Abstract
OBJECTIVE
To evaluate the fetal renal artery impedance in the context of inflammation-associated preterm birth (PTB).
STUDY DESIGN
We conducted a prospective Doppler assessment of the fetal renal artery impedance in 70 singleton fetuses. The study group consisted of 56 premature fetuses (28.1 [25.3–30.6] weeks at enrollment). Gestational age (GA) reference ranges were generated based on fetuses with uncomplicated pregnancies (n=14). Doppler studies included renal artery pulsatility index (PI), resistance index (RI), systolic/diastolic (S/D) ratio and presence-or-absence of end-diastolic blood flow. We assessed amniotic fluid (AF) inflammation by proteomic profiling (SELDI-TOF). Data were interpreted in relationship to amniotic fluid index (AFI), cord blood interleukin-6 (IL-6) and erythropoietin (EPO) levels. The cardiovascular and metabolic profiles of the neonates were investigated in the first 24 hours of life.
RESULTS
Fetuses delivered by mothers with intra-amniotic inflammation had higher cord blood IL-6 but not EPO levels. Fetal inflammation did not affect either renal artery PI,RI,S/D ratio or end-diastolic blood flow. Neonates delivered in the context of intraamniotic inflammation had higher serum blood urea nitrogen levels, which correlated significantly with AF IL-6 levels. The renal artery RI and SD ratio were inversely correlated with the AFI independent of GA, cord blood IL-6 and status of the membranes.
CONCLUSION
The fetus is capable of sustaining normal renal artery impedance despite inflammation. Resistance in the renal vascular bed affects urine output independent of inflammation.
INTRODUCTION
Preterm birth (PTB) remains one of the leading causes of perinatal morbidity and mortality worldwide.1,2 Several distinct pathophysiological pathways are proposed to be involved in triggering PTB.3 Myometrial stretching, oxidative stress, decidual hemorrhage, and infection are thought to play the most significant roles.4
Both clinically symptomatic and asymptomatic intrauterine infection induce an intra-amniotic inflammatory response that includes the release of multiple cytokines and chemokines, which in turn trigger histological chorio-amnionitis, preterm contractions and/or rupture of the membranes.5,6 The current working model in prematurity attendant intra-amniotic infection/inflammation is that a systemic fetal inflammatory response is initiated when pathogens gain access to the fetus and stimulate the production of cytokines.7,8 This inflammatory response is a well-recognized risk factor for increased perinatal morbidity and mortality after adjusting for gestational age (GA) at birth.9
There is strong evidence that abnormalities exist in both vascular anatomy and vasomotor regulation of arterial tone under pathological circumstances (i.e chorioamnionitis), and particularly if the process of fetal inflammation is initiated in utero.10,11,12,13,14 Thus, we postulate that as intra-uterine infection/inflammation can affect fetal brain and cardiac vascular function, the vasomotor regulation of the fetal kidney may also become significantly altered prior to birth. 10,15
Fetal metabolic stress, such as that caused by inflammation, triggers apoptosis in a variety of tissues, including the nervous system and the kidney.16 The purpose of this study was to test the hypothesis that disturbances in renal artery blood flow resistance occur in utero as part of a complex adaptive fetal response to inflammation.
MATERIALS AND METHODS
Study population and research design
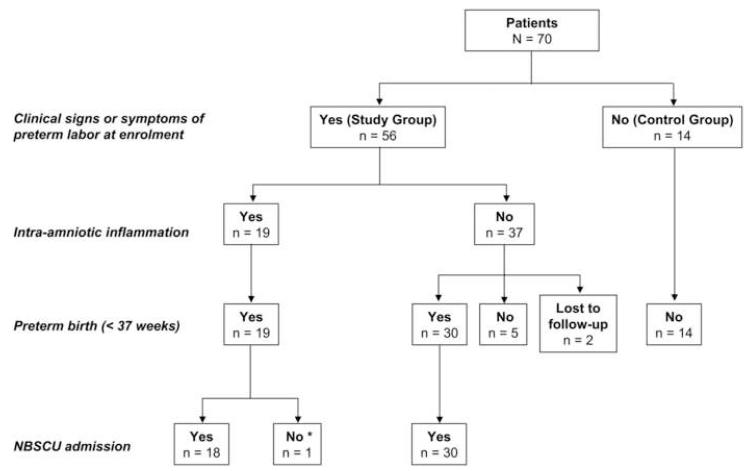
We evaluated fetal renal artery blood flow hemodynamics in 70 fetuses. A flow diagram of our study population is presented in Figure 1. Fifty-six fetuses (study group) were delivered by mothers who had a clinically indicated amniocentesis to rule out intra-amniotic infection/inflammation. Gestational age renal artery blood flow velocity reference ranges were generated based on fetuses with uncomplicated pregnancies (n=14) (control group). All our control fetuses were carried by asymptomatic healthy women undergoing ultrasound as part of their routine prenatal care. We enrolled our study subjects consecutively based on the availability of 3 investigators (HA, MOB, CSB). The Yale University Human Investigation Committee approved our research protocol and written informed consent was obtained from all the participants.
Figure 1. Enrollment flowchart of patients assigned to the study and control groups.
* Demise prior to admission to Newborn Special Care Unit (NBSCU).
Women in the study group presented to Yale New Haven Hospital between October 2004 and February 2008, with one or several symptoms including advanced cervical dilation (>3 cm) (n=17), preterm labor symptoms (n=29) or preterm premature rupture of the membranes (PPROM) (n=27). Gestational age was established based on either the last menstrual period or a first or second trimester ultrasound evaluation. Eligible women had a singleton fetus at ≥ 22.1 or <34 weeks GA without evidence of structural abnormalities, at the time of assessment or birth. Women with maternal medical complications (i.e. hypertension, preeclampsia, diabetes, thyroid disease), viral infections [human immunodeficiency virus (HIV), hepatitis B or C], anhydramnios, fetal intra-uterine growth restriction (IUGR-estimated fetal weight <10th percentile for GA) and fetuses with abnormal karyotypes and/or congenital anomalies were excluded.
Preterm labor was defined as presence of regular uterine contractions associated with advanced cervical dilatation or effacement at <37 weeks of gestation. We confirmed rupture of the membranes either by “pooling” on speculum examination, positive “nitrazine” and “ferning” tests, or by a positive amnio-dye test. Clinical chorioamnionitis was diagnosed in the presence of maternal fever (>37.8°C), maternal leukocytosis (>15,000 cells/mm3), uterine tenderness, foul smelling amniotic fluid (AF) or visualization of pus at the time of the speculum exam, maternal or fetal tachycardia.17
In all study cases, assessment of the renal artery Doppler velocity was performed immediately prior to the amniocentesis procedure. Following amniocentesis and fetal renal artery Doppler assessment, each patient was followed prospectively to delivery. Clinical care of the patients was left to the discretion of the clinical team. In PPROM patients, digital examinations were not allowed. Women with PPROM received corticosteroids for lung maturity if <32 weeks and antibiotic therapy (ampicillin/erythromycin or clindamycin) if <34 weeks GA.18 Per our institutional protocol, women were monitored with vital sign assessment (temperature, blood pressure, pulse) every 4 hours and by cardiotocography at least twice daily for the presence of fetal heart abnormalities and/or uterine contractions. Induction of labor or a surgical delivery was recommended for clinical indications such as: clinical chorioamnionitis, AF laboratory results suggestive of intra-amniotic infection/inflammation, breech presentation, prolapsed umbilical cord, presence of fetal heart rate abnormalities (fetal bradycardia, recurrent, late, severe and prolonged variable deceleration) and/or GA ≥34 weeks.
Amniotic fluid was retrieved by ultrasound-guided amniocentesis under sterile conditions. Following retrieval, AF was cultured for aerobic and anaerobic bacteria, Ureaplasma and Mycoplasma species. The clinical laboratory performed the glucose and lactate dehydrogenase (LDH) measurements, the Gram stain and the AF white blood cell count (WBC). An AF glucose cut-off of ≤15 mg/dL and LDH levels ≥419 U/L and/or a positive Gram stain were considered suggestive of intra-amniotic infection/inflammation.19,20 These results were available for clinical care.
Mass restricted (MR) scoring for diagnosis of intra-amniotic inflammation
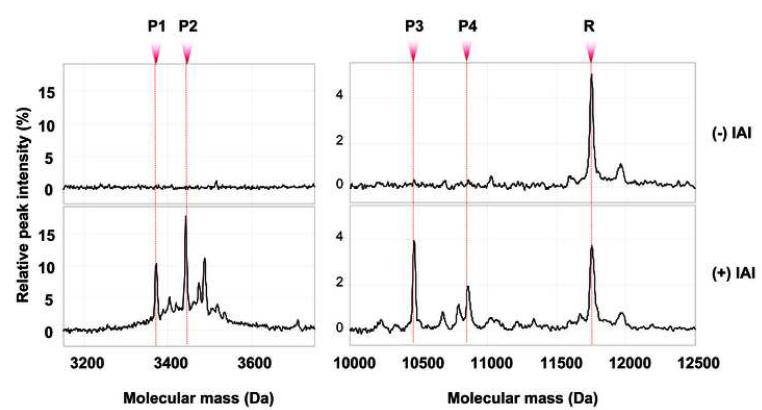
For research purposes, to confirm or exclude intra-amniotic inflammation, we used SELDITOF (surface-enhanced laser desorption ionization time of flight) mass spectrometry (PBSIIC, Ciphergen Biosystems, Fremont, CA). Following AF retrieval, a proteomic profile [Mass Restricted (MR) score] was immediately generated. The criteria and methodology for assessing intra-amniotic inflammation were as previously reported.21,22 Peaks composing the MR score were identified by their conspicuous aspect at or in proximity of their known respective masses: 3377.0 and 3448.1 Da (corresponding to neutrophil defensins 2 and 1, respectively) and at 10,443.8 and 10,834.5 Da (corresponding to calgranulins C and A respectively) (Figure 2). The MR score provides qualitative information regarding the presence or absence of intra-amniotic inflammation and ranges from 0 to 4, depending upon the presence or absence of each of the four protein biomarkers. A categorical value of 1 is assigned if a biomarker peak is present and 0 if absent. An MR score of 3-4 indicates the presence of “severe” intra-amniotic inflammation while a score. The presence or absence of the R peak corresponding to the β2-microglobulin [11,731 Da] was specifically searched for in all our proteomic tracings (study group).23 One investigator (IAB) performed all the SELDI-TOF assays and scored the samples blinded to either clinical presentation or outcome.
Figure 2. Representative SELDI-TOF mass spectrometry profiles of the amniotic fluid of a woman without and one with intra-amniotic inflammation [Mass Restricted (MR) score = 3 or 4].
The MR score ranges from 0 to 4, depending upon the presence or absence of each of the 4 protein biomarkers (defensin 2, 1 and calgranulin C and A). A value of 1 was assigned if a biomarker peak was present and 0 if absent. MR score 0 (zero) indicates “no” inflammation. MR score 3-4 indicates “severe” inflammation. Biomarkers: P1= defensin 2 [3,377 Da], P2= defensin 1 [3,448 Da], P3= calgranulin C [10,443 Da], P4= calgranulin A [10,834 Da], R = beta2 microglobulin [11,731 Da]. The R peak is not part of the MR score. IAI= intra-amniotic inflammation; Da = Daltons
Doppler ultrasonography
We performed our evaluation of the fetal renal artery blood flow with either the Voluson 730 system or the Voluson Expert E8 (General Electric Medical Systems, Milwaukee, WI, USA), equipped with a 4-8 MHZ curved array transducer. All women were placed in the semi-recumbent position. Fetal renal artery Doppler velocimetry was assessed by 3 investigators (HA, MOB, CSB) within 30 minutes before amniocentesis. When necessary, we used harmonic imaging to provide clear contrast between tissue structures. All measurements were performed during periods of fetal apnea and in the absence of movement.24 In the study group and at the time of renal artery blood flow assessment, 47 fetuses (84%, 47/56) were in the “spine up” position. In 3 fetuses the spinal position was intermediate. In 30 fetuses (54%) we evaluated the right fetal renal artery. The other 26 fetuses (46%) had Doppler evaluation of the left renal artery. The renal artery Doppler results were not used for clinical management.
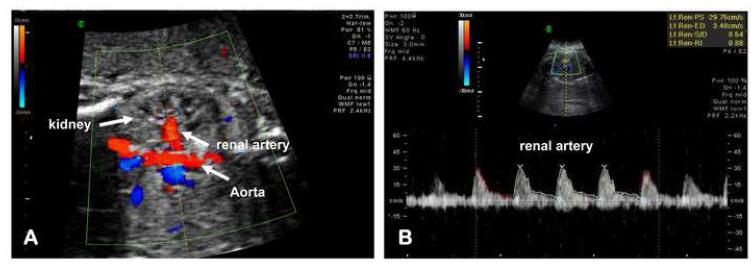
The technique for the fetal renal artery Doppler blood flow velocity waveform assessment was similar to the method previously published by Haugen et al. 25 Briefly, we first obtained a frontal plane image of the fetal abdomen to allow identification of the abdominal aorta and its bifurcation at the level of the fetal kidneys. Using color flow Doppler a straight segment of the renal artery was identified as it approached the kidney parenchyma from the descending aorta. Subsequently, we optimized the sector angle and depth of the penetration. The scanning plane was adjusted to obtain an insonation angle as closed to 0° as possible but always <30°. To preserve the end-diastolic component of the of the renal artery waveform we used appropriate low-filter (50-75Hz) settings. The Doppler gate (1.5 to 2.0 mm) was placed within the lumen of the renal artery away from the aorta and before any emergent branches (Figure 3A). Renal artery pulsatility index (PI), resistance index (RI), systolic/diastolic (S/D) ratio, and presence or absence of end-diastolic blood flow were assessed. Three consecutive waveforms from each fetus were traced and the average values used for the final analysis (Figure 3B). Immediately after Doppler evaluation, renal artery data were introduced in a computerized perinatal research data base by investigators (HA, MOB, ATD) without knowledge of the maternal or neonatal clinical outcome, presence or absence in the AF of proteomic biomarkers characteristic for inflammation or the results of the AF and cord blood IL-6 levels. The intra- and inter-observer coefficient of variations for the renal artery Dopplers were less than 10%. For each woman we performed fetal weight estimations (EFW) by combining biparietal diameter, head and abdominal circumference and femur length. 26 The AF volume was estimated using the AF index (AFI).27
Figure 3. Technique for evaluation and assessment of the fetal renal artery blood flow.
(A) A frontal plane image of the fetal abdomen to allow identification of the abdominal aorta and its bifurcation at the level of the fetal kidneys. Renal artery blood flow was sampled within the lumen of the renal artery away from the aorta and before any emergent branches. The velocity waveform was recorded at fast speed with a low pass filter. (B) Representative Doppler flow tracing of the renal artery in a patient with intra-amniotic inflammation.
Histological evaluation of the placenta for acute inflammation
Placental tissue from 49 study group patients was available for examination. Neutrophil infiltration of the chorionic plate and amnio-chorion membranes was used to establish presence or absence of histological chorioamnionitis, using well recognized histological stages and grading systems. 28,29 The pathologist who examined the placentas was unaware of the results of fetal renal artery blood flow measurements, proteomic results and AF or cord blood analytes.
Umbilical cord blood
Umbilical cord blood was obtained by aseptic puncture of the clamped umbilical vein at the time of delivery (n=39). Cord blood samples (umbilical artery and vein) were collected in pre-heparinized 1-cc. syringes, capped and transported to the laboratory. The acid-base status was determined within 10 min. of delivery with the ABL 800 FLEX blood gas analyzer (Radiometer Medical A/S, Denmark). Immediately following collection, the cord blood was centrifuged at 1000 × g for 15 minutes. Serum was aliquoted in sterile polypropylene tubes and stored at −80°C until IL-6 and EPO levels were examined.
Evaluation of cardiovascular and metabolic profiles of the neonates
Within the first 24 hours of life, neonatal cardiovascular and metabolic profiles were assessed through evaluation of the temperature, blood pressure, heart rate, serum blood urea nitrogen (BUN), creatinine and urinary output levels. The neonatal serum analyte data was evaluated in relationship to the volume of fluid administered to the newborn in the first 24 hours of life.
Immunoassays for IL-6 and erythropoietin (EPO)
ELISA for human IL-6 (Pierce-Endogen, Rockford, IL) and EPO (R&D Systems, Minneapois, MN) was performed in duplicate according to manufacturers’ instructions by investigators (IAB, ATD) unaware of the AF and umbilical cord blood sample origin. The minimal detectable concentration for IL-6 was 1-pg/mL and the inter- and intra-assay coefficients of variation <10%. The minimal detectable concentration for EPO was 0.6 mIU/mL. The inter- and intra-assay coefficients of variation were <10% for EPO. The results of the AF, cord blood IL-6 and cord blood EPO levels for several fetuses were previously used in studies aimed to explore the fetal adaptive response to intrauterine infection/inflammation.22,23,30
Statistical analysis
The Kolmogorov-Smirnov test was used for data normality testing. Comparisons between groups included Student t-tests, Mann Whitney tests and One-Way ANOVA, as appropriate. Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc., Chicago, IL) and MedCalc (Broekstraat, Belgium) statistical software. Proportions were compared with Chi square or Fisher’s exact test, as appropriate. Statistical analysis was completed following logarithmic transformation of the AF and umbilical cord blood IL-6 levels. Stepwise logistic regression was used for multivariable analysis using a P<0.05 for variable entry and a p≥0.1 for variable removal. A sample size calculation was performed to estimate the required number of participants. Based on available data, in order to detect a 5% difference in renal artery RI between fetuses delivered in the presence or absence of intra-amniotic inflammation, with 80% power and α = 0.05, it was anticipated that a minimum of 18 subjects would be required.31 A P value of <0.05 was used to indicate significance.
RESULTS
Out of the 56 women who presented with clinical signs or symptoms of preterm labor (study group), 19 (34%) had AF proteomic profiles characteristic of intra-amniotic inflammation. All 19 women had a clinically indicated preterm delivery (Figure 1). In 37 women (66%) AF infection/inflammation was excluded based on the amniocentesis results. Of these, 30 still delivered preterm. Only 5 women in the study group (14%) delivered fetuses at term. In the absence of intra-amniotic infection/inflammation 2 women were discharged and subsequently lost to follow-up. All 14 women in the control group delivered healthy fetuses at term.
Following birth, 48 preterm neonates were admitted to the Yale Newborn Special Care Unit (NBSCU). One neonate from the cohort of women diagnosed with intraamniotic infection/inflammation expired immediately after birth (GA: 23 1/7 weeks). Two premature neonates delivered by women with no intra-amniotic infection/inflammation expired following admission to NBSCU (GA: 22 1/7 and 23 5/7 weeks, respectively).
The clinical characteristics of the 70 women included in our analysis are presented in Table 1. Control women were of lower parity, more frequently Hispanic, had longer ultrasound-to-delivery intervals and larger neonates at birth. There was no difference in GA at enrollment between the study and control groups. We present the clinical characteristics of the 56 study group women in Table 2. Women with intraamniotic inflammation (MR score 3-4) were characterized by an earlier GA at both amniocentesis and delivery, a shorter amniocentesis-to-delivery interval, more frequent prenatal exposure to antibiotics, lower neonatal birthweights and lower Apgar scores at 1 minute.
Table 1.
Characteristics of the enrolled women (n = 70)
| Variable | Study Group n=56 |
Control Group n=14 |
P value |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years* | 27.5 [6.3] | 25.1 [6.4] | 0.204 |
| Parity † | 1 [0 – 1] | 2 [1 – 2] | 0.011 |
| Gravidity † | 2 [1 – 3] | 3 [1 – 4] | 0.428 |
| Race § Caucasian African-American Hispanic Other |
25 (45) 15 (27) 9 (16) 7 (12) |
2 (14) 1 (7) 8 (57) 3 (21) |
0.005 |
| Gestational age enrollment, weeks † | 28.1 [25.3 – 30.6] | 30.2 [28.0 – 31.5] | 0.094 |
| PPROM § | 27 (48) | 0 (0) | <0.001 |
| Outcome characteristics | |||
| Ultrasound-to-delivery interval, days † | 4 [1 – 23] | 62 [53 – 82] | <0.001 |
| Gestational age delivery, weeks † | 30.6 [27.4 – 33.3] | 39.4 [38.2 – 40.2] | <0.001 |
| Birthweight, grams † | 1,670 [973 – 2,133] | 3,245 [3,060 – 3,580] | <0.001 |
| Cesarean delivery § | 20 (36) | 4 (29) | 0.758 |
| Preterm delivery <34 weeks ‡ | 44 (79) | 0 (0) | <0.001 |
| Preterm delivery <37 weeks ‡ | 49 (88) | 0 (0) | <0.001 |
Data presented as mean and standard deviation [SD] and analyzed with Student t-test
Data presented as median [interquartile range] and analyzed with Mann-Whitney test
Data presented as n (%) and analyzed by Chi square test
Data presented as n (%) and analyzed by Fisher’s exact test
Table 2.
Demographic and clinical characteristics of the Study Group (n=56)
| Variable | INTRA-AMNIOTIC INFLAMMATION |
P value | |
|---|---|---|---|
| NO n=37 |
YES n=19 |
||
| Demographic characteristics | |||
| Age, years * | 27.9 [5.6] | 26.7 [7.6] | 0.522 |
| Parity † | 1 [0 – 1] | 1[0 – 1] | 0.933 |
| Gravidity † | 2 [1 – 3] | 2 [1 – 3] | 0.622 |
| Gestational age enrollment, weeks † | 30.3 [27.4 – 31.6] | 27.1 [24.7 – 28.9] | 0.014 |
| Ruptured membranes ‡ | 20 (54) | 7 (37) | 0.348 |
| History of preterm birth § | 10 (27) | 4 (21) | 0.751 |
| Steroid exposure during pregnancy ‡ | 30 (86) | 18 (95) | 0.243 |
| Prenatal antibiotic treatment ‡ | 25 (67) | 18 (95) | 0.041 |
| Outcome characteristics | |||
| Amniocentesis-to-delivery, days † | 4.9 [1.2 – 27.9] | 0.3 [0.2 –1.1] | < 0.001 |
| Gestational age delivery, weeks † | 32.3 [30.1 – 34.2] | 27.2 [25.3 – 29.3] | < 0.001 |
| Birthweight, grams † | 1,870 [1,450 – 2,270] | 955 [805 – 1,344] | < 0.001 |
| Cesarean delivery ‡ | 12 (32) | 8 (42) | 0.674 |
| Umbilical artery pH * | 7.30 [0.05] | 7.33 [0.08] | 0.407 |
| Umbilical artery base excess, mmols/L * | - 3.9 [3.4] | - 5.0 [3.9] | 0.526 |
| Apgar score at 1 minute † | 8 [7 – 9] | 7 [4 – 8] | 0.035 |
| Apgar score at 5 minutes † | 9 [8 – 9] | 8 [7 – 9] | 0.150 |
Data presented as mean and standard deviation [SD] and analyzed with Student t-test
Data presented as median [interquartile range] and analyzed with Mann-Whitney test
Data presented as n (%) and analyzed by Chi square test
Data presented as n (%) and analyzed by Fisher’s exact test
Intra-amniotic inflammation was defined based on the proteomic Mass Restricted (MR) score. An MR score 0 (zero) indicates “no” inflammation. MR score 3-4 indicates “severe” inflammation (see reference 21&22).
The results of the AF analysis and histological examination of the placenta are presented in Table 3. Women with intra-amniotic inflammation had lower glucose but higher LDH levels, higher WBC counts and a higher frequency of positive Gram stain and microbial culture results. Amniotic fluid IL-6 levels were significantly higher in women with MR score 3-4. The SELDI peak corresponding to β2-microglobulin was detected in all 56 samples of AF analyzed in this study. Histological examination of the placenta showed that women with MR score 3-4 more frequently had neutrophil infiltration of the chorionic plate, amnion, chorio-decidua and umbilical cord.
Table 3.
Amniotic fluid analysis (n=56) and histological examination of the placenta (n=49) for the Study Group.
| Variable | INTRA-AMNIOTIC INFLAMMATION |
P value | |
|---|---|---|---|
| NO n=37 |
YES n=19 |
||
| Amniotic fluid | |||
| Glucose, mg/dL † | 28 [23-40] | 5 [2-16] | <0.001 |
| LDH activity, U/L † | 159 [115-194] | 534 [434-768] | <0.001 |
| WBC count, cells/mm3 † | 4 [2-9] | 488 [110-1,670] | <0.001 |
| Positive Gram stain § | 0 (0) | 8 (42) | <0.001 |
| Positive cultures § | 0 (0) | 12 (63) | <0.001 |
| IL-6, ng/mL † | 0.5 [0.2-1.1] | 32.1 [11.2-57.8] | <0.001 |
| Placenta | n=30 | n=19 | |
| Chorioamnionitis, stage II-III ‡ | 8 (27) | 14 (74) | 0.003 |
| Amnionitis, grade 2-4 ‡ | 6 (20) | 11 (58) | 0.016 |
| Chorio-deciduitis grade 2-4 ‡ | 13 (43) | 16 (84) | 0.011 |
| Funistis, grade 1-4 ‡ | 9 (30) | 11 (58) | 0.034 |
Data presented as median [interquartile range] and analyzed with Mann-Whitney test
Data presented as n (%) and analyzed by Chi square test
Data presented as n (%) and analyzed by Fisher’s exact test
Intra-amniotic inflammation was defined based on the proteomic Mass Restricted (MR) score. An MR score 0 (zero) indicates “no” inflammation. MR score 3-4 indicates “severe” inflammation (see reference 21&22).
Abbreviations: LDH: lactate dehydrogenase activity; WBC: white blood cell.
Table 4 summarizes the ultrasonographic findings at the time of enrollment for all 70 fetuses included in our analysis. We found that fetuses delivered by mothers with intra-amniotic inflammation had significantly lower ultrasound estimated fetal weights in comparison to the fetuses delivered by mothers without intra-amniotic inflammation and controls. We further determined that there were no significant differences in the renal artery PI, RI or S/D ratio between groups. In multivariate analysis, these findings remained without significance after correction for GA and estimated fetal weight. Interestingly, we determined that more fetuses of mothers with symptoms of preterm labor without intra-amniotic inflammation had absent renal artery end-diastolic blood flow compared with fetuses of mothers with intra-amniotic inflammation and controls.
Table 4.
Ultrasound findings at enrolment (n=70)
| Variable | INTRA-AMNIOTIC INFLAMMATION |
Control Group n=14 |
P value |
|
|---|---|---|---|---|
| NO n=37 |
YES n=19 |
|||
| Ultrasonographic fetal characteristics | ||||
| Estimated fetal weight, grams ¶ | 1,505 [873 – 1,852] |
955 [756 – 1,077] |
1,608 [1,299 – 1,951] |
0.011 |
| Amniotic fluid index, mm ¶ | 112 [68-148] | 128 [77-156] | 147 [134-156] | 0.141 |
| Renal artery Doppler studies | ||||
| Pulsatility index ¶ | 2.2 [1.7-2.4] | 2.1 [1.7-2.5] | 2.1 [1.9-2.2] | 0.881 |
| Resistance index ¶ | 0.9 [0.8-0.9] | 0.9 [0.8-0.9] | 0.8 [0.8-0.9] | 0.093 |
| Systolic / Diastolic ratio ¶ | 8.2 [5.1-14.5] | 8.1 [5.9-13.2] | 6.6 [6.1-7.2] | 0.172 |
| Absent end-diastolic flow § | 13 (35) | 2 (11) | 0 (0) | 0.010 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA
Data presented as n (%) and analyzed by Chi square test
Intra-amniotic inflammation was defined based on the proteomic Mass Restricted (MR) score. An MR score 0 (zero) indicates “no” inflammation. MR score 3-4 indicates “severe” inflammation (see reference 21&22).
Fetuses delivered by mothers with MR scores 3-4 had higher umbilical cord IL-6 but not EPO levels compared with fetuses delivered by women without intra-amniotic inflammation (Table 5). There was a direct relationship between cord blood IL-6 levels and severity of histological chorioamnionitis independent of GA (regression: R=0.660, P<0.001; chorioamnionitis: P<0.001; GA at delivery: P=0.091).
Table 5.
Interleukin-6 and Erythropoietin Umbilical cord values for 39 neonates. Cardiovascular and metabolic profiles of the neonates admitted in the NBSCU in the first 24 hours of life (n=48).
| Variable | INTRA-AMNIOTIC INFLAMMATION |
P value | |
|---|---|---|---|
| NO | YES | ||
| Umbilical cord | n=20 | n=19 | |
| Interleukin-6, pg/mL ¶ | 5.8 [5.1-8.3] | 18.9 [7.6-69.2] | 0.003 |
| Erythropoietin, mIU/mL ¶ | 6.9 [5.1-16.8] | 8.6 [2.7-15.2] | 0.911 |
|
Neonatal metabolic and
cardiovascular status |
n=30 | n=18 | |
| Blood urea nitrogen, mg/dL ¶ | 14.0 [10.5-21.5] | 25.5 [21.5-39.0] | < 0.001 |
| Creatinine, mg/dL ¶ | 0.8 [0.8-0.9] | 0.8 [0.7-0.9] | 0.742 |
| Temperature, °C ¶ | 37.2 [36.9-37.4] | 36.9 [36.9-37.1] | 0.205 |
| Mean arterial blood pressure, mmHg ¶ | 35.0 [30.7-38.2] | 29.5 [25.0-32.0] | 0.002 |
| Heart rate, beats/minutes ¶ | 162 [150-170] | 170 [164-180] | 0.040 |
| Urinary output, cc/kg/hr ¶ | 1.9 [1.3-2.8] | 3.1 [1.1-4.1] | 0.322 |
| Intravenous fluids, cc/kg/day ¶ | 76.15 [68.1-85.9] | 74.8 [69.1-91.4] | 0.765 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA.
NBSCU= Newborn Special Care Unit. Intra-amniotic inflammation was defined based on the proteomic Mass Restricted (MR) score. An MR score 0 (zero) indicates “no” inflammation. MR score 3-4 indicates “severe” inflammation (see references 21 & 22).
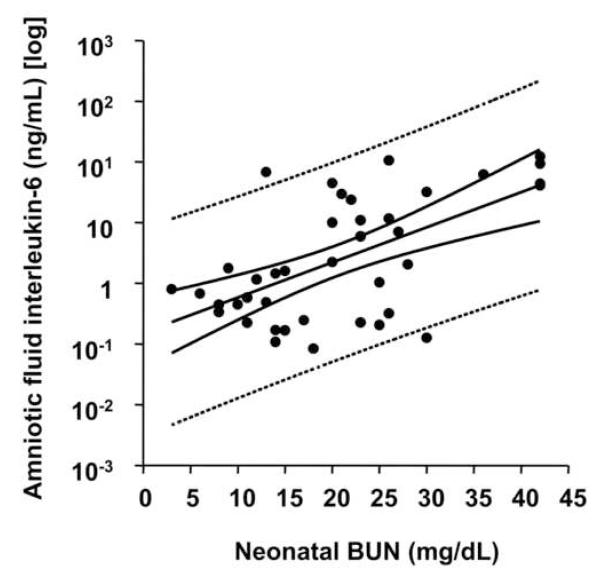
Of all neonates admitted to NBSCU, only one received inotropic support (study group – no inflammation). All neonates had negative blood culture results. We further determined that in the first day of life neonates delivered in the context of intra-amniotic inflammation had significantly higher serum BUN levels. Furthermore, cardiovascular assessment showed that neonates delivered by mothers with AF proteomic profiles characteristic for inflammation had lower mean arterial pressures and elevated heart rates. However, in multivariate analysis after correction for GA at delivery, only the differences in serum BUN maintained significance when correlated with the presence of intra-amniotic inflammation as determined by SELDI-TOF methodology (regression: R=0.706, P<0.001; MR score 3-4: P=0.010). Additionally, serum BUN significantly correlated with AF IL-6 levels (R=0.610, P<0.001) (Figure 4) independent of GA or amniocentesis-to-delivery interval.
Figure 4. Relationship of amniotic fluid interleukin-6 (IL-6) and neonatal blood urea nitrogen (BUN).
Distribution of amniotic fluid IL-6 levels in logarithmic format (y axis) versus the neonatal serum blood urea nitrogen (BUN) (x axis) in the neonates admitted to New Born Special Care Unit who had an evaluation of the serum blood urea nitrogen (BUN, n=40) in the first day of life. The regression line and 95% confidence and prediction intervals (dotted lines) are also shown.
There was no correlation between neonatal BUN and cord blood IL-6 levels (R=0.094, P=0.591). Although the cord blood IL-6 was significantly elevated in fetuses delivered by women with intra-amniotic inflammation, we found no relationship between the intensity of the fetal inflammatory response and renal artery Doppler indices (IL-6 vs. Doppler: PI: R=0.270, P=0.129; RI: R=0.214, P=0.190; S/D ratio: R=0.052; P=0.754). These relationships maintained after correction for GA and the ultrasound to delivery interval.
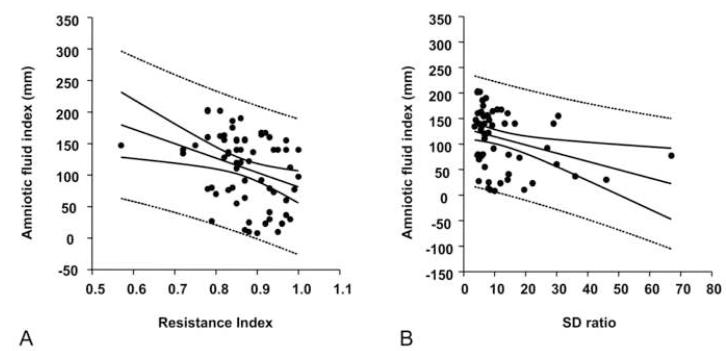
Fetal urine production is an important determinant of the AF volume. We evaluated the relationships between renal artery Doppler indices and AFI by performing a stepwise logistic regression analysis with RI, PI, S/D ratio, and absent end-diastolic flow as dependent variables, and AFI, cord blood IL-6 and EPO levels, status of the membranes, ultrasound evaluation-to-delivery interval and GA as independent variables. We found that both the RI (R= −0.325, P=0.008) (Figure 5A) and S/D ratio (R=−0.322, P=0.011) (Figure 5B) were inversely correlated with the AFI independent of GA and status of the membranes. The relationship between RI and AFI remained even when the analysis was restricted to the study group (R=−0.297, P=0.032).
Figure 5. Relationships of amniotic fluid amniotic fluid index (AFI) and renal artery Doppler indices in the Study group (n=56).
(A) Distribution of amniotic fluid index (y axis) versus the resistance index (RI) (x axis) (B) Distribution of amniotic fluid index (y axis) versus the systolic/diastolic (S/D) ratio (x axis). The regression line and 95% confidence and prediction intervals (dotted lines) are also shown.
DISCUSSION
We found that a systemic fetal inflammatory response, defined as an increased cord blood IL-6 level, is not associated with detectable changes of the renal artery blood flow impedance, in utero. Thus, our original hypothesis that the fetal renal artery impedance is significantly altered in the context of intra-amniotic infection/inflammation is rejected. Further, we show that the process of intra-amniotic inflammation is linked to elevated neonatal serum BUN levels in the first day of life. In previous studies we have shown that the maternal and fetal inflammatory response to a microbial attack was not binary (“disease present” or “disease absent”), but rather, a gradient progressing from the absence of, to “minimal”, and then “severe” disease. 7,22 However, the results of the current study suggest that the slope of maternal and fetal inflammation is not paralleled by a gradual deterioration of the neonate toward acute renal failure or hemodynamic instability. Lastly, spectral Doppler of the renal artery circulation confirms that the increased renal arterial impedance, as defined by an elevated arterial RI and S/D ratio is an important regulator of the AF volume independent of GA, cord blood IL-6 levels or status of the membranes.
The present research was motivated by the observation that in adults, the systemic inflammatory response is an entity complicated by multiple organ dysfunction, including that of the kidneys.32 Dysfunction of sensory neurons and excess inflammatory cytokines (IL-6, tumor necrosis factor-α, IL-1β, granulocyte colony-stimulating factor) released from activated neutrophils are central themes of the mechanism responsible for the clinical manifestations of septic shock syndrome.33,34 For a better understanding of the human adaptive response to inflammation, a number of investigators including us, have used animal models 35,36,37,38 Taken together, animal studies demonstrate that along with a complex set of changes, sepsis is associated with a decline in vascular tone, accompanied by marked regionalization of vital organ blood flow.35,39 For example, the kidney circulation follows a biphasic pattern: first an increase followed by a decrease in renal artery blood flow. Interestingly, even with an initial increase in the renal artery circulation, animals acquired oliguria and increased creatinine levels.35 However, knowledge of the fetal vascular adaptive response to intrauterine infection/inflammation proved to be difficult to extrapolate to humans.
Several investigators have looked into the mechanisms responsible for adaptation of the human fetus to inflammation. 4,5,8,10,22,40,41,42,43,44,45 The results of the ultrasound studies have been mixed. While some investigators demonstrated abnormal placental perfusion and fetal circulation in the context of histological chorioamnionitis and amniorrhexis, others could not confirm these results, underscoring the complexity of the fetal adaptive response to inflammation.42,45,46,47 The RI and S/D ratio of the fetal renal artery are considered the most reliable reflection of the renal vascular resistance.48 A previous study suggests that an increase in the impedance of the renal artery vessels is one of the most important regulators of fetal urine production and hence, amniotic fluid volume.49 Based on our results we propose that this relationship is preserved in pregnancies complicated by PTB, independent of GA, fetal or AF inflammation and status of the membranes.
Several women had evidence of histological chorioamnionitis in the absence of intra-amniotic inflammation. Previous work by our group and others demonstrated that placental histology by itself is a poor predictor in diagnosing intra-amniotic inflammation 13,50 with an accuracy of only 58%. Another possible explanation for the differential maternal and fetal inflammatory response may rest with our genetic makeup.4 Mothers and fetuses have a genetic predisposition toward either a hyper- or a hypo-reactive immune system. The implications of this dissimilarity make a strong case for the variant maternal, placental and fetal inflammatory response observed in this study.
The finding that the process of intra-amniotic inflammation is associated with an increased serum neonatal BUN level in the first day of life is novel. Our Doppler data, corroborated with the neonatal urinary output and serum creatinine results suggest that the elevated BUN levels in infants delivered by women with intra-amniotic infection/inflammation is not the consequence of renal hypoxia, but rather, the expression of an enhanced catabolic state triggered by inflammation.51 There are a number of markers that may have a role in predicting postnatal renal function, including β2-microglobulin and EPO.52,53 Recognition of the β2-microglobulin peak in all our AF samples and similar cord blood EPO levels at birth irrespective of inflammation argues in support of the above theory.
Hemodynamic disturbances among premature infants are well-recognized risk factors for lengthy critical care stay and poor short- and long-term neonatal outcome.12,54 Following correction for GA we showed that fetal inflammation was not associated with hemodynamic instability in our premature infants. This observation is in partial variance with the findings of Yanowitz et al. who reported that 2 to 4 hours after delivery, premature neonates delivered by mothers with clinical chorioamnionitis were hypotensive and had higher right ventricular output.12 Yet, no changes in the cerebral Doppler indices were identified. The dissimilarity between these two reports may originate in the study design. In our study, delivery of the fetus was indicated immediately once intra-amniotic infection/inflammation was detected. Because Yanowitz et al. evaluated neonates delivered by mothers who were managed expectantly their data may reflect a more advanced stage of fetal, and thus, neonatal inflammation. This explanation is supported by the observation that in Yanowitz’s study cord blood IL-6 levels were higher in comparison to our findings.
Compelling evidence suggests that intra-amniotic inflammation, histologic choriamnionitis and hemodynamic abnormalities are risk factors for perinatal injury that includes white matter disorders and development of periventricular leukomalacia. 55 Based on previous studies which conclude that oliguric acute renal failure occurs in approximately 26 % of the septic premature neonates, we cannot exclude the possibility that overwhelming fetal sepsis may be associated with abnormal renal artery blood flow and hence kidney hypoxia.56 Our results are consistent with the absence of hemodynamic disturbances in the context of a lower intensity of the fetal inflammatory response. This brings into discussion the issue of whether a reasonable approach is removal of the fetus from the septic environment, before the occurrence of irreversible fetal damage. Because the decision to actively intervene and deliver a fetus, subject to a hostile uterine process, should take into account the risks and benefits for the mother and her fetus, the value of this approach remains to be proven.
CONDENSATION.
Doppler flow velocimetry of the fetal renal artery shows that in pregnancies complicated by intra-amniotic inflammation the resistance in the renal vascular bed remains normal but impacts on urine output despite an increased fetal inflammatory state.
ACKNOWLEDGEMENTS
We are indebted to the nurses, fellows and residents at Yale New Haven Hospital, Department of Obstetrics, Gynecology and Reproductive Sciences and to all patients who participated in the study. CSB is supported by National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Grant RO3 HD 50249 and the Yale WRHR Career Development Center (K12 HD 1027766). This work was also supported from (NIH/NICHD) Grant RO1 HD 047321 (IAB). The funding source had no involvement in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
REFERENCES
- 1.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 3.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001;15:78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 4.Buhimschi CS, Rosenberg VA, Dulay AT, Thung S, Sfakianaki AK, Bahtiyar MO, Buhimschi IA. Multidimensional system biology. Genetic and proteomic biomarkers of adverse pregnancy outcome in preterm birth. Am J Perinatol. 2008;25:175–87. doi: 10.1055/s-2008-1061497. [DOI] [PubMed] [Google Scholar]
- 5.Buhimschi IA, Zambrano E, Pettker CM, Bahtiyar MO, Paidas M, Rosenberg VA, et al. Using proteomic analysis of the human amniotic fluid to identify histological chorioamnionitis. Obstet Gynecol. 2008;111:403–412. doi: 10.1097/AOG.0b013e31816102aa. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Fung W, Moore RM, Pandey V, Fox J, Stetzer B, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod. 2006;74:29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 7.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, Rosenberg VA, Thung SF, Zhao G, et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res. 2007;61:318–24. doi: 10.1203/01.pdr.0000252439.48564.37. [DOI] [PubMed] [Google Scholar]
- 8.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–8. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 10.Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–6. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lee SY, Ng DK, Fung GP, Chow CB, Shek CC, Tang PM, et al. Chorioamnionitis with or without funisitis increases the risk of hypotension in very low birthweight infants on the first postnatal day but not later. Arch Dis Child Fetal Neonatal Ed. 2006;91:F346–8. doi: 10.1136/adc.2005.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanowitz TD, Baker RW, Roberts JM, Brozanski BS. Low blood pressure among very-low-birth-weight infants with fetal vessel inflammation. J Perinatol. 2004;24:299–304. doi: 10.1038/sj.jp.7211091. [DOI] [PubMed] [Google Scholar]
- 13.Salafia CM, Ghidini A, Sherer DM, Pezzullo JC. Abnormalities of the fetal heart rate in preterm deliveries are associated with acute intra-amniotic infection. J Soc Gynecol Investig. 1998;5:188–91. doi: 10.1016/s1071-5576(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 14.Itoh K, Aihara H, Takada S, Nishino M, Lee Y, Negishi H, Itoh H. Clinicopathological differences between early-onset and late-onset sepsis and pneumonia in very low birth weight infants. Pediatr Pathol. 1990;10:757–68. doi: 10.3109/15513819009064710. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo G, Capponi A, Arduini D, Turri E, Romanini C. Uterine and fetal blood flows in pregnancies complicated by preterm labor. Gynecol Obstet Invest. 1996;42:163–6. doi: 10.1159/000291938. [DOI] [PubMed] [Google Scholar]
- 16.Brines M, Cerami A. Discovering erythropoietin’s extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246–50. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 17.Hauth JC, Gilstrap LC, 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol. 1985;66:59–62. [PubMed] [Google Scholar]
- 18.ACOG Committee on Practice Bulletins-Obstetrics ACOG Practice Bulletin No. 80: premature rupture of membranes. Clinical management guidelines for obstetriciangynecologists. Obstet Gynecol. 2007;109:1007–19. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RK, Clark P, Locksmith Gregory J, Duff P. Performance characteristics of putative tests for subclinical chorioamnionitis. Infect Dis Obstet Gynecol. 2001;9:209–214. doi: 10.1155/S1064744901000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol. 1996;175:1336–1341. doi: 10.1016/s0002-9378(96)70051-0. [DOI] [PubMed] [Google Scholar]
- 21.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–81. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 22.Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhimschi IA, Zhao G, Rosenberg VA, Abdel-Razeq S, Thung S, Buhimschi CS. Multidimensional proteomics analysis of amniotic fluid to provide insight into the mechanisms of idiopathic preterm birth. PLoS ONE. 2008;3:e2049. doi: 10.1371/journal.pone.0002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mari G, Abuhamad AZ, Uerpairojkit B, Martinez E, Copel JA. Blood flow velocity waveforms of the abdominal arteries in appropriate- and small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 1995;6:15–8. doi: 10.1046/j.1469-0705.1995.06010015.x. [DOI] [PubMed] [Google Scholar]
- 25.Haugen G, Godfrey K, Crozier S, Hanson M. Doppler blood flow velocity waveforms in the fetal renal arteries: variability at proximal and distal sites in the right and left arteries. Ultrasound Obstet Gynecol. 2004;23:590–3. doi: 10.1002/uog.976. [DOI] [PubMed] [Google Scholar]
- 26.Chien PF, Owen P, Khan KS. Validity of ultrasound estimation of fetal weight. Obstetrics & Gynecology. 2000;95:856–860. doi: 10.1016/s0029-7844(00)00828-0. [DOI] [PubMed] [Google Scholar]
- 27.Moore TR, Cayle JE. The amniotic fluid index in normal human pregnancy. Am J Obstet Gynecol. 1990;162:1168–73. doi: 10.1016/0002-9378(90)90009-v. [DOI] [PubMed] [Google Scholar]
- 28.Naeye RL. Disorder of the Placenta, Fetus and Neonate: Diagnosis and Clinical Significance. St. Louis; Mosby: 1992. Disorders of the placenta and decidua; pp. 118–247. [Google Scholar]
- 29.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 30.Buhimschi CS, Turan OM, Funai EF, Azpurua H, Bahtiyar MO, Turan S, et al. Fetal Adrenal Gland Volume and Cortisol/Dehydroepiandrosterone Sulfate Ratio in Inflammation-Associated Preterm Birth. Obstet Gynecol. 2008;111:715–722. doi: 10.1097/AOG.0b013e3181610294. [DOI] [PubMed] [Google Scholar]
- 31.Oz AU, Holub B, Mendilcioglu I, Mari G, Bahado-Singh RO. Renal artery Doppler investigation of the etiology of oligohydramnios in postterm pregnancy. Obstet Gynecol. 2002;100:715–8. doi: 10.1016/s0029-7844(02)02203-2. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler AP. Recent developments in the diagnosis and management of severe sepsis. Chest. 2007;132:1967–76. doi: 10.1378/chest.06-2535. [DOI] [PubMed] [Google Scholar]
- 33.Shimaoka M, Park EJ. Advances in understanding sepsis. Eur J Anaesthesiol Suppl. 2008;42:146–53. doi: 10.1017/S0265021507003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia Garcia A, Lopez Messa J, Aparicio Duque R. Peripheral polyneuropathy complicating conditions of sepsis and multi-organ failure. Electromyogr Clin Neurophysiol. 1991;31:181–6. [PubMed] [Google Scholar]
- 35.Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest. 2003;124:1053–9. doi: 10.1378/chest.124.3.1053. [DOI] [PubMed] [Google Scholar]
- 36.Conger JD, Falk SA, Guggenheim SJ. Glomerular dynamics and morphologic changes in the generalized Shwartzman reaction in postpartum rats. J Clin Invest. 1981;67:1334–46. doi: 10.1172/JCI110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravishankar V, Buhimschi CS, Booth CJ, Bhandari V, Norwitz E, Copel J, Buhimschi IA. Fetal nucleated red blood cells in a rat model of intrauterine growth restriction induced by hypoxia and nitric oxide synthase inhibition. Am J Obstet Gynecol. 2007;196:482.e1–8. doi: 10.1016/j.ajog.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188:203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- 39.Payen D. Septic shock and regional circulations. Rev Prat. 1993;43:26–30. [PubMed] [Google Scholar]
- 40.Dulay AT, Buhimschi IA, Zhao G, Luo G, Abdel-Razeq S, Cackovic M, et al. Nucleated red blood cells are a direct response to mediators of inflammation in newborns with early-onset neonatal sepsis. Am J Obstet Gynecol. 2008;198:426.e1–9. doi: 10.1016/j.ajog.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubiel M, Seremak-Mrozikiewicz A, Breborowicz GH, Drews K, Pietryga M, Gudmundsson S. Fetal and maternal Doppler velocimetry and cytokines in high-risk pregnancy. J Perinat Med. 2005;33:17–21. doi: 10.1515/JPM.2005.002. [DOI] [PubMed] [Google Scholar]
- 42.Turan OM, Turan S, Funai EF, Buhimschi IA, Copel JA, Buhimschi CS. Fetal adrenal gland volume: a novel method to identify women at risk for impending preterm birth. Obstet Gynecol. 2007;109:855–62. doi: 10.1097/01.AOG.0000258282.47919.41. [DOI] [PubMed] [Google Scholar]
- 43.Yücel N, Yücel O, Yekeler H. The relationship between umbilical artery Doppler findings, fetal biophysical score and placental inflammation in cases of premature rupture of membranes. Acta Obstet Gynecol Scand. 1997;76:532–5. doi: 10.3109/00016349709024578. [DOI] [PubMed] [Google Scholar]
- 44.Mari G, Abuhamad AZ, Uerpairojkit B, Martinez E, Copel JA. Blood flow velocity waveforms of the abdominal arteries in appropriate and small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 1995;6:15–8. doi: 10.1046/j.1469-0705.1995.06010015.x. [DOI] [PubMed] [Google Scholar]
- 45.Carroll SG, Papaioannou S, Nicolaides KH. Doppler studies of the placental and fetal circulation in pregnancies with preterm prelabor amniorrhexis. Ultrasound Obstet Gynecol. 1995;5:184–8. doi: 10.1046/j.1469-0705.1995.05030184.x. [DOI] [PubMed] [Google Scholar]
- 46.Santolaya J, Sampson M, Nobles G, Font G, Ramakrishnan V, Warsof SL. Doppler evaluation of the fetoplacental circulation in the latent phase of preterm premature rupture of membranes. J Ultrasound Med. 1991;10:327–30. doi: 10.7863/jum.1991.10.6.327. [DOI] [PubMed] [Google Scholar]
- 47.Leo MV, Skurnick JH, Ganesh VV, Adhate A, Apuzzio JJ. Clinical chorioamnionitis is not predicted by umbilical artery Doppler velocimetry in patients with premature rupture of membranes. Obstet Gynecol. 1992;79:916–8. [PubMed] [Google Scholar]
- 48.Andriani G, Persico A, Tursini S, Ballone E, Cirotti D, Lelli Chiesa P. The renal-resistive index from the last 3 months of pregnancy to 6 months old. BJU Int. 2001;87:562–4. doi: 10.1046/j.1464-410x.2001.00085.x. [DOI] [PubMed] [Google Scholar]
- 49.Oz AU, Holub B, Mendilcioglu I, Mari G, Bahado-Singh RO. Renal artery Doppler investigation of the etiology of oligohydramnios in postterm pregnancy. Obstet Gynecol. 2002;100:715–8. doi: 10.1016/s0029-7844(02)02203-2. [DOI] [PubMed] [Google Scholar]
- 50.Pettker CM, Magloire LK, Sfakianaki AK, Hamar B, Buhimschi IA, Buhimschi CS. The value of placental microbial evaluation in diagnosing intra-amniotic infection and inflammation. Obstet Gynecol. 2007;109:739–49. doi: 10.1097/01.AOG.0000255663.47512.23. [DOI] [PubMed] [Google Scholar]
- 51.Blantz RC. Pathophysiology of pre-renal azotemia. Kidney Int. 1998;53:512–23. doi: 10.1046/j.1523-1755.2003_t01-1-00784.x. [DOI] [PubMed] [Google Scholar]
- 52.Nicolini U, Spelzini F. Invasive assessment of fetal renal abnormalities: urinalysis, fetal blood sampling and biopsy. Prenat Diagn. 2001;21:964–9. doi: 10.1002/pd.212. [DOI] [PubMed] [Google Scholar]
- 53.Mitra A, Bansal S, Wang W, Falk S, Zolty E, Schrier RW. Erythropoietin ameliorates renal dysfunction during endotoxaemia. Nephrol Dial Transplant. 2007;22:2349–53. doi: 10.1093/ndt/gfm216. [DOI] [PubMed] [Google Scholar]
- 54.Bassan H, Feldman HA, Limperopoulos C, Benson CB, Ringer SA, Veracruz E, et al. Periventricular hemorrhagic infarction: risk factors and neonatal outcome. Pediatr Neurol. 2006;35:85–92. doi: 10.1016/j.pediatrneurol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Mittendorf R, Montag AG, MacMillan W, Janeczek S, Pryde PG, Besinger RE, Gianopoulos JG, Roizen N. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol. 2003;188:1438–4. doi: 10.1067/mob.2003.380. [DOI] [PubMed] [Google Scholar]
- 56.Mathur NB, Agarwal HS, Maria A. Acute renal failure in neonatal sepsis. Indian J Pediatr. 2006;73:499–502. doi: 10.1007/BF02759894. [DOI] [PubMed] [Google Scholar]