Abstract
Background
Sleep-disordered breathing (SDB) has been increasingly recognized as a possible risk factor for adverse perioperative outcomes in non-bariatric surgeries. However, the impact of SDB on postoperative outcomes in patients undergoing bariatric surgery remains less clearly defined. We hypothesized that SDB would be independently associated with worse postoperative outcomes.
Methods
Data were obtained from the Nationwide Inpatient Sample database, and included a total of 91,028 adult patients undergoing bariatric surgeries from 2004 to 2008. The primary outcomes were in-hospital death, total charges and length of stay. There were two secondary outcomes of interest: respiratory and cardiac complications. Regression models were fitted to assess the independent association between SDB and the outcomes of interest.
Results
SDB was independently associated with decreased mortality (OR 0.34, 95% CI 0.23-0.50, p<0.001), total charges (-$869, p<0.001), and length of stay (-0.25 days, p<0.001). SDB was independently associated with significantly increased odds ratio of emergent endotracheal intubation (OR 4.35, 95% CI 3.97-4.77, p<0.001), noninvasive ventilation (OR 14.12, 95% CI 12.09-16.51, p<0.001), and atrial fibrillation (OR 1.25, 95% CI 1.11-1.41, p<0.001). Emergent intubation occurred significantly earlier in the postoperative course in patients with SDB. Although non-SDB patients had an overall lower risk of emergent intubation compared to SDB patients, their outcomes were significantly worse when they did get emergently intubated.
Conclusions
In this large nationally representative sample, despite the increased association of SDB with postoperative cardiopulmonary complications, the diagnosis of SDB was negatively, rather than positively, associated with in-hospital mortality and resource use.
Keywords: Sleep-disordered breathing, bariatric surgery, obstructive sleep apnea, postoperative complications, intubation, respiratory failure, death, length of stay, cost
Introduction
Sleep-disordered breathing (SDB) is increasingly recognized as a possible risk factor for adverse perioperative outcomes [1-6]. Several studies have reported worse postoperative outcomes in SDB patients such as increased rates of hypoxemia, endotracheal intubation, respiratory failure, intensive care unit transfers, increased hospital length of stay (LOS), encephalopathy, and postoperative infections [2-3, 5-9]. Clinicians may expect SDB to be associated with increased risk of adverse postoperative outcomes after bariatric surgery. However, to the best of our knowledge, rates of postoperative complications after bariatric surgery have not been systematically compared in patients with and without SDB in a large, nationally representative sample.
To that end, we examined the association of SDB with several postoperative outcomes in patients undergoing bariatric surgery. We analyzed the Nationwide Inpatient Sample (NIS) database to quantify the impact of the diagnosis of SDB on in-hospital death, total charges, LOS, respiratory outcomes, and cardiac outcomes. We hypothesized that the diagnosis of SDB would be independently associated with worse postoperative outcomes, after controlling for comorbidities and demographic characteristics.
Methods
Data Source
Data were obtained from the NIS database, which is one of several databases that form the Healthcare Utilization Project. The NIS is the largest all-payer database in the United States and has been used in a variety of research studies [9-11]. The NIS contains information on approximately 8 million hospitalizations per year from 1,050 hospitals in 44 states. The data approximates a 20% stratified sample of hospitals in the United States. The data has been collected on an annual basis since 1988 [12]. The database includes a record for every hospital discharge, regardless of payer, at included hospitals during a given year.
This study was approved by the University of Chicago’s Institutional Review Board (BSD/UCH IRB approval # 10-567-E).
Patient Cohort
Our cohort was derived by including all hospital admissions in adults (age 18 or more) for bariatric surgeries in the NIS database from the years 2004 to 2008. We selected the most recent 5 years in the NIS database to avoid significant changes in practice patterns. At the time of data extraction, 2008 was the most recent year with data available in the NIS database. Patients were stratified based on the diagnosis of SDB. The ICD-9-CM codes used to characterize SDB are described in Appendix 1. The ICD-9-CM codes used to identify the bariatric surgery procedures are also described in Appendix 1.
Patient Data
Patient demographics included age, sex, self-reported race/ethnicity, Charlson Comorbidity Index (CCI), income by quartile, health insurance source (i.e. Medicare, Medicaid, private), teaching or non-teaching hospital status, and United States region (Northeast, South, West, Midwest/Central). The information about race is missing in approximately 27% of cases because some participating states restrict race data. The CCI is a tool to assign severity to a patient’s comorbid conditions. Common comorbid conditions are assigned varying weights, and the sum of the patient’s score indicates their cumulative comorbid condition and higher scores indicate increased comorbidity [13]. Income was divided into quartiles with 1 being the poorest quartile and 4 being the wealthiest quartile. Income data were obtained from zip codes and demographic data from Nielson online demographic services [14].
The primary outcomes compared between SDB and non-SDB patients included in-hospital death, cost in total hospital charges, and LOS. Secondary respiratory outcomes included emergent endotracheal intubation and mechanical ventilation, continuous positive airway pressure/noninvasive ventilation (CPAP/NIV) during hospitalization, tracheostomy, pneumonia, and respiratory failure. Secondary cardiac outcomes included atrial fibrillation and percutaneous coronary procedures.
Data Extraction
In-hospital death, total charges, and LOS are variables available in the NIS database. Secondary outcomes were derived using ICD-9-CM and Clinical Classifications Software (CCS) codes (Appendix 1) [15].
Statistical Analysis
For unadjusted comparisons between SDB and non-SDB patients, continuous variables (age, LOS, and total charges) were presented as the mean and standard deviation and were compared using the student’s t-test. All other categorical variables were summarized as percentages and compared using the chi-square test. Generalized linear models with log-link and gamma distributed errors were fitted to assess the independent association between SDB and length of stay and total charges in order to adjust the difference over other independent variables. We used the iteratively reweighted least squares method for maximum likelihood estimation of the model parameters. There is a substantial statistical literature that recommends using generalized linear model techniques when modeling health care costs and LOS due to unique features of such data, namely that they tend to be differentially dispersed around the mean (heteroskedastic) and prone to large outliers (right-skewed) [16-17]. Mean adjusted LOS and total charges for patients with and without SDB were then estimated from the generalized linear models. We constructed logistic regression models for outcomes that were dichotomous to determine the independent association of SDB and the outcomes of interest. Independent variables introduced in the generalized linear models and logistic regression models included age by quartiles, sex, type of health insurance, income by quartiles, CCI, type of surgical procedure, year of the surgical procedure, hospital teaching status, United States region where the hospital is located, and weekend admission. We included weekend admission as an independent variable given its association with worse outcomes in some studies. Race was not included in the models because it was missing for approximately 27% of admissions. Body mass index is not available in the NIS database.
The percentage of patients that were dropped from the regression models due to a missing variable was quite negligible, between 3.0-3.5%. Although multiple imputation could have been performed for missing variables, we elected not do it since missing data was small and statistical power was not an issue. In order to measure model discrimination and the goodness of fit of the models, several statistics were examined including deviance, Pearson chi-square, Akaike (AIC), Bayesian Information Criterion (BIC), and pseudo R2 statistics.
Stata version 11.0 (StataCorp LP) statistical software was used for all analyses.
Results
Demographics
Our cohort included a total of 91,028 patients undergoing bariatric surgeries from 2004 to 2008. Patient demographics are summarized in Table 1. The prevalence of CCI score 0-1, indicating a very low burden of comorbid disease, was significantly higher among non-SDB patients (84.4% vs. 80.4%, p<0.01). In general, SDB patients were slightly older with more men, had higher CCI scores, and had a higher percentage of Medicare coverage.
Table 1.
Demographic data for bariatric surgery patients based on sleep-disordered breathing (SDB) status.
| Variables | All patients | No SDB | SDB | p-value |
|---|---|---|---|---|
| N, (%) | 91,028 | 57,832 (64%) | 33,196 (36%) | |
| Age, mean (SD) | 44.2 (11.8) | 43.5 (12.3) | 45.4 (10.6) | <0.01 |
| Female, % | 80 | 85.5 | 70.3 | <0.01 |
| Race, % | ||||
| White | 75.5 | 74.6 | 77.1 | 0.01 |
| Black | 12.8 | 12.8 | 12.7 | 0.65 |
| Hispanic | 6.9 | 7.5 | 5.9 | <0.01 |
| Other | 4.8 | 5.1 | 4.3 | <0.01 |
| Charlson Comorbidity Index, % | ||||
| CCI 0 | 51.5 | 55.3 | 44.9 | <0.01 |
| CCI 1 | 31.4 | 29.1 | 35.5 | <0.01 |
| CCI 2 | 6.7 | 5.5 | 8.9 | <0.01 |
| CCI 3 | 6.1 | 6.4 | 5.7 | <0.01 |
| CCI ≥ 4 | 4.3 | 3.9 | 5 | <0.01 |
| Insurance type, % | ||||
| Medicare | 10.2 | 9.5 | 11.4 | <0.01 |
| Private | 73.3 | 72.8 | 74.2 | <0.01 |
| Medicaid | 5.9 | 61 | 5.7 | 0.01 |
| Other | 4.2 | 4.6 | 3.5 | <0.01 |
| Income quartile, % | ||||
| First | 21.5 | 22.3 | 20.2 | <0.01 |
| Second | 26.7 | 26.7 | 26.9 | 0.55 |
| Third | 26.3 | 26.3 | 26.5 | 0.45 |
| Fourth | 25.4 | 24.8 | 26.5 | <0.01 |
| Teaching hospital, % | 58.1 | 58.1 | 58.2 | 0.66 |
| Hospital region, % | ||||
| Northeast | 32.4 | 33.9 | 29.7 | <0.01 |
| South | 35.6 | 35.1 | 36.6 | <0.01 |
| West | 11.6 | 11.3 | 12.1 | <0.01 |
| Midwest/North Central | 20.4 | 19.8 | 21.6 | <0.01 |
Unadjusted comparisons
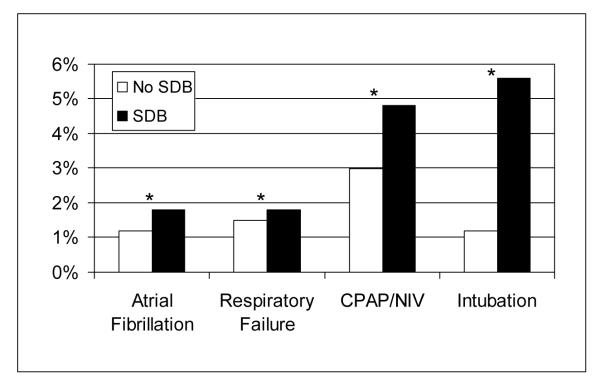
In the unadjusted comparisons (Table 2), SDB patients experienced significantly less in-hospital death (0.1% vs. 0.3% p <0.01%), incurred significantly less total charges ($37,934 vs. $39,977, p <0.001), and had significantly shorter LOS (5.78 vs. 7.10 days, p <0.01). In contrast, SDB patients experienced significantly increased rates of emergent intubation and mechanical ventilation (5.6% vs. 1.2%, p <0.01), CPAP/NIV use (4.8% vs. 0.3%, p <0.01), respiratory failure (1.8% vs. 1.5%, p <0.01), and atrial fibrillation (1.8% vs. 1.2%, p <0.01) (Figure 1). Conversely, SDB patients experienced significantly lower rates of pneumonia (0.6% vs. 1.0%, p <0.01) and tracheostomy placement (0.08% vs. 0.13%, p=0.02). Rates of coronary procedures did not differ significantly between SDB patients and non-SDB patients.
Table 2.
Unadjusted outcomes of bariatric surgery patients based on sleep-disordered breathing (SDB) status
| Outcomes | All patients | No SDB | SDB | p-value |
|---|---|---|---|---|
| N | 91,028 | 57,832 | 33,196 | |
| In-hospital death, % | 0.2 | 0.3 | 0.1 | <0.01 |
| Total charges, dollars | 39,909 (48,694) | 39,977 (49,133) | 37,934 (33,448) | <0.001 |
| Length of stay, days | 7.05 (6.58) | 7.10 (6.63) | 5.78 (4.53) | <0.01 |
| Respiratory complications, % | ||||
| Emergent Intubation | 2.8 | 1.2 | 5.6 | <0.01 |
| CPAP/NIV | 1.9 | 0.3 | 4.8 | <0.01 |
| Respiratory failure | 1.6 | 1.5 | 1.8 | <0.01 |
| Pneumonia | 0.9 | 1 | 0.6 | <0.01 |
| Tracheostomy | 0.12 | 0.13 | 0.08 | 0.02 |
| Cardiac complications, % | ||||
| Atrial fibrillation | 1.5 | 1.2 | 1.8 | <0.01 |
| Coronary procedures | 0.1 | 0.1 | 0.1 | 0.59 |
Total charges and length of stay presented as mean (SD), other variables presented as percentage, CPAP/NIV: continuous positive airway pressure/non-invasive ventilation.
Figure 1.
Unadjusted outcomes for atrial fibrillation, respiratory failure, CPAP/NIV use, and emergent intubation in patients with and without SDB. * p <0.01
Adjusted comparisons
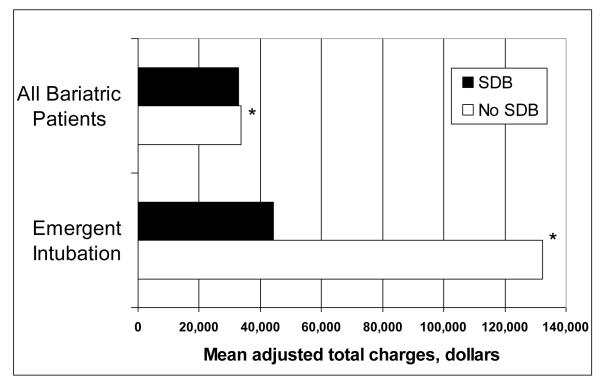
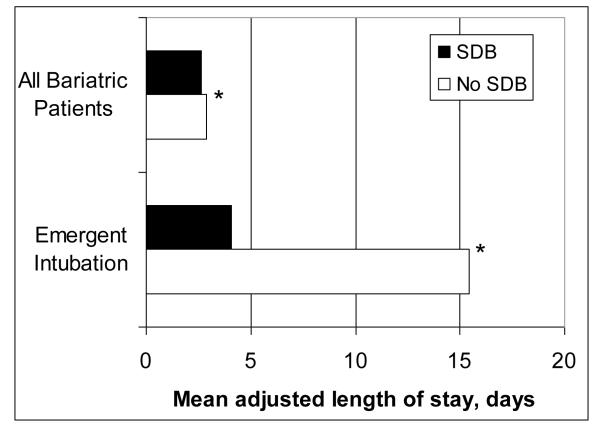
In generalized linear models after adjusting for the covariates, SDB was independently associated with decreased mortality (OR 0.34, 95% CI 0.23 – 0.50, p <0.001). SDB was independently associated with small but a statistically significant decrease in estimated mean total charges by $869 (p< 0.001) and estimated mean LOS by 0.25 days (p< 0.001) (Table 3, Figures 2 and 3).
Table 3.
Multivariable regression modeling to estimate adjusted risk of outcomes associated with sleep-disordered breathing (SDB)
| Outcomes | Regression coefficient or OR for SDB |
95% Confidence Interval |
p-value |
|---|---|---|---|
| In-hospital death | 0.34 | 0.23 – 0.50 | <0.001 |
| Total charges | −0.03 | −0.04, −0.02 | <0.001 |
| Length of stay | −0.09 | -0.10, −0.07 | <0.001 |
| Respiratory complications | |||
| Emergent Intubation | 4.35 | 3.97 – 4.77 | <0.001 |
| CPAP/NIV | 14.12 | 12.09 – 16.51 | <0.001 |
| Respiratory failure | 1.07 | 0.96 – 1.20 | 0.24 |
| Pneumonia | 0.61 | 0.51 – 0.72 | <0.001 |
| Tracheostomy | 0.51 | 0.32 – 0.81 | 0.004 |
| Cardiac complications | |||
| Atrial fibrillation | 1.25 | 1.11 – 1.41 | <0.001 |
| Coronary procedures | 0.72 | 0.38 – 1.38 | 0.329 |
Generalized linear modeling for continuous variables (length of stay and total charges) and multivariable logistic regression for dichotomous variables were used to estimate regression coefficients or odds ratio for the presence of SDB after adjusting for age, sex, Charlson comorbidity index, insurance status, geographic region, teaching institution, income, weekend admission, year of surgery, and surgical procedure code. CPAP/NIV: continuous positive airway pressure/non-invasive ventilation.
OR: odds ratio.
Figure 2.
Mean adjusted total charges in all patients undergoing bariatric surgery (n=91,028) and the subset of bariatric patients that required emergent intubation (n=2,572) based on SDB status. * p <0.001
Figure 3.
Mean adjusted length of stay in all patients undergoing bariatric surgery (n=91,028) and the subset of bariatric surgery patients that required emergent intubation (n=2,572) based on SDB status. * p <0.001
SDB was independently associated with a significantly increased odds ratio of emergent endotracheal intubation and mechanical ventilation (OR 4.35, 95% CI 3.97 – 4.77, p <0.001), CPAP/NIV (OR 14.12, 95% CI 12.09 – 16.51, p <0.001), and atrial fibrillation (OR 1.25, 95% CI 1.11 – 1.41, p <0.001). In contrast, SDB was associated with decreased odds ratio of pneumonia and tracheostomy (Table 3).
Outcomes of patients requiring emergent endotracheal intubation and mechanical ventilation
In total, 2,572 patients required emergent intubation and mechanical ventilation (1,855 SDB patients and 717 non-SDB patients). In unadjusted analyses, we examined the impact of emergent intubation and mechanical ventilation on outcomes between SDB and non-SDB patients (Table 4). In-hospital death, total charges, LOS, respiratory failure, and pneumonias increased significantly more in non-SDB patients requiring emergent intubation and mechanical ventilation than SDB patients requiring the same intervention. Unadjusted analysis demonstrated that emergent intubation in patients with SDB was associated with an increase in in-hospital death by 1.1%, an increase in total charges by $11,191, an increase in the LOS by 1.6 days, an increase in respiratory failure by 13.9%, and an increase in pneumonias by 1.7%. In contrast, emergent intubation in non-SDB patients was associated with significantly worse outcomes such as an increase in in-hospital death by 13.7%, an increase in total charges by $102,438, an increase in the LOS by 12.7 days, an increase in respiratory failure by 59.5%, and an increase in pneumonias by approximately 20.0% (Table 4).
Table 4.
Outcomes based on the diagnosis of sleep-disordered breathing (SDB) and need for emergent endotracheal intubation in bariatric surgery patients.
| Outcomes | No Sleep-Disordered Breathing | Sleep-disordered Breathing | ||||
|---|---|---|---|---|---|---|
| No Intubation | Intubation | p | No Intubation | Intubation | p | |
| Number of cases | 57,115 | 717 | 31,341 | 1,855 | ||
| In-hospital death, % | 0.15 | 13.81 | <0.01 | 0.04 | 1.13 | <0.01 |
| Total charges, dollars | 32,033 (26,451) |
134,471 (142,897) |
<0.01 | 32,798 (18,731) |
43,989 (46,829) |
<0.01 |
| Length of stay, days | 2.8 (3.84) |
15.47 (19.93) |
<0.01 | 2.47 (1.84) |
4.05 (4.92) |
<0.01 |
| CPAP/NIV, % | 0 | 26.22 | <0.01 | 0 | 85.01 | <0.01 |
| Respiratory failure, % | 0.75 | 60.25 | <0.01 | 1.02 | 14.93 | <0.01 |
| Pneumonia, % | 0.75 | 20.78 | <0.01 | 0.52 | 2.26 | <0.01 |
| Intubation on Day 0 or Day 1, % |
NA | 63.0 | NA | 90.0 | ||
Day 0 is day of surgery, Day 1 is post-operative day 1.
CPAP/NIV: continuous positive airway pressure/non-invasive ventilation.
NA: Not applicable
We constructed regression models to assess whether emergent endotracheal intubation in non-SDB patients was independently associated with worse outcomes (Table 5). After adjusting for age, sex, CCI, hospital teaching status, and year of surgery, non-SDB patients who required emergent intubation had significantly worse in-hospital death (OR 10.99, 95% CI 6.76 – 18.18, p <0.001), respiratory failure (OR 8.33, 95% CI 6.80 – 10.20, p <0.001), and pneumonia (OR 10.75, 95% CI 7.46 – 15.63, p <0.001). Similarly, the mean adjusted total charges and mean adjusted LOS was significantly higher in non-SDB patients requiring emergent intubation (Figure 2 and Figure 3).
Table 5.
Multivariable regression modeling to estimate adjusted risk of outcomes in patients requiring emergent endotracheal intubation. No-SDB status in patients requiring intubation was independently associated with worse outcomes.
| Outcomes in patients requiring intubation |
Regression coefficient or OR for non-SDB |
95% Confidence Interval |
p-value |
|---|---|---|---|
| In-hospital death | 10.99 | 6.76 – 18.18 | <0.001 |
| Total charges | 1.09 | 1.00 – 1.19 | <0.001 |
| Length of stay | 1.34 | 1.22 – 1.45 | 0.009 |
| Respiratory failure | 8.33 | 6.80 – 10.20 | <0.001 |
| Pneumonia | 10.75 | 7.46 – 15.63 | <0.001 |
Generalized linear modeling for continuous variables (length of stay and total charges) and multivariable logistic regression for dichotomous variables were used to estimate regression coefficients or odds ratio in patients without SDB after adjusting for age, sex, Charlson comorbidity index, insurance status, teaching institution, and year of surgery.
OR: odds ratio.
The use of CPAP/NIV during the hospital course was significantly lower in non-SDB patients requiring emergent intubation and mechanical ventilation. Notably, CPAP/NIV use during the hospitalization was not recorded in any patient who did not require emergent intubation and mechanical ventilation regardless of the SDB status (Table 4). A significantly higher proportion of patients with SDB required emergent intubation and mechanical ventilation on the day of surgery or the first post-operative day compared to non-SDB patients (90% vs. 63%, p <0.001).
Discussion
In our analysis of the largest nationally representative sample of patients undergoing bariatric surgery, we found an increased rate of postoperative cardiopulmonary complications in patients with SDB. Specifically, SDB was independently associated with increased risk of emergent endotracheal intubation, CPAP/NIV use, and atrial fibrillation. These findings differ from what has been previously reported [18-20]. In a retrospective single-center study, Weingarten et al found that SDB was not associated with an increased likelihood of pulmonary complications in patients undergoing bariatric surgery [20]. However, 93% of their patients received postoperative positive airway pressure therapy for SDB and were closely monitored in the postoperative setting with continuous pulse oximetry, which likely explains the low complication rates. Although we have no information regarding the perioperative management of SDB in the NIS sample, we suspect it was suboptimal as CPAP/NIV use was not recorded in the vast majority of patients with SDB during their hospitalization. In an analysis by the Longitudinal Assessment of Bariatric Surgery (LABS) consortium, SDB was also not significantly associated with their composite end point of death, deep venous thrombosis/pulmonary embolism, surgical reintervention, or delayed discharge in the adjusted model [18]. These studies were performed in tertiary care academic centers whereas 42% of our patients had their surgery performed in a non-teaching hospital [18-20]. Academic centers may have used multidisciplinary perioperative processes and pathways to identify and treat SDB more often than non-teaching hospitals.
Despite the fact that SDB was associated with increased rates of emergent endotracheal intubation, CPAP/NIV use, and atrial fibrillation, we were surprised to find that it was negatively associated with rates of in-hospital death, total charges, or LOS. Other studies have demonstrated that SDB is not a risk factor for death in the postoperative bariatric setting [18, 20-22], but why this association was in the opposite direction of what we expected is unclear. The NIS database includes up to 15 diagnostic codes for each hospitalization. One possibility may be that when a patient dies or has a prolonged hospital stay additional diagnoses are added to their hospitalization which can push SDB off the list. Another possible putative mechanism by which SDB could decrease mortality is ischemia preconditioning. Indeed, studies have reported that in patients with acute myocardial infarction, those with SDB and intermittent hypoxemia during sleep have less severe cardiac injury and better coronary collateral circulation [23-26]. Therefore, it is plausible that SDB does not increase in-hospital cardiovascular mortality in the immediate postoperative setting despite the increased risk of upper airway complications. Although chronically untreated severe SDB is strongly associated with increased cardiovascular mortality [27-30], it remains unclear whether untreated SDB in the acute postoperative setting can lead to increased cardiovascular mortality. It is also possible that the lower mortality rate and lower levels of resource utilization are due to more appropriate care of SDB patients with CPAP/NIV while the population of patients without a diagnosis of SDB includes patients with SDB experiencing adverse outcomes because of failure to identify and treat SDB. The higher rates of emergent endotracheal intubation in patients with SDB, however, suggest that it is unlikely that the patients with SDB received better postoperative care. In addition, the vast majority of patients undergoing bariatric surgery did not require emergent intubation and in this cohort, CPAP/NIV use during the hospitalization was not recorded in any patient (Table 4). Low rates of CPAP/NIV utilization may be due to underreporting in the NIS database. Another possibility is that postoperative CPAP/NIV may have been underutilized due to the concern that it can increase the risk of aerophagia and anastomotic leak [31-33]. Although postoperative CPAP does not increase the risk of disrupting the anastomotic integrity after upper gastrointestinal tract surgery [34], its safety during the immediate postoperative period may have not been widely recognized by bariatric surgeons during the years included in our analysis (2004 to 2008). Our findings that SDB was not associated with increased LOS and in-hospital mortality are in line with a recent single-center study of presurgical patients [35]. The goal of this prospective observational study was to determine if a prior diagnosis of SDB or a positive screen for SDB was associated with increased mortality in 14,962 presurgical patients. Surprisingly, despite higher rates of ICU admissions, patients with SDB did not have an increase in LOS or in 30-day mortality in the postoperative period [35].
Our analysis of patient outcomes in relationship to emergent endotracheal intubation yielded interesting results. We found that SDB patients that required emergent intubation were intubated much earlier in their postoperative course. As expected, both SDB and non-SDB patients requiring emergent intubation had worsened outcomes compared to their counterparts who did not require emergent intubation. However, non-SDB patients that required intubation had substantially worse outcomes than SDB patients requiring intubation. This finding suggests that upper airway complications may be the main cause for emergent intubation in patients with SDB. We speculate that in patients with SDB, the primary reason for emergent intubation was related to a rapidly reversible upper airway complication in the setting of sedative and opioid administration in the immediate postoperative period. This speculation is supported by the fact that in patients with SDB, emergent intubation occurred earlier in their postoperative course. Additionally, the fact that emergent intubation in patients with SDB led to a significantly less increase in in-hospital death, total charges, LOS, and pneumonias as compared to non-SDB patients requiring intubation supports this speculation. It is well known that patients with SDB have elevated pharyngeal closing pressures as well as increased upper airway instability and collapsibility in the postoperative setting as compared to matched controls thus predisposing them to upper airway complications [36-37]. Our data demonstrates that although an increase in postoperative complications should be anticipated in SDB patients undergoing bariatric surgery, the overall prognosis with a complication like emergent endotracheal intubation is better than if they did not have SDB. Our analysis revealed that non-SDB patients requiring emergent intubation were intubated later in their post-operative course and had higher rates of pneumonia, and we suspect their intubation was indicative of a global decline or complication that took much longer to recover from resulting in worse outcomes. Another possibility is that clinicians may have had a lower threshold for intubating patients with known SDB in the setting of postoperative respiratory complications.
Our study has several limitations that are inherent to analysis of large administrative databases such as the inability to confirm the diagnosis and severity of SDB, and lack of data on CPAP therapy at home or during the postoperative period. It is also likely that CPAP/NIV was often not coded even when they were used during the postoperative period. We could not ascertain whether the diagnostic codes for SDB were part of the admission diagnoses or discharge diagnoses, therefore it is conceivable that respiratory complications could have lead to a presumptive diagnosis of SDB. We also do not have clinical information on all potential confounders, such as body mass index. Moreover, the NIS database also lacks information about readmissions as well as long-term postoperative outcomes.
We found the prevalence of SDB to be 36% among patients undergoing elective bariatric surgery in the NIS database. We suspect the majority of the cases of SDB were of significant severity that warranted clinical attention, which is why they were coded for in the database. Although most recent studies have reported a prevalence of severe OSA in patients undergoing bariatric surgery ranging from 27-36%, there is likely a component of underreporting in the NIS database since the reported prevalence of moderate and severe SDB in patients undergoing bariatric surgery is 44-48% [20, 38-40]. The lower than expected prevalence of SDB in patients undergoing bariatric surgery can in part be related to a significantly higher prevalence of premenopausal women undergoing surgery. Moreover, it is important to consider that body mass index explains only 4-26% of the variance of SDB severity as measured by the apnea-hypopnea index [41-43]. Therefore, we believe that the NIS database does not represent a significant underreporting of clinically significant cases of SDB.
In summary, using a large nationally representative database, we have reported that in patients undergoing bariatric surgery, SDB is independently associated with significant postoperative cardiopulmonary complications but not with increased in-hospital mortality, total charges, and LOS. Although non-SDB patients had an overall lower risk of emergent intubation compared to SDB patients, their outcomes were significantly worse when they did get emergently intubated. Given the overall low complication rates, prospective large scale multicenter randomized controlled trials are needed to assess the impact of SDB treatment on patient outcomes during the postoperative period in patients undergoing bariatric surgery.
Acknowledgment
Authors Affiliation: Department of Medicine, University of Chicago, Chicago, Illinois (Drs. Mokhlesi, Hovda, Vekhter, Arora, and Meltzer) and the Department of Anesthesia, University of Toronto, Toronto, Canada (Dr. Chung).
Funding: Supported by the University of Chicago Institute for Translational Medicine and the Clinical and Translational Science Awards (CTSA) program (UL1 RR024999).
Role of the Sponsor: The sponsor had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Acknowledgments
Funding: Supported by the University of Chicago Institute for Translational Medicine and the Clinical and Translational Science Awards (CTSA) program (UL1 RR024999). Dr. Arora is supported by National Institutes on Aging (K23 AG033763). Dr. Meltzer is supported by a Midcareer Career Development Award from the National Institutes of Health (1 K24 AG031326-01).
Abbreviations
- CCI
Charlson comorbidity index
- CI
Confidence interval
- CPAP/NIV
Continuous positive airway pressure/Noninvasive ventilation
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LOS
Length of stay
- NIS
Nationwide inpatient sample
- OR
Odds ratio
- SDB
Sleep-disordered breathing
Appendix 1.
The ICD-9-CM codes used to characterize sleep-disordered breathing (SDB)
327.23 Obstructive Sleep Apnea
327.20 Organic Sleep Apnea
327.29 Organic Sleep Apnea Not Elsewhere Classified
780.51 Insomnia with Sleep Apnea
780.52 Hypersomnia with Sleep Apnea
780.57 Sleep Apnea
The ICD-9-CM codes used to identify bariatric surgery procedures (the percentage of each surgical procedure are included in parentheses)
44.38 Laparascopic gastric bypass (44.5%)
44.39 Open gastric bypass (25.8%)
44.95 Laparascopic gastric band (16%)
44.31 Open high gastric bypass (6.2%)
43.89 Sleeve gastrectomy (3%)
44.68 Laparoscopic gastroplasty (2.4%)
44.69 vertical banded gastroplasty (2.1%).
Codes used for data extraction of secondary outcomes
Respiratory Outcomes
216 Intubation/Mechanical Ventilation based on Clinical Classifications Software (CCS):
CCS codes provide a classification scheme that facilitates analyzing data regarding procedures and diagnoses. CCS codes condense ICD-9-CM codes into fewer clinically meaningful groups that are easier to analyze. CCS code 216 encompasses all of the following ICD-9-CM codes: emergent endotrachael intubation not related to anesthesia and surgery (96.04), continuous invasive mechanical ventilation of unspecified duration (96.70), continuous invasive mechanical ventilation < 96 hours (96.71), or invasive mechanical ventilation > 96 hours (96.72).
Respiratory Failure/Respiratory Distress
518.82 Acute Respiratory Insufficiency, Acute respiratory distress syndrome
518.81 Acute Respiratory Failure
518.5 Pulmonary Insufficiency Following Surgery and Trauma
518.4 Acute Edema of Lung, unspecified
Pneumonias
486 Pneumonia, Organism Unspecified
482 Other Bacterial Pneumonia
482.8 Pneumonia Due to Other Specified Bacteria
482.9 Bacterial Pneumonia Unspecified
485 Bronchopneumonia, Organism Unspecified
484 Pneumonia in Infectious Diseases Classified Elsewhere
507 Pneumonitis Due to Solids and Liquids
507.0 Due to Inhalation of Food or Vomitus
507.8 Due to Other Solids and Liquids
93.90 Continuous positive airway pressure (CPAP)/Noninvasive Ventilation (NIV)
Tracheostomy
31.1 Temporary Tracheostomy
31.2 Permanent Tracheostomy
Cardiac Outcomes
427.31 Atrial Fibrillation
Cardiac Procedures
88.55 Coronary Arteriography Using a Single Catheter
88.56 Coronary Arteriography Using Two Catheters
88.57 Other and Unspecified Coronary Arteriography
37.23 Combined Right and Left Heart Cardiac Catheterization
37.22 Left Heart Cardiac Catheterization
88.54 Combined Right and Left Heart Angiocardiography
36.09 Other Removal of Coronary Artery Obstruction
00.66 Percutaneous Transluminal Coronary Angioplasty [PTCA] or Coronary Atherectomy
36.06 Insertion of Non-Drug-Eluting Coronary Artery Stent(s)
36.07 Insertion of Drug-Eluting Coronary Artery Stent(s)
Footnotes
Conflict of Interest Disclosures: Babak Mokhlesi has served as a consultant for Philips/Respironics but has no conflicts of interest relevant to the present study.
Margaret D. Hovda has no conflicts of interest relevant to the present study to declare.
Benjamin Vekhter has no conflicts of interest relevant to the present study to declare.
Vineet M. Arora has no conflicts of interest relevant to the present study to declare.
Frances Chung has no conflicts of interest relevant to the present study to declare.
David O. Meltzer has no conflicts of interest relevant to the present study to declare.
Author contribution: Dr. Mokhlesi had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mokhlesi, Arora, Chung, Meltzer.
Acquisition of data: Mokhlesi, Vekhter, Hovda.
Analysis and interpretation of data: Mokhlesi, Hovda, Vekhter, Chung, Arora, Meltzer.
Drafting of the manuscript: Mokhlesi, Hovda, Chung, Arora, Meltzer.
Critical revision of the manuscript for important intellectual content: Mokhlesi, Hovda, Vekhter, Chung, Arora, Meltzer.
Statistical analysis: Vekhter, Mokhlesi, Arora, Meltzer.
Obtained funding: Mokhlesi.
Administrative, technical, or material support: Meltzer.
Study supervision: Mokhlesi, Meltzer.
References
- 1.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76(9):897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 3.Hwang D, Shakir N, Limann B, Sison C, Kalra S, Shulman L, et al. Association of sleep-disordered breathing with postoperative complications. Chest. 2008;133(5):1128–34. doi: 10.1378/chest.07-1488. [DOI] [PubMed] [Google Scholar]
- 4.Kaw R, Chung F, Pasupuleti V, Mehta J, Gay PC, Hernandez AV. Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. Br J Anaesth. 2012 doi: 10.1093/bja/aes308. [DOI] [PubMed] [Google Scholar]
- 5.Kaw R, Pasupuleti V, Walker E, Ramaswamy A, Foldvary-Schafer N. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141(2):436–41. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 6.Mador MJ, Goplani S, Gottumukkala VA, El-Solh AA, Akashdeep K, Khadka G, et al. Postoperative complications in obstructive sleep apnea. Sleep Breath. 2012 doi: 10.1007/s11325-012-0750-y. [DOI] [PubMed] [Google Scholar]
- 7.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56(11):819–28. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 8.Flink BJ, Rivelli SK, Cox EA, White WD, Falcone G, Vail TP, et al. Obstructive Sleep Apnea and Incidence of Postoperative Delirium after Elective Knee Replacement in the Nondemented Elderly. Anesthesiology. 2012;116(4):788–96. doi: 10.1097/ALN.0b013e31824b94fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memtsoudis S, Liu SS, Ma Y, Chiu YL, Walz JM, Gaber-Baylis LK, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112(1):113–21. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 10.Memtsoudis SG, Bombardieri AM, Ma Y, Walz JM, Chiu YL, Mazumdar M. Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27(5):306–11. doi: 10.1177/0885066611411410. [DOI] [PubMed] [Google Scholar]
- 11.Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152–9. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HCUP [Accessed November 10, 2012];Healthcare Cost and Utilization Project (HCUP) 2012 Jun; cited; Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp#Whatis.
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen demographic services [Accessed July 2012]; http://www.claritas.com/sitereports/default.jsp.
- 15.HCUP C [Accessed November 10, 2012];Healthcare Cost and Utilization Project (HCUP) 2012 Aug; cited; Available from: http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 16.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–94. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 17.Nelder J, Wedderburn R. Generalized linear models. J Royal Stat Soc. 1972;135:370–84. [Google Scholar]
- 18.Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen C, Tejirian T, Lewis C, Yadegar J, Dutson E, Mehran A. Postoperative CPAP and BiPAP use can be safely omitted after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4(4):512–4. doi: 10.1016/j.soard.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Weingarten TN, Flores AS, McKenzie JA, Nguyen LT, Robinson WB, Kinney TM, et al. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106(1):131–9. doi: 10.1093/bja/aeq290. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez AZ, Jr., Demaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239(5):698–702. doi: 10.1097/01.sla.0000124295.41578.ab. discussion -3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen NT, Masoomi H, Laugenour K, Sanaiha Y, Reavis KM, Mills SD, et al. Predictive factors of mortality in bariatric surgery: data from the Nationwide Inpatient Sample. Surgery. 2011;150(2):347–51. doi: 10.1016/j.surg.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Berger S, Aronson D, Lavie P, Lavie L. Endothelial Progenitor Cells in Acute Myocardial Infarction and Sleep-disordered Breathing. Am J Respir Crit Care Med. 2013;187(1):90–8. doi: 10.1164/rccm.201206-1144OC. [DOI] [PubMed] [Google Scholar]
- 24.Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses. 2006;66(6):1069–73. doi: 10.1016/j.mehy.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Lavie L, Lavie P. Coronary collateral circulation in sleep apnea: a cardioprotective mechanism? Chest. 2010;137(3):511–2. doi: 10.1378/chest.09-2657. [DOI] [PubMed] [Google Scholar]
- 26.Shah N, Redline S, Yaggi HK, Wu R, Zhao CG, Ostfeld R, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2012 doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 27.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 28.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 29.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Vasquez TL, Hoddinott K. A potential complication of bi-level positive airway pressure after gastric bypass surgery. Obes Surg. 2004;14(2):282–4. doi: 10.1381/096089204322857717. [DOI] [PubMed] [Google Scholar]
- 32.Yamada S, Nishimiya J, Kurokawa K, Yuasa T, Masaka A. Bilevel nasal positive airway pressure and ballooning of the stomach. Chest. 2001;119(6):1965–6. doi: 10.1378/chest.119.6.1965. [DOI] [PubMed] [Google Scholar]
- 33.Frangos SG, Schwartz DR. Continuous positive airway pressure and postoperative hypoxemia. JAMA. 2005;293(22):2714. doi: 10.1001/jama.293.22.2714-a. author reply -5. [DOI] [PubMed] [Google Scholar]
- 34.Weingarten TN, Kendrick ML, Swain JM, Liedl LM, Johnson CP, Schroeder DR, et al. Effects of CPAP on gastric pouch pressure after bariatric surgery. Obes Surg. 2011;21(12):1900–5. doi: 10.1007/s11695-011-0419-9. [DOI] [PubMed] [Google Scholar]
- 35.Lockhart EM, Willingham MD, Abdallah AB, Helsten DL, Bedair BA, Thomas J, et al. Obstructive sleep apnea screening and postoperative mortality in a large surgical cohort. Sleep Med. 2013 Mar 13; doi: 10.1016/j.sleep.2012.10.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82(4):1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 37.Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology. 2009;110(4):908–21. doi: 10.1097/ALN.0b013e31819c74be. [DOI] [PubMed] [Google Scholar]
- 38.Sareli AE, Cantor CR, Williams NN, Korus G, Raper SE, Pien G, et al. Obstructive sleep apnea in patients undergoing bariatric surgery--a tertiary center experience. Obes Surg. 2011;21(3):316–27. doi: 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 39.Ravesloot MJ, van Maanen JP, Hilgevoord AA, van Wagensveld BA, de Vries N. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur Arch Otorhinolaryngol. 2012;269(7):1865–71. doi: 10.1007/s00405-012-1948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carneiro G, Florio RT, Zanella MT, Pradella-Hallinan M, Ribeiro-Filho FF, Tufik S, et al. Is mandatory screening for obstructive sleep apnea with polysomnography in all severely obese patients indicated? Sleep Breath. 2012;16(1):163–8. doi: 10.1007/s11325-010-0468-7. [DOI] [PubMed] [Google Scholar]
- 41.Dempsey JA, Skatrud JB, Jacques AJ, Ewanowski SJ, Woodson BT, Hanson PR, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122(3):840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 42.Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16(2):118–22. [PubMed] [Google Scholar]
- 43.Katz I, Stradling J, Slutsky AS, Zamel N, Hoffstein V. Do patients with obstructive sleep apnea have thick necks? Am Rev Respir Dis. 1990;141(5 Pt 1):1228–31. doi: 10.1164/ajrccm/141.5_Pt_1.1228. [DOI] [PubMed] [Google Scholar]