Abstract
Sleep helps emotional memories consolidate and may promote generalization of fear extinction memory. We examined whether extinction learning and memory might differ in the morning and evening due, potentially, to circadian and/or sleep-homeostatic factors. Healthy men (N=109) in 6 groups completed a 2-session protocol. In Session 1, fear conditioning was followed by extinction learning. Partial reinforcement with mild electric shock produced conditioned skin conductance responses (SCR) to 2 differently colored lamps (CS+), but not a third color (CS−), within the computer image of a room (conditioning context). One CS+ (CS+E) but not the other (CS+U) was immediately extinguished by un-reinforced presentations in a different room (extinction context). Delay durations of 3 hr (within AM or PM), 12 hr (morning-to-evening or evening-to-morning) or 24 hr (morning-to-morning or evening-to-evening) followed. In Session 2, extinction recall and contextual fear renewal were tested. We observed no significant effects of the delay interval on extinction memory but did observe an effect of time-of-day. Fear extinction was significantly better if learned in the morning (p=.002). Collapsing across CS+ type, there was smaller morning differential SCR at both extinction recall (p=.003) and fear renewal (p=.005). Morning extinction recall showed better generalization from the CS+E to CS+U with the response to the CS+U significantly larger than to the CS+E only in the evening (p=.028). Thus, extinction is learned faster and its memory is better generalized in the morning. Cortisol and testosterone showed the expected greater salivary levels in the morning when higher testosterone/cortisol ratio also predicting better extinction learning. Circadian factors may promote morning extinction. Alternatively, evening homeostatic sleep pressure may impede extinction and favor recall of conditioned fear.
Keywords: Sleep, fear conditioning, extinction, circadian rhythm, sleep homeostasis, cortisol, testosterone
Introduction
Current behavioral therapies for anxiety disorders employ controlled exposure to the feared object or situation, an experience leading to the formation of a therapeutic “extinction memory” that something that once signaling danger no longer does so (Craske et al., 2008; McNally, 2007). Of interest to clinicians treating anxiety disorders are ways to strengthen this therapeutic learning and prevent preexisting fear memories from provoking relapse (Craske et al., 2008). One potential moderator that has not been fully examined is whether the time-of-day during which a therapy session is delivered might influence treatment outcome. This is important because extinction is an emotional memory that might be augmented by sleep and/or hormones that exhibit circadian fluctuations (Diekelmann & Born, 2010; Walker & Stickgold, 2006).
Using a validated fear conditioning and extinction paradigm (Milad et al., 2007), we have previously reported that overnight sleep, compared to over-day waking, promotes generalization of extinction learning (Pace-Schott et al., 2009). This finding could be explained by a time-of-day effect, whereby extinction generalization is better expressed in the morning. Animal experiments demonstrate that the magnitude of both fear conditioning and extinction can show circadian rhythmicity (Chaudhury & Colwell, 2002). Nonetheless, an experimental model of exposure therapy that controlled for time-of-day effects showed sleep-dependent enhancement of extinction memory and generalization (Pace-Schott et al., 2012a). Moreover, inter-session habituation, a non-associative process related to extinction memory, was augmented by a nap versus an equal duration of waking at the same time-of-day (Pace-Schott et al., 2011).
Because the aforementioned studies from our group have used very different procedures, the current study sought to determine whether time-of-day and/or duration of delay between extinction learning and recall would influence conditioned fear responses using the original paradigm (Milad et al., 2007; Pace-Schott et al., 2009). All participants underwent differential fear conditioning and extinction training either in the morning or evening. Subsequently, participants in both groups underwent extinction recall and renewal tests 3, 12, or 24 hr after extinction learning.
Salivary levels of cortisol and testosterone were obtained when fear conditioning and extinction were encoded and recalled. This was done because cortisol (Kalsbeek et al., 2012; Pannain & Van Cauter, 2007) and testosterone (Diver et al., 2003; Leymarie et al., 1974; Piro et al., 1973) both show a morning acrophase and evening nadir, and both modulate fear responses and emotional memory (Ackermann et al., 2012; de Quervain et al., 2009; Montoya et al., 2012). For example, high levels of glucocorticoids (cortisol in humans) enhance acquisition and consolidation of emotionally salient memories (Bentz, Michael, de Quervain, & Wilhelm, 2010; Blundell, Blaiss, Lagace, Eisch, & Powell, 2011; de Quervain, Aerni, Schelling, & Roozendaal, 2009; de Quervain et al., 2011; Soravia et al., 2006) but block their retrieval (de Quervain, 2008; de Quervain et al., 2009). Similarly, exogenous testosterone can decrease fear (Hermans et al., 2007; van Honk et al., 2005) and modulate social emotions (Bos et al., 2012; van Honk et al., 1999).
Participants were restricted to males for 4 reasons. First, human extinction memory varies with levels of estradiol and phase of the menstrual cycle in females (Milad et al., 2006; Milad et al., 2010; Zeidan et al., 2011). Second, unlike estrogen, testosterone shows a clear circadian rhythm in males and hence might help explain time of day differences. Third, testosterone can be rapidly converted to estrogen in the brain (Cornil & Charlier, 2010) and endogenous estrogen has been shown to enhance extinction recall (Milad, et al., 2009; Milad et al., 2010). And fourth, human sex differences have been reported for the effects of endogenous and exogenous cortisol on the acquisition of conditioned fear (Bentz et al., 2013; Jackson et al., 2006; Zorawski, et al., 2005). Given such complex interactions, study of the simpler case in males was chosen as a first step.
Based upon preliminary findings (Pace-Schott et al., 2012), we predicted that extinction learning and recall would both be better in the morning than in the evening. We also hypothesized that acquisition of extinction would vary positively with endogenous levels of cortisol and/or testosterone.
Methods
Participants
Healthy young adult males (n=109) aged 18–27 yrs (mean 20.8, SD 2.6) were enrolled. Detailed inclusion and exclusion criteria are provided in Supplementary Methods. This study was conducted in accordance with the principles of Declaration of Helsinki, procedures were approved by the University of Massachusetts, Amherst and the Partners Healthcare Institutional Review Boards and all participants provided written informed consent.
Pre-study week
During the week prior to the experiment, 97 participants were instructed to keep a regular sleep schedule consisting of a minimum of 7 hours in bed each night, bedtime no later than 2:00 AM and no daytime napping. Compliance with these instructions was monitored with the Evening-Morning Sleep Questionnaire (EMSQ) diary and actigraphy (see Supplementary Methods). Participants were also asked to abstain from alcohol, recreational drugs and, on study days, caffeine. The 12 remaining participants were only required to follow study restrictions on the 2 study days plus the prior day and night due to differing compensation (see Supplementary Methods). All participants completed the Epworth Sleepiness Scale (ESS, Johns, 1991), the Pittsburgh Sleep Quality Index (PSQI, Buysse et al., 1989) and the Morningness-Eveningness Questionnaire (MEQ, Horne & Ostberg, 1976). During one day of the pre-study week, participants provided 6 saliva samples at specified times for hormone assays.
Experimental schedule
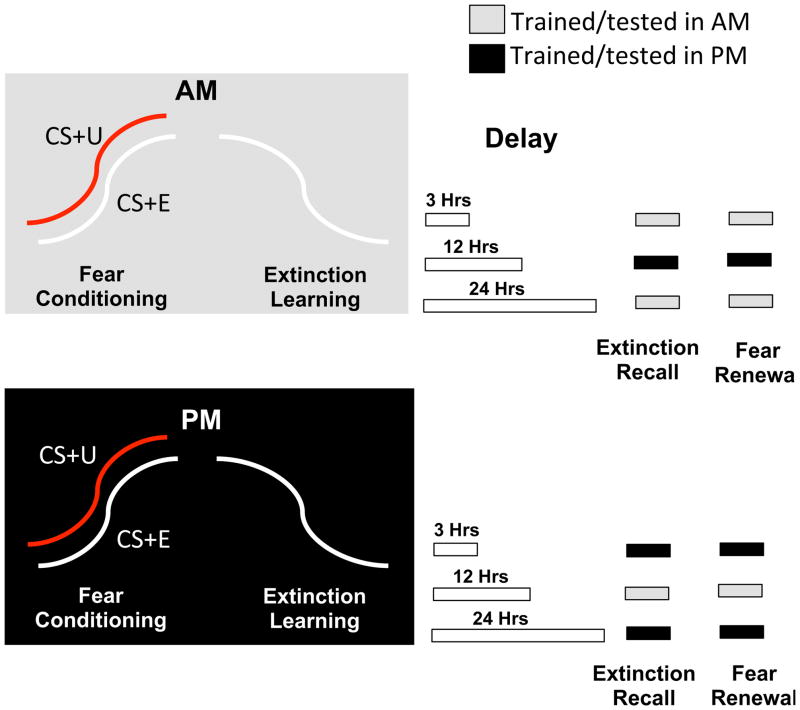
The protocol included five experimental phases that took place over two Sessions (Sessions 1 and 2) with Habituation, Fear Conditioning, Extinction Learning occurring in Session 1 and Extinction Recall and Fear Renewal in Session 2. There were two groups: a morning and an evening group. The morning group underwent Habituation, Fear Conditioning, and Extinction Learning between 7 and 10 AM. Subsequently, Extinction Recall and Fear Renewal were tested at either 3 (n=17), 12 (n=17), or 24 hours (n=18) following Extinction Learning. For the evening group, Habituation, Fear Conditioning and Extinction Learning occurred between 7 and 10 PM. Subsequently, Extinction Recall and Fear Renewal tests again took place 3 (n=19), 12 (n=19), or 24 hours (n=19) after Extinction Learning (Figure 1).
Figure 1.
The experimental protocol consisted of 6 groups 3 of which received Fear Conditioning and Extinction Learning in the evening (black) and 3 in the morning (grey). During Fear Conditioning, conditioned fear responses were established to 2 different colors (CS+) by a mild electric shock. Immediately afterward, during Extinction Learning, conditioned responses to one CS+ (CS+E) but not the other (CS+U) were extinguished. Within the 3 groups trained in the morning and the 3 trained in the evening, one in each then underwent a 3, 12 or 24-hr delay during which they retained fear and extinction memories. After this delay, they were then tested for Extinction Recall and, immediately afterward, for contextual Fear Renewal. The 3 and 24-hr delays following morning and evening training allowed both training and testing to occur at the same approximate time of day (“time-congruent” participant grouping) and these are indicated in the figure by bars of the same shading as their training session placed above the phases of Session 2.
Stimuli and experimental protocol
Stimuli were identical to those described previously (Linnman et al., 2011; Milad et al., 2009; Milad et al., 2007a; Milad et al., 2007b; Pace-Schott et al., 2009; Zeidan et al., 2011). The unconditioned stimulus (US) was a mild electric shock, with a level selected by the subject to be “highly annoying but not painful” (Orr et al., 2000). Conditioned stimuli (CS) were digital photographs of 3 differently colored lamps (blue, red or yellow) displayed on a computer screen within the image of two different rooms, one of which served as the “conditioning context” and the other as the “extinction context”. During Habituation, participants viewed each possible combination of the three CS colors and two contexts. During Fear Conditioning, 2 of the 3 colors (CS+) were followed by the US after 5 of 8 presentations of each, with 16 unreinforced presentations of the third color (CS−) pseudorandomly interspersed. During Extinction Learning, one CS+ color (CS+E) appeared 16 times in the extinction context unaccompanied by the US along with 16 interspersed CS−. The other CS+ color was not presented and hence remained unextinguished (CS+U). During Extinction Recall, the 8 CS+E and 8 CS+U were presented in the extinction context without any US presentations and with 16 interspersed CS−. During Fear Renewal, the 8 CS+E and 8 CS+U were similarly presented but in the conditioning context. (See Supplementary Methods for details.) Thus, participants saw the conditioning context during Habituation, Conditioning and Fear Renewal phases whereas they saw the extinction context during the Habituation, Extinction Learning and Extinction Recall phases. The Stanford Sleepiness Scale (SSS, Hoddes et al., 1973) was administered at the beginning and end of each session.
Skin conductance response (SCR)
Skin conductance level was recorded using the MP150 system (BIOPAC Systems, Inc., Goleta, CA). A skin conductance response (SCR) was calculated for each trial as the mean skin conductance level in microSiemens (μS) during the last 2 sec of context presentation, which preceded onset of the CS, subtracted from the maximum skin conductance level during the 6 sec of CS presentation. SCRs were square-root transformed; if the untransformed SCR was negative, the negative sign was retained after calculating the square root of the SCR’s absolute value (Orr et al., 2000). The outcome variable was differential SCR calculated by subtracting the SCR to the CS− from the SCR to its sequentially corresponding CS+ trial. For example, the first CS− to appear in a particular phase is subtracted from the first CS+ to appear, the second CS− is subtracted from the second CS+ and so on.
Retrospective US expectancy ratings
Retrospective ratings of shock expectancy were obtained after Fear Renewal. Following (Vervliet et al., 2005), participants retrospectively rated their expectancy of being shocked by each CS color at its first 2 and last 2 presentations during each experimental phase (except Habituation) using 24 separate 100-mm visual analog scales (VAS). The two poles of the VAS were “Didn’t expect a shock at all” and “Definitely expected to be shocked” and the raw dependent measure was distance in mm from the former. The outcome variable was a differential shock expectancy score calculated by subtracting, from each CS+ expectancy, the expectancy for the CS− at the same point in time.
Salivary cortisol and testosterone
Saliva samples were obtained immediately before and after each of the 2 sessions and analyzed for cortisol (all samples) and testosterone (first sample per session). Six additional samples were obtained for cortisol analysis on one pre-study day (see Supplementary Methods) to compute an area-under-curve (AUC) baseline (Pruessner et al., 2003) by which raw cortisol values were divided for individual normalization. Unbound cortisol and testosterone levels were assayed by Salimetrics, LLC (State College, PA) using enzyme immunoassay. Due to their known circadian rhythm, relationships between hormone levels and differential SCR were analyzed separately within morning and evening samples.
Statistical Analyses
Differential SCR was compared between ”Time-of-Day” (morning and evening) and “Delay” (3, 12 and 24 hr), both of which were between-subjects factors, using two separate participant “groupings”. The first such grouping was termed “all participant” (N=109) and was used to analyze the Time-of-Day factor in Session 1 (Fear Conditioning and Extinction Learning) which took place in either the morning (n=52) or evening (n=57). The all-participant grouping was also used to analyze the Delay factor. Collapsing across Time-of-Day, there were three levels of Delay: 3 hr (n=36), 12 hr (n=36) and 24 hr (n=37). The second grouping, termed “time-congruent” participants, avoided the potentially confounding effect of opposing times of day at Sessions 1 and 2 that occurred in the 12-hr delay participants. Time-congruent participants (n=73) consisted of those for whom both sessions took place at the same approximate time of day (i.e., 3 and 24-hr delays). Analyses of Time-of-Day effects at Extinction Recall and Fear Renewal (Session 2) were carried out using these time-congruent participants. However, in order to compare the effects of all 3 durations of Delay on extinction recall, the preceding analysis, described above, included all 3 delay groups.
Within each experimental phase (except Habituation), mixed analyses of variance (ANOVA) were performed, each of which contained both between-subject factors: Time-of-Day (at the respective phase analyzed) and Delay. Within-subject variables were “Trial” (trials 1–8 of each CS+) that was nested within “CS+Type” (CS+E or CS+U) for analyses of Extinction Recall and Fear Renewal. At Session 1, “Delay” represented groups destined to have a 3, 12 or 24-hr delay before Session 2. When one of the self-report measures (e.g., ESS, PSQI) significantly differed (un-paired t-test) between individuals tested in the morning and evening in the all-participant or time-congruent participant grouping, it was added as a covariate to the ANOVA analyzing differential SCR for that grouping. Significance was set at p<.05, the Greenhouse-Geisser correction was applied to all within-subject main effects and their interactions, and Bonferroni-Dunn tests were used for post-hoc comparisons of self-report measures. Additional analyses are described in Supplementary Methods.
Results
Self-report measures
Participants who had Session 1 (Fear Conditioning and Extinction Learning) in the morning versus the evening did not differ in age, ESS, MEQ, weekly alcohol, or EMSQ total sleep opportunity 2 nights before Session 1 (Supplementary Table 1). The most prominent Time-of-Day difference for self-report measures was a shorter total sleep opportunity before morning Session 1 (all participant grouping, p<.0001) and before morning Session 2 in the 24-hr delay group--the only time-congruent participants who slept between sessions (p=.003). These Time-of-Day differences in total sleep opportunity were likely to have occurred because individuals unaccustomed to rising early had allowed themselves less sleep opportunity before their morning sessions.
Psychophysiological Measures
Effects of Delay
Fear Conditioning and Extinction Learning
We did not observe any significant differences during the Fear Conditioning and Extinction Learning phases between groups of participants destined to undergo the different testing delays. This was the case in both the all-participant and time-congruent participant groupings.
Extinction Recall and Fear Renewal
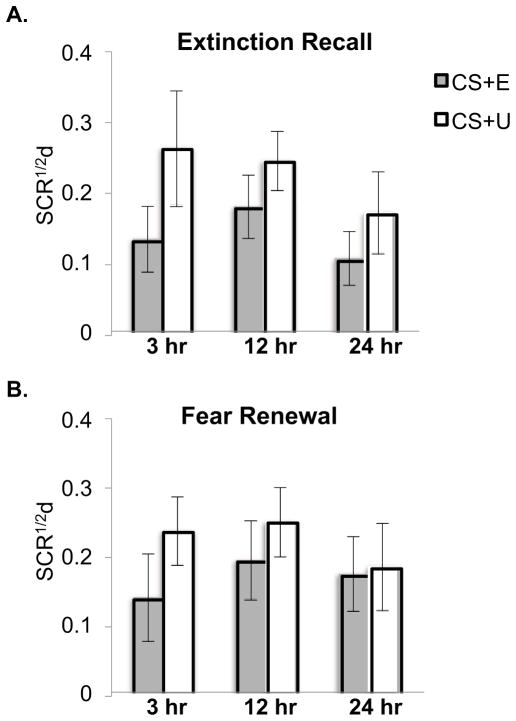
At Extinction Recall and Fear Renewal phases, there were no main effects of Delay among all-participant (Figures 2A and B) or time-congruent participant groupings. Therefore, there was no evidence for any effect of delay duration on the magnitude of extinction recall or fear renewal across periods of 3, 12 and 24 hr.
Figure 2.
The duration of the delay following Fear Conditioning and Extinction Learning had no significant effect on the magnitude of subsequent Extinction Recall or Fear Renewal. A. Mean differential skin conductance response across 8 trials of Extinction Recall following each delay. B. Mean differential skin conductance response across 8 trials of Fear Renewal following each delay. CS+E: conditioned response extinguished at Extinction Learning, CS+U: conditioned response not extinguished, SCR1/2d: differential SCR computed using square-root transformed SCRs (= SCR to CS+ minus SCR to temporally closest CS−). Error bars depict standard error of the mean.
Effects of Time-of-Day
Fear Conditioning
There were no main effects of Time-of-Day on differential SCR during the Fear Conditioning phase among all participants [F(1,103)=.05, p=.83] or only those destined to undergo the 2 time-congruent delays [F(1,69)=1.93, p=.17]. Similarly, there were no Time-of-Day × Trial interactions in either grouping of participants (p=.13 and .41 respectively). As is normally seen in human differential fear conditioning, the response to the CS+ diminished across trials appearing as a main effect of Trial among all participants [F(6,618)=15.66, p< .0001] and time-congruent participants [F(6,414)=10.45, p< .0001]. Differential responding to the CS+ and CS− during Fear Conditioning was clearly evident when the mean SCR of the 2 reinforced CS+ was compared with the mean SCR of their 2 sequentially corresponding CS−. There was both a main effect of CS Type (i.e., CS+ vs. CS−) [F(1,103)=208.22, p<.0001] and a CS Type × Trial interaction [F(6,618)=3.28, p<.006].
Extinction Learning
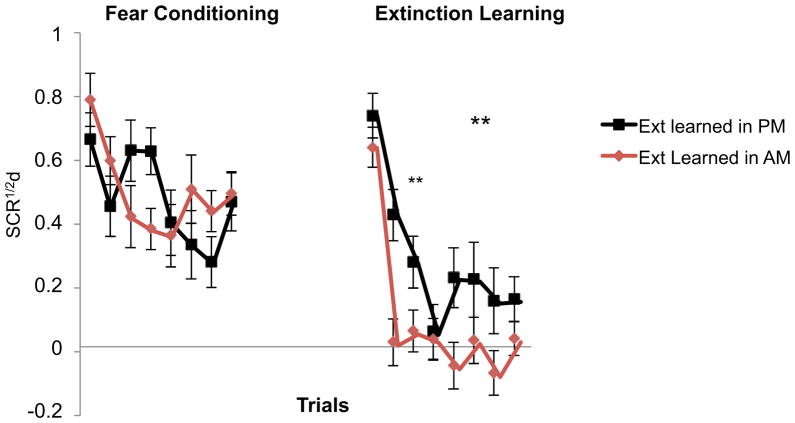
During Extinction Learning, there was a significant main effect of Time-of-Day, showing that the differential SCRs to the CS+E were larger in the evening than the morning. However, post-hoc analysis revealed that this difference was mainly driven by the initial pair of Extinction Learning trials raising the possibility that differing degrees of habituation during Fear Conditioning rather than differences in Extinction Learning per se produced this main effect (see Supplementary Results and Figure S1).
Therefore, to further examine Time-of-Day effects during Extinction Learning itself, a median split of all participants was performed on the initial pair of Extinction-Learning trials in order to isolate, in the upper half of this split, only those individuals who began Extinction Learning with large differential-SCR responses to the CS+E. The upper half of this split (N=54) contained 33 participants who underwent Extinction Learning in the evening and 21 in the morning. In this subgroup, Fear Conditioning continued not to differ by Time-of-Day [F(1,49)=.02, p=.89] (Figure 3A, left panel). Additionally, the initial pair of Extinction Learning trials no longer differed by Time-of-Day [F(1,52)=.84, p=.36). However, with subsequent pairs of Extinction Learning trials, differential SCRs were significantly smaller in the morning [F(1,48)=10.69, p=.002] (Figure 3, right panel), indicating that extinction learning was enhanced in the morning compared to the evening. Although there was no Time-of-Day × Trial interaction across all 8 pairs of extinction learning trials [F(7,364)=.86, p=.52], the Time-of-Day × Trial interaction became significant when the first 2 pairs of trials were analyzed alone [F(1,52)=5.02, p=.029], confirming more rapid initial extinction learning in the morning. The main effect of Trial remained significant [F(7,336)=11.17, p=.0001].
Figure 3.
Fear conditioning did not differ between morning and evening whereas extinction was significantly better learned in the morning. The starting point of Extinction Learning was equated by examining the upper half of a median split based upon the mean differential SCR of first two trials of Extinction Learning (N=33 evening, 21 morning). Each data point represents the mean differential SCR to two successive CS+. During Fear Conditioning, these were trial-by-trial averages of the 2 different CS+ (i.e., mean of first to-be CS+E and first to-be CS+U, mean of second to-be CS+E and second to-be CS+U, etc.). During Extinction Learning, data points are means for successive pairs of CS+E. Significance indicated for the Time-of-Day main effect (large asterisks) and trial-by-trial (small asterisks). SCR1/2d: differential SCR, ** p < .01. Error bars depict standard error of the mean.
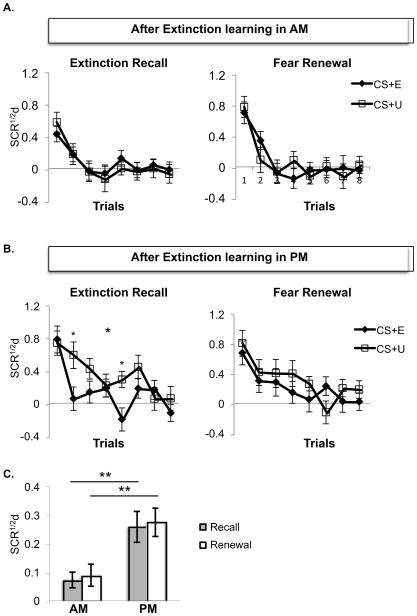
Extinction Recall
In the time-congruent subjects, the morning group exhibited significantly smaller differential SCRs to the CS+ relative to the evening group (main effect for Time-of-Day [F(1,69)=9.65, p=.003], Figure 4C) as well as a significant Time-of-Day × CS+Type interaction [F(1,69)=4.36, p=.041]. This interaction reflected differential SCRs to the CS+E and CS+U that did not differ in the morning [F(1,36)=.13, p=.73] (Figure 4A, left panel), but did so in the evening [F(1,36)=5.21, p=.028], and significantly so for trials 2 (p=.021) and 5 (p=.002) (Figure 4B, left panel). This interaction also reflected the fact that the Time-of-Day main effect was significant for the CS+U [F(1,69)=9.61, p=.003], but not the CS+E [F(1,69)=1.28, p=.26]. Time-of-Day did not interact with Trial (p=.15) or show any higher-order interactions. There was a significant main effect of Trial [F(7,483)=13.65, p=.0001].
Figure 4.
Extinction Recall in the morning showed generalization of extinction memory whereas recall in the evening preserved the differentiation of the extinguished and unextinguished CS+ and favored expression of a greater overall degree of conditioned fear. A. Differential SCR to the CS+E and CS+U at Extinction Recall and Fear Renewal in the morning. B. Differential SCR to the CS+E and CS+U at Extinction Recall and Fear Renewal in the evening. Significance indicated for the CS+E vs. CS+U main effect (large asterisk) and trial-by-trial (small asterisk). C. Comparison of differential SCR in the morning and evening for the average of all 8 trials during Extinction Recall and during Fear Renewal phases collapsing across CS+E and CS+U. SCR1/2d: differential SCR, * p < .05, ** p < .01. Error bars depict standard error of the mean.
Fear Renewal
At Fear Renewal, differential SCR to the CS+ continued to be significantly smaller in the morning group relative to the evening group (main effect of Time-of-Day [F(1,69)=8.49, p=.005], Figure 4C). The Time-of-Day × CS+Type interaction was no longer significant p=.31 (Figure 4A and 4B, right panels). Time-of-Day did not interact with Trial (p=.89) or show any higher-order interactions. There was a significant main effect of Trial [F(7,483)=14.48, p=.0001].
In summary, extinction was better learned and extinction recall was better generalized in the morning. At Extinction Recall, the latter effect was driven by a lowering of CS+U responses to levels shown to the CS+E. Responding to the CS+U remained lower in the morning during Fear Renewal following a change of context. For comparisons of individual groups (e.g., morning vs. evening within the 3-hr delay subjects), see Supplemental Results. Co-varying self-report measures that significantly differed by Time-of-Day (Supplementary Table 1) did not change findings of superior extinction learning and recall generalization in the morning (see Supplementary Results). Specifically, co-varying total sleep opportunity prior to the two sessions did not alter results nor did co-varying PSQI, SSS, and estimated daily caffeine (see Supplementary Results).
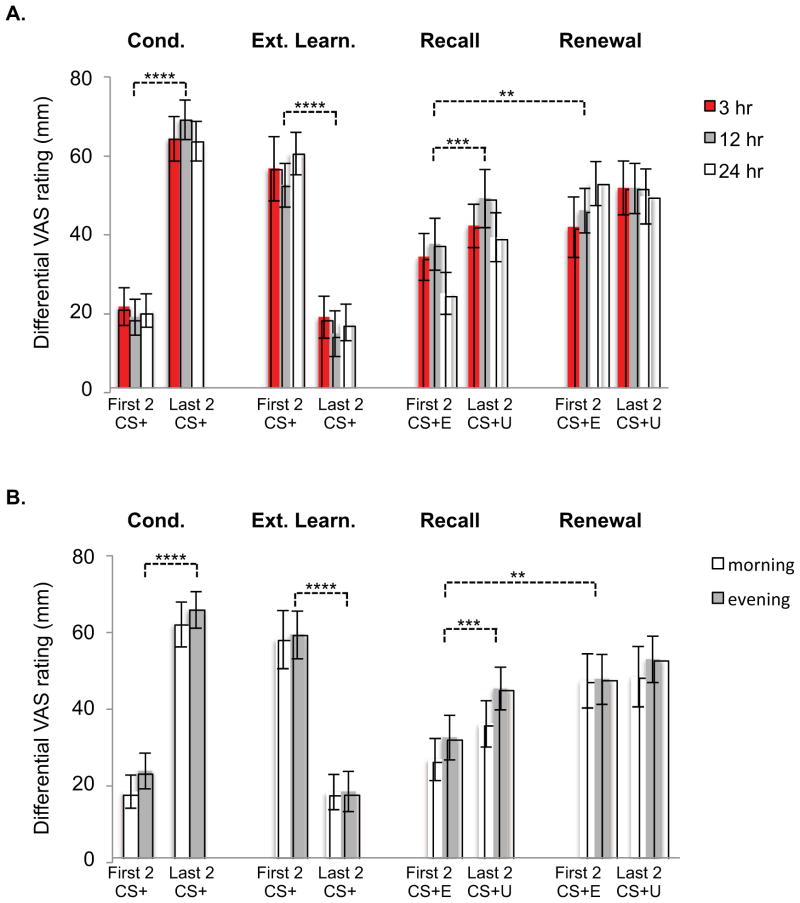
Retrospective shock expectancy
Participants’ differential shock expectancy reflected acquisition of differential conditioning, extinction learning, differentiation of the extinguished and unextinguished CS+ at Extinction Recall and renewal of fear to the CS+E at Fear Renewal (Figure 5). Nonetheless, there were no significant main effects of Delay (Figure 5A) or Time-of-Day (Figure 5B), nor did these factors interact with the four aforementioned learning and memory processes. Notably, among all participants, a significant positive correlation was found between shock expectancy for the first 2 CS+E at Extinction Recall and the mean differential SCR for the first 2 (R=.29, p=.002) and the first 4 (R=.26, p=.006) trials of Extinction Recall. Therefore retrospective self-report varied in parallel with psychophysiological indices of extinction memory.
Figure 5.
Retrospective differential shock expectancy ratings obtained from participants at the end of their second session. Only major learning and memory processes are illustrated. Learning processes include acquisition of differential fear conditioning (comparison of ratings for first and last 2 presentations at Fear Conditioning) and extinction learning (comparison of ratings for first and last 2 presentations of CS+E during Extinction Learning). Memory processes include differentiation of the first 2 presentations of the CS+E in comparison to the CS+U during Extinction Recall and greater shock expectancy for the first 2 presentations of the CS+E at Fear Renewal in comparison to their initial presentations at Extinction Recall (contextual fear renewal). A. Entire sample divided by Delay Duration for which there were no main effects or interactions with processes depicted. B. Time-congruent subsample subjects divided by Time-of-Day for which there were no main effects or interactions with processes depicted. Differential VAS rating is equal to retrospective shock expectancy for the 2 CS+ minus that for the 2 CS− from the same point in time. ** p < .01, *** p < .001, **** p <.0001. Error bars depict standard error of the mean.
Salivary hormone correlates of differential SCR
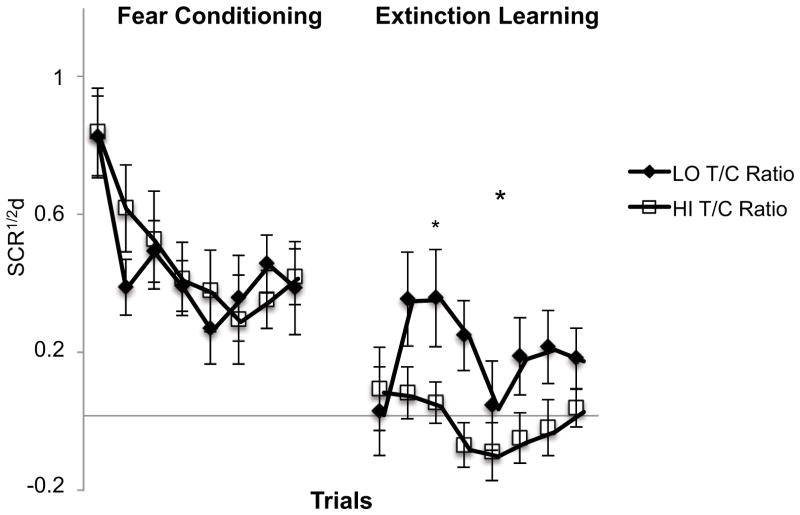
As expected, salivary cortisol and testosterone levels were significantly higher in the morning vs. evening prior to both sessions (Table 1). However, there were no significant correlations between cortisol or testosterone levels and differential SCR at any experimental phase measured within the morning or the evening sessions. We then conducted an analysis to test the ratio of testosterone to cortisol (T/C ratio) based upon literature showing that the endogenous T/C ratio can be predictive of emotional responses to fear and threat (Montoya et al., 2012). This analysis revealed that a higher T/C ratio predicted better extinction learning, i.e., smaller differential SCR, in the morning (Figure 6). There was a significant main effect of T/C ratio (Low vs. High based on a median split) [F(1,37)=9.53, p=.004] and the T/C ratio negatively correlated with the mean differential SCR during the Extinction Learning phase (r=.37, p=.02).
Table 1.
Mean and standard deviation salivary cortisol, cortisol normalized to diurnal area under curve (AUC), testosterone, testosterone/cortisol (T/C) ratio and Pearson correlation of cortisol and testosterone levels at Sessions 1 and 2.
| Session | Morning | Evening | p |
|---|---|---|---|
| Cortisol (μg/dL) | |||
| S1 (Conditioning, Extinction) | .508 (.260) | .122 (.072) | <.0001 |
| S2 (Recall, Renewal) | .410 (.294) | ,076 (.068) | <.0001 |
| Cortisol/AUC | |||
| S1 | .260 (.166) | .055(.033) | <.0001 |
| S2 | .197(.162) | .039 (.060) | <.0001 |
| Testosterone (pg/mL) | |||
| S1 | 141.28 (40.09) | 109.21 (33.65) | .0004 |
| S2 | 152.92 (45.58) | 89.19 (29.30) | <.0001 |
| T/C ratio (%, above units) | |||
| S1 | 333 (137) | 1084 (538) | <.0001 |
| S2 | 452 (255) | 1451 (673) | <.0001 |
| T/C correlation | |||
| S1 | r=.57, p=.0001 | r=.57, p=.0003 | |
| S2 | r=.46, p=.002 | r=.38, p=.029 |
S1 = Session 1, S2 =Session 2
Figure 6.
Association of endogenous testosterone/cortisol ratio levels, obtained during the morning, with fear and extinction learning. Participants with preconditioning high salivary testosterone to cortisol (T/C) ratio (based upon median split of 39 participants with testosterone data who had Session 1 in the morning) show better extinction learning. Significance indicated for the T/C ratio main effect (large asterisk) and trial-by-trial (small asterisk). SCR1/2d: differential SCR, * p < .05 trial, ** p < .01. Error bars depict standard error of the mean.
Discussion
In the present study we show that time-of-day plays a strong role in both extinction learning and its recall. In the morning, extinction learning was superior and, following a delay, extinction recall was better generalized from an extinguished fear-conditioned stimulus to an un-extinguished stimulus. Fear responses also remained lower in the morning following contextual renewal of conditioned fear. Extinction recall was not influenced by the delay between extinction learning and extinction recall. Shock expectancy measures demonstrated participants’ ability to retrospectively recall fear conditioning, extinction learning, extinction recall and contextual fear renewal, but did not show time-of-day effects. Levels of salivary cortisol and testosterone showed the expected significantly greater morning values. However only the testosterone to cortisol (T/C) ratio predicted psychophysiological measures at the same time of day, with higher T/C predicting better extinction learning in the morning.
Circadian rhythms (controlled by the endogenous clock in the suprachiasmatic nucleus) and/or sleep-homeostatic (relating to the duration of prior waking) may account for superior extinction learning and recall generalization in the morning. It is not possible to dissociate circadian and homeostatic factors in humans without techniques such as forced desynchrony (e.g., Dijk & Duffy, 1999; Murray et al., 2009). However, circadian effects on fear conditioning as well as extinction learning and memory have been demonstrated in rodents (Cain et al., 2008; Chaudhury et al., 2002; Gerstner & Yin, 2010; Hopkins & Bucci, 2010). Moreover, fear conditioning disrupts sleep, particularly REM (Pawlyk et al., 2008; Sanford et al., 2010), and this disruption is ameliorated by extinction (Wellman et al., 2008). Although, time-of-day effects on un-learned physiological responses have been reported in humans (Hot et al., 2005; Hot et al., 1999; Miller & Gronfier, 2006), However, Figures 3 and S1 clearly illustrates that fear conditioning did not differ when performed in the morning vs. the evening as would be expected if Time-of-Day differences in autonomic reactivity were to explain differences in extinction learning and extinction recall. Moreover when those individuals destined to undergo the 3 different delay durations were analyzed separately, there was no evidence of lower fear conditioning in the morning but continued evidence of greater extinction learning (see Supplementary Results).
A circadian mechanism might involve associations between extinction and the circadian rhythms of cortisol or testosterone (Diver et al., 2003; Kalsbeek et al., 2012; Leymarie et al., 1974; Pannain et al., 2007; Piro et al., 1973). However, levels of neither hormone predicted extinction learning or memory within the morning or evening. One possible explanation is that variations between their endogenous morning peak and evening nadir are sufficiently large to produce the observed time-of-day effect on extinction, but the variation within each time of day is insufficient to reveal such differences. Nonetheless, the T/C ratio was positively associated with extinction learning during morning peak levels. How might this ratio influence extinction learning? Mutually inhibitory influences of the hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes are mediated by their hormonal end products, testosterone and cortisol (Montoya et al., 2012). Moreover, studies in humans suggest that high endogenous cortisol promotes fearful responding whereas high endogenous testosterone opposes fear (Montoya et al., 2012). High T/C may, therefore, be associated with a tendency to rapidly extinguish fear. However, current results cannot disambiguate such interactive effects of these two hormones from other time-of-day influences that might promote morning extinction learning and recall.
Little is known about the effects of normally occurring levels of sleep homeostatic pressure on emotional learning and memory. Nonetheless, sleep deprivation studies strongly suggest that experimentally elevated sleep pressure may impair fear memory in rats (Fu et al., 2007; Graves et al., 2003); Kumar and Jha, 2012), emotion regulation in humans (Yoo et al., 2007) and, when REM is deprived, extinction memory in both rats and humans (Fu et al., 2007; Silvestri, 2005; Spoormaker et al., 2011). Therefore poorer extinction learning and recall in the evening may reflect weakening of the fear extinction network (see Graham & Milad, 2011; Milad & Quirk, 2012) due to processes underlying sleep homeostasis such as increased extracellular adenosine (Porkka-Heiskanen & Kalinchuk, 2011) or cytokines (Krueger et al., 2011). For example, in the rat, adenosine A1 receptor activation has been shown to impair both fear conditioning (Corodimas & Tomita, 2001) and extinction of appetitive conditioning (Kuzmin et al., 1999). Additional studies should be conducted to examine the neural mechanisms by which sleep homeostasis may influence the function of the fear extinction network during extinction learning and recall.
Using a 12-hr delay, we previously suggested that overnight sleep promoted extinction generalization in the morning, but not the evening (Pace-Schott et al., 2009). Absence of time-of-day differences for extinction learning supported this hypothesis. However, in the current study, morning favored both extinction learning and extinction recall. Additionally, in the 3-hr delay groups, a time-of-day effect appeared with no intervening sleep and, in the 24-hr groups, it appeared despite both groups having slept. Moreover, unlike the previous study, there was no time-of-day effect on extinction generalization in the 12-hr group (see Supplementary Results). In these 12-hr subjects, extinction may have been better learned in the morning to evening group but better recalled in the evening to morning group resulting in their apparently equal recall. The possibility also exists that, due to random selection of subjects, the 12-hr delay sample acquired fear conditioning especially strongly in the morning as evidenced by a statistical trend toward higher fear conditioning in the morning not seen in the other delay-duration groups (see Supplementary Results). This might, in turn, have obscured differences at Extinction Recall 12 hr later.
Notably, however, neither study showed effects on responses to the CS+E at extinction recall. Therefore, regardless of whether sleep or time-of-day was primary, effects in both studies may represent greater generalization of extinction from the CS+E to the CS+U in the morning, enhanced fear (CS+U) relative to extinction (CS+E) memory in the evening, or both.
The current study examined only males whereas Pace-Schott et al. (2009) examined both genders. Males alone from that previous study showed better morning extinction recall (but not learning) with a trend-level group main effect (p=.053) and Group × CS+E interaction (p=.057). Neither effect, however, was seen in females. Similarly, 41 females studied with the abbreviated version of the current protocol showed no time-of-day effects on either extinction learning or its recall (see Supplementary Results). It is likely, therefore, that sex differences contributed to discrepancies between these two studies and superior morning extinction learning and recall may be characteristic only of males, possibly in association with interactive effects of testosterone and cortisol. Differing populations, data acquisition systems, experimental environments and dependent variables may have also contributed to differing results between studies.
Subsequent studies should investigate how sex differences, menstrual cycle phase, and gonadal hormones may interact with circadian and sleep-homeostatic factors. Differing populations, data acquisition systems, experimental environments and dependent variables may have also contributed to differences between the current results and Pace-Schott et al. (2009).
The present findings could have important clinical implications. For example, they suggest that exposure therapy may be more efficacious if delivered in the morning. Notably, the superior extinction recall in the morning relative to the evening was resistant to contextual fear renewal. The generalizability of extinction across cues and contexts is also clinically relevant and needs further testing and replication. Future studies hold potential for clinically important findings.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bentz D, Michael T, de Quervain DJ, Wilhelm FH. Enhancing exposure therapy for anxiety disorders with glucocorticoids: from basic mechanisms of emotional learning to clinical applications. Journal of Anxiety Disorders. 2010;24:223–230. doi: 10.1016/j.janxdis.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Bentz D, Michael T, Wilhelm FH, Hartmann FR, Kunz S, von Rohr IR, de Quervain DJ. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Lagace DC, Eisch AJ, Powell CM. Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiology of Learning and Memory. 2011;95:453–460. doi: 10.1016/j.nlm.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behavioural Brain Research. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. Journal of Neuroendocrinology. 2010;22:664–673. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corodimas KP, Tomita H. Adenosine A1 receptor activation selectively impairs the acquisition of contextual fear conditioning in rats. Behavioral Neuroscience. 2001;115:1283–1290. doi: 10.1037//0735-7044.115.6.1283. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ. Glucocorticoid-induced reduction of traumatic memories: implications for the treatment of PTSD. Progress in Brain Research. 2008;167:239–247. doi: 10.1016/S0079-6123(07)67017-4. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Frontiers in Neuroendocrinology. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Bentz D, Michael T, Bolt OC, Wiederhold BK, Margraf J, Wilhelm FH. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clinical Endocrinology. 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Han L, Feng S, Tian S, Hu B. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–1192. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Graham BM, Milad MR. The study of fear extinction: implications for anxiety disorders. American Journal of Psychiatry. 2011;168:1255–1265. doi: 10.1176/appi.ajp.2011.11040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress differentially modulates fear conditioning in healthy men and women. Biological Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and Cellular Endocrinology. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA, Davis CJ. Involvement of cytokines in slow wave sleep. Progress in Brain Research. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leymarie P, Roger M, Castanier M, Scholler R. Circadian variations of plasma testosterone and estrogens in normal men. A study by frequent sampling. Journal of Steroid Biochemistry. 1974;5:167–171. doi: 10.1016/0022-4731(74)90124-1. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv Emot. 2012;36:65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Vijayakumar S, Murphy M, Ahmed N, Spencer RMC, Milad M, Orr S, Pitman RK. Time-of-day influences on fear conditioning, extinction learning and extinction recall. Sleep. 2012;35(Supplement):A100. [Google Scholar]
- Pannain S, Van Cauter E. Modulation of endocrine function by sleep-wake homeostasis and circadian rhythmicity. Sleep Medicine Clinics. 2007;2:147–159. [Google Scholar]
- Piro C, Fraioli F, Sciarra F, Conti C. Circadian rhythm of plasma testosterone, cortisol and gonadotropins in normal male subjects. Journal of Steroid Biochemistry. 1973;4:321–329. doi: 10.1016/0022-4731(73)90056-3. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep Med Rev. 2011;15:123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiology and Behavior. 2005;84:343–349. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, Roozendaal B, de Quervain DJ. Glucocorticoids reduce phobic fear in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Schroter MS, Andrade KC, Dresler M, Kiem SA, Goya-Maldonado R, Wetter TC, Holsboer F, Samann PG, Czisch M. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Human Brain Mapping. 2011 doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory, and plasticity. Annual Review of Psychology. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorawski M, Cook CA, Kuhn CM, LaBar KS. Sex, stress, and fear: individual differences in conditioned learning. Cogn Affect Behav Neurosci. 2005;5:191–201. doi: 10.3758/cabn.5.2.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.