions to trauma-related stimuli and engage in active mammalian defensive reactions (e.g., freeze, flight, attachment cry), whereas another subtype engages in passive mammalian defense (playing dead), which implies emotional and bodily anesthesia.
Severe and chronic dissociative symptoms tend to develop in the context of severe and chronic childhood traumatization, which includes profound attachment disruptions (Dalenberg et al., 2012; Diseth, 2006; Nijenhuis and Den Boer, 2009; Nijenhuis et al., 2002; Ogawa et al., 1997; Trickett et al., 2011). In a Positron Emission Tomography (PET) study, female DID patients listened as ANP and as EP (in Reinders et al. (2006b) referred to as a neutral identity state (NIS) and trauma-related identity state (TIS)) to autobiographical neutral and trauma scripts while their psychophysiological and brain activation was monitored (Reinders et al., 2003, 2006b). As ANP, the patients in this study reacted similarly to the neutral and the trauma memory scripts. This finding suggests low emotional involvement in trauma-related stimuli, which is consistent with TSDP. In this study, EP (subtype active defense), as compared to the ANP, showed significant activation of many areas also observed in PTSD patients while being confronted with a personalized trauma script (Lanius et al., 2001; Rauch et al., 1996; Shin et al., 2001). EP but not ANP demonstrated strong psychophysiological reactions to the trauma script. Thus, EP but not ANP was psychobiologically aroused. ANP showed a brain activation pattern similar to patients with depersonalization disorder (Simeon et al., 2000) and PTSD patients with negative dissociative symptoms to trauma-related stimuli (Lanius et al., 2002, 2006).
According to the sociocognitive view (also referred to as fantasy model, Dalenberg et al., 2012), DID is caused by high fantasy proneness, role-playing, suggestibility, and iatrogenic suggestion (Giesbrecht et al., 2008; Lilienfeld et al., 1999; Merckelbach and Muris, 2001; Merckelbach et al., 2002; Merskey, 1992; Spanos, 1994). Few suggestions would suffice to generate dissociative parts in suggestible, fantasy prone individuals (Spanos, 1996). However, a recent symptom provocation functional brain imaging study provided evidence suggesting that DID is not linked to fantasy proneness. Reinders et al. (2012) found that neither high nor low fantasy prone mentally healthy women instructed and motivated to simulate ANP and EP were able to enact the psychophysiological and neural activation patterns of the genuine ANP and EP.
A study by Hermans et al. (2006) used backward masking to expose DID patients to angry and neutral faces for 25 ms. Attentional bias scores were calculated by subtracting the reaction times (RTs) needed to color-name the mask that immediately followed a neutral face from the RTs needed to color-name the mask that immediately followed an angry face. A positive attentional bias score (i.e., longer RT for angry than neutral faces) was interpreted as vigilance, and a negative one (i.e., longer RT for neutral than angry faces) as avoidance (Bakvis et al., 2009; Putman et al., 2004; Van Honk et al., 1998, 2000). Hermans et al. (2006) found that as ANP but not as EP, DID patients had a negative attentional bias in that their RT to angry faces was faster than that to neutral faces. Healthy controls instructed and motivated to role-play ANP and EP did not show this negative bias. Taken together, these behavioral data also contradict the sociocognitive view of DID.
The findings from Reinders et al. (2003, 2006b, 2012) and Hermans et al. (2006) support the hypotheses derived from TSDP that as EP engaging in active defense, DID patients are fixed in traumatic memories and demonstrate unusually strong cortical, subcortical and vegetative reactions (i.e., hyperarousal) to reminders of traumatic experiences. As ANP on the other hand, they react to trauma-related cues in a depersonalized and detached manner (i.e., hypoarousal). In addition, these differences between ANP and EP exist already at a preconscious level, that is, with respect to pre-attentive reactivity to external and internal stimuli.
EP's preconscious fixation on perceived threat (Hermans et al., 2006) is hypothesized to be associated with neural networks related to perceptual and emotional processing of the angry faces. Reactions to emotional faces compared to neutral faces are expected to be associated with greater activation in early visual areas (striate cortex) and higher order visual areas (extrastriate cortex) including face-sensitive regions in the fusiform gyrus (Vuilleumier and Pourtois, 2007). Functional imaging studies have identified additional areas in the extrastriate occipito-temporal region involved in the visual analysis of faces (i.e., lateral inferior occipital cortex, sulcus temporalis superior [STS]) (Haxby et al., 2000). Amaral and colleagues have demonstrated that enhanced activity within the visual cortex as reaction to emotional stimuli is mainly driven by the amygdala, which has strong anatomical connections to visual areas (Amaral et al., 1992). One of the main contributions of the amygdala is to support rapid reaction to potential or actual sources of danger (Davis and Whalen, 2001; LeDoux, 1998; Phan et al., 2002). Activity within the amygdala can occur even if the threatening stimuli are presented below the level of awareness (Morris et al., 1998; Whalen et al., 1998). Amygdala responsitivity and associated vigilance are abnormally enhanced in PTSD (Armony et al., 2005; Rauch et al., 2000; Shin et al., 2004). This hypervigilance fits clinical observations that as EP engaged in active defense, patients are continuously scanning the environment for threat cues. Engagement in active defense may thus be associated with enhanced activation in motor-related areas, which was found in the study of Reinders et al. (2006b) as well (i.e., basal ganglia, cerebellum). This proposal also fits the observations that the cortical motor system is activated during emotional processing in humans (Hajcak et al., 2007; Oliveri et al., 2003), which prepares the individual for an appropriate motor reaction (Baumgartner et al., 2007).
In most previous functional imaging studies with masked stimuli investigating PTSD patients, the analysis was mainly restricted to the amygdala as a key brain structure for emotional processing (Armony et al., 2005; Hendler et al., 2003; Rauch et al., 2000). This focus on the amygdala reflected a particular a priori interest in the role of this brain structure in fear. However, Sakamoto and colleagues conducted a whole-brain analysis (Sakamoto et al., 2005). In this study, PTSD patients showed significantly higher activations to masked traumatic images in the left parahippocampal gyrus and the tail of the left hippocampus.
Per definition neutral faces do not express a clear emotion, thus can be perceived as emotionally ambiguous. Like anxiety disorder patients, and consistent with clinical observations, as EP, DID patients may have difficulty tolerating uncertainty or ambiguity (Grillon et al., 2008; Holaway et al., 2006) and may tend to interpret ambiguous stimuli in negative ways (Bishop, 2007; Eysenck et al., 1991).
The current functional magnetic resonance imaging (fMRI) study aims to examine the underlying neural activation patterns involved in ANP-dependent and EP-dependent preconscious reactivity. Based on the mentioned theoretical and empirical grounds, we specifically hypothesized that compared to (i) ANP in DID patients, and (ii) EP in controls, EP in DID patients have a different pattern of neural activity in response to subliminally presented faces, particularly more activity in primary and higher-order visual areas, face-sensitive areas including extrastriate occipito-temporal regions, limbic structures including the amygdala and hippocampal/parahippocampal region, and motor-related areas comprising the cortical motor system, basal ganglia, and cerebellum. We also hypothesized that (iii) these differences are more pronounced following angry faces, that (iv) EP in DID patients have longer RTs to these faces than ANP in DID patients and than EP in controls, and that (v) comparisons of ANP and EP in controls yield different neural and behavioral reactivity patterns than comparisons of ANP and EP in DID patients.
2. Methods and materials
2.1. Participants
Fifteen female outpatients who met the DSM-IV American Psychiatric Association (American Psychiatric Association, 1994) criteria for DID were enrolled in the study. They were recruited from private practitioners of psychiatry and psychotherapy and psychiatric outpatient departments in Switzerland and Germany. The clinical diagnosis was independently checked by clinical experts in dissociative disorders (E. Weder [EW] and E. Zimmermann [EZ]) using the German version of the Structured Clinical Interview for DSM-IV Dissociative Disorders (SCID-D) (Steinberg, 1993), the (SKID-D) (Gast et al., 2000). All patients had to be involved in a treatment phase involving exposure to trauma-related memories (Steele et al., 2005; Van der Hart et al., 2006). Exclusion criteria were comorbid psychosis, drug abuse or addiction, antisocial or histrionic personality disorder, and a neurological or organic brain disease. Two patients were free of medication. All other patients were medicated predominantly with antidepressant medication.
Fifteen female actors who were motivated to simulate ANP and EP served as controls. They did not differ significantly from the patients in age (controls: M = 43.2 years, SD = 10.4; patients: M = 43.3 years, SD = 9.1; t(28) = 0.019, p > .05) and educational level (controls: M = 4.7, SD = 1.2; patients: M = 4.1, SD = 1.5; t(26.099) = − 1.341, p > .05; the educational level was assessed by a 7-point Likert scale based on the common European educational system). The controls were interviewed by EW and EZ using the SKID-D (Gast et al., 2000). They also completed the German version of the Posttraumatic Diagnostic Scale (PDS) (Ehlers et al., 1996) and the Beck Depression Inventory II (BDI-II) (Hautzinger et al., 2006) to ensure that none of the controls had a dissociative disorder, PTSD, and/or major depression. The actors watched a video showing a DID patient talking to her therapist. In the video, the therapist invites the patient to alternate between ANP and EP. Based on detailed written information on TSDP (Van der Hart et al., 2006), the actors were instructed and motivated to create an ANP and EP using a list of properties (e.g., name, sex, age). ANP should be a dissociative part without personalized memories of traumatizing events and EP a dissociative part with personalized traumatic memories. The actors were requested to practice simulating ANP and EP as often as they deemed necessary to adequately enact these roles but at least three times before the fMRI measurement. Patients completed as ANP and EP the State Anxiety Inventory (STAI-S) (Laux et al., 1981) immediately after the MRI measurement, as did the controls to check if the actors had understood and followed the instructions to simulate an ANP and EP.
Each subject was informed about risks and inconveniences associated with the experiment before written informed consent was obtained. All procedures were approved by the local ethical committee and were conducted in accordance with the standards set by the Declaration of Helsinki. All participants received a financial compensation of 80 Swiss Francs for their participation.
2.2. Stimuli and experimental design
A backward masking paradigm was used to investigate preconscious mental reactivity to masked faces. The Karolinska Directed Emotional Faces (KDEF) served as photographic stimuli. They involved neutral, happy, fearful, and angry facial expressions, including approximately half male and half female subjects (Lundquist et al., 1998). The selection of the facial pictures used in the study was based on a rating of the intensity and genuineness of the displayed emotions (Van Balen, 2005). In addition to the faces, houses and scrambled images were presented. Scrambled stimuli were created in Fourier space by setting a low level of phase-coherence (Reinders et al., 2005, 2006a) in face pictures and served as baseline stimuli. All pictures were matched for luminance, contrast, brightness, and spatial frequency information (Rainer et al., 2001; Reinders et al., 2005, 2006a).
The pictures were generated by the software Presentation (version 14.1, http://www.neurobs.com) on a computer (Intel Core 2 Duo CPK, 60-Hz refresh rate) outside the scanner room. A DLP beamer (Plus U2-1110) projected them on a half-transparent screen, which could be seen via a mirror system placed on the head coil. All blocks of pictures were shown three times in a pseudorandomized order (18 blocks in total). Order effects were controlled by using two playlists (P1, P2), which were randomly assigned to ANP and EP. Each block consisted of 10 subliminal pictures (16.7 ms) and 11 black-and-white dotted masks (2.5 s). The masks, also used in previous studies (Henke et al., 2003a, 2003b), immediately preceded and followed the subliminal stimuli. This procedure ensured that the pictures could not be consciously perceived. The duration of the mask (equivalent to the interstimulus interval) was jittered by ± 1 s in randomized steps of 0.5 s. Every block lasted for 27.5 s and was separated by a 2.5 s mask (interblock interval), resulting in a total time of 9 min per run. Fig. 1 depicts the temporal sequence of events in a block.
Fig. 1.
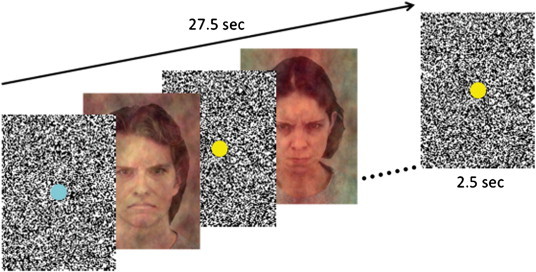
Experimental design. Example stimuli (KDEF, identity number M14 and F20, Lundquist et al., 1998), masks, and fixation dots are presented from one block displayed during the fMRI measurement.
A button press task (based on Reinders et al., 2005, 2006a) was used to measure condition-dependent RTs. Each mask contained a colored dot (yellow or turquoise). The color of the dot on the masks that preceded the experimental pictures was different from the color of the dot on the masks that followed these pictures. The participants were instructed to immediately press a button when they noticed that the color of the dot had changed. To direct the participants' gaze to the center of the faces, the dots on the masks were positioned at the place that corresponded with the center between the eyebrows of the faces. Each participant was first tested as ANP, and then as EP. The patient switched between dissociative parts of the personality outside the scanner room with little guidance from the research clinician. Inadvertent switches to a different dissociative part than the intended ANP or EP during the fMRI measurement were checked by asking the participants after the run what dissociative part had been present during the run. If there had been a switch to or a co-activation of an unintended dissociative part, the run was repeated, which was the case in one ANP and two EPs. A LED light of the response box in the scanner room switched on and off in synchrony with the participants' button presses. The authors observed that irregular flashing of this light was a good indicator of co-awareness of and/or switching to an unintended dissociative part during the experiment in DID patients. DID patients behaving like this explained that they had major difficulty to execute the button press task in an adequate fashion. For example, they reported that an unintended dissociative part wanted to participate in the task but was not or not fully aware of task instructions. Therefore, the authors closely watched the regularity of the LED flashing. It appeared that DID patients with irregular patterns of button presses were precisely the patients who were removed from the statistical analysis for other methodological reasons (see later).
2.3. Determination of awareness
The level of awareness of the masked images was determined at the very end of the experiment, outside of the scanner, using a subjective and an objective test (Cheesman and Merikle, 1984). The subjective test involves the participant's self report. Thus, the ANPs and EPs were asked what they had seen while lying in the scanner. The objective test is a forced-choice task, and constitutes the ‘gold-standard’ for the determination of awareness (Cheesman and Merikle, 1984; Greenwald et al., 1996; Holender, 1986). The subjective and objective tests demonstrated that the participants had not consciously seen the experimental images (see Supplementary Findings 1 and Inline Supplementary Table S1). A light sensor (Vishay Semiconductors) was used to examine the beamer's capacity to project pictures within the refresh rate of the computer's graphic card (NVIDIA Quadro FX 1700, 60-Hz) (see Supplementary Findings 2).
Inline Supplementary Table S1.
Table S1.
Objective determination of awareness (forced-choice task) in ANP.
| DID patients |
Controls |
|
|---|---|---|
| (n = 15) | (n = 14) | |
| Hits (%) | 50.71 ± 9.66 | 51.28 ± 9.67 |
DID, Dissociative Identity Disorder; mean percentage hit ± 1 SD is reported.
The level of awareness of the masked images was determined at the very end of the experiment, outside of the scanner, using a subjective and an objective test (Cheesman and Merikle, 1984). The subjective test involves the participant's self report. Thus, the ANPs and EPs were asked what they had seen while lying in the scanner. The objective test is a forced-choice task, and constitutes the ‘gold-standard’ for the determination of awareness (Cheesman and Merikle, 1984; Greenwald et al., 1996; Holender, 1986). The subjective and objective tests demonstrated that the participants had not consciously seen the experimental images (see Supplementary Findings 1 and Inline Supplementary Table S1). A light sensor (Vishay Semiconductors) was used to examine the beamer's capacity to project pictures within the refresh rate of the computer's graphic card (NVIDIA Quadro FX 1700, 60-Hz) (see Supplementary Findings 2).
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.07.002.
2.4. Image acquisition and data preprocessing
fMRI scanning was performed at the University Hospital of Zurich with a 3-T Philips Achieva whole-body magnetic resonance imaging equipped with an eight-channel Philips SENSE head coil. A total of 325 T2*-weighted echo planar image volumes, with blood-oxygen-level-dependent (BOLD) contrast (imaging parameter: echo time = 30 ms, repetition time = 1.7 s, flip-angle = 79°, FOV = 220 × 220 × 107 mm, slice thickness = 2.4 mm, slice gap = 1 mm, acquired voxel size = 2.75 × 2.75 × 2.4 mm, slices per volume = 32, SENSE factor = 2), were acquired during a single run. Initial ‘dummy’ volumes were obtained to ensure BOLD saturation. The data analysis was performed with the parametric mapping software SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Standard imaging pre-processing and statistical analysis procedures were applied. To account for movement artifacts, the functional images were realigned to the mean volume and coregistered onto the subject specific T1 image. This T1 image was normalized using the unified segmentation approach (Ashburner and Friston, 2005). The resulting normalization matrix was applied to the functional volumes, which transformed them into MNI space (new voxel size = 2 × 2 × 2 mm). Data were spatially smoothed with an 8-mm full width at half-maximum (FWHM) Gaussian kernel. In line with the experimental design, the BOLD data was modeled with a block design convolved with the standardized canonical haemodynamic response function (HRF). In one ANP of a DID patient, we observed huge imaging artifacts. One ANP of a DID patient reported that she had fallen asleep during the measurement. For one patient's EP, we found massive movement artifacts and one patient's EP was unable to complete the measurement. In view of our repeated measures ANOVA, the data of these four patients were omitted casewise. The final brain imaging statistical analysis was performed with data of 11 participants in the patient group and 15 in the control group.
A model with six condition and six movement regressors (with the realignment parameters) was aligned for each participant for ANP and EP separately at the first level analysis. The current analyses are restricted to the contrasts Neutral-Scramble (N-S) and Angry-Scramble (A-S). The results of the other contrasts will be published elsewhere. At the second level, the data were analyzed using a factorial design that consisted of two independent variables resulting in a 2 × 4 ANOVA with repeated measures on the second factor: Group (two levels: DID/CON), Condition (four levels: ANP N-S/ANP A-S/EP N-S/EP A-S). The analysis was based on a whole-brain voxel-wise comparison. For the main effect of condition, main effect of group, and interaction effect, we employed an uncorrected statistical threshold (i.e., voxel level of significance uncorrected [unc.] for multiple testing for the whole brain) of p < .001 with respect to our a priori defined regions. The selection of these regions is based on previous studies outlined in the introduction section (Hajcak et al., 2007; Haxby et al., 2000; Reinders et al., 2006b; Sakamoto et al., 2005; Vuilleumier and Pourtois, 2007; Whalen et al., 1998). To avoid type-2 errors, statistical thresholds of similar sizes have been used in affective and clinical neuroscience research (Felmingham et al., 2008; Phelps et al., 2001). Where no a priori hypothesis was available, we only accepted brain areas which reached a corrected p-value (p < .05). Corrected p-values are reported based on the family-wise error (FWE) correction at cluster level (Friston et al., 1994, 1996).
The participants were measured as ANP and EP in the patient group (DIDanp/DIDep) and in the control group (CONanp/CONep). The following eight planned comparisons were performed: DIDanp–DIDep N-S, CONanp-CONep N-S, DIDanp–CONanp N-S, DIDep–CONep N-S, DIDanp–DIDep A-S, CONanp-CONep A-S, DIDanp–CONanp A-S, DIDep–CONep A-S. Planned comparisons were not orthogonal. Statistical thresholds for a priori defined regions for these planned comparisons were adjusted for multiple testing using Bonferroni correction (p < .05/8 = p < .000125). All tests were one-sided, thus, were performed twice to assess positive differences in the BOLD signal in one and in the inverse contrast. Again, where no a priori hypothesis was available, we only accepted brain areas that survived FWE correction at cluster-level (p < .05). A cluster-size threshold of 7 voxels was applied. Only the first peak of a cluster and only the most significant finding of a brain area are reported in the Tables 2 to 5. The exact location of all clusters was defined using the Harvard–Oxford cortical and subcortical structural atlases (Desikan et al., 2006) and by visual inspection on a high-resolution T1-weighted image in FSL (http://www.fmrib.ox.ac.uk/fsl). The cingulate subregions were named according to Vogt's cytoarchitectonic division (Vogt, 2005).
Table 1.
Descriptive statistics of state anxiety.
| STAI-S | Mean | SD |
|---|---|---|
| DID (n = 13) | ||
| DIDanp | 49.92 | 11.64 |
| DIDep | 52.90 | 14.83 |
| CON (n = 15) | ||
| CONanp | 48.87 | 13.22 |
| CONep | 49.80 | 10.15 |
STAI-S, state anxiety inventory; DIDanp, ANP DID group; DIDep, EP DID group; CONanp, ANP control group; CONep, EP control group.
Table 2.
Main effect condition and interaction effect.
| Brain area | MNI coordinatesa |
||||||
|---|---|---|---|---|---|---|---|
| Side | x | y | z | kE | F value | ||
| Main effect condition | Putamen | L | − 24 | 6 | 0 | 73 | 8.70 |
| Parahippocampal gyrus (posterior part) | R | 18 | − 36 | − 10 | 33 | 7.90 | |
| Interaction effect | Parahippocampal gyrus (anterior part) | R | 16 | − 10 | − 24 | 29 | 9.60 |
| Middle temporal gyrusb | R | 62 | − 38 | − 8 | 17 | 7.31 | |
R/L, left or right hemisphere; kE, cluster size in voxels (one voxel is 2 × 2 × 2 mm).
MNI coordinates (in mm) refer to the maximum of signal change in each region.
Ventral bank of the sulcus temporalis superior.
Table 3.
ANP/EP effects within groups in response to masked angry and neutral faces as compared to scrambled faces (A-S, N-S).
| Brain area | Side | MNI coordinatesa |
kE | T value | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Condition A-S | |||||||
| DIDanp–DIDep | n.s | ||||||
| DIDep–DIDanp | Parahippocampal gyrus (anterior part) | R | 20 | − 14 | − 26 | 9 | 4.20 |
| CONanp–CONep | n.s. | ||||||
| CONep–CONanp | n.s | ||||||
| Condition N-S | |||||||
| DIDanp–DIDep | n.s | ||||||
| DIDep–DIDanp | Parahippocampal gyrus (anterior part) | R | 16 | − 12 | − 26 | 11 | 4.27 |
| CONanp–CONep | Superior frontal gyrus | L | − 20 | 28 | 54 | 140 | 4.94⁎ |
| aMCC/pMCC | R | 2 | 12 | 36 | 277 | 4.65⁎ | |
| Precentral gyrus (premotor cortex) | L | − 42 | − 4 | 46 | 25 | 4.26 | |
| Amygdala | R | 26 | − 6 | − 22 | 16 | 4.15 | |
| Middle temporal gyrus (temporooccipital part) | R | 58 | − 56 | 2 | 7 | 4.03 | |
| CONep–CONanp | n.s. | ||||||
R/L, left or right hemisphere; kE, cluster-size in voxels (one voxel is 2 × 2 × 2 mm); n.s., not significant; DIDanp, ANP DID group; DIDep, EP DID group; CONanp, ANP control group; CONep, EP control group; aMCC, anterior midcingulate cortex; pMCC, posterior midcingulate cortex.
MNI coordinates (in mm) refer to the maximum of signal change in each region.
Corrected for multiple comparisons using cluster-level statistics, p < .05.
Table 4.
ANP/EP effects between groups in response to masked angry and neutral faces as compared to scrambled faces (A-S, N-S).
| Brain area | Side | MNI coordinatesa |
kE | T value | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Condition A-S | |||||||
| DIDanp–CONanp | n.s. | ||||||
| CONanp–DIDanp | n.s | ||||||
| DIDep–CONep | Precentral gyrus (primary motor cortex) | L | − 36 | − 14 | 40 | 21 | 4.31 |
| CONep–DIDep | n.s. | ||||||
| Condition N-S | |||||||
| DIDanp–CONanp | n.s. | ||||||
| CONanp–DIDanp | n.s. | ||||||
| DIDep–CONep | Brainstem (dorsal part)c | L | − 12 | − 26 | − 18 | 1729 | 5.44⁎ |
| Parahippocampal gyrus (anterior part) | R | 16 | − 10 | − 24 | 35 | 5.29 | |
| Middle frontal gyrus | R | 40 | 32 | 32 | 267 | 5.26⁎ | |
| Middle frontal gyrus | L | − 28 | 32 | 48 | 136 | 5.26⁎ | |
| Middle temporal gyrus | R | 62 | − 38 | − 8 | 81 | 4.86⁎ | |
| Pre-SMA | L | − 2 | 4 | 62 | 159 | 4.85⁎ | |
| Precentral gyrus (primary motor cortex) | R | 42 | − 10 | 44 | 386 | 4.83⁎ | |
| pMCC/dPCC | 0 | − 26 | 32 | 274 | 4.74⁎ | ||
| DMPFC | R | 2 | 56 | 24 | 166 | 4.53⁎ | |
| Middle temporal gyrusb | R | 60 | − 22 | − 12 | 54 | 4.50 | |
| Precentral gyrus (primary/premotor cortex) | L | − 36 | − 14 | 42 | 46 | 4.34 | |
| STS | L | − 58 | − 16 | − 6 | 13 | 4.32 | |
| Lateral occipital cortex (inferior part) | R | 54 | − 68 | 0 | 24 | 4.21 | |
| Occipital pole (peristriate cortex) | R | 28 | − 96 | − 2 | 7 | 4.12 | |
| CONep–DIDep | n.s. | ||||||
R/L, left or right hemisphere; kE, cluster-size in voxels (one voxel is 2 × 2 × 2 mm); n.s., not significant; DIDanp, ANP DID group; DIDep, EP DID group; CONanp, ANP control group; CONep, EP control group; Pre-SMA, pre-supplementary motor area; pMCC, posterior midcingulate cortex; dPCC, dorsal posterior cingulate cortex; DMPFC, dorsomedial prefrontal cortex; STS, sulcus temporalis superior.
MNI coordinates (in mm) refer to the maximum of signal change in each region.
Ventral bank of the sulcus temporalis superior.
Cluster includes Brainstem R, Parahippocampal gyrus L, Lingual gyrus R/L, Temporal occipital fusiform gyrus L, Occipital fusiform gyrus L.
Corrected for multiple comparisons using cluster-level statistics, p < .05.
2.5. Data analysis: behavioral reactions
An attentional bias (AB) score was calculated by subtracting the mean value of the RTs for the three scrambled face blocks (S) from the mean value for the RTs of the three neutral face blocks (N) and the angry face blocks (A), respectively. The data of the participant who fell asleep and the one whose EP was not able to finish the measurement were excluded. The final statistical analysis was performed with data of 13 participants in the patient group and 15 in the control group. We calculated a 2 × 4 ANOVA with repeated measures on the second factor: Group (two levels: DID/CON), Condition (four levels: ANP N-S/ANP A-S/EP N-S/EP A-S) in SPSS18. For the main effect of condition, main effect of group, and interaction effect, p-values were set at .05. The following eight planned comparisons were performed: DIDanp–DIDep N-S, CONanp-CONep N-S, DIDanp–CONanp N-S, DIDep–CONep N-S, DIDanp–DIDep A-S, CONanp-CONep A-S, DIDanp–CONanp A-S, DIDep–CONep A-S. Planned comparisons were not orthogonal. Therefore, Bonferroni correction was applied and p-values were set at .00625, one-tailed.
Furthermore, the following four post-hoc t-tests were calculated to ensure that a RT difference can be explained by a face-specific effect: DIDanp N-S versus DIDanp A-S, DIDep N-S versus DIDep A-S, CONanp N-S versus CONanp A-S, CONep N-S versus CONep A-S. Bonferroni adjusted p-values were set at .0125, one-tailed.
2.6. Data analysis: state anxiety
A total value of the STAI-S (sum of obtained scores in the questionnaire) was calculated for each participant. The data of the participant who fell asleep and the one whose EP was not able to finish the measurement were excluded. The final statistical analysis was performed with data of 13 participants in the patient and 15 in the control group.
We calculated a 2 × 2 ANOVA with repeated measures on the second factor: Group (two levels: DID/CON), Type of dissociative part (two levels: ANP/EP) in SPSS18. For the main effect of group, main effect of type of dissociative part, and interaction effect, p-values were set at .05.
3. Results
3.1. Behavioral data
There was a significant interaction effect of group by condition (F(1,26) = 4.82, p < .05, partial η2 = .16). The main effect of group and the main effect of condition did not reach a significant threshold (p > .05). In AB N-S, Bonferroni corrected planned comparisons revealed a RT difference between DIDanp and DIDep (t(12) = − 3.15, p < .00625, d = 1.31). In AB A-S, planned comparisons did not reveal any significant results (p > .00625). Nevertheless, there is a clear positive AB N-S and a tendency to a positive AB A-S in DIDep (Fig. 2).
Fig. 2.
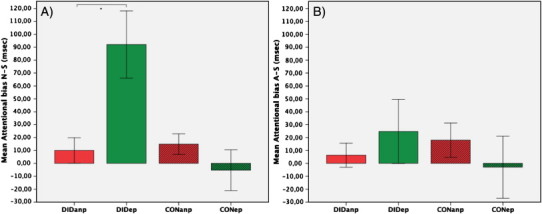
Mean attentional bias (AB) score (reaction times [RTs] for emotional faces minus RTs for scrambled faces) for (A) the neutral faces (AB N-S) and (B) the angry faces (AB A-S) in ms (± SEM). A positive AB indicates vigilance, a negative AB indicates avoidance, *p < .00625 (Bonferroni corrected).
We observed a significant longer RT in DIDep N-S compared to DIDep A-S (t(12) = 2.69, p < .0125, d = 0.73). All other post-hoc tests did not reach the critical threshold (p > .0125). Fig. 3 depicts the mean and standard error of RT (A-S)–(N-S) in DIDanp, DIDep, CONanp, and CONep.
Fig. 3.
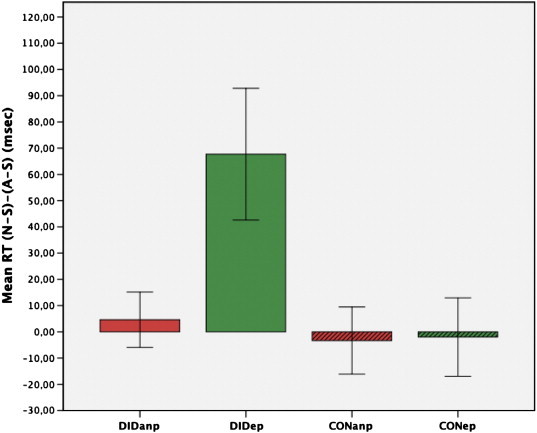
Mean reaction time (N-S)–(A-S) of ANP and EP in DID and CON (± SEM).
3.2. State anxiety
There was neither a significant main effect of group, nor a significant main effect of type of dissociative part, nor an interaction effect of group by type of dissociative part (p > .05). Table 1 summarizes the descriptive statistics of the STAI-S score in DIDanp, DIDep, CONanp, and CONep.
3.3. Neural data
3.3.1. Repeated measures ANOVA
We found a significant main effect of condition (putamen, posterior part of the parahippocampal gyrus) and a significant interaction effect of group by condition (parahippocampal gyrus, middle temporal gyrus) (Table 2). There was no significant main effect of group.
3.3.2. Planned comparisons
Within-group comparisons of two different types of dissociative parts of the personality (i.e., ANP–EP comparisons) are listed in Table 3. ANP–EP comparisons between groups are given in Tables 4 and 5.
Table 5.
Dissociative-part effects between groups in response to masked neutral faces as compared to scrambled faces (N-S).
| Brain area | Side | MNI coordinatesa |
kE | T value | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Condition N-S | |||||||
| DIDep–CONep | Brainstem | L | − 12 | − 26 | − 18 | 42 | 5.44⁎ |
| Middle frontal gyrus | R | 40 | 32 | 32 | 35 | 5.26⁎ | |
| Middle frontal gyrus | L | − 28 | 32 | 48 | 9 | 5.26⁎ | |
| Lingual gyrus | L | − 26 | − 54 | − 6 | 37 | 5.20⁎ | |
R/L, left or right hemisphere; kE, cluster-size in voxels (one voxel is 2 × 2 × 2 mm); DIDep, EP DID group; CONep, EP control group.
MNI coordinates (in mm) refer to the maximum of signal change in each region.
FWE correction for whole-brain multiple comparisons, p < .05 (kE = 7).
3.3.2.1. Within-group ANP–EP comparisons
In the angry and neutral face condition, DIDep had more activation in the parahippocampal gyrus than DIDanp (DIDep–DIDanp N-S/A-S, Table 3). This activation was not found for ANP versus EP in controls. The neutral faces but not the angry faces evoked a significantly increased right amygdala activity as well as in several cortical regions in CONanp compared to CONep (CONanp–CONep N-S, Table 3).
3.3.2.2. Between-group ANP–EP comparisons
In the angry face condition and compared to CONep, DIDep was associated with more activation in the precentral gyrus (DIDep–CONep A-S, Table 4). In the neutral face condition (DIDep–CONep N-S, Table 4), the same contrast demonstrated increased neural activation for DIDep. Multiple large clusters reached our predefined statistical thresholds. The first cluster with a peak value in the left dorsal brainstem includes several mainly left lateralized areas in the occipito-temporal junction (lingual gyrus, temporal occipital fusiform gyrus, and occipital fusiform gyrus) and the left parahippocampal gyrus (Fig. 4). Within this cluster, brainstem and lingual gyrus survived FWE correction for whole-brain multiple comparisons (p < .05, Table 5). DIDep had more activation in several a priori defined regions (middle temporal gyrus, STS, lateral occipital cortex, occipital pole). As this type of dissociative part, DID patients also had more activation in several motor-related areas (pre-supplementary motor area, precentral gyrus).
Fig. 4.
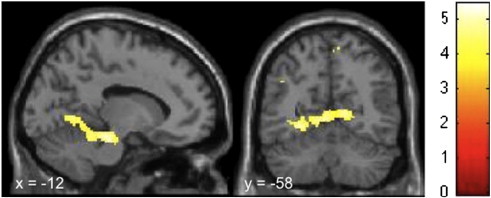
Brain regions showing significantly higher activation during preconscious exposure to neutral faces as compared to scrambled faces in DIDep compared to CONep (DIDep–CONep, N-S). The sagittal view depicts areas in the dorsal brainstem, occipitotemporal junction, and parahippocampal gyrus. Activation in the visual cortex can be seen in the coronal view. Corresponding regions, cluster-sizes, MNI coordinates, and T-values can be found in Table 4.
4. Discussion
This is the first fMRI study of neural activation patterns to preconsciously perceived facial expressions for two different prototypes of dissociative parts of the personality (ANP and EP) in DID patients. As generally hypothesized, we found different neural and behavioral activation patterns for ANP and EP in DID patients and in controls.
Consistent with our first hypothesis, as EP, DID patients demonstrated more activation in the right parahippocampal gyrus during the masked presentation of neutral and angry faces than they had as ANP (see Table 3). The parahippocampal gyrus has been implicated in recall of autobiographical memories (Fink et al., 1996), with a right hemispheric predominance (Tulving et al., 1994), and in re-experiencing symptoms in PTSD (Osuch et al., 2001; Sakamoto et al., 2005). The observed enhanced activation in the parahippocampal gyrus corresponds with core features of EP, that is, their fixation in traumatic memories, their tendency to perceive safe individuals as dangerous, and their tendency to reactivate traumatic memories when confronted with reminders of traumatic experiences. However, we did not find the hypothesized differences for ANP and EP in DID patients with respect to visual areas, face sensitive areas, amygdala, and motor areas. This negative finding may at least in part relate to limitations of the present study, which will be discussed below.
Differences in neural activation patterns were much more pronounced for EP in DID patients compared to EP in controls. But in contrast with our third hypothesis, EP's subliminal perception of neutral and not angry faces revealed these strong differences. In reaction to subliminally presented angry faces, EP in DID showed enhanced activity in the precentral gyrus (see Fig. 4). We also observed increased activity in the temporal pole of the superior temporal gyrus. This area is known to participate in the analysis of faces too, particularly in processing the semantic knowledge of a face (Haxby et al., 2000). We are reluctant to discuss this activity any further, as it did not reach the statistical threshold for non-a priori defined regions. Masked neutral faces evoked activation in a cluster of brain areas including the dorsal brainstem, parahippocampal gyrus, and mainly left lateralized areas positioned in the occipito-temporal junction (see Fig. 4), as well as several motor-related areas (see Table 4).
Taken together, the findings of the current study suggest that as EP, DID patients deeply engaged in subliminally presented faces, particularly in neutral faces. DIDep's dorsal brainstem activity furthermore indicates increased arousal (Jones, 2003) and associated vigilance in reaction to subliminally perceived neutral faces.
The occipito-temporal junction is a face-sensitive region (Gorno-Tempini et al., 2001; Haxby et al., 2000; Nakamura et al., 2000), and the occipital fusiform gyrus contributes at a very early phase in the face-processing stream and generates the initial representation of a face (Pitcher et al., 2007). The mainly left lateralized activation pattern is in line with previous findings of left hemispheric involvement in subliminal perception of faces (Henke et al., 1994). Activation of motor areas could indicate defensive reactions to perceived threat.
Given the integral role of the amygdala in automatic processing of threatening stimuli (Öhman, 2005; Vuilleumier, 2005), the reason for the lack of amygdalar activity in our study deserves a closer look. PTSD neuroimaging studies have led to inconsistent findings with regards to amygdala activation. Studies employing masked-faces paradigm (Rauch et al., 2000) or visual imagery (Shin et al., 1997) demonstrated exaggerated amygdala responses in PTSD patients compared to healthy controls, although studies conducting script-driven imagery failed to reveal increased amygdala activity in PTSD subjects (Bremner et al., 1999a, 1999b; Shin et al., 1999). Furthermore, amygdala engagement during the processing of fearful faces is a reliable and consistent finding in the fMRI literature, whereas amygdala enhancement as reaction to angry or neutral faces has been reported less consistently (Fusar-Poli et al., 2009). The amygdala can habituate during repeated exposure to emotional stimuli (Breiter et al., 1996; Fischer et al., 2003; Schwartz et al., 2003; Wright et al., 2001). Time courses of left and right amygdala activity (mean beta values within the left and right amygdala, data not shown) did not reveal evidence of amygdala habituation during the whole experimental period, neither in DID patients nor in controls. However, the amygdala is not only restricted to signaling of fear, but is also involved in the evaluation of salient (Sander et al., 2003) and novel stimuli (Blackford et al., 2010). It has been shown that the amygdala is activated most strongly at the beginning of a stimulus series (Büchel et al., 1998). Hence, the blockwise manner, in which our stimuli were presented, might explain the non significant amygdala activity in our study. It could also be possible that the amygdala of DID patients was consistently overactivated even before negative stimuli were presented. This idea accords with results of other imaging studies showing that anxious individuals have increased anticipatory activity in the amygdala preceding stimuli with prior known negative, neutral, or ambiguous emotional valence (Brühl et al., 2011; Nitschke et al., 2009).
Comparisons in which ANP's brain activation was contrasted to other conditions did not give significant results (i.e., DIDanp–DIDep, DIDanp–CONanp; see Tables 3 and 4). This finding indicates a relatively decreased BOLD signal all over the brain for this type of dissociative part, suggesting low involvement in subliminally presented faces.
There was increased activation in many a priori defined brain regions for EP in DID patients compared to EP in controls, but fewer differences for ANP in DID patients compared to EP in these patients. We therefore checked post hoc if these differences also existed for ANP in DID patients compared to EP in controls (DIDanp–CONep N-S, data not shown). We found enhanced activity in the dorsal brainstem, lingual gyrus (with some voxels extending to the temporal occipital fusiform gyrus), and motor-related areas such as the putamen and the (pre-)supplementary motor area. While this pattern resembles the one for DIDep, it was less pronounced. It thus seems that ANP's decreased involvement in consciously perceived trauma-related cues (Reinders et al., 2003, 2006b) has roots in ANP's subdued preconscious reactivity to trauma-related cues.
This study is the first to document that foremost as EP, DID patients specifically focus on, and seem to be alarmed by preconsciously perceived neutral faces. Consistent with the neural findings and our fourth hypothesis, EP in DID patients also showed significantly slower RTs to neutral faces and a tendency to slower RTs to angry faces compared to ANP in DID patients and EP in controls (see Fig. 2). This face- and dissociative part-specific effect could also be observed in the direct comparison between RTs related to neutral and angry faces. This comparison yielded a significant longer RT in the neutral face condition in EP of DID patients only.
Emotionally neutral faces may be threatening to them for a variety of reasons. First, it can be hard to disambiguate these expressions (“what does this face mean?”), particularly following emotional neglect (“this person may not care about me”) and abuse (“this person seems calm, but for how long, what emotion will he or she show next?”). Consistent with this interpretation, patients with borderline personality disorder (BPD) regarded neutral faces as threatening, and demonstrated a hyperactivated amygdala when supraliminally confronted with these faces (Donegan et al., 2003). BPD, DID, and dissociative symptoms are all intimately related to a context of unstable and disrupted interpersonal relationships (Benjamin, 1993; Dutra et al., 2009; Kelley et al., 2002; Korol, 2008; Linehan, 1993; Ogawa et al., 1997). As the type of dissociative part of the personality that is fixed in the traumatic past, EP may regard neutral faces as untrustworthy and threatening, and thus become hypervigilant when confronted with them, and prepare motor defensive reactions. Neutral faces can also express affective unavailability (of caretakers), a condition that all DID patients in the study reported (neglect and abuse by family members). The quality of the early caregiving relationship is linked to dissociation in that affective parental unavailability and disorganized attachment in childhood are major predictors of dissociative symptoms in adulthood (Dutra et al., 2009; Ogawa et al., 1997). Our results fit findings of grave effects of still faces on children (Mesman et al., 2009; Tronick et al., 1978), particularly in individuals who are neglected, abused, and insecurely attached. They generally add to the evidence for a pivotal role of emotional neglect and emotional unavailability of caretakers in DID.
Our data contrast with the findings of Hermans et al. (2006), who reported longer RTs to angry compared to neutral faces in EP of DID patients. This conflicting finding might be related to several methodological differences between these studies in relation to stimulation, such as the facial and masking stimuli, the design of the subliminal presentation, and the presentation time. While our study presented facial stimuli for 16.7 ms, Hermans et al. presented these stimuli for 25 ms. Cognitive theories of anxiety maintain that the attentional bias toward threatening material occurs at a preconscious level (Cisler and Koster, 2010). The stage of sensory reactivity at which this bias emerges in DID has not been investigated systematically to date. There is neurophysiological evidence showing that the signals transmitted by neurons in the visual cortex increase as a function of stimulus length (Rolls et al., 1999). In other words, the shorter the presentation time, the less sensory signals for the discrimination of a face are provided. Neutral faces have an uncertain emotional valence and, therefore, require deeper processing demands. It might be speculated that the slightly shorter presentation time in our study particularly increased preconscious fixation in EP on neutral facial expressions, as EP is focused on threat or potential threat cues (Van der Hart et al., 2006). Future studies are needed to test this hypothesis.
Our fifth and last hypothesis was that the identified behavioral and neural differences for ANP and EP in DID patients would not be matched by controls, who were instructed and motivated to simulate ANP and EP. Controls showed a tendency to inverse RTs and neural activation patterns for these different prototypical parts. That is, as ANP, the actors tended to react like EP in DID patients, and as EP like ANP in these patients. The actors were thus unable to simulate DID with respect to behavioral and neural reactivity, which contradicts the sociocognitive model of DID. Compared to EP, as ANP, controls had amygdala activity in the neutral face condition (see Table 3), but neither brainstem activity nor a longer RT. Whereas the neutral faces were thus salient (Davis and Whalen, 2001; LeDoux, 1998) for ANP-simulating controls, they did not arouse them or attract much preconscious attention, as happened for authentic EP. The current findings add to the psychobiological evidence (Hermans et al., 2006; Reinders et al., 2012) that DID is neither an effect of suggestion and fantasy, nor of role-playing.
The findings have strong implications for the clinical context in dealing with DID patients and suggest that therapists of DID patients must be emotionally and behaviorally engaged. Therapeutic neutrality will probably scare them, particularly as EP, triggering and reinforcing conditioned emotional and defensive reactions. As EP, these patients will tend to perceive an emotionally neutral therapist as an emotionally unavailable caretaker. These effects may not be immediately visible when an ANP is dominant due to ANP's mental avoidance and under-engagement. However, ANP and EP can be activated in parallel (Van der Hart et al., 2006), so that the therapist's neutrality can nonetheless affect the patient as one or more EPs. This interpretation is consistent with clinical observations (Van der Hart et al., 2006). For example, ANP may report that EP is negatively affected by the therapist's neutrality. It may also happen that ANP does not notice or report this emotionality in an EP, but that EP responds in the described emotional sense in a later stage, while expressing that she/he felt rejected, confused, or afraid when the therapist was emotionally un(der)engaged.
This study has several limitations. Our sample size was relatively small, which was due to the difficulty in finding DID patients who are able to alternate between ANP and EP at request and to remain activated, particularly as EP, for a substantial period of time in an fMRI environment. Patients who can perform this feat are the ones who have been in treatment for at least several years. Because treatment of DID fosters integration between the different dissociative parts and integration of traumatic memories, studies such as ours are prone to underestimate naturally existing biopsychosocial differences between these subsystems of the personality. In order to check if the actors had understood and followed the instructions to simulate an ANP and EP, patients and controls completed as ANP and EP the STAI-S (Laux et al., 1981) immediately after the fMRI measurement. Explorative data analysis revealed that, in contrast to the behavioral and neural data, no inverse simulation pattern could be observed (see Table 1). That is, as ANP, the actors tended to react like ANP in DID patients, and as EP like EP in these patients. However, no significant differences were observed between and within groups. Therefore, the STAI-S does not seem to be an appropriate measurement to examine adherence to simulation instructions. In future studies, other assessments such as self-report should be included. DID patients have considerable comorbidity (Ellason et al., 1996). Future studies will need to evaluate axis I and axis II comorbidity and address covariations between this comorbidity and patterns of neural activation. Another limitation of the study is that only two of our patients were free of medication. Medication washout is not feasible with DID patients. However, medication does not explain the observed differences between ANP and EP in DID patients.
In conclusion, the current study shows that two prototypical parts of the personality in DID patients, ANP and EP, have different biopsychosocial reaction patterns to backward masked neutral and angry faces that controls were unable to simulate. Fixed in active defense, as EP, DID patients engage in early and automatic scanning of facial expressions. Avoiding threat cues, as ANP, they are underinvolved in the faces. These results and interpretations are consistent with clinical observations and TSDP, but inconsistent with the sociocognitive model of DID.
Acknowledgments
This research was supported by the Forschungskredit of the University of Zurich. A.A.T. Simone Reinders is supported by the Netherlands Organization for Scientific Research (www.nwo.nl), NWO-VENI grant no. 451-07-009. We would like to thank the colleagues of Prof. Jäncke's lab for their helpful comments and Franz Liem and Thomas Reber for their technical support. Special thanks go to the patients and their therapists for participating in the study.
Competing interests
None declared.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Yolanda R. Schlumpf, Email: y.schlumpf@psychologie.uzh.ch.
Ellert R.S. Nijenhuis, Email: e.nijenhuis@home.nl, enijenhuis@me.com.
Sima Chalavi, Email: sima.chalavi@gmail.com.
Ekaterina V. Weder, Email: praxis@ev-weder.li.
Eva Zimmermann, Email: eva.zimmermann@bluewin.ch.
Roger Luechinger, Email: luechinger@biomed.ee.ethz.ch.
Roberto La Marca, Email: r.lamarca@psychologie.uzh.ch.
A.A.T. Simone Reinders, Email: a.a.t.s.reinders@gmail.com.
Lutz Jäncke, Email: l.jaencke@psychologie.uzh.ch.
Appendix A. Supplementary data
Supplementary Findings.
References
- Amaral D.G., Price J.L., Pitkanen A., Carmichael S.T. Anatomical organization of primate amygdaloid complex. In: Aggleton W., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- American Psychiatric Association . 4th ed. American Psychiatric Association; Washington, DC: 1994. Diagnostic and Statistical Manual of Mental Disorders DSM-IV. [Google Scholar]
- Armony J.L., Corbo V., Clement M.H., Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. The American Journal of Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bakvis P., Roelofs K., Kuyk J., Edelbroek P.M., Swinkels W.A., Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. 2009;50:1001–1011. doi: 10.1111/j.1528-1167.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner T., Willi M., Jancke L. Modulation of corticospinal activity by strong emotions evoked by pictures and classical music: a transcranial magnetic stimulation study. Neuroreport. 2007;18:261–265. doi: 10.1097/WNR.0b013e328012272e. [DOI] [PubMed] [Google Scholar]
- Benjamin L.S. 2nd ed. Guilford; New York: 1993. Interpersonal Diagnosis and Treatment of Personality Disorders. [Google Scholar]
- Bishop S.J. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Blackford J.U., Buckholtz J.W., Avery S.N., Zald D.H. A unique role for the human amygdala in novelty detection. NeuroImage. 2010;50:1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H.C., Etcoff N.L., Whalen P.J., Kennedy W.A., Rauch S.L., Buckner R.L., Strauss M.M., Hyman S.E., Rosen B.R. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Narayan M., Staib L.H., Southwick S.M., McGlashan T., Charney D.S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. The American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brühl A.B., Rufer M., Delsignore A., Kaffenberger T., Jancke L., Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Research. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- Büchel C., Morris J., Dolan R.J., Friston K.J. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cheesman J., Merikle P.M. Priming with and without awareness. Perception & Psychophysics. 1984;36:387–395. doi: 10.3758/bf03202793. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Koster E.H. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalenberg C.J., Brand B.L., Gleaves D.H., Dorahy M.J., Loewenstein R.J., Cardena E., Frewen P.A., Carlson E.B., Spiegel D. Evaluation of the evidence for the trauma and fantasy models of dissociation. Psychological Bulletin. 2012;138:550–588. doi: 10.1037/a0027447. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diseth T.H. Dissociation following traumatic medical treatment procedures in childhood: a longitudinal follow-up. Development and Psychopathology. 2006;18:233–251. doi: 10.1017/S0954579406060135. [DOI] [PubMed] [Google Scholar]
- Donegan N.H., Sanislow C.A., Blumberg H.P., Fulbright R.K., Lacadie C., Skudlarski P., Gore J.C., Olson I.R., McGlashan T.H., Wexler B.E. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Dutra L., Bureau J.F., Holmes B., Lyubchik A., Lyons-Ruth K. Quality of early care and childhood trauma: a prospective study of developmental pathways to dissociation. The Journal of Nervous and Mental Disease. 2009;197:383–390. doi: 10.1097/NMD.0b013e3181a653b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A., Steil R., Winter H., Foa E.B. University, Warneford Hospital; Oxford: 1996. Deutsche Übersetzung der Posttraumatic Diagnostic Scale (PDS) [Google Scholar]
- Ellason J.W., Ross C.A., Fuchs D.L. Lifetime axis I and II comorbidity and childhood trauma history in dissociative identity disorder. Psychiatry. 1996;59:255–266. doi: 10.1080/00332747.1996.11024766. [DOI] [PubMed] [Google Scholar]
- Eysenck M.W., Mogg K., May J., Richards A., Mathews A. Bias in interpretation of ambiguous sentences related to threat in anxiety. Journal of Abnormal Psychology. 1991;100:144–150. doi: 10.1037//0021-843x.100.2.144. [DOI] [PubMed] [Google Scholar]
- Felmingham K., Kemp A.H., Williams L., Falconer E., Olivieri G., Peduto A., Bryant R. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychological Medicine. 2008;38:1771–1780. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- Fink G.R., Markowitsch H.J., Reinkemeier M., Bruckbauer T., Kessler J., Heiss W.D. Cerebral representation of one's own past: neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Wright C.I., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Research Bulletin. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Worsley K.J., Frackowiak R.S.J., Mazziotta J.C., Evans A.C. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A., Poline J.B., Price C.J., Frith C.D. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S., Benedetti F., Abbamonte M., Gasparotti R., Barale F., Perez J., McGuire P., Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gast U., Hofmann A., Oswald T., Zündorf F. Deutsche Fassung. Manual und Interviewheft. Hogrefe; Göttingen: 2000. Das Strukturierte Klinische Interview für DSM-IV Dissoziative Störungen (SKID-D) [Google Scholar]
- Giesbrecht T., Lynn S.J., Lilienfeld S.O., Merckelbach H. Cognitive processes in dissociation: an analysis of core theoretical assumptions. Psychological Bulletin. 2008;134:617–647. doi: 10.1037/0033-2909.134.5.617. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Pradelli S., Serafini M., Pagnoni G., Baraldi P., Porro C., Nicoletti R., Umita C., Nichelli P. Explicit and incidental facial expression processing: an fMRI study. NeuroImage. 2001;14:465–473. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- Greenwald A.G., Draine S.C., Abrams R.L. Semantic activation without conscious identification in dichotic listening, parafoveal vision, and visual masking: a survey and appraisal. Science. 1996;273:1699–1702. doi: 10.1126/science.273.5282.1699. [DOI] [PubMed] [Google Scholar]
- Grillon C., Lissek S., Rabin S., McDowell D., Dvir S., Pine D.S. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. The American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Molnar C., George M.S., Bolger K., Koola J., Nahas Z. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44:91–97. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Keller F., Kühner C. Harcourt Test Services; Frankfurt a. M.: 2006. Beck Depressions-Inventar (BDI-II) Revision. [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hendler T., Rotshtein P., Yeshurun Y., Weizmann T., Kahn I., Ben-Bashat D., Malach R., Bleich A. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. NeuroImage. 2003;19:587–600. doi: 10.1016/s1053-8119(03)00141-1. [DOI] [PubMed] [Google Scholar]
- Henke K., Landis T., Markowitsch H.J. Subliminal perception of words and faces. International Journal of Neuroscience. 1994;75:181–187. doi: 10.3109/00207459408986302. [DOI] [PubMed] [Google Scholar]
- Henke K., Mondadori C.R., Treyer V., Nitsch R.M., Buck A., Hock C. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia. 2003;41:863–876. doi: 10.1016/s0028-3932(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Henke K., Treyer V., Nagy E.T., Kneifel S., Dursteler M., Nitsch R.M., Buck A. Active hippocampus during nonconscious memories. Consciousness and Cognition. 2003;12:31–48. doi: 10.1016/s1053-8100(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Hermans E.J., Nijenhuis E.R., Van Honk J., Huntjens R.J., Van der Hart O. Identity state-dependent attentional bias for facial threat in dissociative identity disorder. Psychiatry Research. 2006;141:233–236. doi: 10.1016/j.psychres.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Holaway R.M., Heimberg R.G., Coles M.E. A comparison of intolerance of uncertainty in analogue obsessive–compulsive disorder and generalized anxiety disorder. Journal of Anxiety Disorders. 2006;20:158–174. doi: 10.1016/j.janxdis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Holender D. Semantic activation without conscious identification in dichotic listening, parafoveal vision, and visual masking: a survey and appraisal. The Behavioral and Brain Sciences. 1986;9:1–23. [Google Scholar]
- Jones B.E. Arousal systems. Frontiers in Bioscience. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Kelley W.M., Macrae C.N., Wyland C.L., Caglar S., Inati S., Heatherton T.F. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Korol S. Familial and social support as protective factors against the development of dissociative identity disorder. Journal of Trauma & Dissociation. 2008;9:249–267. doi: 10.1080/15299730802048744. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Densmore M., Boksman K., Gupta M.A., Neufeld R.W., Gati J.S., Menon R.S. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. The American Journal of Psychiatry. 2001;158:1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Williamson P.C., Boksman K., Densmore M., Gupta M., Neufeld R.W., Gati J.S., Menon R.S. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biological Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Bluhm R., Lanius U., Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. Journal of Psychiatric Research. 2006;40:709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Laux L., Glanzmann P., Schaffner P., Spielberger C.D. Theoretische Grundlagen und Handanweisung. Beltz Test GmbH; Weinheim: 1981. Das State-Trait-Angstinventar. [Google Scholar]
- LeDoux J.E. Touchstone; New York: 1998. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. [Google Scholar]
- Lilienfeld S.O., Lynn S.J., Kirsch I., Chaves J.F., Sarbin T.R., Ganaway G.K., Powell R.A. Dissociative identity disorder and the sociocognitive model: recalling the lessons of the past. Psychological Bulletin. 1999;125:507–523. doi: 10.1037/0033-2909.125.5.507. [DOI] [PubMed] [Google Scholar]
- Linehan M.M. Guilford Press; New York, N.Y.: 1993. Cognitive-behavioral Treatment of Borderline Personality Disorder. [Google Scholar]
- Lundquist D., Flykt A., Öhman A. 1998. The Karolinska Directed Emotional Faces — KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institute. [Google Scholar]
- Merckelbach H., Muris P. The causal link between self-reported trauma and dissociation: a critical review. Behaviour Research and Therapy. 2001;39:245–254. doi: 10.1016/s0005-7967(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Merckelbach H., Devilly G.J., Rassin E. Alters in dissociative identity disorder. Metaphors or genuine entities? Clinical Psychology Review. 2002;22:481–497. doi: 10.1016/s0272-7358(01)00115-5. [DOI] [PubMed] [Google Scholar]
- Merskey H. The manufacture of personalities. The production of multiple personality disorder. The British Journal of Psychiatry. 1992;160:327–340. doi: 10.1192/bjp.160.3.327. [DOI] [PubMed] [Google Scholar]
- Mesman J., Van IJzendoorn M.H., Bakermans-Kranenburg M.J. The many faces of the Still-Face Paradigm: a review and meta-analysis. Developmental Review. 2009;29:120–162. [Google Scholar]
- Morris J.S., Ohman A., Dolan R.J. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kawashima R., Sato N., Nakamura A., Sugiura M., Kato T., Hatano K., Ito K., Fukuda H., Schormann T., Zilles K. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123(Pt 9):1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- Nijenhuis E.R.S., Den Boer J.A. Psychobiology of traumatisation and trauma-related structural dissociation of the personality. In: Dell P.F., O'Neil J.A., editors. Dissociation and the Dissociative Disorders: DSM-V and Beyond. Routledge; New York: 2009. pp. 337–367. [Google Scholar]
- Nijenhuis E.R.S., Van der Hart O., Steele K. The emerging psychobiology of trauma-related dissociation and dissociative disorders. In: D'Haenen H., Den Boer J.A., Willner P., editors. Biological Psychiatry. Wiley; London: 2002. pp. 1079–1098. [Google Scholar]
- Nitschke J.B., Sarinopoulos I., Oathes D.J., Johnstone T., Whalen P.J., Davidson R.J., Kalin N.H. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. The American Journal of Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J.R., Sroufe L.A., Weinfield N.S., Carlson E.A., Egeland B. Development and the fragmented self: longitudinal study of dissociative symptomatology in a nonclinical sample. Development and Psychopathology. 1997;9:855–879. doi: 10.1017/s0954579497001478. [DOI] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Oliveri M., Babiloni C., Filippi M.M., Caltagirone C., Babiloni F., Cicinelli P., Traversa R., Palmieri M.G., Rossini P.M. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Experimental Brain Research. 2003;149:214–221. doi: 10.1007/s00221-002-1346-8. [DOI] [PubMed] [Google Scholar]
- Osuch E.A., Benson B., Geraci M., Podell D., Herscovitch P., McCann U.D., Post R.M. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biological Psychiatry. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Wager T., Taylor S.F., Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., O'Connor K.J., Gatenby J.C., Gore J.C., Grillon C., Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitcher D., Walsh V., Yovel G., Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Current Biology. 2007;17:1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Putman P., Hermans E., Van Honk J. Emotional stroop performance for masked angry faces: it's BAS, not BIS. Emotion. 2004;4:305–311. doi: 10.1037/1528-3542.4.3.305. [DOI] [PubMed] [Google Scholar]
- Rainer G., Augath M., Trinath T., Logothetis N.K. Nonmonotonic noise tuning of BOLD fMRI signal to natural images in the visual cortex of the anesthetized monkey. Current Biology. 2001;11:846–854. doi: 10.1016/s0960-9822(01)00242-1. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Van der Kolk B.A., Fisler R.E., Alpert N.M., Orr S.P., Savage C.R., Fischman A.J., Jenike M.A., Pitman R.K. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Whalen P.J., Shin L.M., McInerney S.C., Macklin M.L., Lasko N.B., Orr S.P., Pitman R.K. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Reinders A.A.T.S., Nijenhuis E.R.S., Paans A.M., Korf J., Willemsen A.T., Den Boer J.A. One brain, two selves. NeuroImage. 2003;20:2119–2125. doi: 10.1016/j.neuroimage.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Reinders A.A.T.S., Den Boer J.A., Buchel C. The robustness of perception. European Journal of Neuroscience. 2005;22:524–530. doi: 10.1111/j.1460-9568.2005.04212.x. [DOI] [PubMed] [Google Scholar]
- Reinders A.A.T.S., Glascher J., De Jong J.R., Willemsen A.T., Den Boer J.A., Buchel C. Detecting fearful and neutral faces: BOLD latency differences in amygdala-hippocampal junction. NeuroImage. 2006;33:805–814. doi: 10.1016/j.neuroimage.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Reinders A.A.T.S., Nijenhuis E.R.S., Quak J., Korf J., Haaksma J., Paans A.M., Willemsen A.T., Den Boer J.A. Psychobiological characteristics of dissociative identity disorder: a symptom provocation study. Biological Psychiatry. 2006;60:730–740. doi: 10.1016/j.biopsych.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Reinders A.A.T.S., Willemsen A.T.M., Vos H.P.J., Den Boer J.A., Nijenhuis E.R.S. Fact or factitious? A psychobiological study of authentic and simulated dissociative identity states. PLoS One. 2012;6:e39279. doi: 10.1371/journal.pone.0039279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., Tovee M.J., Panzeri S. The neurophysiology of backward visual masking: information analysis. Journal of Cognitive Neuroscience. 1999;11:300–311. doi: 10.1162/089892999563409. [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Fukuda R., Okuaki T., Rogers M., Kasai K., Machida T., Shirouzu I., Yamasue H., Akiyama T., Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. NeuroImage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sander D., Grafman J., Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Wright C.I., Shin L.M., Kagan J., Whalen P.J., McMullin K.G., Rauch S.L. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Kosslyn S.M., McNally R.J., Alpert N.M., Thompson W.L., Rauch S.L., Macklin M.L., Pitman R.K. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Archives of General Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Shin L.M., McNally R.J., Kosslyn S.M., Thompson W.L., Rauch S.L., Alpert N.M., Metzger L.J., Lasko N.B., Orr S.P., Pitman R.K. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. The American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Whalen P.J., Pitman R.K., Bush G., Macklin M.L., Lasko N.B., Orr S.P., McInerney S.C., Rauch S.L. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Orr S.P., Carson M.A., Rauch S.L., Macklin M.L., Lasko N.B., Peters P.M., Metzger L.J., Dougherty D.D., Cannistraro P.A., Alpert N.M., Fischman A.J., Pitman R.K. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Simeon D., Guralnik O., Hazlett E.A., Spiegel-Cohen J., Hollander E., Buchsbaum M.S. Feeling unreal: a PET study of depersonalization disorder. The American Journal of Psychiatry. 2000;157:1782–1788. doi: 10.1176/appi.ajp.157.11.1782. [DOI] [PubMed] [Google Scholar]
- Spanos N.P. Multiple identity enactments and multiple personality disorder: a sociocognitive perspective. Psychological Bulletin. 1994;116:143–165. doi: 10.1037/0033-2909.116.1.143. [DOI] [PubMed] [Google Scholar]
- Spanos N.P. American Psychological Association; Washington: 1996. Multiple Identities & False Memories: A Sociocognitive Perspective. [Google Scholar]
- Steele K., Van der Hart O., Nijenhuis E.R. Phase-oriented treatment of structural dissociation in complex traumatization: overcoming trauma-related phobias. Journal of Trauma & Dissociation. 2005;6:11–53. doi: 10.1300/J229v06n03_02. [DOI] [PubMed] [Google Scholar]
- Steinberg M. American Psychiatric Press; Washington, DC: 1993. Structured Clinical Interview for DSM-IV Dissociative Disorders (SCID-D) [Google Scholar]
- Trickett P.K., Noll J.G., Putnam F.W. The impact of sexual abuse on female development: lessons from a multigenerational, longitudinal research study. Development and Psychopathology. 2011;23:453–476. doi: 10.1017/S0954579411000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick E., Als H., Adamson L., Wise S., Brazelton T.B. The infant's response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Craik F.I., Moscovitch M., Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Balen L. Rijksuniversiteit Groningen; Groningen: 2005. Activation and Localization of the Emotional Circuits in the Brain: An fMRI Study in Patients with Schizophrenia and Healthy Volunteers. [Google Scholar]
- Van der Hart O., Nijenhuis E.R.S., Steele K. W.W. Norton & Company; New York, London: 2006. The Haunted Self: Structural Dissociation and the Treatment of Chronic Traumatization. [Google Scholar]
- Van Honk J., Tuiten A., Van den Hout M., Koppeschaar H., Thijssen J., De Haan E., Verbaten R. Baseline salivary cortisol levels and preconscious selective attention for threat. A pilot study. Psychoneuroendocrinology. 1998;23:741–747. doi: 10.1016/s0306-4530(98)00047-x. [DOI] [PubMed] [Google Scholar]
- Van Honk J., Tuiten A., Van den Hout M., Koppeschaar H., Thijssen J., De Haan E., Verbaten R. Conscious and preconscious selective attention to social threat: different neuroendocrine response patterns. Psychoneuroendocrinology. 2000;25:577–591. doi: 10.1016/s0306-4530(00)00011-1. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M.A. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C.I., Fischer H., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Findings.


