Abstract
Schizophrenia is a chronic debilitating brain disorder characterized by a complex set of perceptual and behavioural symptoms that severely disrupt and undermine the patient’s psychological well-being and quality of life. Since the exact disease mechanisms remain essentially unknown, holistic animal models are indispensable tools for any serious investigation into the neurobiology of schizophrenia, including the search of remedies, prevention, and possible biological markers. This review provides some practical advice to those confronted with the task of evaluating their animal models for relevance to schizophrenia that inevitably involves behavioural tests with animals. To a novice, this challenge is not only a technical one, as it also entails attention to interpretative issues concerning validity and translational power. Here, we attempt to offer some guidance to help overcome these obstacles by drawing on our experience on diverse animal models of schizophrenia based on genetics, strain difference, brain lesions, pharmacological induction, and early life developmental manipulations. The review pays equal emphasis on the general (theoretical) considerations in experimental design and the illustration of the problematics related to test parameters and data analysis of selected exemplar behavioural tests. Finally, the individual difference of behavioural expression in relevant tests observed in wild type animals may offer an alternative approach to explore the mechanism of schizophrenia-related behavioural dysfunction at the molecular, cellular and structural levels that are of more immediate relevance to cell and tissue research.
Keywords: attention, antipsychotics, behaviour, cognition, depression, learning, memory, mouse, psychosis, rat, schizophrenia, translational research
1. INTRODUCTION
With a lifetime prevalence of 1%, schizophrenia is not the most common mental disorder, but it is certainly one of the most debilitating human diseases according to the World Health Organization. Since its identification as a distinct disease entity by Emil Kraepelin over a century ago, our current understanding of schizophrenia is still incomplete and available treatment far from satisfactory. However, we are now in a better position to develop plausible theories on the biological causes and mechanisms of the disease because of advances made in the characterization of the disease in terms of its genetics, epidemiology, neurochemistry, physiology, histopathology and neuropsychology. Schizophrenia is now generally considered as a neurodevelopmental brain disorder, partly shaped by genes and partly by environmental factors, with the involvement of multiple dysfunctional neural circuits (Brown 2011; Owen 2012; Piper et al. 2012). The use of behaving animals provides an effective holistic approach to test and explore new ideas on the disease’s neurobiology and therapy under highly controlled conditions and the use of invasive manipulations that otherwise cannot be performed in human subjects (O’Donnell 2011; Geyer and Gross 2012). A recent survey has summarized more than 87 animal models of schizophrenia (www.schizophreniaforum.org), and the number is still on the rise. Laboratory rodents, including rats and mice, are the most common species. Supported by the rich background knowledge on rodent behaviour, they are the ideal vertebrate species for establishing holistic models transcending all levels of biological explanation.
1.1 Positive vs negative symptoms, and cognitive deterioration
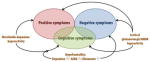
The complex symptoms of schizophrenia are typically divided into clusters. The current positive-negative dichotomy in the classification of schizophrenia symptoms has been emphasized since the 80’s (e.g., Andreasen and Olsen 1982). Positive symptoms are characterized by excess of functions normally not experienced by healthy people; they are experiences and behaviours added to a person’s normal way of functioning. These include hallucinations and delusions (see Table 1), which are typically seen in acute psychosis. On the other hand, negative symptoms refer to the loss or diminution of normal functions (see Box 1). This conceptual distinction is nowadays primarily used as a descriptive device carrying no pathophysiologic implications, unlike its first inception by early 19th century English neurologists (John Russell Reynolds and John Hughlings Jackson) when it had implied some form of functional inter-dependence (see Berrios 1985). However, the positive-negative distinction of schizophrenia symptoms is also justified on several important grounds, including functional imaging data (Liddle et al. 1992), neurotransmitters involved, and responsiveness to conventional pharmacotherapy (See section below).
Table 1.
Overview on common behavioural paradigms to evaluate rodent models of schizophrenia. Their relevance to specific symptoms, methods and procedures, pros and cons are summarized. Readers may consult the relevant references listed in the last column for detailed descriptions of each test.
| Test and behaviour measured | Relevance to schizophernia (SZ) and symptoms to be modelled | Translationability | Advantages | Disadvantages | Critical test parameters | Confounding variables | References |
|---|---|---|---|---|---|---|---|
| Latent inhibition (LI): measures selective attention conducted in various Pavlovian conditioning paradigms |
|
|
|
|
|
|
Weiner 2003; Weiner and Arad 2009 |
| Prepulse inhibition (PPI): measures sensory gating |
|
|
|
|
|
|
Geyer et al. 2001; Swerdlow et al. 2008 |
| 5-choice serial reaction time task (5-CSRTT): measures sustain attention, vigilance and impulsivity |
|
|
|
|
|
|
Chudasama and Robbins 2004; Bari et al. 2008 |
| Working memory (WM): measures the capability to hold information online to solve complex cognitive tasks |
|
|
|
|
|
|
Dudchenko 2004; Young et al. 2012 |
| Object and spatial recognition memory: measures familiarity judgement through the animals’ natural tendency to explore new environments/objects |
|
|
|
|
|
|
Ennaceur and Delacour 1988; Deacon and Rawlins 2006; Sanderson et al. 2007; Singer et al. 2007 |
| Reversal learning (RL) and attentional set shifting task (ASST): Two problems solving tasks based on two-choice discrimination learning |
|
|
|
|
|
|
Birrell and Brown, 2000; Young et al. 2012 |
| Forced swim test (FST): placing animals an inescapable cylinder filled with water is a test of “lost helplessness” |
|
|
|
|
|
|
Porsolt et al. 1978; Cryan et al. 2005 |
| Sucrose preference test: measures the rewarding (hedonic) effect of sucrose consumption |
|
|
|
|
|
|
Pothion et al. 2004 |
| Social interaction test: measures social interaction between an experimental subject an conspecific |
|
|
|
|
|
|
Crawley 2007a |
| Open field test: measures spontaneous locomotor activity in an open field arena |
|
|
|
|
|
|
Henry et al. 2011 |
BOX 1. Contrasting positive and negative symptoms of schizophrenia and the key impairment in cognitive function.
Readers should consult the latest Diagnostic and Statistical Manual of Mental Disorders (DSM-5 to be released later this year), and International Classification of Diseases (ICD-11 is scheduled to complete in 2015) for the most up-to-date clinical definitions.
| Positive Symptoms | Negative Symptoms |
|---|---|
|
|
| Cognitive Deterioration | |
| |
| |
The recent emphasis on cognitive deterioration in schizophrenia patients refers to impairments in attention, memory and executive function. They are part of the negative symptomatology by description but are often singled out, because unlike the rest of the negative symptoms, they focus on deteriorations of the patient’s ability to process and store information. Hence, cognitive symptoms undermine the patient’s intellectual capability to lead a normal independent life (e.g., to earn a living), whereas the other negative symptoms on affect and motivation diminish the desire or drive to pursue a normal productive life (e.g., neglect of basic personal hygiene). Both aspects impose major hurdles to rehabilitation and the return to functional independence; but unfortunately they are often difficult to diagnose and the most resistant to current medication (Coyle et al. 2010). Indeed, the pressing medical need for effective treatment against negative and cognitive symptoms is high on the public health agenda. The NIMH has organized the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative to spearhead the drug discovery process (Green et al. 2004), including the creation of a consensus cognitive battery for clinical trials – focusing on speed of processing, attention/vigilance, working memory, learning and memory in the verbal and visual domains, reasoning and problem solving, and social cognition – which will foster a similar focus in preclinical animal models (Young et al. 2009, 2012).
The cognitive symptoms also receive special attention because they can be readily linked to the traditional research in cognitive neuropsychology in human and animals. The possibility that specific alterations in information processing might underlie the emergence of other symptoms (such as disorganized thoughts) has been raised on theoretical grounds (e.g., Gray et al. 1991; Frith 1992; Swerdlow et al. 1994; Weiner 2003). Such theories have also identified tests/paradigms critical to the hypothesized core psychological dysfunctions that can be applied to animals (and humans), thus providing translational readouts linked to positive symptoms. Elucidating the neural basis underlying normal and abnormal performance on such tests may also shed light on the pathophysiology of the specific symptoms. The prepulse inhibition (Swerdlow et al. 1994) and latent inhibition paradigms (Gray et al. 1991; Weiner 2003) are two classical examples in this tradition, and their practical application will be examined in subsequent sections.
1.2 Pharmacological hypotheses of schizophrenia
The dopamine hypothesis of schizophrenia (Carlsson 1988; Carlsson and Carlsson 1990) rests on a set of critical findings. First, drugs that induce dopamine release can produce psychotic-like experience in healthy humans, and exacerbate symptoms when administered to schizophrenics (Snyder 1976). Second, the ability to block dopamine D2 receptor appears to predict clinical efficacy against some schizophrenia symptoms (Seeman et al. 1976; Seeman 1987). Third, dopamine super-sensitivity and elevated levels of high-affinity dopamine D2 receptors have been reported in schizophrenia patients (Seeman 2011). Taken together, the argument is far stronger than when these critical findings are separately considered. For instance, (i) many non-dopaminergic drugs can also alter perception and produce hallucinogenic experience in human, e.g., LSD, psilocybin, phencyclidine, ketamine, muscimol, scopolamine, and even caffeine. (ii) There are effective antipsychotics whose main pharmacological action is not dopamine receptor blockade, e.g., clozapine. (iii) Schizophrenia patients exhibit non-dopaminergic neurochemical and structural abnormalities, e.g., reduction in cortical levels of the inhibitory neurotransmitter GABA. Nevertheless, since its inception half a century ago, the dopamine hypothesis has evolved into a “final common pathway” – as an explanatory framework for a host of risk factors, including pregnancy and obstetric complications, stress and trauma, drug use, and genes (Howes and Kapur 2009). One implication of this dominating position of the dopamine hypothesis is that new theories and models seek to make connection with the dopamine hypothesis as evidence for validity (see below). To this end, evaluation of the motor stimulating effect of systemic amphetamine has been a popular test. And, amphetamine-induced hyperlocomotion in wild type animals is also considered as a standard model of schizophrenia.
If the dopamine hypothesis is the current first pillar to the neurobiology of schizophrenia, then the glutamate hypothesis would be the second pillar. The glutamate hypothesis addresses one critical inadequacy of the dopamine hypothesis: unlike positive symptoms, negative and cognitive symptoms are not responsive to dopamine blockade (Coyle et al. 2010; Javitt 2010). This is an empirical failure, not necessarily a theoretical one, because the involvement of dopamine in cognitive processes is well known, including reward processes, attention, and memory. The emphasis of the glutamate hypothesis on the reduction in NMDA receptor function can be readily linked to deficiency in neuroplasticity and thus cognitive deterioration. Sometimes, this emphasis may make us overlook the relevance of the NMDA receptor to positive symptoms. The noncompetitive NMDA receptor antagonists, phencyclidine and ketamine, are potent psychomimetics that can induce the full range of positive, negative, and cognitive symptoms in healthy humans (Farber 2003). Therefore, phencyclidine- and ketamine-induced behavioural impairments could be relevant to models of all symptom classes; and those impairments that do not benefit from dopamine receptor blockade might be considered more relevant to negative/cognitive symptoms (e.g., Geyer et al. 2003). By contrast, the latter behavioural impairments might be expected to respond better to clozapine – the standard second generation (atypical) antipsychotic drug with some efficacy against negative/cognitive symptoms in patients.
1.3 Inferring schizophrenia-like disturbances in animals
Despite being a highly heritable disease, there are as yet no reliable biological (molecular or genetic) markers for schizophrenia. The clinical diagnostic criteria (e.g., DMS and ICD) are formulated based on the presentation of the disease in humans derived from interviews with the patient and family. Many of the key clinical signs and symptoms are psychological and behavioural in nature and related to subjective feelings, perceptual experiences and beliefs. It is obvious that we cannot approach our animal subjects in the same manner. Even if animals did suffer from auditory hallucinations and delusions of grandeur, we would not be able to describe or measure them. Hence, we should not expect animal models to recapitulate the full clinical description of schizophrenia in humans. This is neither possible nor is it our objective. There are no definitive ‘standard’ or ‘perfect’ animal models, e.g., for evaluation of drug efficacy. As a collection of tools, the value of animal models lies in them being informed approximation of isolated components of the disease. A more practical approach is to target specific symptoms or clusters of symptoms, and this has been the recommended approach by many (e.g., Arguello and Gogos 2006; Geyer 2008; Nestler and Hyman 2010).
Connections between human symptoms and measurable behaviour in animals can only be inferred. Crossing the species barrier requires the interpretation/theorization of human symptoms into psychological dysfunction that can be precisely defined and effectively measured in animals with the appropriate tests. Such tests may belong to the traditions of animal behaviour and cognition developed independently (e.g., latent inhibition), or were adapted from human neuropsychological tests (e.g., the 5-choice serial reaction time test in animals captures elements of the continuous performance test; and the assessment of intra- and extra-dimensional shifts captures critical aspects of the Wisconsin Card Sort test) and psychophysics (e.g., prepulse inhibition) with varying degrees of procedural modifications. Thus, operational measures or indices of “schizophrenia-like” behavioural disturbances can be derived from behaving animals, allowing meaningful inter-species translation of concepts and communication between preclinical and clinical findings.
1.4 Concerning validity of animal models
Validity is commonly evaluated from several independent aspects: face, construct and predictive validity (Nestler and Hyman 2010; van der Worp et al. 2010; Yanagi et al., 2012) in relation to the disease of interest. Face validity is fulfilled when the model is able to reproduce critical features of the disease pathology. Because schizophrenia lacks reliable anatomical, genetic, molecular, or neurochemical biomarkers, recapitulating the clinical/behavioural phenomenology (symptoms) of the disease is critical. Construct (or etiologic) validity points to the similarity between the mechanisms underlying the behaviour in the model and that underlying the behaviour in the modelled diseased. It is therefore an experimental substantiation of a theory, which provides an a priori framework for interpreting the data generated. In a narrower sense (as in psychopharmacology), predictive validity refers to the ability to correctly identify the efficacy of a putative therapeutic. In addition, predictive validity also implies external validity with respect to extrapolation of findings across species, between laboratories, and all relevant confounding factors – i.e., generalizability (van der Staay et al. 2009). Predictive/external validity depends on internal validity which deals with the quality and confidence of the evaluative process in terms of replicability, control of confounds, and support for a causal interpretation between the experimental manipulation and outcome (van der Staay et al. 2009). On the other hand, predictive validity does not necessarily depend on face validity. For instance, reports that antipsychotic drugs reliably disrupt conditioned active avoidance learning in wild type rats have led to the widespread use of this test to screen drugs, yet the behavioural readout bears little resemblance to therapeutic efficacy – the test simply serves as a behavioural index of dopamine suppression (Ogren and Archer 1994). Hence, an experimenter may wish to emphasize one type of validity over another, and this should not diminish the value of a given model so long that due caution is exercised in the interpretation. On the other hand, external and internal validity are always relevant and several practical considerations in the planning and conduction of experiments bear deciding impacts, and deserve to be briefly summarized.
Choice of species/strain/sex of subjects
Even between rats and mice, there are critical differences in test parameters, including drug dosage, and ability. For example, assessing reaction to low doses of amphetamine typically employs 1 mg/kg in rats and 2.5 mg/kg in mice (van den Buuse 2010), and some variation between mouse strains exists. Rats are far better swimmers than mice, and training with mice involving swimming (e.g., Morris water maze) demands extra attention to minimize floating. Consideration over the use of inbred vs outbred strains can be critical when evaluating environmental manipulations. Attention to possible confounding effects in specific mouse strains should be exercised. C57BL/6 mice suffer from age-dependent hearing loss in the high frequency range, and adult C3H/He mice are blind, etc. Sex difference exists in a variety of behaviours and is known to exist in many models of schizophrenia (e.g., Wu et al. 2012). Comparison between sexes is generally encouraged whenever practical (Kim et al. 2010), especially because notable sex differences are known in schizophrenia (e.g., Abel et al. 2010).
Breeding strategy
Experiments with genetic mouse models or early life interventions typically require the experimenter to breed their animals in-house. Besides following the standard practice, it is important to establish sufficient number of breeding pairs to avoid possible litter effects (Zorrilla 1997). Altered maternal behaviour should also be considered, measured, and if necessary, controlled and balanced across litters with a cross-fostering approach to validate critical initial findings (Kim et al. 2010). If animals were obtained from outside sources, varying degree of transport stress might be involved, and adequate acclimatization to the laboratory should be allowed before any manipulation or experimentation.
Housing conditions
Daily keeping of animals destined for behavioural evaluation should be maintained to a high standard. Housing is an important early life environmental factor that may interfere with manipulations modelling developmental aspects of schizophrenia. Post-weaning Isolation itself has been used to trigger schizophrenia-like behavioural disturbance in later life (e.g., Weiss and Feldon 2001). Similarly, deviations from standard pre-weaning animal care may affect maternal-infant interaction that can lead to behavioural abnormalities in adulthood including altered maternal behaviour across generations that further imply epigenetic mechanisms (Champagne and Meaney 2001). Furthermore, environmental enrichment can produce profound behavioural, neurochemical and neuroanatomical changes in mice (e.g., Zhu et al., 2006). The presence of any form of environmental enrichment should therefore be noted and standardized across studies.
Age
The age at which the experimental manipulation is induced as well as the age of behavioural evaluation is critical. Schizophrenia typically emerges in later adolescence and early adulthood. Models with etiological relevance should particularly pay attention to the onset of behavioural abnormalities. For example, the neonatal hippocampal lesions model of schizophrenia (Tseng et al. 2009) captures this developmental delay. The maternal gestational infection model developed based on epidemiological evidence also revealed a developmental delay in behavioural deficiency resembling that seen in the human disease (Meyer and Feldon 2009), which in addition is further modulated by the precise gestational day on which the infection-like challenge is administered to the pregnant dam (Meyer et al. 2006).
Compatibility and organization of multiple tests
It is common that animals are to be subjected to multiple tests. This helps minimize the number of animals needed thus complying with the 3R principle of animal testing (Russell and Burch 1959). In addition, it allows within-subject comparison between tests in a manner that is otherwise not possible (e.g., correlative analysis). However, due care must be exercised to minimize confounds due to transfer effects between tests. Spontaneous behaviour that might be highly sensitive to extensive handling should be measured when the animals are behaviourally naïve, e.g., the elevated plus maze test of anxiety. Tests that are more stressful in general and requiring extended training across many days should be carried later. Tests involving exposure to drugs should ideally be performed in drug-naïve subjects even though they might not be behaviourally naïve, yet re-use of drugged animals should clearly be avoided.
Replication and statistical power
Appropriate sample sizes should be determined by evidence-based power analysis. However, this might be difficult with novel experimental manipulations. In practice, only a limited number of animals can be tested at a given round. In such cases, separately evaluating different experimental groups is unacceptable, and balanced replications are essential. The latter further enables examination of variability across replications – an important determinant of internal validity. When evaluating statistical significance, one should not overlook the importance of effect size, especially in the context of therapeutic efficacy studies. Often referees from journals and grant reviewing bodies request the use of more than one test paradigm for a given behavioural function, say working memory test to be performed in the water maze as well as the T-maze. This essentially addresses external validity, allowing a test of the generalization of a specific finding. It ought to be encouraged; and it would be ideal if the tests can be performed in the same animals.
Test sensitivity
Whenever a new test is introduced to a laboratory, it may be necessary to ascertain its sensitivity to known manipulations (see Table 1). For instance, the sensitivity of the prepulse inhibition test to dopamine receptor agonists (viz., apomorphine and amphetamine) and NMDA receptor antagonists (viz., MK-801 and phencyclidine) is crucial. A prepulse inhibition set-up that is unable to capture these psychopharmacological actions is unlikely to be useful as a test of schizophrenia-related sensorimotor deficiency. For tests of cognitive function, such as working memory and executive function, it is common to obtain independent confirmation that they are sensitive to lesions of the hippocampal and prefrontal cortex at least once in one’s laboratory. These considerations help to establish the necessary internal validity of a given test.
1.5 A few words on genetic models
Mutant mouse models are powerful tools in schizophrenia research (Papaleo et al. 2012). Although no clinically reliable molecular or genetic diagnostic markers are available for schizophrenia, a host of susceptibility or risk-factor genes have been identified. Databases exist to maintain the growing lists of schizophrenia-related genes: The Schizophrenia Gene Resource (SZGR) that deposits genetic data from all available sources (bioinfo.mc.vanderbilt.edu/SZGR/) (Jia et al. 2010), the SzGene Database (www.szgene.org/) (Allen et al. 2008), and the VSD mutation and polymorphism database (www.chgb.org.cn/vsd.htm) (Zhou et al. 2004). Relevant genes can be studied in mouse models with a deletion (knockout) or mutation of a specific gene (e.g., DISC 1 and NRG1, Desbonnet et al. 2009) or an insertion of a transgene leading to over-expression of the target gene (e.g., RSG4, Schwendt et al 2012). The new mutant lines are typically comprehensively phenotyped, with special a priori interest in behavioural tests relevant to schizophrenia. For a general guide to explorative behavioural phenotyping in mutant mice, the readers are encouraged to consult Crawley (2007b). Such mutant mouse models allow us to evaluate the aetiological significance of separate mutations, or if one wishes, combinations of mutations. The analysis of such mutant models clearly should not stop at the behavioural level, but should also be extended to the underlying physiological and biochemical mechanisms under the guidance of the uncovered behavioural phenotypes. They can also be studied with a developmental perspective including the possible interaction with known environmental risk factors in the context of a “two-hit” model (Bayer et al. 1999; Robertson et al. 2006).
Genetic models are also useful tools for the collection of proof-of-concept data in the search for new therapeutics. They can provide the first approximation of antagonistic or agonistic action of a specific drug target by genetic disruption or over-expression, especially when compounds with the desired pharmacological specificity are not available. Diverse Cre-LoxP conditional gene expression systems further permit tissue-specific (conditional) genetic manipulations that cannot be easily achieved by pharmacological routes (e.g., Kos 2004), and the tetracycline-controlled system (“Tet-on” or “Tet-off”) enables us to turn genes on and off at our discretion (Lewandoski 2001; Zhu et al. 2002). More advanced tools including viral vectors, engineered zinc finger nucleases, or small hairpin RNA-expressing constructs, provide further possibilities to manipulate the expression of a gene in a specific and controlled manner (Belizário et al. 2012).
2. BEHAVIOURAL EVALUATION
2.1 Preliminary matters
Any behavioural readout obtained is a reflection of the functional status of the animal’s entire central nervous system in the defined experimental conditions. Hence, it is essential to ascertain that the subjects are in good health. This is particularly relevant to the evaluation of mutant mouse models, but actually applies to any independent interventions. The rule of thumb is to check for gross abnormalities that will obviously interfere with the behavioural tests in question. Most if not all tests relevant to schizophrenia are designed to obtain data related to psychological functions, rather than sensory or motor output. Yet, all tests rely on the animals being able to detect stimuli in the relevant senses (visual, auditory, olfactory, or tactile) and to be physically able to perform (to move, swim, or press a lever). Crawley (2007b) has provided a comprehensive guide for examination of general health including the presence of neurological and physical abnormalities, ranging from impaired sensory abilities to motor dysfunctions. Despite this, it is not unusual that a few animals might still fail to perform in a test even though the majority of other subjects in the same group are behaving as expected. In this case, it would not be sensible to continue testing with such outliers. It is essential to compare the drop-out rate between experimental groups. Significant differences from controls might reflect unforeseen effects that warrant further investigation.
2.2 Overview of exemplar paradigms
Next, we wish to provide a critical guide to the common behavioural tests for assessing rodent models of schizophrenia (summarized in Table 1). Readers are referred to relevant publications for detailed methodological descriptions. On-line resources such as Current Protocols offers a list of over 90 behavioural protocols prepared by experts in the field (www.currentprotocols.com/WileyCDA/CurPro3Category/L1-3800,L2-3803.html). Below, we focus instead on paradigm-specific parametric variation, common confounding variables, critical issues related to data analysis and interpretation.
2.2.1 Attentional Dysfunction
The MATRICS initiative identifies attention as a key domain of cognitive deficiency in schizophrenia (Young et al. 2009). Effective allocation of cognitive resources depends on attention. Its functional relevance may be considered under three subdomains (Parasuraman 1998): Selective attention describes the process by which certain stimuli are selected to be in the centre of attention while others remain in the periphery of attention; sustained attention refers to the continuous allocation of attentional resources on particular stimuli for prolonged periods and is also known as vigilance; and divided attention or attentional control whereby attention is sustained despite distractors or focuses on multiple tasks at the same time. Schizophrenia patients are deficient in all three domains. In animals, they can be assessed by the tests below.
2.2.1.1 Latent inhibition – a measure of learned inattention
Attention can have profound effects on learning, but attention itself is also subjected to modification by one’s past experience (Mackintosh 1973). Latent inhibition (LI) is an exemplar phenomenon whereby the subjects learn to tune down attention to a specific stimulus that consequently slows down learning about the predictive significance of that stimulus. Thus, LI is measured by its impact on learning. It was first discovered in the context of Pavlovian associative learning (Lubow and Moore 1959), but can also be observed in instrumental learning, e.g., two-way signalled active avoidance (Weiner 2001). Pavlovian learning refers to the acquisition of a new response to a stimulus (e.g., a tone) after it has been paired in space and time with the presentation of an unconditional stimulus (US, e.g., food) that readily elicits a specific response (e.g., salivation). Following CS-US pairing, the tone (now a conditioned stimulus, CS) comes to elicit salivation by itself – a conditioned response (CR). LI refers to the observation that pre-exposure to the tone-CS alone without any consequence prior to CS-US pairing impedes subsequent Pavlovian learning. LI is an important demonstration that learning is modulated by attention. A stimulus with a history of non-significance (i.e., non-reinforcement) commands less attention than a novel stimulus (as it appears to another subject who has not been pre-exposed to it), so learning proceeds more slowly in pre-exposed than non-pre-exposed animals. Schizophrenia patients are impaired in this form of attention modulation (Baruch et al. 1988). Importantly, this impairment in LI is expressed as faster learning compared to healthy controls, who are expressing the negative effect of stimulus pre-exposure on learning. Hence, the LI impairment cannot be attributed to a general learning deficit per se, but a deficiency in learned inattention. LI is connected to the dopamine hypothesis of schizophrenia. In human and animals, LI is disrupted by amphetamine (Weiner et al. 1988; Gray et al. 1992), and antipsychotic drugs can restore the deficit in LI (Weiner and Arad 2009). Moreover, antipsychotic drugs can readily potentiate the expression of LI, a property that allows the use of the LI paradigm as a drug screening test for potential new antipsychotics (Moser et al. 2000).
The demonstration of LI requires a 2-stage procedure: pre-exposure and conditioning/learning, which may continue without a break or be separated by a day. The magnitude of LI is determined by the intricate balance between the amount of pre-exposure and the strength of conditioning. Conditioning is strong when the intensity of the US (e.g., shock intensity or size of food reward) is high, and/or the number of CS-US pairings is high. Low strength of conditioning in combination with high number of CS pre-exposures would generate strong LI in controls, which is congenial for a hunt of LI-disruption. Conversely, strong conditioning and/or low number of pre-exposure reduces the LI effects in normal subjects, and is suitable for the hunt of an LI-enhancing effect resembling antipsychotic drugs. As explained in Box 2, alteration in LI may assume different forms; and their distinction and interpretation require a detailed statistical examination of a two-way interaction. Changes in the magnitude of LI (defined as weaker learning in the pre-exposed (PE) condition relative to the non-pre-exposed (nPE) control condition can be achieved solely by enhancing or reducing the stimulus pre-exposure effect, i.e., without changing performance in the nPE subjects (situations a and d in Box 2). However, the absence or presence of LI can also be influenced by effects largely attributed to the nPE condition as illustrated in situations b and e in Box 2. Finally, there are situations in which the combined bidirectional changes of learning in the PE and nPE conditions lead to an abolition (situation c) or potentiation (situation f) of LI.
Box 2. Qualitative distinction between different forms of disruption or potentiation of LI.
LI is defined by weaker learning in the pre-exposed (PE) relative to the non-pre-exposed (nPE) condition. Alterations in the expression of LI may assume one of a number of possibilities. These can be distinguished by isolating whether the changes in LI are mediated by changes in PE (a and d), nPE (b and e) or both conditions (c and f). In addition, according to the “two-headed” LI model by Weiner (2003) there are two basic forms of LI abnormalities: (i) LI disruption demonstrated under parametric conditions that produce robust LI in the controls (examples a–c), and (ii) LI augmentation (or persistence) demonstrated under parametric conditions that fail to yield LI in the controls (examples d to f). LI disruption can result from insensitivity to CS-pre-exposure which leads to increased learning in the PE group indicative of impaired attentional processing (a). This form of LI disruption is produced by amphetamine and considered an animal model of the positive symptoms. Alternatively, LI can be disrupted due to a deficit in Pavlovian learning as indicated by reduced performance in the nPE group (b). A combination of both effects is possible (c) given that the cognitive processes taking place in pre-exposure and conditioning are independent from each other. LI augmentation can result from (d) increased sensitivity to CS-preexposure leading to reduced performance in the PE vs. nPE group against a lack of LI in the controls. This shape of LI augmentation is typical for antipsychotics and associated with antipsychotic properties. An LI augmentation can also be achieved via a strengthening of conditioned responding in the nPE group indicative of enhanced associative learning (e). Again, a combination of both effects is possible (f).
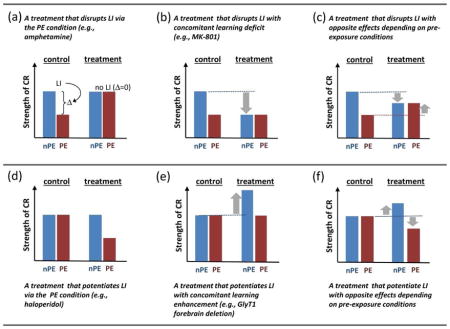
Potentiation of LI is considered an indication of antipsychotic potential – a property shared by existing antipsychotic drugs. However, Weiner (2003) has proposed that the expression of LI under conditions that are insufficient to generate the effect in normal subjects can also be considered as a cognitive aberration. According to Weiner’s “two-headed” theory of LI, this represents abnormally persistent LI, which may be linked to the negative symptoms of schizophrenia. In practice, it is therefore important to first assess LI using parameters that generate robust LI in controls. If this does not detect a disruption of LI in the treatment group, one may not conclude that the expression of LI is normal. Further experimentation with test parameters that generate weak or no LI in controls is necessary to test if LI might be potentiated. If so, the outcome may reflect an antipsychotic-like (therapeutic) effect, or negative symptomatology. To distinguish between them, additional evaluation is necessary to clarify if the model or treatment in question may give rise to other effects resembling negative symptoms or cognitive deteriorations in schizophrenia.
In evaluating effects on LI by pharmacological interventions, a distinction between drug actions on the preexposure or conditioning stage of the experiment is made. To dissect them, a four-group design with at least 24h separating pre-exposure or conditioning is necessary. The compound is administered (i) before preexposure only, (ii) before CS-US conditioning only, or (iii) before both stages, in comparison with vehicle injection on both days. Based on such comparison, for instance, it has been deduced that the LI-disruptive and LI-enhancing effects of amphetamine and haloperidol, respectively, are both attributed to the drug’s action on the conditioning day. The drugs therefore affect the ‘expression’ of LI rather the acquisition of LI if the critical drug action is confined to the pre-exposure day (see Moser et al. 2000).
2.2.1.2 Prepulse inhibition – a measure of sensory gating
Prepulse inhibition (PPI) is a form of sensory gating regulating stimulus access to higher cognitive resources. It is typically demonstrated using the acoustic startle reflex system. PPI of the startle reflex is operationally defined as the attenuation of the startle reaction to an intense acoustic pulse stimulus when it is shortly preceded by a low-intensity non-startle-eliciting prepulse stimulus. Typically both pulse and prepulse stimuli are in the form of white noise or pure tone, but other sensory modality, such as tactile stimulation in the form of an air puff of different intensity, can also be used. PPI is a robust cross-species phenomenon. Almost identical procedures and stimuli are used in both human and animals (Swerdlow et al. 1999). This and its relative ease of implementation make it one of the most popular high-throughput translational paradigms in schizophrenia research. It is commonly held that PPI stems from an innate pre-attentional sensory filter that protects the on-going processing of the antecedent prepulse from interference by the subsequent pulse stimulus (Graham 1975). It involves the active inhibition of the processing of the pulse and therefore the associated startle response is substantially reduced. PPI is impaired in schizophrenia patients; and it is disrupted by psychomimetic drugs. Although the ability of antipsychotic drugs to enhance PPI expression is not consistently demonstrated, they can effectively nullify the PPI-disruptive effect of psychomimetic drugs (Geyer et al. 2001). The attenuation of PPI in schizophrenia is theoretically linked to the perceptual symptoms of schizophrenia. The excessive intrusion of stimuli resulting from poor sensory gating is hypothesized to lead to sensory overload and cognitive fragmentation that characterizes positive symptomatology (Braff et al. 2001). There are recent suggestions that at least some forms of PPI deficiency might reflect cognitive/negative symptoms (Geyer 2006), which have received some empirical support in animal research (e.g., Csomor et al. 2008; Singer et al. 2013).
While the PPI test itself is straightforward and requires minimal expertise and familiarity with the dedicated apparatus, the analysis and interpretation of the data require attention to several critical details.
Indexation of PPI
It is customary to index PPI by a measure of percent inhibition. The reduction of startle magnitude on prepulse+pulse condition compared with pulse-alone condition is divided by the latter and expressed in percent, i.e., %PPI = (prepulse+pulse startle – pulse-alone) × 100%. This proportion measure is sensitive to independent changes in startle reactivity obtained on pulse-alone condition in humans (Csomor et al. 2006) as well as rodents (Yee et al. 2005). Interpretation of this measure would not be straightforward in the presence of a notable treatment effect on baseline startle reaction. To avoid spurious conclusion, it is recommended that the reactivity data must also be examined (Swerdlow et al. 2000). The startle magnitude should be expressed as a function of increasing prepulse intensity, beginning from the pulse-alone condition. The resulting downward sloping curve obtained from different treatment conditions can then be compared. Because the data distribution of startle reactivity is typically highly skewed (Csomor et al. 2008), a logarithmic transformation is recommended prior to analysis of variance (ANOVA). Furthermore, since difference of log-transformed values corresponds to a ratio between the untransformed values, interpretation of log-transformed data is conceptually similar to %PPI. To allow greater freedom in circumventing confounding difference in baseline startle reactivity, we have been advocating the incorporation of multiple pulse intensities (e.g., 100, 110 and 120 dB) to allow the ad hoc matching of the startle reaction between groups. This approach has been successfully applied to the interpretation of data confounded by baseline startle difference (see Singer et al. 2009a; Singer and Yee 2012). It is a practical approach compared with the laborious method used by earlier researchers, which in effect forced all subjects to show a comparable response by defining the pulse stimulus intensity with respect to individual subject’s startle threshold (Hoffman and Searle 1965, 1968), which would be considered impractical now.
Body weight confound
Body weight is another potential confounding factor in the indexation of PPI, because startle reaction in mice and rats is typically indexed by measuring whole body motion detected by a piezoelectric sensor. Systematic difference in body weight might exist between sexes, mouse strains, or between mutants and wild type controls. In such cases, the reactivity scores should be corrected for weight difference.
Reaction elicited by the prepulse stimulus
Most researchers do not examine the data obtained on prepulse-alone control conditions even when they have been included in the test protocol. We have published a series of papers demonstrating and explaining the potential value of these measures (Yee et al. 2004a, 2004b; Yee and Feldon 2009), including the distinction between different pharmacological forms of PPI disruption. Although still controversial, prepulse data should be routinely examined and reported.
2.2.1.3 The five-choice serial reaction time task – measures of sustain attention, vigilance and impulsivity
Attention deficits in schizophrenia patients are most frequently observed in the continuous performance test (CPT) that taxes sustained attention. The five-choice serial reaction-time task (5-CSRTT) developed by Robbins and colleagues 30 years ago (Carli et al. 1983) is an attempt at translating the CPT to rats. It has since been extended to mice. It is a powerful test allowing the examination of dissociable psychological functions besides sustained attention, including response speed, sedation, locomotion, motivation, or impulsivity (Robbins 2002). This single test therefore can be regarded as a multi-dimensional test with multi-dimensional performance measures. It offers an opportunity to examine inter-relation between the different functional dimensions, but they might not be easily untangled. Alternatively, there are tests that would allow one to study separately the effects on impulsivity, locomotion and motivation etc (see Crawley 2007b).
The 5-CSRTT requires a specially designed operant chamber equipped with an array of 5 nose-poke apertures. Each aperture can be illuminated, signalling to the animal when and where to make a nose-poke response in order to obtain a food reward from an automated pellet dispenser located on the opposite side of the five nose-poke response units. The task requires the animal to scan the five nose-poke response units and to make a nose-poke into the unit that is briefly illuminated. A full description of the apparatus and standard procedure is provided by Bari et al. (2008). Necessary expertise is required for training mice or rats on the 5-CSRTT. Attempts have been made to simplify the test by reducing the number of choices from 5 to 2 (the minimal) so that it can be implemented in a standard operant chamber (Dillon et al. 2009; Bitanihirwe et al. 2011). This provides a more expedient test and retains sensitivity to the negative effect of scopolamine (Bitanihirwe et al. 2011) – a cholinergic antagonist at the muscarinic receptor that also disrupts performance on the 5-CSRTT (e.g., Pattij et al. 2007).
2.2.2 Working memory & short-term memory tests
The MATRICS initiative has identified working memory and problem solving as the two inter-linked cognitive domains most relevant to schizophrenia cognitive impairment (Young et al. 2009). Working memory refers to a memory system supporting the active maintenance and manipulation of information stored in a transient limited capacity memory buffer, which is essential for goal-directed actions, reasoning and comprehension. Working memory tasks require monitoring and manipulation of information or behaviours in the setting of interfering processes and distractions. Efficient working memory performance depends on integration, processing, disposal, and retrieval of task-dependent information that also require the support of effective central executive and attention control. This integrative description of working memory function is heavily influenced by the theoretical foundation laid down by Baddeley (1986, 1992) based largely on human data. Hence, some of Baddeley’s finer distinctions, such as that between verbal and visual buffers, would not apply to animal studies.
Recent clinical data suggest that the primary deficit underlying the impairment of working memory in schizophrenia is a reduction in the memory buffer capacity rather than the ability to retain information over time. Hence, fewer pieces of information can be held on-line at any one time to support problem solving and to guide goal-directed actions. With an emphasis on memory span, the MATRICS initiative has included two (human) tasks aimed to measure working memory span (Young et al. 2009). Consequently, translational paradigms in rodents should be able to assess working memory span. This can be easily implemented in the radial arm maze task originally invented by Olton and Samuelson (1976) (see below). However, the radial arm maze has been somewhat neglected recently in favour of the water maze test of working memory for ease of implementation (e.g., Hodges et al. 1996). The water maze paradigm is effective in assessing memory retention but not memory span. The latter might contribute to the failure in translating findings from animal studies to effective therapeutic against working memory deficiency in humans (Young et al. 2009). The argument for the need to assess both retention and span capacity is strengthened by claims that they can be dissociated both anatomically and pharmacologically (Tarantino et al. 2011).
2.2.2.1 The radial arm maze test of spatial working memory
The initial conception of the radial arm maze test of working memory is simple (Olton and Samuelson 1976). The maze consists of eight arms radiating from a central platform; and a food reward is placed at the end of each arm. The subject is free to roam about and collect food reward. Rodents would eventually learn to visit each arm once and collect all rewards without unnecessary entries to visited arms in which the food reward has been collected. Performance is indexed by re-entry errors – entering arms that have already been visited on a given trial. For this reason, the animals need to remember a list of visited (or unvisited) arms relevant to the current test trial. The relevant information is said to be “trial-dependent”, and thus imposes demand specifically on the working memory system as explained above. Information learned from preceding trials can potentially interfere with performance on the current trial – a form of proactive interference. Animals need to suppress irrelevant memories and only access those relevant to the solution of the current task. Hence, typically only one trial is performed per day to avoid excessive proactive interference from one trial to the next. The amount of proactive interference can be increased by shortening the time between trials.
Rats typically would not develop a spatial sequential strategy, but mice could when given the opportunity, e.g., starting with a random arm and then going through the rest either in a clockwise or anti-clockwise direction. The latter can be easily prevented by inserting an interval between choices when the animal is confined to the central platform by doors blocking entry to all arms (Wenk 2004; Tarantino et al. 2011). It is therefore essential to incorporate an inter-choice interval in the procedures. By varying the duration of the inter-choice intervals, the experimenter can assess retention capacity. Typically, animals that are already familiarized with the 8-arm task can be challenged with an extended inter-choice interval (e.g., 15 min to 1 h) after four rewards have been collected. Afterwards they are returned to the maze to complete the task by collecting the four remaining rewards. Performance in the second half of the trial can provide a test of temporal retention capacity. During the second-half of the test, the number of re-entry errors to arms already visited in the first-half of the test can be contrasted with re-entry errors to arms visited within the second-half, because avoidance of arms entered earlier is more demanding in terms of temporal retention.
Indeed, a temporal retention effect can be deduced from the classic radial arm maze procedure (Dubroqua et al. 2012). Working memory errors can be classified depending on the number of arms visited (disparity) between two visits into the same arm. For example, in the sequence of arm entries: ➀ ⟨ ➄ ⟨ ➂ ⟨ ➁ ⟨ ➁ ⟨ ➃ ⟨ ➈ ⟨ ➆ ⟨ ➃ ⟨ ➇ (where re-entry errors are underlined), the re-entry into arm ➁ in the fifth choice is an error with disparity=0, whereas the subsequent re-entry into arm ➃ is an error with disparity = 2. The frequency of errors is expected to rise with increasing disparity (see, Dubroqua et al. 2012). Analysis of error frequency as a function of disparity provides a snapshot of working memory performance as a function of memory retention demand imposed by time and retroactive interference, which refer to resistance of memory decay (forgetting) and distractions by intervening events between learning and recall, respectively.
By contrast, it is straightforward to assess memory span capacity in the radial arm maze by varying the number of arms. If necessary, arms with more than 8 arms can be easily constructed. The limit of the memory span has been estimated to be between 24 and 32 in rats (Cole and Chappell-Stephenson 2003). Typically with an 8-arm radial maze, animals can be first trained using only four arms arranged in the shape of a “+”. They can then be transferred to the alternative ×-shaped configuration comprising the other four arms to assess transfer of the acquired rules. This part serves as a control procedure that involves no change in the required memory capacity. Finally, all 8 arms (in the shape of “✳”) are used (Singer et al. 2012).
Another advantage of the radial arm maze is that it readily allows the concurrent evaluation of both working and reference memory. The procedures are the same except that 4 arms are selected to be never baited. Avoidance of these four arms reflects reference memory – i.e., constant absence of food. Concurrent working memory is required to efficiently collect the rewards from the remaining 4 arms. This was first exploited by Jarrard in his investigation of hippocampal dependent learning (Jarrard 1986). Inclusion of reference memory testing may provide an index of hippocampus-dependent episodic memory which is also impaired in schizophrenia patients (Leavitt and Goldberg 2009).
2.2.2.2 Morris water maze
The Morris water maze is a popular test of spatial memory and needs little introduction (see Morris 1981). Here, it is important to emphasize that the use of the water maze to test working memory was a relatively recent introduction (e.g., Hodges et al. 1996). It is an escape task performed in a large circular water tank, in which the animals can free themselves from the water by climbing onto an escape platform hidden just under the water surface. Good memory of the hidden platform location facilitates escape. By changing the position of the escape platform from one day to the next, the procedure captures the important element of working memory demand. On each day’s first exposure to the water maze, the animals essentially reach the platform by chance because its location is not known. The next trial allows improvement of escape performance, in terms of escape latency and path distance. The improvement or “saving” compared to first trial performance serves as a measure of rapid one-trial learning, which arguably reveals the function of the working memory buffer. It typically takes about 4–5 days for normal rats or mice to show a significant improvement from trial 1 to 2.
Because the animals need to remember only one escape location, there is no room for manipulating memory span. Assessment of temporal retention can be easily assessed by varying the delay between trials 1 and 2. It is also useful to examine if animals show any tendency to return to yesterday’s platform location on the first trial of each test day. Such tendency would imply the presence of a significant proactive interference effect. Working memory performance can be weak due to high susceptibility to proactive interference.
The surface area of the water maze should be sufficiently large: >1 m diameter for a mouse pool, and > 2 m diameter for a rat pool. The testing room that houses the pool should allow sufficient spatial extension, but not be a tight fit. Extra-maze cues should be abundant and distinct. Intra maze cues (markings on the inside wall of the water maze) are unnecessary and might even prevent the animals from adopting a spatial strategy. Compared to rats, mice are not effective swimmers. To minimise stress, it is wise to expose mice to water before being introduced to the pool, e.g., in a bucket with a large escape out of the water. Despite such care, floating is not uncommon in mice. It may reflect a lack of motivation to escape from the water. Hence, any floaters must be excluded from further testing and data analysis and the rate of drop-outs compared between groups. Floating is readily identified by examination of the swim path, swim speed, and mismatch between latency and distance measures. Short swim path combined with maximal escape latency (i.e., failure to escape within the maximum time allowed) may suggest floating. To completely overcome floating in mice, the dry-land equivalent of the water maze – the ‘cheese board’ maze (Kesner et al. 1989) – is a viable alternative (see Llano Lopez et al. 2010). Another alternative that might be a compromise of the wet and dry approach is the “paddle pool” (Deacon and Rawlins 2002).
2.2.2.3 Tests of object and spatial recognition memory, and alternation behaviour
The memory buffer that supports efficient working memory, which emphasizes the active maintenance and manipulation of stored information, is also relevant to short-term recognition memory that allows discrimination based on familiarity judgement. Such memory is need for the distinction between visited and un-visited arms in the radial arm maze test to support errorless performance on a given trial. Deficits in recognition memory in schizophrenia patients (Coleman et al. 2002; Gabrovska et al. 2003; Shipman et al. 2009) have been translated from tests originally developed to assess either object or spatial recognition memory in animals (see Box 3).
Box 3. Tests of object and spatial recognition memory, and alternation behaviour.
(a) Novel object recognition test: The animal is first exposed to two identical copies of an object (A1 and A2) in the ‘sample phase’. After a delay (from 5 min up to 24 h) the animal is presented with a choice to explore either a third copy of the now familiar object (A3) or a novel object (B) in the ‘test phase’. The presence of object novelty detection is indexed by an explorative preference for the novel over the familiar object (Ennaceur and Delacour 1988). (b) Object location test: This test involves the integration of information about the spatial location of objects. The sample phase is the same as before, but the test phase involves the displacement of one of the two objects that are equally familiar. Preferential exploration for the displaced object indicates that the animal is sensitive a change in the spatial location of an object (Singer et al. 2007). (c) Spatial novelty preference task in the Y-maze: The animal is first exposed to two arms of the Y-maze (the Start and Familiar arms) while access to the remaining arm (the Novel arm) is blocked during the sample phase. After a delay, the animal is returned to the Y-maze with now access to all three arms. Preference for the Novel arm over the Familiar and Start arms provides a measure of spatial familiarity judgement (Sanderson et al. 2007). (d) Spontaneous alternation task in the T-maze: To begin the first trial (Trial 1), the animal is placed on the start arm and allowed to enter one of the two goal arms, either by free choice or assigned by the experimenter by blocking the alternative choice. The animal is then briefly confined to the chosen goal arm. After a delay, the animal is given another identical trial (Trial 2) in which the animal is allowed to choose freely between the two goal arms. An alternating response is scored when it enters the arm not visited on the preceding trial; a perseverative response is scored when it re-enters the arm just visited before the current trial. The animal can be subjected to multiple trials and the total number of alternations can be compared between groups as well as against chance performance. Normal mice have a strong natural tendency to alternate providing a measure of spatial familiarity judgment (Deacon and Rawlins 2006).
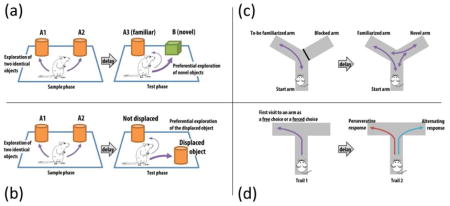
As summarized in Box 3, various tests have been devised by making use of the rodent’s innate curiosity to explore novel environments or discrete objects. These tests are easy to implement. Data extraction can be automatically performed with tracking software that allows accurate detection of the animal’s nose with prior calibration with an experienced observer. The animals are first familiarized to a distinct object or place in a “sample” (or “study”) phase, followed by a “test” (or “choice”) phase in which the animals have access to the familiarized object/place as well as a novel object/place. Invariably, the behavioural readouts from these tests refer to the animals’ spontaneous preference for the novel over the familiar object/place. Memory retention over time can be manipulated by varying the delay interval between the sample and test phases in order to assess forgetting. For repeated testing, a novel set of objects or a new testing room with new distal cues are necessary. Due to the nature of the test, it is apparent that novelty-seeking and anxiety are potential confounding factors for all the tests summarized in Box 3. For example, poor performance may arise due to neophobia – the fear of novel things or experience, rather than deficient familiarity judgement. Similarly, a lack of motivation (i.e., avolition or apathy) to seek novel stimulation or “novelty-seeking” may also lead to performance deficits. Hence, exclusion of such potential confounds is necessary to substantiate specific claims for altered recognition memory function.
Because these tests neither require lengthy pre-training nor impose stress on the animals, they can be routinely used as a first screening before considering whether additional tests focusing more specifically on working memory function should be performed (e.g., the water maze or radial arm maze). The latter is critical because conclusions regarding whether working memory function has been altered cannot be based on tests of novelty preference alone. In the way these novelty preference tests are typically run, there is no obvious requirement for active maintenance and manipulation of stored information – the defining elements of working memory. For example, the animals are not motivated to forget information at the end of a trial as in the radial arm maze test. The spontaneous preference for novelty therefore cannot be equated with working memory.
Psychologically, spontaneous novelty preference depends on the interplay between habituation and dishabituation processes related to exploration (Chemero and Heyser 2005, 2012). Habituation is defined as the decrement in the response to a specific stimulus resulting from its repeated presentation, not attributable to sensory adaptation or sensory/motor fatigue (Thompson and Spencer 1966; Randkin et al. 2009). Because the animals have been habituated with the familiar object/place in the sample phase, they predictably explore less the familiar object/place compared with the novel object/place in the test phase. Spontaneous (non-rewarded) alternation behaviour in the T-maze can be similarly explained (see Box 3, Sanderson et al. 2010; Sanderson and Bannerman 2012), especially the first alternation trial when the novel arm has never been visited (Deacon and Rawlins 2006). Exploration to a familiarized object/place may resume when sufficient time is allowed to elapse between sample and test phases – an effect known as dishabituation. Hence, the absence of a preference towards the novel object in control subjects at longer delays could potentially be due to dishabituation to the familiar object, which reinstates exploration towards the familiarized object in the test phase (Honey and Good 2000). According to the two-process theory of habituation (Thompson and Spencer 1966), the emergence of dishabituation is more than the dissipation of habituation, but also involves the modulation of the complementary sensitization process. Consequently, poor novelty preference does not necessarily imply memory loss because it could also be interpreted as a stronger dishabituation effect. Conversely, stronger novelty preference may stem from weaker dishabituation rather than a promnesic (namely, resistance to forgetting) effect (Singer et al. 2007).
Given the pivotal role of habituation in novelty detection that underlies familiar judgement and recognition memory, it is not surprising that deficits in recognition memory (Coleman et al. 2002; Gabrovska et al. 2003; Shipman et al. 2009) and habituation impairments (Geyer and Braff 1987; Parwani et al. 2000) have both been reported in schizophrenia patients. There are preclinical models showing that disruption of either NMDA or AMPA receptor functions can impair spontaneous novelty preference (Grayson et al. 2007; Karasawa et al. 2008; Barkus et al. 2012); and reduced prefrontal dopamine activity may underlie these behavioural deficits (Neill et al. 2010). In particular, the integrity of nigro-straital dopamine projection to the prefrontal cortex has been specifically linked to object recognition memory (Chao et al. 2013). Finally, atypical rather than typical antipsychotics may reverse spontaneous object recognition deficit induced by NMDA receptor blockade (Grayson et al. 2007; Karasawa et al. 2008).
However, the neural basis of spontaneous novelty preference is far from understood. Although damage to the hippocampus can reliably disrupt performance on tests of spontaneous novelty preference involving spatial information (paradigms B-D depicted in Box 3), the precise involvement of the hippocampus in novel object recognition remains a matter of on-going debate (see Brown and Aggleton 2001; Singer et al. 2007). One suggestion is that the hippocampus is only involved in object recognition memory at long retention intervals (Clark et al. 2000; Hammond et al. 2004). Hence, impaired spontaneous preference for novelty should not be equated with hippocampal dysfunction. The user is recommended to verify empirically whether their specific spontaneous object memory test is sensitive to hippocampal damage or not.
2.2.3 From reversal learning to attentional set shifting – measures of cognitive flexibility in problem-solving
The study of problem solving has a long tradition in human neuropsychology. It invariably involves reaching a desired goal by overcoming a barrier through higher cognitive function such as reasoning, mental imagery, introspection, working memory, utilization of feedback to test strategies and hypothesis. Typical human neuropsychological tests include the Wisconsin Card Sort Test (Berg 1948) and adaptation of the Tower of Hanoi puzzle (e.g., “Stockings of Cambridge” test from the Cambridge Automated Neuropsychological Testing Battery (CANTAB; www.camcog.com/) that is particularly useful in assessing frontal lobe function (Robbins 1996). In rodents, the simplest problem we can pose is two-choice discrimination. This can be implemented on a T-maze, in an operant chamber between two levers, or choice of two objects (e.g., see Meyer et al. 2005). Rodents readily learn such (S1+ → reward vs S2− → no reward) discriminations. Effective problem solving places special demand on cognitive flexibility. This can be challenged by subjecting the animals to reversal learning in which the solution to the problem is reversed (S1− → no reward vs S2+ → reward). The ability to recognize the unexpected change in the reward contingency associated with the two choices and to switch to a new cognitive set to govern response (according to the new contingency) is crucial to reversal learning.
The characteristic set shifting deficits in schizophrenia is exemplified by the poor performance in the Wisconsin Card Sort Test (see Box 4) – a deficiency attributable to underactivity of the dorsolateral prefrontal cortex, commonly known as hypofrontality syndrome (Weinberger and Berman 1996). The test is presented as a sorting problem task in which the subjects are required to sort pictorial stimuli (presented physically in the form of cards, or on a computer screen) according to one of four possible dimensions (see Box 4) based on error feedback alone. The test taxes the speed and readiness to shift one’s sorting strategy from one relevant dimension to a previously irrelevant dimension – i.e., attentional set-shifting. A rodent analogue of this test has been developed by many laboratories. One version employs odour cues and digging media as the two possible dimensions to guide the solution of a two-choice discrimination task (Birrel and Brown 2000). This allows the distinction between intra-dimensional shift (IDS) and extra-dimensional shift (EDS) in the same test design as illustrated in Box 4. The test design enables the within-subject evaluation of IDS and EDS as well as the contrast between intra-dimensional reversal and intra-dimensional reversal learning.
Box 4.
In the Wisconsin Card sort test (a), The subject is required to sort cards into piles in front of four stimulus cards. The matching is done according to a rule which the participant has to work out. The cards could be matched by number, colour or shape of the symbols. This can be seen in the illustration above: 1, 2, 3 or 4 / circles, stars, squares or crosses / red, green, blue or yellow. Hence the two red crosses can be matched by ‘two’ or ‘red’ or ‘cross’. The participant will be told “correct” or “incorrect” depending on whether they guess the rule correctly or not. The rule is applied for a run of trials and then changed without warning. The rodent analogue of the test (b) based on two-choice discrimination is represented here with colour and shape as the two possible relevant dimensions. If the relevant dimension is the same between the problems learned in the training set and the new test problem set, the shift or transfer involved is called intra-dimensional shift (IDS). If there is a change in the relevant dimension (e.g., from shape to colour) then it is called extra-dimensional shift (EDS). In the test designed by Birrel and Brown (2000), the two dimensions are odour and digging medium (see text).
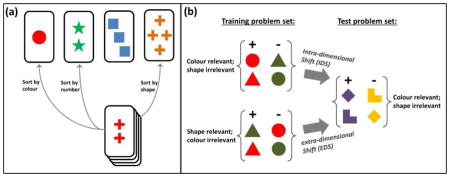
Seven distinct phases can be identified in the task design by Birrel and Brown (2000):
First, animals are trained to discriminate between two stimuli by odour (odour A → reward, odour B → no reward) always presented in one digging medium only.
New digging media (x, y) are then introduced but remain irrelevant to the discrimination problem (A in medium x or y → reward, B in medium x or y → no reward).
Next, the odour reward contingency is reversed (A in medium x or y → no reward, B in medium x or y → reward).
Keeping odour as the relevant dimension so far, new sets of discrimination problems with new odours are introduced with digging medium still being irrelevant (e.g., odour C in medium x or y → reward, odour D in medium x or y → no reward). This intra-dimensional shift involves the consistent use of the same dimension to guide discrimination although never-experienced odours are used. Efficient transfer here indicates that the animals have learned to focus their attention to the relevant dimension.
As in phase (iii), the reward contingency of the new odours is now reversed (odour D in medium x or y → reward, odour C in medium x or y → no reward).
A new set of odour stimuli (E and F) is introduced. To gain reward, however, the discrimination must now be guided by the identity of the digging medium while odours are now rendered irrelevant, i.e., odour E or F in medium x → reward, odour E or F in medium y → no reward. The animals are now required to learn to attend to the digging medium for the first time. This is defined as an extra-dimensional shift (EDS). EDS should take longer to learn compared with shifting within the same stimulus dimension, i.e., intra-dimensional shift (IDS). Such an EDS/IDS difference is expected in the controls.
Finally, the reward contingency associated with the digging media is reversed with odour continued being irrelevant, i.e., odour E or F in medium x → no reward, odour E or F in medium y → reward.
Although such an extended series of tests is labour intensive, it can generate a comprehensive evaluation of problem solving at the interface between attention and executive function. Although an animal model that yields a specific deficit in EDS learning may match closely with the impression obtained in schizophrenia patients (Tyson et al. 2004), the facilitation and perseveration of reversal learning (Weiner 1990; Gray et al. 1991) may be linked to positive and negative symptoms (Crider 1997), respectively. Indeed, the conceptual similarity between reversal learning and latent inhibition has been emphasized by Weiner (1990), with both behavioural expressions being highly sensitive to changes in mesolimbic dopaminergic transmission.
Recent attempts to develop new rodent tests for executive function and problem-solving have emphasized convenience to avoid long training sessions and vast experimenter efforts typical of the tests discussed above. One approach is to construct relatively simple ethologically (species-specific) relevant problems that a mouse or rat can resolve by trial-and-error similar to the famous early experiments designed by Thorndike (1911) for studying intelligence in cats. A mouse ‘puzzle box’ that might be useful in evaluating executive function and problem-solving deficits relevant to schizophrenia has been developed recently by Ben Abdallah et al. (2011). The test has been shown to be sensitive to lesions, genetic and pharmacological models of schizophrenia, although its sensitivity to antipsychotic drugs remains untested. The test does not require any pre-training, and it can be completed within three days, with a new problem of increasing difficulty presented per day across three trials. It may be a first choice for high-throughput screening in the direction of cognitive flexibility, executive function and problem-solving before considering performing more sophisticated, yet labour intensive, paradigms.
2.2.4 Flattened affect and social deficits related to negative symptoms
Negative symptoms of schizophrenia related to mood and affect are generally not easy to evaluate in rodents. Poverty of speech for instance is impossible to recapitulate in rodents. There are however established behavioural tests for the evaluation of anhedonia and asociality, which are prominent negative symptoms of schizophrenia. These tests are also routinely used as preclinical models of depression. This is not surprising since flattened affect, defined as diminished or absent of emotional expression or reaction, is a key feature of depression and a negative symptom of schizophrenia. Hence, the construct validity of these tests should not be taken as specific to schizophrenia.
2.2.4.1 Force swim test
The forced swim test was developed by Porsolt et al. (1977) as a simpler alternative to the classical “learned helplessness” paradigm to induce behavioural despair (Seligman 1972). Rats or mice become immobile after attempting in vain to escape from an inescapable cylinder filled with water. Immobility is typified by the complete absence of struggling except the minimal effort to keep the head above the water – the animals are essentially floating. Although image analysis software can now provide image analysis algorithm to detect immobility, manual observation under blind conditions, by multiple experienced raters with high inter-rater reliability, would be preferred. This could be time consuming, but such care is necessary to ensure test reliability. Any automated immobility system should first be calibrated with human observers beforehand.
Rats are better swimmers than mice and in order to induce sufficient floating, they are typically tested twice (about 6–10 min each test) separated by 24 h. On the second occasion, floating should emerge more rapidly by the end of the second minute. Hence, it is common to focus on data collected between the third to sixth minutes. Mice, on the other hand, have a stronger tendency to give up escaping and begin floating during the first test. Therefore one test is often sufficient, yet an additional test 24 h later may provide further validation of the results.
The immobility induced by forced swimming is thought to reflect a state of “despair” and anti-depressant drugs are effective in reducing immobility by conferring a resistance to the development of despair. The forced swim test is therefore first and foremost a test of anti-depressant activity rather than a test of depression as such. As originally emphasized by Porsolt (1977), increased locomotor activity is an important confound of the measure of immobility (as an index of “behavioural despair”) that must be excluded (e.g., by the open field test). Unlike the “learned helplessness” paradigm, the use of the forced swim test as a behavioural model of depression is not recommended. It should primarily be used to assess antipsychotic efficacy against negative symptoms. It has been shown that typical but not atypical antipsychotics can reverse the increase in immobility time induced by NMDA receptor blockade in the forced swim test (Castagné et al. 2009; Chindo et al. 2012). This outcome does not readily translate to patients, because neither typical nor atypical antipsychotics substantially improve flattened affect in schizophrenia; and if anything, better efficacy has been claimed for atypical rather than typical antipsychotic drugs. Moreover, the NMDA receptor blockade is also known to reduce immobility in the forced swim test indicative of antidepressant action (Engin et al. 2009) – an effect that has been attributed to a shift of glutamatergic neurotransmission from NMDA to AMPA receptors (Maeng et al. 2008). Hence, the effects of NMDA receptor blockade on the forced swim test can assume either direction, which might be problematic in terms of internal validity.
2.2.4.2 Sucrose preference – a measure of hedonic value to sweetness
Anhedonia is not only a core negative symptom of schizophrenia but also a key feature of depression. It is defined as the diminished ability to experience pleasure from activities or stimuli that are usually enjoyable or rewarding (American Psychiatric Association, 2000). In human, the orbitofrontal cortex is implicated in hedonic experience (Kringelbach 2005). A relatively simple paradigm frequently used to measure anhedonia in rodents is the sucrose preference test. Consumption of sucrose elicits positive (hedonic) reactions in both humans and animals (Berridge 2000) such that animals will readily work for sucrose reward, i.e., it is an effective positive reinforcer to motivate operant learning.
In rodents, the rewarding effect of sucrose can be evaluated by a simple two-choice sucrose preference test in which the animals can freely choose to drink either sucrose solution or normal water form two similar drinking tubes. It is best implemented in the home cage with single caging facilities. Consistent with the glutamate hypothesis of schizophrenia, blockade of NMDA receptors can reduce the preference for sucrose in rats and this effect can be reversed by the atypical antipsychotic, clozapine (Vardigan et al. 2010).
The sucrose preference test is prone to confounding effects on perception of sweetness. Poor (or enhanced) discrimination is a confounding factor for a reduction (or increase) in sucrose preference. This possibility is often ignored, but a sweetness discrimination test can be easily performed as recommended by Willner et al. (1990). Alternatively, the parallel examination of quinine aversion has been recommended, and specific changes in the hedonic response to sucrose can only be concluded if no change in quinine aversion is observed (Vardigan et al. 2010). It is because anhedonia as a clinical symptom does not imply enhanced aversion. Indeed, the experiences of pleasant and unpleasant events are mediated by distinct subregions within the orbitofrontal cortex in humans (Small et al. 2001).
2.2.4.3 Social approach behaviour
Social withdrawal or asociality may be considered as another expression of anhedonia or lack of motivation. Although the repertoire and complexity of social behaviour in laboratory rodents is very limited compared to primates, they do initiate and engage in social interaction with conspecifics when given the opportunity. Early laboratory tests of social interaction in rodents were developed in the context of social phobia and their potential for screening anxiolytic drugs (File and Hyde 1978). This reminds us that anxiety and fear towards an unfamiliar conspecific is a potential confounding factor of many social interaction tests. However, there is a current need for paradigms that are relevant to a number of psychiatric disorders marked by social withdrawal including schizophrenia, depression and autism. An automated high-throughput test has been developed (Nadler et al. 2004; Crawley 2007a), which has become increasingly popular in recent years because of its apparent ease of implementation. However, this is far from a test of social interaction. It is in principle a preference test similar to the sucrose preference test. Hence, Crawley’s test may be viewed as a test of social anhedonia. In a familiarized enclosure, animals are confronted with a free choice to spend time in a compartment housing an unfamiliar con-specific imprisoned inside a wire cage or another interconnected compartment with an empty wire cage. The tendency to approach the compartment with a con-specific (quantified by time spent and number of entries) is taken as an index of the motivation to engage in social interaction. While this generation may be true, the same preference may emerge simply because the compartment with a conspecific simply contains a richer and more varying source of stimulation. The empty wire cage in the other compartment is not an appropriate control for comparison, and neither is a cage containing a toy or a dead conspecific. The automated test proposed by Crawley (2007a) also includes a preference test in the same apparatus between a familiar and a novel conspecific. A tendency to visit the latter is considered a measure of social memory. The rationale is similar to the spontaneous object memory test – in which animals exhibit a tendency to explore novel rather than familiar objects. However, this parallelism may be questionable, and therefore also the translational power and face validity of the test. In human social interaction, the preference may more likely be towards familiar conspecifics, e.g., participants in scientific conferences. Again, social anxiety is a potential confound, e.g., reduced social approach following social defeat (Nestler and Hyman 2010).
In short, Crawley’s social approach test is not a satisfactory test of social interaction. Complex social interaction is neither quantified nor described, but merely reduced to social preference or social approach response. This over-simplification in design and measurement may also be responsible for the test’s low statistical power. It is not uncommon that a sample size exceeding 20 may be necessary to generate significant social preference or recognition in normal control animals. The test’s construct validity with respect to schizophrenia is mainly derived from its sensitivity to NMDA receptor antagonists and responsiveness to some antipsychotic drugs (Castagné et al. 2009). This remains unsatisfactory. Future advances in social neuroscience may provide more valid translational tests applicable to rodents.
2.2.5 Locomotor activity test
Observation of rodent activity in an open field is one of the most standard and easy-to-implement behavioural tests. It is typically studied by placing the animal in a featureless open field for a fixed period of time (from 15 min to 2h), and distance travelled across consecutive time bins represents the most common open field dependent measure. Although specific behaviour such as rearing, grooming, or stereotyped behaviours are still best quantified by experienced human observers under blind conditions, current image tracking system and software readily provides a high resolution record of the locomotor pattern in the horizontal direction that can be subjected to advanced dynamic motion and pattern analyses developed based on reversed translational studies from rodents to men (e.g., Young et al. 2007; Perry et al. 2009).
Rodents are nocturnal animals, so they are more active when tested in the dark phase than the light phase. When the animals are exposed to the open field arena for the first time, the drive to explore is the strongest and therefore particularly conducive for the stimulation of locomotor activity; and we may use the term “motor and exploratory behaviour” to refer to the underlying construct being measured. This drive to explore will diminish over the course of the observation period as novelty wanes, and habituation of exploratory motor activity ensues. Long term habituation can be observed when animals are exposed to the same open field repeatedly, and this indicates that the animals have acquired the memory of having been in the same apparatus before. Habituation may be weakened (i.e., spontaneous recovery) simply due to passage of time, i.e., forgetting. Explorative locomotor response may also resume due to external stimulation, a phenomenon called dishabituation.
The measurement of locomotor activity in an open field can be confounded by anxiety. An anxious subject may appear less active. For the purpose of studying locomotor activity, the open field should not be an anxiogenic environment – one reason as to why a circular open field is discouraged. Potential confounding effects on anxiety can be assessed by comparing the percent time or distance moved in the central zone of the open field arena. If anxiogenic (reduced exploration to the centre) or anxiolytic (increased exploration to the centre) effects are suspected, verification by additional tests of anxiety, such as the elevated plus maze test, are warranted.
The relevance of open field activity to preclinical schizophrenia research is based on the fact that psychostimulant drugs (e.g., amphetamine and phencyclidine) reliably induce hyperlocomotor activity. Conversely, antipsychotic drugs tend to produce sedative effects in the open field, i.e., reduce activity. At sufficiently high doses, haloperidol can even induce catatonia - a state of neurogenic motor immobility. According to some researchers, therefore, hyperactivity may approximate psychotic agitation (positive symptoms), whereas stereotypy approximates negative motor symptoms, respectively. The underlying rationale is that enhanced dopaminergic activity may correlate with hyperactivity, based on the premise that the reverse causal relationship is true (at least within specific dose range). The face validity of this dichotomy receives some partial support from analogous explorative behaviour in human (Henry et al., 2010, 2011). Motor hyperactivity in bipolar and schizophrenia patients appears to correlate with symptom ratings of mania and psychosis, yet they can be distinguished by characteristic motor signatures (Perry et al, 2009; Minassian et al. 2010). In terms of negative symptoms, behavioural pattern analysis in human open field analogues can also identify prominent withdrawal, limited motor activity, and inattention to the environment (see Henry et al. 2010).
2.2.5.1 Novelty-induced Locomotor
Like amphetamine, novelty evokes dopamine release in the striatum which is thought to stimulate locomotor activity. This also explains habituation of locomotor activity over time. Hence, novelty-induced hyperactivity may be more readily linked to increased dopamine activity. However, this may stem from a lack of locomotor habituation – which may reflect a learning deficit, e.g., the hyperactivity effect associated with hippocampal lesions may partly stem from spatial memory deficit, which does affect spatial familiarity judgement. Of course, this could be relevant to schizophrenia since structural abnormalities are most readily detected in the temporal lobe, including circuitry abnormalities in the hippocampus (e.g., Gothelf et al. 2000; Shepherd et al. 2012). A common approach is to contrast novelty-induced activity against home cage activity. An increase in activity specific to novel environment is consistent with a dopaminergic interpretation.
2.2.5.2 Changes in the locomotor response to amphetamine
Low doses of amphetamine reliably stimulate locomotor activity, but the drug can induce stereotypic behaviour at sufficiently high doses. The precise relevant low and high doses are typically higher in mice (low doses ≈2 mg/kg; high doses ≈ 20mg/kg; e.g., Yates et al. 2007) compared to rats (low doses ≈1 mg/kg; high doses ≈ 10 mg/kg, e.g., Tai et al. 1991). The dose-dependent profile of amphetamine is attributed to the differential activation between the mesolimbic and nigrostriatal dopamine pathways. Within the striatum, the motor stimulant and motor stereotypic effects are demarcated between the ventral and dorsal striatum, respectively. Due to the critical dependency on dosage, inclusion of multiple doses would be most desirable. This is because change in amphetamine responsiveness may involve a horizontal shift in the dose response curve rather than a vertical shift. Otherwise, additional measures might help to clarify interpretation. For instance, a reduction in amphetamine-induced hyperlocomotor activity that is accompanied by an increase in stereotypy may imply a shift of the dose-response function to the left. Hence, it is always prudent to keep video record for such supplementary analysis (e.g., scoring of stereotypic behaviour) rather than relying on tracking data alone that are limited to the evaluation of horizontal movements.
Valid conclusions of drug-dependent treatment effects can only be made if baseline activity (i.e., vehicle control) has been properly taken into account. If a between-subject design is used, this would simply involve an examination of the critical treatment × drug interaction. If this achieves statistical significance, one can then evaluate how the psychostimulant drug induces, exacerbates, reduces or reverses any baseline treatment difference. However, this analytic approach may not be applicable to experiments in which drug challenge is a within-subjects factor, whereby animals were first observed after vehicle solution injection (pre-drug phase) before psychostimulant administration (drug phase). A substantial baseline difference of activity in the pre-drug phase can limit interpretation of psychostimulant-induced hyperactivity, if the treatment effect in both phases is in the same direction. Statistically, this may be addressed by analysis of covariance with pre-drug activity levels as covariate. Procedurally, one should allow sufficient locomotor habituation in the pre-drug phase (up to 30 min) to minimize baseline difference just prior to drug administration.
2.2.5.3 Behavioural sensitization
Exposure to amphetamine once is sufficient to substantially potentiate the response to a subsequent challenge. This is known as behavioural sensitization, and it is a very robust effect in rodents (Robinson and Becker 1986). The second drug challenge is typically administered after a few days of withdrawal from the first exposure. The “endogenous sensitization” hypothesis of schizophrenia asserts that the development of behavioural sensitization to amphetamine may capture similar physiological and structural changes in schizophrenia (see Laruelle 2000). In addition to acute amphetamine challenge, animal models with particular relevance to dopaminergic dysfunction of schizophrenia and its therapy ought to be evaluated for the development of sensitization.
3. Concluding remarks
3.1 What tests suit me best?
From a pure explorative perspective, the more tests are performed the more comprehensive and reliable would be the eventual characterization of a given animal model. This of course applies to behavioural as much as any other biological assays. However, such an exhaustive approach is not always practical; even if at all possible, it will take years to accomplish. One is therefore confronted with difficult choices between possible tests, especially at the early stage of a project when one has few preliminary data to offer guidance. One may ask whether there are a minimal number of tests that ought to be conducted, and whether certain tests might be preferable to others. There are no standard answers to such questions. The selection of tests should be firmly grounded on the hypothesis behind one’s model. Behavioural tests whose neural basis (in terms of critical brain circuits and pharmacological profile) are well defined and investigated, such as prepulse inhibition, latent inhibition, and working memory, would offer more precise opportunities to formulate testable mechanistic hypothesis, either as a model of the disease or its therapy. Confirmation as well as refutation of a clear and well-defined hypothesis can spawn new and more refined hypotheses to guide further evaluation.
Tests of motor responsiveness to systemic amphetamine and phencyclidine challenge can offer direct (although more grossly defined) connection to the dopamine and glutamate hypotheses of schizophrenia. Such tests are simple to implement and evaluate in any laboratories with minimal requirements on video recording. Psychostimulant tests also offer quick outcomes with possible distinction between positive or negative/cognitive symptomatology; and the behavioural readout can be readily related to physiological and neurochemical data. Reaction to psychostimulant is a practical first test to generate preliminary data before exploring the possibility to develop new tests or to seek consultation or collaboration with behavioural experts.
3.2 Deviations from an idealized profile
These are not unexpected given that our idealized pattern of outcome is derived from our imperfect understanding of schizophrenia. As one’s repertoire of tests increase, it is not unusual to obtain readouts across tests that seemingly offer incompatible interpretations, suggesting the presence of both psychotic-like and antipsychotic-like properties in a model. We have ourselves encountered such dilemma when evaluating the link between brain adenosine imbalance and schizophrenia-related behavioural traits (Shen et al. 2012), and the antipsychotic potential of glycine transporter 1 blockade (Singer et al. 2009b; Singer et al. 2011). Often this may only seem perplexing because of one’s tendency to place a model against either one or the other end of this artificial dichotomy. In such cases, one should not be pressured into hiding one of the other (seemingly) contradictory outcomes for the sake of adherence to predetermined dogmatic stance, which may be counterproductive to progress.
For instance, while the dopamine releasing drug, amphetamine, exacerbates psychotic symptoms in patients, it has been shown to confer benefits for some cognitive symptoms when co-administered with existing antipsychotic drugs (e.g., Goldberg et al. 1991). Conversely, the typical antipsychotic drug, haloperidol, is effective in suppressing positive florid symptoms, yet it may worsen negative and cognitive symptoms at the same time (e.g., Volavka et al. 1996; Saeedi et al. 2006). Hence, what might initially be perceived as internal inconsistency within a model could perhaps find correspondence with documented clinical experiences. The model may offer new insights into the intricate balance of dopamine function in this case, for instance, between antipsychotic efficacy against the positive symptoms derived from suppression of mesolimbic dopamine neurotransmission and improved working memory performance by stimulation of prefrontal dopamine activity in humans.
3.3 Future Perspectives
There is obvious need for better behavioural models and tests in animals to forge strong and effective connections between preclinical and clinical research that will improve and catalyse translational research in schizophrenia with clinical deliveries as the ultimate goal. The overview above readily illustrates that the need is most acute for negative symptoms in affect and asociality. Face validity is typically low, readouts are prone to experimental confounds, and interpretations difficult. The difficulties in developing new effective treatments against negative symptoms are partly attributed to the shortcomings of existing models as well as their interpretation. Perhaps the search of the cognitive underpinnings of such symptoms would offer new insights for the development of new and better tests or models. Indeed, the behavioural despair induced in the Porsolt forced swim test is based on the concept of learned helplessness, which is cognitive in nature, whereby the animals learn to give up and surrender to the inescapability of the adverse situations.
We see that the application of cognitive tests such as latent inhibition and prepulse inhibition has been rather powerful and successful. These tests recapitulate specific attentional dysfunctions in schizophrenia and provide meaningful conceptual as well as neurobiological links to positive symptoms, which would not have been feasible if one attempts to model directly hallucinations and delusional thoughts in animals. Recent extensions of these behavioural models to negative symptomatology have been explored by distinguishing between different (pharmacologically defined) forms of abnormalities. For example, abnormally persistent LI and deficient PPI induced by NMDA receptor blockade are specifically linked to negative symptoms because they can discriminate between atypical and typical antipsychotics (Geyer and Ellenbroek 2003; Geyer et al. 2008; Weiner and Arad 2009).
Another obvious hurdle in the development of new negative symptoms models is the lack of clinical effective drugs to control such symptoms. Hence, non-responsiveness to current antipsychotic drugs as such might not invalidate the value of a given model. Ironically, the lack of response to current antipsychotic drugs might lend predictive validity. However, most behavioural models claiming relevance to negative symptoms are based on demonstration of specific responsiveness to clozapine, because clozapine is generally considered more effective against negative symptoms than first generation antipsychotic drugs, such as haloperidol. Since the clinical efficacy of clozapine in the treatment of negative symptoms is still disappointing, this criterion may not readily lead us to better drugs, or even false positives that fail to translate to the clinics (see Young et al. 2009).
Breakthroughs in animal models are expected from advances in the causative and mechanistic descriptions of schizophrenia, including the genetics and epidemiology of the disease (Brown 2011; Owen 2012; Piper et al. 2012). Evaluation of individual differences (in wild type animals) revealed on schizophrenia-related behavioural tests can offer new opportunities in the search of the neural basis (genetic, neurochemical or neuroanatomical) of extreme schizophrenia-like and anti-schizophrenia behavioural traits without necessarily defined a specific “disease-causing” manipulations. This top-down approach from behaviour to brain has, for instance, provided further insights into the construct and predictive validity of the prepulse inhibition model (Singer et al. 2013; Peleg-Raibstein et al. 2013). As reviewed above, through back translation, animal tests such as open field test of locomotor and explorative activity have been evaluated in patients which has yielded new translational evidence as well as methods of data analysis (Young et al. 2007; Perry et al. 2009; Henry et al. 2010). Attempts to translate the water maze and radial arm maze to human application with the help of virtual reality (Astur et al. 2004) will facilitate meaningful inter-species translation of concepts and communication between preclinical and clinical findings. Animal models will continue to play an important role as an integrative platform in this respect.
References
- Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JPA, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic Meta-Analyses and Field Synopsis of Genetic Association Studies in Schizophrenia: The SzGene Database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. Revised. [Google Scholar]
- Andreasen NC, Olsen S. Negative vs positive schizophrenia. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Arguello PA, Markx S, Gogos JA, Karayiorgou M. Development of animal models of schizophrenia. Dis Model Mech. 2010;3:22–26. doi: 10.1242/dmm.003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford: Clarendon Press; 1986. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, Kiselycznyk C, Schmitt W, Sanderson DJ, Rawlins JN, Saksida LM, Bussey TJ, Sprengel R, Bannerman D, Holmes A. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2012;62:1263–1272. doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task (1988) J Nerv Ment Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: The basis of the “two hit hypothesis. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Belizário JE, Akamini P, Wolf P, Strauss B, Xavier-Neto J. New routes for transgenesis of the mouse. J Appl Genet. 2012;53:295–315. doi: 10.1007/s13353-012-0096-y. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM, Fuss J, Trusel M, Galsworthy MJ, Bobsin K, Colacicco G, Deacon RM, Riva MA, Kellendonk C, Sprengel R, Lipp HP, Gass P. The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp Neurol. 2011;227:42–52. doi: 10.1016/j.expneurol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:1522. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Taste reactivity: measuring hedonic impact in human infants and animals. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berrios GE. Positive and Negative Symptoms and Jackson: A Conceptual History. Arch Gen Psychiatry. 1985;42:95–97. doi: 10.1001/archpsyc.1985.01790240097011. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Dubroqua S, Singer P, Feldon J, Yee BK. Sensorimotor gating and vigilance-dependent choice accuracy: a within-subject correlative analysis in wild-type C57BL/6 mice. Behav Brain Res. 2011;217:178–187. doi: 10.1016/j.bbr.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001;49:171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990;16:425–432. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- Castagné V, Moser PC, Porsolt RD. Preclinical behavioral models for predicting antipsychotic activity. Adv Pharmacol. 2009;57:381–418. doi: 10.1016/S1054-3589(08)57010-4. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Chao OY, Pum ME, Huston JP. The interaction between the dopaminergic forebrain projections and the medial prefrontal cortex is critical for memory of objects: Implications for Parkinson’s disease. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.01.003. in Press. [DOI] [PubMed] [Google Scholar]
- Chemero A, Heyser CJ. Object exploration and a problem with reductionism. Synthese. 2005;17:403–423. [Google Scholar]
- Chindo BA, Adzu B, Yahaya TA, Gamaniel KS. Ketamine-enhanced immobility in forced swim test: a possible animal model for the negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:310–316. doi: 10.1016/j.pnpbp.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology (Berl) 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MR, Chappell-Stephenson R. Exploring the limits of spatial memory using very large mazes. Learn Behav. 2003;31:349–368. doi: 10.3758/bf03195996. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Cook S, Matthysse S, Barnard J, Lo Y, Levy DL, Rubin DB, Holzman PS. Spatial and object working memory impairments in schizophrenia patients: a Bayesian item-response theory analysis. J Abnorm Psychol. 2002;111:425–435. doi: 10.1037//0021-843x.111.3.425. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Balu D, Benneyworth M, Basu A, Roseman A. Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin Neurosci. 2010;12:359–382. doi: 10.31887/DCNS.2010.12.3/jcoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007a;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse. John Wiley & Sons, Inc; Hoboken, New Jersey: 2007b. [Google Scholar]
- Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swim test. Neurosci Biobehav Rev. 2005;29:547–568. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Quednow BB, Stadler RR, Feldon J, Vollenweider FX. The monotonic dependency of prepulse inhibition of the acoustic startle reflex on the intensity of the startle eliciting stimulus. Beh Brain Res. 2006;174:143–150. doi: 10.1016/j.bbr.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav Neurosci. 2002;116:472–478. doi: 10.1037//0735-7044.116.3.472. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Waddington JL, O’Tuathaigh CM. Mutant models for genes associated with schizophrenia. Biochem Soc Trans. 2009;37:308–312. doi: 10.1042/BST0370308. [DOI] [PubMed] [Google Scholar]
- Dillon GM, Shelton D, McKinney AP, Caniga M, Marcus JN, Ferguson MT, Kornecook TJ, Dodart JC. Prefrontal cortex lesions and scopolamine impair attention performance of C57BL/6 mice in a novel 2-choice visual discrimination task. Behav Brain Res. 2009;204:67–76. doi: 10.1016/j.bbr.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Dubroqua S, Serrano L, Boison D, Feldon J, Gargiulo PA, Yee BK. Intact working memory in the absence of forebrain neuronal glycine transporter 1. Behav Brain Res. 2012;230:208–214. doi: 10.1016/j.bbr.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Farber NB. The NMDA receptor hypofunction model of psychosis. Ann NY Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Essays in cognitive psychology series. Lawrence Earlbarm Associates Ltd; Hove, UK: 1992. The cognitive neuropsychology of schizophrenia. [Google Scholar]
- Gabrovska VS, Laws KR, Sinclair J, McKenna PJ. Visual object processing in schizophrenia: Evidence for an associative agnostic deficit. Schizophr Res. 2003;59:277–286. doi: 10.1016/s0920-9964(02)00168-8. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Are cross-species measures of sensorimotor gating useful for the discovery of procognitive cotreatments for schizophrenia? Dialogues Clin Neurosci. 2006;8:9–16. doi: 10.31887/DCNS.2006.8.1/mgeyer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotox Res. 2008;14:71–78. doi: 10.1007/BF03033576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Gross G, Hofmann FB. Handbook of Experimental Pharmacology. Vol. 213. Springer-Verlag; Berlin Heidelberg: 2012. Novel Antischizophrenia Treatments. [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Bigelow LB, Weinberger DR, Daniel DG, Kleinman JE. Cognitive and behavioral effects of the coadministration of dextroamphetamine and haloperidol in schizophrenia. Am J Psychiatry. 1991;148:78–84. doi: 10.1176/ajp.148.1.78. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Soreni N, Nachman RP, Tyano S, Hiss Y, Reiner O, Weizman A. Evidence for the involvement of the hippocampus in the pathophysiology of schizophrenia. Eur Neuropsychopharmacol. 2000;10:389–395. doi: 10.1016/s0924-977x(00)00097-3. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Gray JA, Feldon J, Rawlins JNP, Hemsley DR, Smith AD. The neuropsychology of schizophrenia. Behav Brain Res. 1991;14:1–84. [Google Scholar]
- Gray NS, Pickering AD, Hemsley DR, Dawling S, Gray JA. Abolition of latent inhibition by a single 5 mg dose of d-amphetamine in man. Psychopharmacology (Berl) 1992;107:425–430. doi: 10.1007/BF02245170. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W Translational Methamphetamine AIDS Research Center (TMARC) Group. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology (Berl) 2011;215:697–707. doi: 10.1007/s00213-011-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neurosci Biobehav Rev. 2010;34:1296–1306. doi: 10.1016/j.neubiorev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Chemero A. Novel object exploration in mice: not all objects are created equal. Behav Processes. 2012;89:232–238. doi: 10.1016/j.beproc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Hodges H, Sowinski P, Fleming P, Kershaw TR, Sinden JD, Meldrum BS, Gray JA. Contrasting effects of fetal CA1 and CA3 hippocampal grafts on deficits in spatial learning and working memory induced by global cerebral ischaemia in rats. Neuroscience. 1996;72:959–988. doi: 10.1016/0306-4522(96)00004-8. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic and temporal factors in the evocationof startle. J Acoust Soc Am. 1968;43:269–282. doi: 10.1121/1.1910776. [DOI] [PubMed] [Google Scholar]
- Honey RC, Good M. Associative components of recognition memory. Curr Opin Neurobiol. 2000;10:200–204. doi: 10.1016/s0959-4388(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior: Implications for current research and theorizing. In: Isaascon RL, Pribram KH, editors. The Hippocampus. Vol. 4. Plenus Press; New York: 1986. [Google Scholar]
- Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 2010;47:4–16. [PubMed] [Google Scholar]
- Jia P, Sun J, Guo AY, Zhao Z. SZGR: a comprehensive schizophrenia gene resource. Mol Psychiatry. 2010;15:453–462. doi: 10.1038/mp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Farnsworth G, DiMattia BV. Double dissociation of egocentric and allocentric space following medial prefrontal and parietal cortex lesions in the rat. Behav Neurosci. 1989;103:956–961. doi: 10.1037//0735-7044.103.5.956. [DOI] [PubMed] [Google Scholar]
- Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465:688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- Kos CH. Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev. 2004;62:243–246. doi: 10.1301/nr2004.jun243-246. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychol Rev. 2009;19:312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Jones T, Hirsch SR, Frackowiak RSJ. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Llano Lopez L, Hauser J, Feldon J, Gargiulo PA, Yee BK. Evaluating spatial memory function in mice: a within-subjects comparison between the water maze test and its adaptation to dry land. Behav Brain Res. 2010;209:85–92. doi: 10.1016/j.bbr.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced preexposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Stimulus selection: learning to ignore stimuli that predict no change in reinforcement. In: Hinde RA, Stevenson-Hinde J, editors. Constraints on learning. London: Academic Press; 1973. pp. 75–96. [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 2009;206:587–602. doi: 10.1007/s00213-009-1504-9. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord. 2010;120:200–206. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Moser PC, Hitchcock JM, Lister S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Rev. 2000;33:275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Phencyclidine in the social interaction test: an animal model of schizophrenia with face and predictive validity. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, editor. Animal Models of Schizophrenia and Related Disorders. Springer; New York: 2011. [Google Scholar]
- Ogren SO, Archer T. Effects of typical and atypical antipsychotic drugs on two-way active avoidance: Relationship to DA receptor blocking profile. Psychopharmacology (Berl) 1994;114:383–391. doi: 10.1007/BF02249327. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann GE. Hippocampus, space, and memory. Behav Brain Sci. 1979;2:313–365. [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: spatial memory in rats. J Exp Psychol: Anim Behav Process. 1976;2:97–116. [Google Scholar]
- Owen MJ. Implications of genetic findings for understanding schizophrenia. Schizophr Bull. 2012;38:904–907. doi: 10.1093/schbul/sbs103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–1220. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. The attentive brain. MIT Press; MA, USA: 1998. [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Sanfilipo M, Chappell PB, Chakravorty S, Gonzenbach S, Ko GN, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–587. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Hauser J, Lopez LH, Feldon J, Gargiulo PA, Yee BK. Baseline prepulse inhibition expression predicts the propensity of developing sensitization to the motor stimulant effects of amphetamine in C57BL/6 mice. Psychopharmacology. 2013;225:341–352. doi: 10.1007/s00213-012-2819-5. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry. 2009;66:1072–1080. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Beneyto M, Burne TH, Eyles DW, Lewis DA, McGrath JJ. The neurodevelopmental hypothesis of schizophrenia: convergent clues from epidemiology and neuropathology. Psychiatr Clin North Am. 2012;35:571–584. doi: 10.1016/j.psc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Hori SE, Powell KJ. Schizophrenia: an integrative approach to modelling a complex disorder. J Psychiatry Neurosci. 2006;31:157–167. [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Russel WMS, Burch RL. The Principles of Humane Experimental Technique. 1959 http://altweb.jhsph.edu/pubs/books/humane_exp/het-toc.
- Saeedi H, Remington G, Christensen BK. Impact of haloperidol, a dopamine D2 antagonist, on cognition and mood. Schizophr Res. 2006;85:222–231. doi: 10.1016/j.schres.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Bannerman DM. The role of habituation in hippocampus-dependent spatial working memory tasks: evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus. 2012;22:981–994. doi: 10.1002/hipo.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson DJ, Gray A, Simon A, Taylor AM, Deacon RM, Seeburg PH, Sprengel R, Good MA, Rawlins JN, Bannerman DM. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–569. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, McHugh SB, Good MA, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: Evidence for Wagner’s dual-process memory model. Neuropsychologia. 2010;48:2303–2315. doi: 10.1016/j.neuropsychologia.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Sigmon SA, McGinty JF. RGS4 overexpression in the rat dorsal striatum modulates mGluR5- and amphetamine-mediated behavior and signaling. Psychopharmacology (Berl) 2012;221:621–635. doi: 10.1007/s00213-011-2606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1:133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther. 2011;17:118–132. doi: 10.1111/j.1755-5949.2010.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Seligman ME. Learned helplessness. Annu Rev Med. 1972;23:407–412. doi: 10.1146/annurev.me.23.020172.002203. [DOI] [PubMed] [Google Scholar]
- Shen HY, Singer P, Lytle N, Wei CJ, Lan JQ, Williams-Karnesky RL, Chen JF, Yee BK, Boison D. Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. J Clin Invest. 2012;122:2567–2577. doi: 10.1172/JCI62378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Shipman SL, Baker EK, Pearlson G, Astur RS. Absence of established sex differences in patients with schizophrenia on a two-dimensional object array task. Psychiatry Res. 2009;166:158–165. doi: 10.1016/j.psychres.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Boison D, Möhler H, Feldon J, Yee BK. Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behav Neurosci. 2007;121:815–825. doi: 10.1037/0735-7044.121.5.815. [DOI] [PubMed] [Google Scholar]
- Singer P, Boison D, Möhler H, Feldon J, Yee BK. Modulation of sensorimotor gating in prepulse inhibition by conditional brain glycine transporter 1 deletion in mice. Eur Neuropsychopharmacol. 2011;21:401–413. doi: 10.1016/j.euroneuro.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Feldon J, Yee BK. Are DBA/2 mice associated with schizophrenia-like endophenotypes? A behavioural contrast with C57BL/6 mice. Psychopharmacology (Berl) 2009;206:677–698. doi: 10.1007/s00213-009-1568-6. [DOI] [PubMed] [Google Scholar]
- Singer P, Feldon J, Yee BK. Interactions between the glycine transporter 1(GlyT1) inhibitor SSR504734 and psychoactive drugs in mouse motor behaviour. Eur Neuropsychopharmacol. 2009b;19:571–580. doi: 10.1016/j.euroneuro.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Singer P, Hauser J, Lopez LL, Peleg-Raibstein D, Feldon J, Gargiulo PA, Yee BK. Prepulse inhibition predicts working memory performance whilst startle habituation predicts spatial reference memory retention in C57BL/6 mice. Behav Brain Res. 2013;242:166–177. doi: 10.1016/j.bbr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Singer P, McGarrity S, Shen HY, Boison D, Yee BK. Working memory and the homeostatic control of brain adenosine by adenosine kinase. Neuroscience. 2012;213:81–92. doi: 10.1016/j.neuroscience.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Yee BK. Reversal of scopolamine-induced disruption of prepulse inhibition by clozapine in mice. Pharmacol Biochem Behav. 2012;101:107–114. doi: 10.1016/j.pbb.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry. 1976;133:197–202. doi: 10.1176/ajp.133.2.197. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer M. Cross-species studies of sensorimotor gating of the startle reflex. Ann N Y Acad Sci. 1999;29:202–216. doi: 10.1111/j.1749-6632.1999.tb09269.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacolog (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CT, Clark AJ, Feldon J, Rawlins JN. Electrolytic lesions of the nucleus accumbens in rats which abolish the PREE enhance the locomotor response to amphetamine. Exp Brain Res. 1991;86:333–340. doi: 10.1007/BF00228956. [DOI] [PubMed] [Google Scholar]
- Tarantino IS, Sharp RF, Geyer MA, Meves JM, Young JW. Working memory span capacity improved by a D2 but not D1 receptor family agonist. Behav Brain Res. 2011;219:181–188. doi: 10.1016/j.bbr.2010.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. Animal Intelligence. Macmillan; NY: 1911. [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson PJ, Laws KR, Roberts KH, Mortimer AM. Stability of set-shifting and planning abilities in patients with schizophrenia. Psychiatry Res. 2004;129:229–239. doi: 10.1016/j.psychres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Van den Buuse M. Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull. 2010;36:246–270. doi: 10.1093/schbul/sbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Staay FJ, Arndt SS, Nordquist RE. Evaluation of animal models of neurobehavioral disorders. Behav Brain Funct. 2009;5:11. doi: 10.1186/1744-9081-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Volavka J, Cooper TB, Czobor P, Meisner M. Effect of varying haloperidol plasma levels on negative symptoms in schizophrenia and schizoaffective disorder. Psychopharmacol Bull. 1996;32:75–79. [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol. 1996;351:1495–503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- Weiner I. Neural substrates of latent inhibition: the switching model. Psychol Bull. 1990;108:442–461. doi: 10.1037/0033-2909.108.3.442. [DOI] [PubMed] [Google Scholar]
- Weiner I. Latent inhibition. Curr Protoc Neurosci. 2001;Chapter 8(Unit 8.13) doi: 10.1002/0471142301.ns0813s16. [DOI] [PubMed] [Google Scholar]
- Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169:257–297. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–386. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Weiner I, Lubow RE, Feldon J. Disruption of latent inhibition by acute administration of low doses of amphetamine. Pharmacol Biochem Behav. 1988;30:871–878. doi: 10.1016/0091-3057(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology (Berl) 2001;156:305–326. doi: 10.1007/s002130100800. [DOI] [PubMed] [Google Scholar]
- Wenk GL. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8.5A) doi: 10.1002/0471142301.ns0805as26. [DOI] [PubMed] [Google Scholar]
- Willner P, Papp M, Phillips G, Maleeh M, Muscat R. Pimozide does not impair sweetness discrimination. Psychopharmacology (Berlin) 1990;102:278–282. doi: 10.1007/BF02245934. [DOI] [PubMed] [Google Scholar]
- Wu YC, Hill RA, Gogos A, van den Buuse M. Sex differences and the role of estrogen in animal models of schizophrenia: Interaction with BDNF. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.024. in Press. [DOI] [PubMed] [Google Scholar]
- Yanagi M, Southcott S, Lister J, Tamminga CA. Animal models of schizophrenia emphasizing construct validity. Prog Mol Biol Transl Sci. 2012;105:411–444. doi: 10.1016/B978-0-12-394596-9.00012-3. [DOI] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Chang DL, Feldon J. The Effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology. 2004a;29:1865–1877. doi: 10.1038/sj.npp.1300480. [DOI] [PubMed] [Google Scholar]
- Yee BK, Chang T, Pietropaolo S, Feldon J. The expression of prepulse inhibition of the acoustic startle reflex as a function of three pulse stimulus intensities, three prepulse stimulus intensities, and three levels of startle responsiveness in C57BL6/J mice. Behav Brain Res. 2005;163:265–276. doi: 10.1016/j.bbr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Yee BK, Feldon J. Distinct forms of prepulse inhibition disruption distinguishable by the associated changes in prepulse-elicited reaction. Behav Brain Res. 2009;204:387–395. doi: 10.1016/j.bbr.2008.11.049. [DOI] [PubMed] [Google Scholar]
- Yee BK, Russig H, Feldon J. Apomorphine-induced prepulse inhibition disruption is associated with a paradoxical enhancement of prepulse stimulus reactivity. Neuropsychopharmacology. 2004b;29:240–248. doi: 10.1038/sj.npp.1300323. [DOI] [PubMed] [Google Scholar]
- Young JW, Amitai N, Geyer MA. Behavioral animal models to assess pro-cognitive treatments for schizophrenia. Handb Exp Pharmacol. 2012;213:39–79. doi: 10.1007/978-3-642-25758-2_3. [DOI] [PubMed] [Google Scholar]
- Young JW, Amitai N, Geyer MA. Behavioral animal models to assess pro-cognitive treatments for schizophrenia. Handb Exp Pharmacol. 2012;213:39–79. doi: 10.1007/978-3-642-25758-2_3. [DOI] [PubMed] [Google Scholar]
- Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: rodent and human studies in the Behavioral Pattern Monitor. Neurosci Biobehav Rev. 2007;31:882–896. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Zhuang YL, Xu Q, Li YD, Shen Y. VSD: a database for schizophrenia candidate genes focusing on variations. Hum Mutat. 2004;23:1–7. doi: 10.1002/humu.10289. [DOI] [PubMed] [Google Scholar]
- Zhu SW, Yee BK, Nyffeler M, Winblad B, Feldon J, Mohammed AH. Influence of differential housing on emotional behaviour and neurotrophin levels in mice. Behav Brain Res. 2006;169:10–20. doi: 10.1016/j.bbr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zheng T, Lee CG, Homer RJ, Elias JA. Tetracycline-controlled transcriptional regulation systems: advances and application in transgenic animal modeling. Semin Cell Dev Biol. 2002;13:121–128. doi: 10.1016/s1084-9521(02)00018-6. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


