Abstract
Changes in tissue oxygen levels trigger molecular signaling pathways that regulate cellular proliferation and differentiation in multiple cell types. The functional role of oxygen signaling in the immune system is not well understood. Rama and colleagues demonstrate that hypoxia induces dendritic-cell maturation; thus they provide a novel mechanistic link between hypoxia/ischemia and the activation of the immune system in the kidney.
Tissue hypoxia as a result of cardiopulmonary dysfunction or reduced oxygen-carrying capacity is a frequently encountered clinical problem, which in the worst-case scenario results in cellular death. Under physiological conditions, tissue oxygen levels provide important cues that are critical for normal embryonic development, cellular differentiation, and stem-cell maintenance. Like any other cell type, immune cells have to be able to respond and to adapt to a low-oxygen environment in order to generate the bioenergetic resources that allow them to survive and to continue to execute cell type-specific functions under hypoxic conditions. Rama et al.1 (this issue) now report that hypoxia, similar to allogeneic stimulation, induced maturation of dendritic cells (DCs), which was associated with an increase in hypoxia-inducible factor-1α (HIF-1α) protein levels and was attenuated by mammalian target of rapamycin (mTOR) inhibition. Although the underlying molecular mechanisms remain to be investigated, the authors' data suggest that renal hypoxia induces an immune/inflammatory response that may promote and/or maintain renal injury (Figure 1).
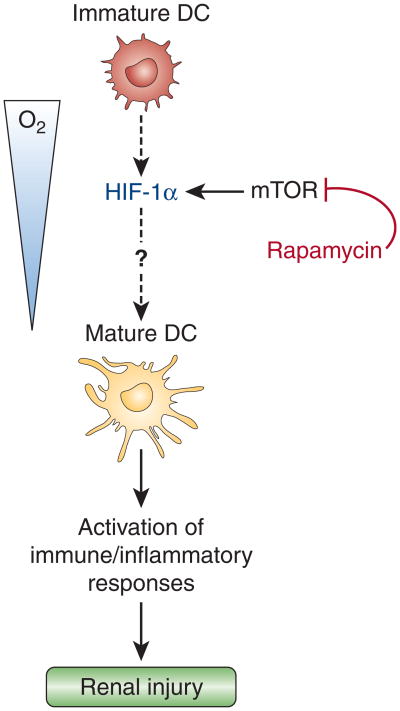
Figure 1. Schematic illustrating the role of hypoxia and hypoxia-inducible factor activation in dendritic-cell maturation.
Rama et al. provide evidence that both hypoxia and the hypoxia mimetic desferrioxamine, a relatively nonspecific inhibitor of hypoxia-inducible factor-α (HIF-α) degradation, induce maturation of dendritic cells (DCs);1 the latter observation suggests that activation of HIF signaling alone is sufficient to increase the percentage of differentiated DCs. Although the molecular mechanisms that underlie this process still need to be identified (dashed line), mammalian target of rapamycin (mTOR) inhibition decreased HIF-1α protein levels, as previously shown in cancer cells,14 and inhibited hypoxic DC maturation.
Dendritic Cells
DCs are an integral part of the immune system. The discovery and further characterization of these cells over the past 35 years led to Ralph Steinman receiving the Lasker Award in 2007. The term “dendritic cell” encompasses multiple cell types that share common characteristics. DCs are potent antigen-presenting cells, are derived from hematopoietic stem cell precursors in the bone marrow, and have also been shown to prime and amplify the innate immune response. They are distributed in a so-called “immature” state in a variety of tissues, including skin, gastrointestinal tract, and kidney. Here they act as sentinels of immune surveillance, extending projections and engulfing antigens by endocytosis, phagocytosis, and pinocytosis. Peptides derived from exogenous antigens or cellular peptides are processed and presented by major histocompatibility complex class II and class I molecules, respectively. Activation of DCs leads to maturation with concomitant migration to draining lymph nodes and upregulation of cell-surface proteins including several costimulatory receptors. Interaction of T lymphocytes with these mature DCs promotes T-cell activation, resulting in lymphocyte proliferation and differentiation into effector cells that express signature cytokines such as interleukin-4, interferon-γ, or interleukin-17.
Immature DCs found in the kidney (renal DCs, or rDCs) have traditionally been difficult to isolate and study. However, it is clear that rDCs exist in several functional subsets based on cell-surface markers (reviewed by John and Nelson2). rDCs are present throughout the interstitium and as such are poised to survey this tissue for foreign antigen and respond to maturation signals from the innate immune system. Rama et al. have proposed short-term hypoxia as a novel maturation signal not only for monocyte-derived DCs, but for rDCs as well.1 This new mechanism for rDC maturation invites interesting speculation as to the role of rDCs in inducing the immune-mediated response to renal hypoxia. The cellular and molecular mechanisms underlying the adaptive and innate immune response to ischemia–reperfusion are an active area of research in many laboratories. For example, CD4+ T cells are required in an ischemia–reperfusion injury (IRI) model of acute renal failure,3 and rDCs have recently been shown to be the predominant source of intrarenal tumor necrosis factor-α in early IRI,4 an observation that points to their pathophysiological importance in ischemic renal injury. Whether hypoxia-induced maturation of rDCs alone leads to their migration to regional lymph nodes, T-cell activation, and additional immune-mediated damage to the kidney requires further investigation.
Molecular oxygen sensing: Is there a role for HIF in dendritic-cell maturation?
Key factors in cellular adaptation to hypoxia are hypoxia-inducible factor-1 and factor-2 (HIF-1 and HIF-2), heterodimeric transcription factors that consist of an oxygen-sensitive α-subunit, which under normoxia is targeted for rapid degradation by the pVHL-E3-ubiquitin ligase, and a constitutively expressed β-subunit (reviewed by Haase5). HIFs regulate the expression of gene products that control cellular energy metabolism and glucose transport, cellular proliferation and differentiation, angiogenesis and erythropoiesis; classic HIF target genes include phosphoglycerate kinase-1 (PGK), glucose transporter-1 (GLUT-1), vascular endothelial growth factor (VEGF), and erythropoietin (EPO) (reviewed by Haase5). Although HIF-α degradation is inhibited in cultured cells when oxygen levels fall below 5% O2, HIF-α proteins are usually not detectable in adult renal tissues, unless HIF-α proteolysis is inhibited pharmacologically, for example by cobalt chloride, or unless a significant decrease in oxygen availability has occurred as a result of acute or chronic ischemia. In hypoxic kidneys HIF-1α has been detected in most cell types, whereas HIF-2α is expressed in cortical interstitial, endothelial, and glomerular cells, as well as in macrophages, but is absent in renal tubular cells (reviewed by Haase5). Hypoxia through HIF-1 activation has been shown to modulate lymphocytic function and T-cell receptor signaling6,7 and strongly impacts on inflammatory-cell recruitment and function.8,9 Along those lines, Rama et al. show that the hypoxic induction of DC maturation coincides with HIF-1α stabilization,1 suggesting that HIF-1 may play a role in this process, pending more rigorous genetic and functional studies that directly examine whether HIF-1α is actually required for DC maturation under hypoxia. Thus, given these and other published observations, it is plausible that alterations of HIF signaling in inflammatory cells may play a fundamental role in immune-mediated renal injury and inflammation. This potential injury-promoting function of HIF in immune cells is in contrast to its proposed cytoprotective role in ischemic renal disease (reviewed by Haase5), which has to be taken into account in the design of therapeutic strategies that aim at HIF-α stabilization to improve disease outcome.
Hypoxia, dendritic cells, and renal disease
Renal oxygen concentration can vary greatly; it is estimated to be about 5% O2 (equivalent to ∼38 mm Hg) in the cortex and can be as low as 1% O2 (∼8 mm Hg) in the renal medulla. As a result of high renal oxygen demand and limited compensatory mechanisms, renal hypoxia/ischemia is a frequently encountered problem in clinical nephrology. Thus the observations by Rama et al.1 have broad implications for the pathogenesis of kidney diseases.
One of the most obvious settings in which hypoxia may play a role in immune-mediated renal damage is the transplantation setting. It is clear that IRI during transplantation contributes to the adaptive and innate immune response. Rama and colleagues1 offer a new potential mechanism whereby IRI may induce early acute allograft rejection. Here, hypoxia induced by removal of the organ and storage for transport could lead to rDC activation in addition to parenchymal damage. With the use of HIF-1α stabilization as a surrogate marker, substantial and widespread hypoxia has been demonstrated in transplant biopsies immediately and even 10–14 days after renal engrafment.10 Once transplanted into the recipient, donor rDCs would mature and migrate to regional lymph nodes, leading to direct allorecognition by the recipient's T-cell repertoire. Direct allorecognition occurs when the recipient's T cells respond to the donor's major histocompatibility complex molecules expressed on the donor's antigen-presenting cells. T cells directed against foreign major histocompatibility complex are present at high frequency (approximately 1%–10%) compared with those directed at nominal peptide antigen (estimated to be <0.001% of the T-cell repertoire). Is hypoxia-induced rDC maturation a major mechanism underlying the direct alloreactive response? Studies evaluating rDC migration and functional capacity following IRI will only begin to evaluate this potential mechanism.
Similarly to IRI in the transplant setting, hypoxia-induced DC activation may contribute to the pathophysiology of acute injury of native kidneys, as CD4+ T cells are required in an IRI model of acute renal failure.3 Aside from immune-mediated glomerular diseases, another important scenario in which hypoxia activation of DCs could be relevant is chronic kidney disease (CKD). In CKD, hypoxia as a result of glomerulosclerosis and capillary rarefaction has long been thought to play an active role in disease progression, as it often precedes the development of fibrosis.11 Although recent data demonstrated an active role for tubular-cell hypoxia and HIF-1 in this process,12 the presence of interstitial inflammatory infiltrates, a hallmark of CKD, illustrates the importance of inflammatory-cell responses in the progression of CKD. This notion is underscored by the observation that blockade of leukocyte recruitment substantially retards the development of chronic renal disease in rodents.13 The finding by Rama and colleagues that low oxygen induces DC maturation1 therefore provokes further probing into the potential mechanistic links among hypoxia, the immune system, inflammation, and CKD progression.
Summary
Tissue oxygen levels play a fundamental role in the regulation of cellular proliferation and differentiation. Although there is mounting evidence that hypoxia modulates immune and inflammatory responses, the role of hypoxic signaling in renal immune-mediated injury is largely unexplored. Rama and colleagues1 propose that hypoxia is a key regulator of DC maturation in the kidney, suggesting a novel mechanism by which oxygen regulates immune responses. Their work will stimulate future investigations into the role of molecular oxygen sensing in immune-cell maturation and function and has implications for acute and chronic renal injuries in the transplantation and non-transplantation settings.
Acknowledgments
VHH is supported by grants from the National Institutes of Health and the American Heart Association. JSM is an ASN–AST John Merrill Grant Scholar of the American Society of Nephrology.
References
- 1.Rama I, Bruene B, Torras J, et al. Hypoxia stimulus: an adaptive immune response during dendritic cell maturation. Kidney Int. 2008;73:816–825. doi: 10.1038/sj.ki.5002792. [DOI] [PubMed] [Google Scholar]
- 2.John R, Nelson PJ. Dendritic cells in the kidney. J Am Soc Nephrol. 2007;18:2628–2635. doi: 10.1681/ASN.2007030273. [DOI] [PubMed] [Google Scholar]
- 3.Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong X, Swaminathan S, Bachman LA, et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 5.Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271–F281. doi: 10.1152/ajprenal.00071.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann AK, Yang J, Biju MP, et al. Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc Natl Acad Sci USA. 2005;102:17071–17076. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 8.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong T, Eltzschig HK, Karhausen J, et al. Leukocyte adhesion during hypoxia is mediated by HIF-1-dependent induction of beta2 integrin gene expression. Proc Natl Acad Sci USA. 2004;101:10440–10445. doi: 10.1073/pnas.0401339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberger C, Pratschke J, Rudolph B, et al. Immunohistochemical detection of hypoxia-inducible factor-1alpha in human renal allograft biopsies. J Am Soc Nephrol. 2007;18:343–351. doi: 10.1681/ASN.2006070792. [DOI] [PubMed] [Google Scholar]
- 11.Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int. 2000;57(Suppl 75):S22–S26. [PubMed] [Google Scholar]
- 12.Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anders HJ, Ninichuk V, Schlondorff D. Progression of kidney disease: blocking leukocyte recruitment with chemokine receptor CCR1 antagonists. Kidney Int. 2006;69:29–32. doi: 10.1038/sj.ki.5000053. [DOI] [PubMed] [Google Scholar]
- 14.Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]