Abstract
Data from our lab indicate that the orexin system is involved in the regulation of both conditioned and unconditioned responding for palatable foods. Anticipation of food rewards activates orexin receptor containing neurons within the paraventricular nucleus of the thalamus (PVT). The PVT regulates mesolimbic dopamine neurochemistry through direct connections with the nucleus accumbens and modulates the processing of cognitive-emotional information, suggesting that the PVT may represent a unique brain region with the capacity to mediate orexinergic effects on brain dopamine and behavior. Here, we tested the hypothesis that PVT orexin signaling mediates mesolimbic dopamine and reward-based feeding. To do this we used a behavioral pharmacological approach in tandem with central genetic manipulation of the orexin-1 receptor in the PVT. Data from these studies indicate that orexin-A action in the PVT increases dopamine levels in the nucleus accumbens. In addition, endogenous orexin signaling in the PVT mediates locomotor activity and hedonic feeding responses. Together these data highlight the PVT as a critical site capable of mediating orexin action on brain dopamine and reward-based feeding.
Keywords: Paraventricular Thalamus, Orexin, Dopamine, Hedonic Feeding
Introduction
The lateral hypothalamic orexin system has received considerable attention for its ability to mediate arousal and reward function (Peyron et al., 1998; Kirouac et al., 2005, Zheng et al., 2007; Borgland et al., 2009; Cason et al., 2010; Choi et al., 2010). Orexinergic projections to the mesolimibic dopamine pathway, via the ventral tegmental area (VTA) of the midbrain, mediate the action of orexin-A on food reward (Aston-Jones et al., 2009). However, the wide range of orexin projections throughout the CNS indicates that orexin-A may signal at alternative sites to manifest its actions on brain dopamine and behavior (Boutrel et al., 2009; Choi et al., 2010).
The paraventricular thalamus (PVT), a midline thalamic nucleus, receives dense innervations from lateral hypothalamic orexin neurons (Peyron et al., 1998; Kirouac et al., 2005) and is involved in the regulation ofcognition, anxiety, emotionality, and addiction behaviors (Huang et al., 2006; Li et al., 2009, 2010a, 2010b and 2011). Glutamatergic fibers arising from the PVT stimulate dopamine efflux in the nucleus accumbens (NAcc) (Jones et al., 1989; Pinto et al., 2003; Parsons et al., 2007). Notably, this effect does not require VTA activation, indicating that the PVT may represent an indirect means for orexin to mediate mesolimbic dopamine release. Previously, we demonstrated that PVT neurons expressing orexin-1 receptors (OX1R) become activated in the presence of cues associated with food rewards (Choi et al., 2010). When viewed collectively, these findings raise the possibility that the PVT may be a critical mediator of orexin’s actions on food reward and mesolimbic dopamine function.
In the current study, we hypothesized that orexin-A acts within PVT neurons to augment mesolimbic dopamine and promote reward-related feeding. Specifically, we predicted that direct application of orexin-A within the PVT would increase mesolimbic dopamine in the nucleus accumbens (NAcc). To do this we utilized behavioral pharmacology in tandem with central genetic manipulation of the orexin-1 receptor (OX1R). Specifically, we measured dopamine and associated metabolites in the NAcc and medial Prefrontal Cortex (mPFC) following intra-PVT orexin administration. In addition, we measured basal locomotor activity, PR responding for high fat pellets and hedonic feeding behavior in rats with reduced PVT OX1R. Results indicate that orexin-A acting in the PVT increases mesolimbic dopamine, and that endogenous OX1R signaling in the PVT mediates hedonic feeding.
Materials and Methods
Animals
Male Long-Evans rats (Harlan; Indianapolis, IN) weighing 300 - 350 g were housed individually in a vivarium on a 12-hr light–dark cycle schedule. Room temperature was maintained at 25 °C. All animals were given ad libitum access to pelleted standard rodent chow and water unless noted. Rats were maintained on chow (Teklad, 3.41 kcal/g, 0.51 kcal/g from fat) unless otherwise noted. The hedonic feeding experiments utilized high-fat diet (HFD) (Research Diets, New Brunswick, NJ, 4.41 kcal/g, 1.71 kcal/g from fat) as the palatable food reinforcer.
Intra-PVT Orexin-A and Dopamine Neurochemistry
After a 1 week habituation period, 6 rats (n=3/group) were deeply anesthetized with a 1 ml/kg dose of (0.22 g Ketamine/0.03 g Xylazine) and placed into a stereotaxic apparatus. Subsequently, an indwelling cannula was lowered into the paraventricular thalamus (PVT) using the following coordinates relative to bregma, AP= −2.8, ML= 0, DV= −5.0. All animals were allowed to recover for 1 week following surgery. All testing was conducted in the animals subjective night phase and each rat was given a 1 μl injection of either saline or 5 nmole orexin-A (Phoenix Pharmaceuticals; Burlingame, CA) into the PVT. Rats were sacrificed 1 hour after the injection via carbon dioxide asphyxiation, and brains were rapidly removed, frozen, and stored at −80 °C. Bilateral micro-punches of the nucleus accumbens (NAcc) and medial prefrontal cortex (mPFC) were isolated from each animal. Although substantial care was taken to minimize contamination by neighboring brain regions, due to the nature and size of each micro-punch our method did not allow us to distinguish subregions (i.e. NAcc core vs. shell) within the NAcc. For high-performance liquid chromatography (HPLC) analysis, an antioxidant solution (0.4 N perchlorate, 1.343 mM ethylenediaminetetraacetic acid (EDTA) and 0.526 mM sodium metabisulfite) was added to the samples followed by homogenization using an ultrasonic tissue homogenizer (Biologics; Gainesville, VA). A small portion of the tissue homogenate was dissolved in 2% sodium dodecyl sulfate (SDS) (w/v) for protein determination (Pierce BCA Protein Reagent Kit; Rockford, IL). The remaining suspension was spun at 14,000 g for 20 min in a refrigerated centrifuge. The supernatant was reserved for HPLC. Samples were separated on a Microsorb MV C-18 column (5 Am, 4.6_250 mm, Varian; Walnut Creek, CA) and simultaneously examined for DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), both of which are markers of dopamine degradation, 5-HT and 5-HIAA. Compounds were detected using a 12-channel coulometric array detector (CoulArray 5200, ESA; Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters; Milford, MA) under the following conditions: flow rate of 1 ml/min; detection potentials of 50, 175, 350, 400 and 525 mV, and; scrubbing potential of 650 mV. The mobile phase consisted of a 10% methanol solution in distilled H2O containing 21 g/l (0.1 M) citric acid, 10.65g/l (0.075 M) Na2HPO4, 176 mg/l (0.8 M) heptanesulfonic acid and 36 mg/l (0.097 mM) EDTA at a pH of 4.1. Unknown samples were quantified against a 6-point standard curve with a minimum R2 of 0.97. Quality control samples were interspersed with each run to ensure HPLC calibration.
Small Hairpin RNA Design and Viral-mediated Knockdown Surgery
Adeno-associated viral (AAV) vector construction and packaging was performed by Vector Biolabs (Philadelphia, PA). Three distinct shRNA sequences designed and directed towards rat orexin-1 receptor were tested in vitro to identify and verify the most effective knockdown sequence to be packaged into an AAV vector for in vivo use. From this effort the following sequences were chosen, scrambled (SC shRNA; 5′-gatccAGTACTGCTTACGATACGGTTCAAGAGACCGTATCGTAAGCAGTACTTTTTTTACGCGTa-3′) and a shRNA directed against rat OX1R (OX1R shRNA; 5′-gatccGCTACTTCATTGTCAACCTGTTCAAGAGACAGGTTGACAATGAAGTAGTTTTTT ACGCGTa-3′). Green fluorescent protein (GFP) was used as a selection marker driven by elongation factor 1 alpha promoter. The titers of the packaged viruses for in vivo injections were in the range of 1 × 1013 GC/ml. After a 1 week habituation period, all rats were deeply anesthetized with a 1 ml/kg dose of (0.22 g Ketamine/0.03 g Xylazine) and placed into a stereotaxic apparatus. Viral injection was performed using a 26-gauge Hamilton (Reno, NV) syringe lowered into the PVT at the following coordinates, AP= −2.8, ML= 0, DV= −5.2 (-6.2 to account for syringe tip bevel). A 4 μl volume of either AAV-SC-shRNA (n=7) or AAV-OX1R-shRNA (n=12) was manually injected over 6 min using a micro-drive syringe mount. All animals were allowed to recover for 1 week during which time they recovered their pre-surgical body weight. Behavioral testing resumed one week following recovery from surgery. Body weights were taken at 16, 52 and 71 days following recovery from surgery.
Immunohistochemistry
A series of AAV-OX1R-shRNA injections were performed (n=4) to verify anatomical specificity. Following recovery, rats were injected with an overdose of pentobarbital and transcardially perfused with saline for 1 min followed by 4% paraformaldehyde for 20 min. The brains were post-fixed overnight and then stored in 30% sucrose with 0.01% glycerol for a minimum of 24 hours. Brains were frozen on dry ice and sectioned at 35 μm intervals and collected (in series of one-in-four sections) in 20% glycerol. Only sections containing PVT were immunolabeled for GFP. After several washes in 0.1M PBS, sections were blocked in 0.2% bovine serum albumin with 0.4% Triton X-100. Rabbit anti-GFP antibody (1:200; Invitrogen; Carlsbad, CA) was applied to the sections for overnight incubation at room temperature. The secondary antibody, Alexxa 488-conjugated goat anti-rabbit IgG (1:200; Invitrogen), was applied to the sections at room temperature for 45 min were mounted onto slides for immunofluorescent visualization. Sections were viewed and collected on a Zeiss Axioplan 2 fluorescence microscope with an AxioCam camera using Axiovision software (Carl Zeiss Microimaging; Thornwood, NY).
Quantitative Real Time Polymerase Chain Reaction (qPCR)
Verification and quantification of OX1R knockdown was performed following all behavioral experiments. SC shRNA (n=7) and OX1R shRNA (n=12) rats were sacrificed via carbon dioxide asphyxiation, and brains were rapidly removed, frozen, and stored at −80°C until processing. The PVT from each animal was micro-dissected using an AHP-1200CPV freezing plane (Thermoelectric Cooling America; Chicago, Illinois), which maintained a constant temperature of −12°C throughout the dissection process. High-quality messenger RNA (mRNA) was isolated by Trizol (Invitrogen) and chloroform (Sigma; St. Louis, Missouri) extraction. Complementary DNA was synthesized from 300 ng of mRNA by oligo DT priming (Invitrogen). Complementary DNA was amplified in triplicate using 8 pg of each specific primer with quantification of the product by SYBR green fluorescence (Applied Biosystems; Foster City, California). Rat orexin-1 receptor expression was normalized to 36B4 (loading control) and GFP (injection/infection control) expression levels.
Locomotor Activity
Locomotor testing was conducted in a home cage activity unit (Lafayette Instruments, Lafayette, IN). During locomotor activity testing, each animal (SC shRNA n=4 and OX1R shRNA n=7) had a home cage fitted with a locomotor activity-monitoring unit and activity was measured over a 24 hr period. The total number of beam breaks (horizontal plane movements) served as an indicator of locomotor activity and data was collected in 12-hr bins (light and dark phases).
Progressive Ratio Responding
Pellet responding was conducted as previously reported (Choi et al., 2010). Training for a 45 mg high fat pellet (38% calories from fat; TestDiet; Richmond, IN) in SC shRNA (n=7) and OX1R shRNA (n=11) rats was carried out over 13 consecutive days for 1 hr per day up to a progressive ratio (PR) schedule of reinforcement. The response requirements of the PR schedule increased through the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 693, 737, 901. PR sessions were terminated for an individual animal when 20 min elapsed before earning the next reinforcer. The last completed bar press was defined as the breakpoint and recorded.
Hedonic Feeding
To assess the effects of PVT OX1R knockdown on reward-based feeding we used a feeding paradigm in which sated animals voluntarily over-consume a palatable test diet (Choi et al., 2010). Two days prior to this experiment, each rat was fed a small amount of high fat diet (HFD; 4.41 kcal/g, 1.71 kcal/g from fat; Research Diets; New Brunswick, NJ) to prevent neophobia. Subsequently, SC shRNA (n=7) and OX1R shRNA (n=11) rats were subjected to a 46-hr food deprivation. Following deprivation, chow food hoppers were weighed, placed on each cage (in the middle of the light phase) and subsequently reweighed each hour for 2 hours. After the second hour of chow access, a separate set of food hoppers containing HFD were weighed and placed on each cage beside the previously placed chow hoppers. Both sets of food hoppers were reweighed after 1 hour.
Statistical Analyses
Data were analyzed using STATISTICA 6.0 for Windows (StatSoft; Tulsa, OK). Data for all experiments were analyzed between subject groups using analysis of variance (ANOVA). Significance was set at P<0.05.
Results
Intra-PVT orexin-A increases NAcc dopamine
To test the hypothesis that PVT orexin signaling mediates mesolimbic dopamine we injected orexin-A directly into the PVT and measured dopamine levels in the NAcc. The 1nm dose of orexin-A (1nmole) had no effect on NAcc dopamine. However, the 5 nmole dose of orexin-A administered directly into the PVT significantly increased dopamine levels in the NAcc (F(1,4)=8.21, P<0.05; Fig. 1B). Following orexin injection, NAcc dopamine levels were 48.83 ng/mg protein +/− 30.85 (1nm) and 66.48 ng/mg protein +/− 12.38 (5nm) compared to saline controls that were 45.51 ng/mg protein +/− 6.13. Additionally, we measured DA efflux in the mPFC following intra-PVT orexin-A administration. Intra-PVT orexin injection increased DA levels in the mPFC (1.51 ng/mg protein +/− 0.97 (1nm); 0.969 ng/mg protein +/− 0.27 (5nm)) compared to saline controls (0.475 ng/mg protein +/− 0.084), however this effect did not reach statistical significance (Fig. 1A).
Figure 1.
Intra-paraventricular thalamic (PVT) orexin-A differentially increases dopamine levels in limbic regions. (A) Illustrates dopamine content in the medial Prefrontal Cortex (mPFC) following 1 or 5 nmole orexin-A administered directly into the paraventricular thalamus (PVT). (B) Depicts significantly elevated dopamine content in the nucleus accumbens (NAcc) following 1 or 5 nmole orexin-A administered directly into the PVT. (* p<0.05).
AAV-OX1R-shRNA mediated OX1R knockdown
A shRNA directed towards rat OX1R was designed and packaged into an AAV to be used for in vivo knockdown of OX1R. Because the PVT resides close in apposition to the third ventricle, it is possible that our viral injections may have spread though CSF to distal brain regions. Thus, we visualized viral infection in the mPFC and VTA, two regions that express OX1R that are located at sites distal from our injections. Neither the mPFC of VTA displayed GFP expression (data not shown). We interpret these anatomical findings to indicate that our injections were localized to the PVT. Specifically, viral infection was visualized at Bregma −3.00 mm and localized to the PVT (Fig. 2A). Total anterior-posterior spread of infection was approximately 1 mm (in either direction or 0.5mm) and spanned from Bregma −2.80 ± 0.5 mm to Bregma −3.7 ± 0.5 mm. Following the completion of behavioral testing, all brains were collected and used for quantitative confirmation of knockdown of PVT OX1R expression. qPCR analysis revealed a significant reduction of OX1R expression relative to SC shRNA group (F(1,17)=37.64, p<0.01; Fig. 2B).
Figure 2.
Verifcation of virus-mediated shRNA injection site and quantification of subsequent orexin-1 receptor (OX1R) knockdown. (A) A representative lower-magnification image of AAV-OX1R-shRNA injection site in the PVT as shown by immunolabeling (green) of green fluorescent protein (GFP) at approximately Bregma −3.00 mm. Scale bar: 500 μm. (B) Quantitative real-time polymerase chain reaction (qPCR) analysis indicates a significant reduction in relative OX1R mRNA expression in PVT micro-dissected tissue samples from OX1R-shRNA treated rats (OX1R shRNA) when compared to scrambled control-shRNA treated rats (SC shRNA; * p<0.01).
PVT OX1R knockdown increases locomotor activity
To assess the potential effects of OX1R knockdown on general arousal we measured basal locomotor activity changes between SC shRNA and OX1R shRNA treated rats. OX1R shRNA rats exhibited increased 12-hr dark phase total activity compared to SC shRNA controls (F(1,9)=5.93, p<0.05; Fig. 3B), indicating that OX1R signaling in the PVT may regulate general arousal mechanisms. Knockdown of OX1R had no effect on body weight in this study at any time point measured following viral infection compared to SC shRNA controls (p>0.05; Fig. 3A).
Figure 3.
OX1R knockdown in the PVT increases dark phase locomotor activity but has no effects on body weight. (A) There are no differences in the changes in body weights, taken at 16, 52 and 71 days post-surgery, between OX1R knockdown and control groups. (B) OX1R knockdown rats exhibit significantly increased locomotor activity, as measured by total beam breaks in a 12-hr dark phase, when compared to control rats (* p<0.05).
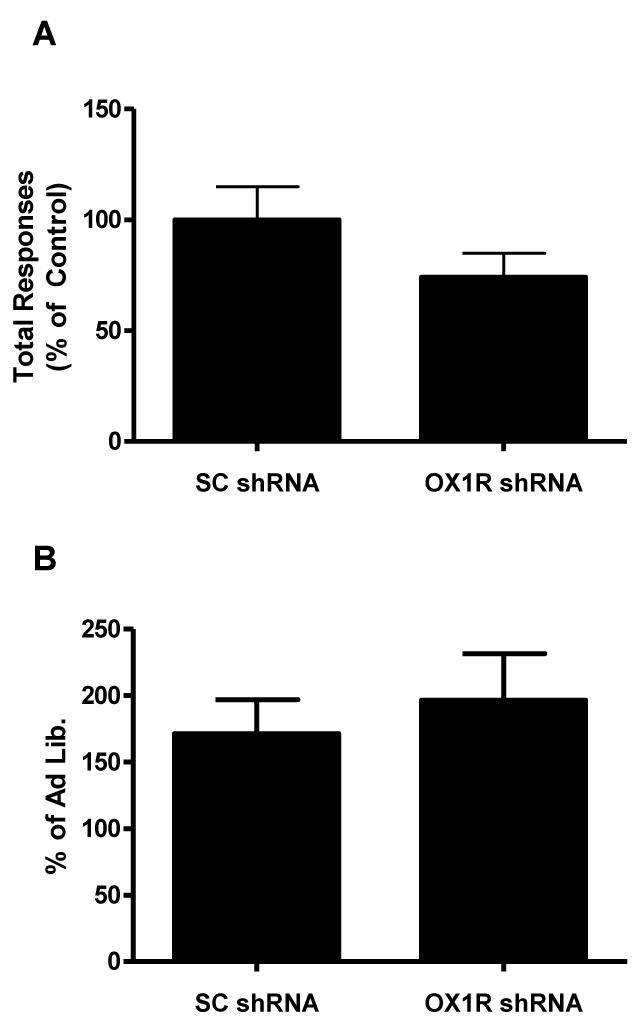
PVT OX1R knockdown does not affect PR responding
Reduction of OX1R shRNA in the PVT had no effect on PR performance, as measured by total responses made to breakpoint (p>0.05; Fig. 4A). To assess if OX1R signaling in the PVT modulates homeostatic responding for food rewards we measured PR responding following a 24 hr deprivation period. Following deprivation, both groups displayed increased PR responding relative to the ad lib feeding condition (p>0.05; Fig. 4B).
Figure 4.
OX1R knockdown in the PVT has no effects on (A) total responses made up to break point under a progressive ratio (PR) schedule to obtain sweet high fat pellets, and on (B) percent change in total responses made in 1 hour under a PR schedule to obtain sweet high fat pellets following an 18-hr food deprivation challenge.
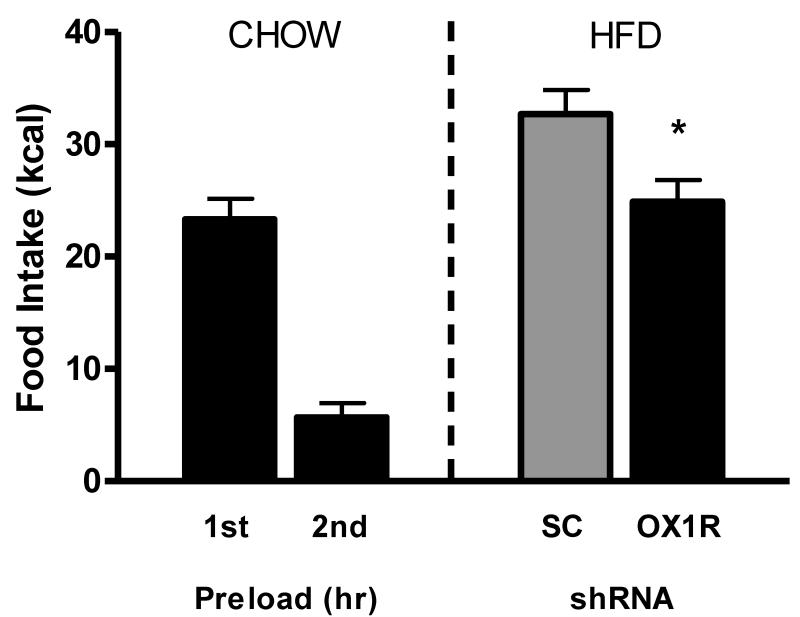
PVT OX1R knockdown attenuates HFD consumption in sated rats
In the current study, we utilized a hedonic feeding model to test the hypothesis that intra-PVT orexin signaling mediates hedonic feeding behavior. As expected, following deprivation both groups of rats displayed rebound hyperphagic feeding responses and subsequent satiation. Importantly, OX1R shRNA rats consumed significantly less HFD relative to SC shRNA controls (F(1,16)=6.85, p<0.05; Fig. 5), indicating that OX1R signaling in the PVT mediates hedonic feeding behavior.
Figure 5.
OX1R knockdown in the PVT attenuates 1-hr high fat diet (HFD) overconsumption following 46-hr food deprivation and 2 hours of chow preload when compared to control rats as measured in kcal (* p<0.05).
Discussion
Data from the current manuscript indicate that 1) intra-PVT orexin-A increases mesolimbic dopamine, 2) OX1R knockdown increases basal locomotor activity, and 3) attenuates hedonic feeding. Together these results implicate the PVT as a critical mediator of orexin effects on mesolimbic dopamine and food reward. Moreover, we propose that the current data support a generalized role for orexin – PVT signaling to promote cognitive arousal secondary to inducing mesolimbic dopamine activity.
It is clear that thalamic relay centers such as the PVT regulate mesolimbic dopamine; however the signaling mechanisms mediating this affect are virtually unknown. Glutamatergic PVT neurons excite and induce dopamine release in the NAcc (Jones et al., 1989; Pinto et al., 2003; Parsons et al., 2007), an effect independent of VTA function. Our results extend these findings and indicate that orexin-A signaling within the PVT is sufficient to increase dopamine levels in the NAcc. There were similar increases, though not significant, in mesocortical dopamine measurements from the mPFC. Of interest, dopamine levels are known to be conditioned predictors of reward expectancy (Schultz et al., 1997); moreover, expectation of reward elicits activation of orexin responsive brain regions that regulate reward behavior (Choi et al., 2010). Thus, it is possible that orexin acts within the PVT to enhance NAcc dopamine tone and arousal in the context of reward expectancy.
It is well established that orexin regulates general arousal (Sakurai et al., 2010). In this study rats with reduced PVT OX1R displayed significant increases in dark phase locomotor activity. These data are consistent with a recent study demonstrating that intra-PVT orexin-A decreases locomotor activity in rats (Li et al., 2009). The ability of orexin-A to attenuate locomotor activity is hypothesized to represent a state of heightened arousal. It is has been suggested that PVT orexin signaling acts to restrict expression of ‘lower priority’ behavioral systems thus affording enhanced vigilance in challenging situations (Li et al., 2009). Our data indicate that reduction in OX1R signaling in the PVT results in increased locomotor activity; thus indicating a potential role for this signaling mechanism in the process of behavioral economics. Collectively, these data support the notion that PVT orexin mediates general arousal and potentially reward behavior.
In terms of reward behavior, recent evidence suggests a role for the PVT in context-induced reinstatement of alcohol-seeking behaviors (Hamlin et al., 2009), and conditioned aversion during drug withdrawal (Li et al., 2011). In the current study, OX1R reduction in the PVT attenuated hedonic high fat feeding. In contrast, reduction of PVT OX1R had no effects on PR responding for food rewards. It is noteworthy that our hedonic feeding model relies on unconditioned feeding responses, thus it is likely that learning or expectancy play minimal roles in this behavior. In contrast, the PR task used here represents a very robust measure of motivation and learning. Thus these two tasks require differing levels of learning and motivation to complete. When viewed generally our results suggest that the ability of PVT OX1R signaling to effect behavior may be dependent on the demand to complete a particular task. In accord with this idea, drug-reward studies indicate that learning reward-stimulus associations involves orexin’s actions in the VTA (Narita et al., 2006; Harris et al., 2007). Thus it is possible that tasks which are high demand in terms of motivational state are regulated by direct orexin-VTA signaling mechanisms which serve to mediate mesolimbic dopamine release. In contrast, orexin action in the PVT, an indirect mediator of mesolimbic dopamine flux, is required for low demand tasks such as unconditioned feeding.
Conclusion
When viewed collectively the present results indicate that orexin signaling in the PVT is capable of modulating brain dopamine and behavior. The ability of orexin to act within PVT neurons to modulate NAcc dopamine represents a novel signaling mechanism by which orexin mediates dopamine neurochemistry and validates the significance of the proposed hypothalamic-thalamic-striatal axis concept for the control of food reward (Kelley et al., 2005). Our behavioral results from knockdown studies support the hypothesis that the PVT OX1R signaling mediates arousal and low demand behaviors such as unconditioned hedonic high fat feeding. Orexin’s unique to regulate dopamine neurochemistry and behavior through actions in the PVT underscores the unique ability of lateral hypothalamic signaling mechanisms to impact reward behavior.
Highlights.
In this manuscript, Choi et al., report that orexin signaling within the paraventricular thalamic nucleus increases dopamine content in the nucleus accumbens. The authors further report that orexin signaling in the PVT modulates locomotor activity and hedonic feeding behavior. These are the first data to implicate PVT orexin signaling in the regulation of mesolimbic function and hedonic feeding.
Acknowledgements
This research was supported by NIH DK066223 to SCB.
Footnotes
Conflict of Interest
The authors have no conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Smith RJ, et al. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, et al. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–111. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, et al. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100(5):419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, et al. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167(1):11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, et al. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29(4):802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, et al. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ghosh P, et al. Prefrontal cortex-projecting glutamatergic thalamic paraventricular nucleus-excited by hypocretin: a feedforward circuit that may enhance cognitive arousal. J Neurophysiol. 2006;95(3):1656–1668. doi: 10.1152/jn.00927.2005. [DOI] [PubMed] [Google Scholar]
- Jones MW, Kilpatrick IC, et al. Regulation of dopamine function in the nucleus accumbens of the rat by the thalamic paraventricular nucleus and adjacent midline nuclei. Exp Brain Res. 1989;76(3):572–580. doi: 10.1007/BF00248914. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, et al. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, et al. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059(2):179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, et al. Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol Biochem Behav. 2009;93(4):506–514. doi: 10.1016/j.pbb.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, et al. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav. 2010;95(1):121–128. doi: 10.1016/j.pbb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, et al. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212(2):251–265. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, et al. Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol Behav. 2011;102(1):42–50. doi: 10.1016/j.physbeh.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MP, Li S, et al. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol. 2007;500(6):1050–1063. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, et al. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459(2):142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Mieda M, et al. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–161. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- W Shultz. Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology. 1997;7(2):191–7. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, et al. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27(41):11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, et al. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]