Abstract
In cattle, the blastocyst hatches from the zona pellucida on days 8 to 9 and then forms a conceptus that grows and elongates into an ovoid and then filamentous shape between days 9 and 16. The growing conceptus synthesizes and secretes prostaglandins and interferon tau. Our hypothesis was that the ovoid conceptus exerts a local effect on the endometrium prior to maternal recognition of pregnancy on day 16 in cattle. In Study One, synchronized cyclic heifers received nothing or 20 in vitro produced blastocysts on day 7, and uteri were collected on day 13. Interferon tau was not detected by radioimmunoassay in the uterine flush of pregnant heifers containing multiple ovoid conceptuses; however, total prostaglandin levels were higher in the uterine lumen of pregnant as compared to cyclic heifers. Microarray analysis revealed that 44 genes were increased in the endometrium of day 13 pregnant as compared to cyclic heifers, and many of those genes were classical Type I IFN-stimulated genes (ISGs). Studies Two and Three determined effects of infusing prostaglandins at the levels produced by the elongating day 14 conceptus into the uterine lumen of cyclic ewes on ISG expression in the endometrium. Results indicated that prostaglandin infusion increased the abundance of several ISGs in the endometrium. These studies support the hypothesis that the day 13 conceptus secretes prostaglandins that act locally in a paracrine manner to alter gene expression in the endometrium prior to pregnancy recognition in cattle.
Introduction
After conception (day 0) in cattle, the zona pellucida-enclosed embryo enters the uterus at the morula stage on days 4 to 5 of gestation and forms a blastocyst. The spherical blastocyst hatches from the zona pellucida on days 9 to 10 and continues to grow, changing from a spherical to ovoid shape between days 12 and 14 during a transitory phase preceding elongation, after which it is termed a conceptus (embryo and associated extra-embryonic membranes) (Betteridge & Flechon 1988). After day 16, the time of maternal recognition of pregnancy in cattle, the elongating conceptus begins the process of implantation and placentation (Guillomot et al. 1981).
Progesterone action via the endometrium of the uterus is critical for conceptus growth and elongation (Spencer et al. 2008, Lonergan 2011, Forde & Lonergan 2012). The major changes in the endometrium required to drive conceptus elongation occur between days 7 and 13 in response to ovarian progesterone, irrespective of whether an appropriately developed embryo/conceptus is present or not (Gray et al. 2001, Forde et al. 2009, Simmons et al. 2009, Forde et al. 2010, Forde et al. 2011a, Forde et al. 2011b, Forde & Lonergan 2012, Forde et al. 2012). The outcome of the progesterone-induced changes in the cyclic and pregnant uterus is to modify the intrauterine milieu, including an increase in select amino acids, glucose, cytokines and growth factors in histotroph, for support of blastocyst growth into an ovoid conceptus and elongation to form a filamentous conceptus (Spencer et al. 2008, Bazer et al. 2010, Forde & Lonergan 2012, Dorniak et al. 2013a). The trophectoderm of the growing and elongating conceptus synthesizes and secretes prostaglandins (PGs) and then interferon tau (IFNT) in ruminants (Lewis 1989, Ulbrich et al. 2009, Forde & Lonergan 2012, Dorniak et al. 2013b). Interferon tau is the signal for maternal recognition of pregnancy in ruminants and is secreted predominantly by the elongating conceptus after day 15 (Roberts et al. 2003, Robinson et al. 2006). As a pregnancy recognition signal, IFNT acts in a paracrine manner on the endometrium to inhibit development of the endometrial luteolytic mechanism required for pulsatile release of PGF2α, thereby ensuring continued production of progesterone by the ovarian CL (Thatcher et al. 1989, Spencer et al. 2007, Bazer et al. 2010). Additionally, IFNT stimulates transcription of a number of genes and activities of several enzymes, in a cell-specific manner within the endometrium, implicated in establishment of uterine receptivity and conceptus elongation and implantation in ruminants (Spencer et al. 2007, Hansen et al. 2010, Dorniak et al. 2013a). The precise role of conceptus-derived PGs remains to be determined in cattle (Ulbrich et al. 2009); however, PGs regulate conceptus growth and elongation in sheep through modulation of endometrial genes important for elongation of the conceptus (Dorniak et al. 2011, Dorniak et al. 2012a).
Comparisons of the endometrial transcriptome in cyclic and pregnant heifers (days 5, 7, 12 and 13) found no difference prior to pregnancy recognition (Forde et al. 2011b, Bauersachs et al. 2012). However, comparisons of day 15 to 18 pregnant and non-pregnant or cyclic endometria revealed conceptus effects on endometrial gene expression, particularly the induction or up regulation of classical IFN-stimulated genes (ISGs) (Bauersachs et al. 2006, Forde et al. 2009, Forde et al. 2011b, Bauersachs et al. 2012, Cerri et al. 2012, Forde & Lonergan 2012). We hypothesized that the ovoid conceptus exerts a local effect on the endometrium during early pregnancy prior to pregnancy recognition in cattle. The rationale is that the detection of local effects of the conceptus on the endometrium on day 13 of pregnancy has been imperceptible to date due to the inability to isolate endometria adjacent to a single small, ovoid conceptus. Consequently, analysis of the endometrium from the entire uterine horn has masked conceptus-induced changes in gene expression in the endometria of day 12 or 13 cyclic and pregnant cattle (Forde et al. 2011b, Bauersachs et al. 2012). In order to test our hypothesis, 20 in vitro-produced embryos were transferred into the uterus of synchronized heifers on day 7 and then obtained and analyzed the uterus containing multiple conceptuses on day 13 as compared to cyclic heifers as a control. Based on the results of that study, we next determined the effects of conceptus-derived PGs on endometrial gene expression using sheep. Collective results support the hypothesis that the ovoid conceptus secretes PGs that act locally in a paracrine manner to alter gene expression in the endometrium during early pregnancy in cattle.
Materials and Methods
Experiment One: design and tissue collection
All experimental procedures involving cattle were licensed by the Department of Health and Children, Ireland, in accordance with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EC and were sanctioned by the Animal Research Ethics Committee of University College Dublin. Unless otherwise stated, all chemicals and reagents were sourced from Sigma (Dublin, Ireland).
The estrous cycles of crossbred beef heifers (n=21, predominantly Charolais cross) were synchronized by insertion of a controlled internal drug release device (CIDR, Pfizer Animal Health, Sandwich, Kent, UK) containing 1.38 g of progesterone for eight days. One day prior to CIDR removal, each heifer received a 2 ml intramuscular injection of a PGF2α analogue (Estrumate, Intervet, Dublin, Ireland; equivalent to 0.5 mg Cloprostenal) to regress the endogenous corpus luteum. All heifers were observed for standing heat (designated as day 0) and were then randomly assigned as either cyclic controls (n=7) or as recipients (n=10) to which 20 in vitro produced blastocysts were transferred on day 7 of the estrous cycle into the uterine horn ipsilateral to the ovary bearing the CL. On Day 13 of the synchronised estrous cycle, all heifers were slaughtered at a commercial abattoir and the reproductive tracts recovered, placed on ice and processed within 30 min. At recovery, each uterine horn was flushed from the distal end near the uterotubal junction towards the common body with 10 ml of PBS to recover uterine lumen fluid and conceptuses. The uterine flush was then clarified by centrifugation at 1,000 × g for 15 min, aliquoted into 1 ml tubes, and snap frozen. The caruncular and intercaruncular endometrium was physically dissected from the underlying myometrium and mixed thoroughly. Aliquots were frozen in liquid nitrogen and stored at -80°C for subsequent RNA extraction.
In vitro cattle embryo production
Unless otherwise stated, all chemicals were purchased from Sigma Chemical Company (Poole, Dorset, UK). The techniques for producing embryos in vitro have been described in detail previously (Rizos et al. 2002). Immature cumulus oocyte complexes (COCs) were obtained by aspirating follicles from the ovaries of heifers and cows collected at slaughter. The COCs were matured for 24 h in TCM-199 supplemented with 10% (v/v) fetal calf serum (FCS) and 10 ng/ml epidermal growth factor (EGF) at 39°C under an atmosphere of 5% CO2 in air with maximum humidity. For IVF, matured COCs were inseminated with frozen-thawed Percoll-separated bull sperm at a concentration of 1×106 spermatozoa/ml. Gametes were co-incubated at 39°C under an atmosphere of 5% CO2 in air with maximum humidity. Semen from the same bull was used for all experiments. At approximately 20 h post insemination, presumptive zygotes were denuded by vortexing and cultured in groups of 50 in 500 μl of synthetic oviduct fluid supplemented with 5% FCS for 7 days. Grade 1 blastocysts were selected for transfer.
Experiments Two and Three: design and tissue collection
Mature Rambouillet ewes (Ovis aries) were observed for estrus (designated as Day 0) in the presence of a vasectomized ram and used in experiments only after exhibiting at least two estrous cycles of normal duration (16-18 days). All experimental and surgical procedures were in compliance with the Guide for the Care and Use of Agriculture Animals in Research and Teaching and approved by the Institutional Animal Care and Use Committee of either Texas A&M University or Washington State University.
Experiment Two
As described previously (Dorniak et al. 2011), ewes (n=20) were checked daily for estrus (Day 0), subjected to a mid-ventral laparotomy and implanted with two Alzet 2ML1 Osmotic Pumps on Day 10 post-estrus using a surgical approach (Bazer et al. 1979, Dunlap et al. 2006). The catheter attached to each pump was inserted about 1 cm into the uterine lumen via the uterotubal junction. Pumps were loaded with vehicle (CX; 2% ethanol in saline), recombinant ovine IFNT (101 μg in vehicle), a PG mixture [PGE2 (251 ng), PGF2α (409 ng), and PGI2 (1,483 ng) in vehicle], or IFNT and PGs (IFNT+PGs) that infused a constant 240 μl into the lumen of each uterine horn each day (n=5 ewes/treatment). Recombinant ovine IFNT was prepared for intrauterine infusion as described previously (Van Heeke et al. 1996). The amount of recombinant ovine IFNT infused each day into the uterus is based on published estimates of daily IFNT production by a Day 14 ovine conceptus, which is about 600 ng per h or 14.4 μg per day (Ashworth & Bazer 1989). Intrauterine infusion of that amount of IFNT mimics effects of the conceptus on endometrial expression of hormone receptors and IFNT-stimulated genes during early pregnancy in ewes (Bazer & Spencer 2006, Dorniak et al. 2011). Prostaglandins were purchased from Cayman Chemical Company (Ann Arbor, MI), and the amount of PGs infused each day into the uterus is based on their daily production by a Day 14 conceptus (Dorniak et al. 2011).
Experiment Three
As described previously (Dorniak et al. 2012a), ewes (n=25) were checked daily for estrus (Day 0) and subjected to a mid-ventral laparotomy and implanted with two Alzet 2ML1 Osmotic Pumps on Day 10 post-estrus. The catheter attached to each pump was inserted about 1 cm into the uterine lumen via the uterotubal junction. Pumps were loaded with vehicle (CX; 2% ethanol in saline), recombinant ovine IFNT (101 μg), PGE2 (251 ng), PGF2α (409 ng), or PGI2 (1,483 ng) (n=5 ewes/treatment). The amount of PGs infused each day into the uterus is based on their daily production by a Day 14 conceptus (Dorniak et al. 2011).
At necropsy on Day 14 for both Experiments two and three, the uterine lumen was flushed with 20 ml of 10 mM Tris (pH 7.2). The volume of the uterine flushing was measured and recorded, and then clarified by centrifugation (3000 × g at 4°C for 15 min). The supernatant was carefully removed with a pipet, aliquoted, frozen in liquid nitrogen, and stored at -80°C. Several sections (∼0.5 cm) from the mid-portion of each uterine horn were fixed in fresh 4% paraformaldehyde in PBS (pH 7.2). After 24 h, fixed tissues were changed to 70% ethanol for 24 h and then dehydrated and embedded in Paraplast-Plus (Oxford Labware, St. Louis, MO). The remaining intercaruncular and caruncular endometrium was physically dissected from myometrium and mixed thoroughly. Aliquots were frozen in liquid nitrogen and stored at -80°C for subsequent RNA extraction.
RNA extraction and Affymetrix GeneChip array analysis
Total RNA was extracted from approximately 100 mg of endometrium (mix of caruncular and intercaruncular) of each cyclic and ET pregnant heifer using Trizol reagent as per manufacturer's instructions (Invitrogen, Carlsbad, CA, USA) and on column DNAse treatment and clean up was performed (Qiagen). RNA quality and quantity was determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and the NanoDrop 1000 (Thermo Fisher Scientific Inc. Wilmington, DE, USA), respectively. Only samples with an RNA Integrity Number of greater than 8.0 were used for microarray analysis. Total RNA was labeled using a Gene Chip One-cycle Target Labeling Kit (Affymetrix, Santa Clara, CA) and then hybridized to the Affymetrix GeneChip® Bovine Genome 1.0 ST Array. Hybridization quality was assessed using GCOS 1.4 (Affymetrix). Preparation of hybridization probes for Affymetrix GeneChip Bovine Genome 1.0 ST Arrays (Affymetrix) was performed using 10 μg of total RNA and the One-Cycle Target Labeling and Control Reagent package (Affymetrix). For the hybridization, wash, and staining process, the GeneChip Hybridization, Wash, and Stain Kit (Affymetrix) and a Fluidic Station 450 (Affymetrix) were used. All steps were done according to the manufacturer's protocol. The processed arrays were scanned with a GeneChip Scanner 3000 (Affymetrix).
Array output was normalized via the robust multiarray method (Irizarry et al. 2003), and probe sets were filtered based on expression calls. Data analysis was conducted using GeneSpring GX software (Agilent Technologies) using ANOVA (P=0.05) with a Benjamini and Hochberg false discovery rate multiple test correction to determine differentially expressed genes.
Radioimmunoassay of IFNT in the uterine flush
A double antibody radioimmunoassay (RIA) for IFNT was developed at Colorado State University (A. Antoniazzi and T. R. Hansen, unpublished results) by using recombinant ovine (ro) IFNT protein and anti-roIFNT polyclonal antibody (1:60,000) from Dr. Fuller Bazer (Texas A&M University, College Station, Texas, USA). Second antibody (diluted 1:25) was anti-rabbit gamma globulin (generated at Colorado State University). Briefly, roIFNT was radioiodinated with 125I using the chloramine T procedure and purified using column chromatography (Sephadex G25; GE Health Care, Piscataway, NJ) using methods generally described previously by Niswender and coworkers (Niswender et al. 1969). Uterine flushings were initially diluted 1:50 in 0.1% PBS and analyzed in the RIA. In the event that IFNT was not detected in diluted samples, uterine flushings were analyzed again at full strength (not diluted). Anti-roIFNT antibody was added to uterine flushing samples, vortexed and incubated at 4°C for 24 hours. Radioactive roIFNT was added, vortexed and incubated for 24 hours at 4°C followed by incubation at 4°C for 72 hour with secondary anti-rabbit gamma globulin antibody. The assay was then terminated with 3 ml of cold PBS and centrifugation at 2800 rpm for 30 minutes. Supernatant was removed and radioactivity of the pellet was determined using a gamma counter. This RIA was optimized for detection of roIFNT in uterine flushings at a sensitivity of 0.1 ng/ml and a range of detection of 0.1 to 13 ng/ml. The intra-assay coefficient of variation was 6.2%, and the inter-assay coefficient of variation was 4.0%.
Real-time PCR analysis
Using methods described previously (Dorniak et al. 2011), total RNA was isolated from samples of endometrium (mix of caruncular and intercaruncular), and reverse transcribed and analyzed by real-time PCR using an ABI prism 7900HT system with Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA). Specific oligonucleotide primers were designed by Oligo 7 program (Molecular Biology Insights, Inc., Cascade, CO) (see Supplemental Table 1 for sequences of the PCR primers, size of PCR products). All primers spanned an intron, and primer specificity and efficiency (-3.6>slope>-3.1) were confirmed using a test amplification run. Each individual sample was run in triplicate under the following conditions: 50°C for 2 min; 95°C for 10 min; 95°C for 15 sec; and 60°C for 1 min for 40 cycles. A dissociation curve was generated at the end of amplification to ensure that a single product was amplified. PCR without template or template substituted with total RNA was used as a negative control to verify experimental results. The threshold line was set in the linear region of the plots above the baseline noise, and threshold cycle (Ct) values were determined as the cycle number at which the threshold line crossed the amplification curve.
Enzyme immunoassay of prostaglandins in the uterine flush
An enzyme immunoassay (EIA) kit (catalog no. 514012; Cayman Chemical, Ann Arbor, MI) was used to measure PG in the uterine flush according to the manufacturer's recommendations. This assay determines the relative amount of total PG in samples using an antiserum that exhibits high cross reactivity for most PG, which allows quantification of all the PG in a given sample with a single assay. Assay sensitivity was 15.6 pg/ml, and the intra- and inter-assay coefficients of variation (CV) were 2.8% and 5.4%, respectively. The data are expressed as total amount of PG in the uterine lumen, which was determined by multiplying assay results by the recovered volume of uterine flushing.
Statistical analysis
All quantitative assay data were subjected to least-squares analyses of variance (ANOVA) using the General Linear Models (GLM) procedures of the Statistical Analysis System (SAS Institute Inc., Cary, NC). For analysis of real-time PCR data, the Ct values of the target mRNA was analyzed for effects of treatment with the GAPDH reference mRNA values used as a covariate. Contrasts were used to determine effects of treatment in Experiment Two (CX vs IFNT, CX vs PGs, PGs vs IFNT+PGs, CX vs IFNT+PGs) and Experiment Three (CX vs IFNT, CX vs PGE2, CX vs PGF2α, CX vs PGI2). Significance (P < 0.10) was determined by probability differences of least squares means (LSM). In all analyses, error terms used in tests of significance were identified according to the expectation of the mean squares for error. With the exception of real-time PCR, data are presented as LSM with standard error (SE). Real-time PCR data are presented as fold change relative to the mRNA levels in control (CX) samples calculated using the LSM Ct values from the statistical analysis.
Results
Experiment One: conceptus recovery on day 13 in heifers
Synchronized recipient heifers (n=10) received 20 in vitro produced (IVP) blastocysts into the uterine horn ipsilateral to the ovary containing a CL on day 7 post-estrus. Conceptuses were recovered in 9 of the 10 heifers on day 13. As summarized in Table 1, the majority of the transferred embryos developed into ovoid or tubular conceptuses and remained in the uterine horn to which they were transferred on day 7. Conceptus size and morphology was not different between the ipsilateral and contralateral uterine horns (data not shown).
Table 1. Summary of conceptus number and measurements recovered from day 13 ET pregnant heifers.
| MEAN | RANGE | |
|---|---|---|
| Conceptuses recovered (number) | 9.3 | 5-14 |
| Ipsilateral uterine horn | 7.7 | 3-13 |
| Contralateral uterine horn | 0.8 | 1-2 |
| Conceptus length (cm) | 1.1+0.1 | 0.2-8.0 |
| Conceptus width (cm) | 0.7+0.1 | 0.2-4.0 |
Study One: IFNT and PGs in the uterine flush
A specific RIA was used to quantify IFNT in the uterine flush of cyclic and pregnant heifers with a sensitivity of 0.1 ng per ml. Although IFNT was easily detectable in uterine flush from day 14 pregnant ewes containing an elongated conceptus (Dorniak et al. 2011), no IFNT was detected in the uterine flush of day 13 ET pregnant heifers containing multiple ovoid or slightly tubular conceptuses (data not shown).
An ELISA was used to quantify the total amount of PGs in the ipsilateral uterine lumen from day 13 cyclic and pregnant heifers; the antiserum used in this assay exhibits high cross reactivity for most PGs (PGE1, PGE2, PGF1α, PGF2α, PGF3α, PGE2 ethanolamide, 6-keto PGF1α, 8-iso-PGF2α, 8-iso PGE2, PGD2, 8-iso-2,3-dinor PGF1α, PGE3, TXB2) that allows quantification of all the PGs in a given sample with a single assay (Cayman Chemical, Ann Arbor, Michigan, USA). The total amount of PGs in the ipsilateral uterine flush of day 13 ET pregnant heifers (95.4±16.1 ng) was higher (P<0.01) than in day 13 cyclic heifers (31.7±16.1 ng).
Experiment One: day 13 bovine conceptus effects on endometrial gene expression
Both caruncular and intercaruncular endometria were obtained from the ipsilateral uterine horn of day 13 ET pregnant or cyclic control heifers. Transcriptional profiling of endometrial total RNA was conducted using the Affymetrix Bovine Gene 1.0 ST array for determination of whole-transcript expression with up to 26 probes per transcript. As summarized in Table 2, 44 expressed transcripts were higher (fold change >1.5, P<0.05) in endometria from day 13 ET pregnant than cyclic heifers, whereas only 14 expressed transcripts were lower (fold change >-1.5, P<0.05) in day 13 ET pregnant than cyclic heifers.
Table 2. Effects of pregnancy by embryo transfer (ET) on endometrial gene expression on day 13 following oestrus.
| Gene symbol | Fold change* | Gene description |
|---|---|---|
| OAS1 | 3.43 | 2′,5′-oligoadenylate synthetase 1, 40/46kDa |
| PLET1 | 3.39 | placenta-expressed transcript 1 protein |
| CA2 | 3.24 | carbonic anhydrase II |
| CYP24A1 | 2.56 | similar to cytochrome P450, family 24, subfamily A, polypeptide 1 |
| ABCC4 | 2.44 | ATP-binding cassette, sub-family C (CFTR/MRP), member 4 |
| BOLA | 2.09 | non-classical MHC class I antigen/MHC class I heavy chain |
| MX1 | 1.97 | myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) |
| MX2 | 1.97 | myxovirus (influenza virus) resistance 2 (mouse) |
| IFI47 | 1.89 | interferon gamma inducible protein 47 |
| FABP3 | 1.86 | fatty acid binding protein 3, muscle and heart (mammary-derived growth inhibitor) |
| MFAP5 | 1.85 | microfibrillar associated protein 5 |
| LOC100296849 | 1.81 | nerve growth factor receptor (TNFRSF16) associated protein 1-like |
| SUMO2 | 1.76 | SMT3 suppressor of mif two 3 homolog 2 (S. cerevisiae) |
| RSAD2 | 1.75 | radical S-adenosyl methionine domain containing 2 |
| OAS2 | 1.70 | 2′-5′-oligoadenylate synthetase 2, 69/71kDa |
| OAS1|OAS2 | 1.69 | 2′,5′-oligoadenylate synthetase 1, 40/46kDa | 2′-5′-oligoadenylate synthetase 2, 69/71kDa |
| LOC533818 | 1.67 | rCG28728-like |
| HLA-DQA1 | 1.67 | major histocompatibility complex, class II, DQ alpha 5 |
| UPK1B | 1.66 | uroplakin 1B |
| PRG4 | 1.66 | proteoglycan 4 |
| GRIK1 | 1.64 | glutamate receptor, ionotropic, kainate 1 |
| USP18 | 1.64 | ubiquitin specific peptidase 18 |
| IGFBP2 | 1.64 | insulin-like growth factor binding protein 2, 36kDa |
| SLC36A2 | 1.63 | solute carrier family 36 (proton/amino acid symporter), member 2 |
| HERC6 | 1.62 | hect domain and RLD 6 |
| SAMD9 | 1.60 | sterile alpha motif domain containing 9 |
| DDX58 | 1.60 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 |
| CRYM | 1.56 | crystallin, mu |
| IFI27 | 1.56 | putative ISG12(a) protein |
| PSPH | 1.54 | phosphoserine phosphatase |
| CYP1A1 | 1.53 | cytochrome P450, subfamily I (aromatic compound-inducible), polypeptide 1 |
| LOC100337391 | 1.51 | predicted protein-like |
| IFI44L|LOC508347 | 1.51 | interferon-induced protein 44-like |
| ISG15 | 1.51 | Interferon-stimulated gene 15 |
| CDH12 | 1.50 | cadherin 12, type 2 (N-cadherin 2) |
| IFIT1 | 1.50 | interferon-induced protein with tetratricopeptide repeats 1 |
| GBP4|LOC507055 | 1.50 | guanylate binding protein 4 | similar to guanylate binding protein 4 |
| SLC4A4 | 1.50 | solute carrier family 4, sodium bicarbonate cotransporter, member 4 |
| MIR2390 | 1.50 | microRNA mir-2390 |
| DPT | 1.50 | dermatopontin |
| SLC5A1 | 1.50 | solute carrier family 5 (sodium/glucose cotransporter), member 1 |
| LOC614522 | 1.50 | similar to transmembrane protein 56 |
| LOC615277 | 1.50 | similar to thioesterase superfamily member 5 |
| CHGA | 1.50 | chromogranin A (parathyroid secretory protein 1) |
| CLDN10 | -1.50 | claudin 10 |
| LOC100296441 | -1.50 | novel |
| HOXA2 | -1.50 | homeobox A2 |
| LY6G6E | -1.50 | lymphocyte antigen 6 complex, locus G6E |
| MIR296 | -1.50 | microRNA mir-296 |
| HIST1H2BN | -1.51 | H2B histone family, member T |
| SYT4 | -1.55 | synaptotagmin IV |
| FREM1 | -1.56 | FRAS1 related extracellular matrix 1 |
| TAT | -1.58 | tyrosine aminotransferase |
| LOC100297468 | -1.61 | similar to CD24 antigen |
| LOC618755 | -1.63 | novel |
| GAT | -1.78 | glycine-N-acyltransferase-like |
| LOC100138068 | -1.80 | ATP-binding cassette, sub-family C, member 4 |
| KERA | -2.22 | keratocan |
Fold change (FC >1.5 for ET versus cyclic) calculated by using the average of multiple probes, where applicable, and adjusted P value <0.05.
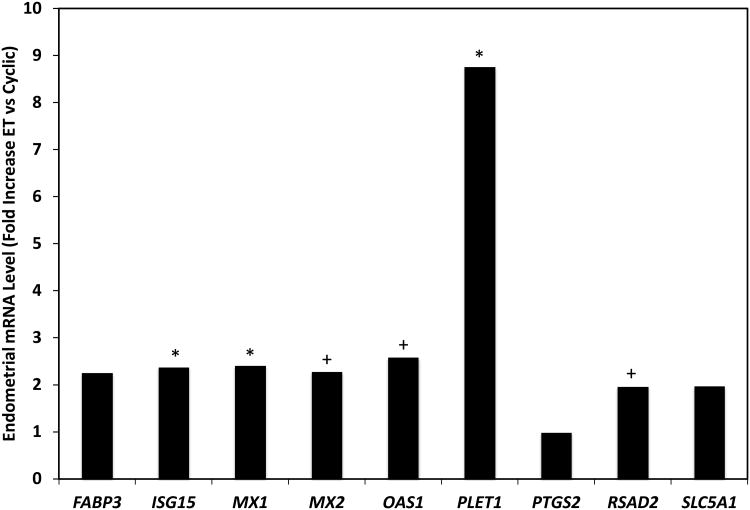
Several differentially expressed genes identified as increased in ET pregnant endometria were validated by qPCR (Figure 1). PLET1, ISG15 and MX1 mRNA was higher (P<0.01) in the endometria of day 13 ET pregnant as compared to cyclic heifers. In addition, MX2, OAS1 and RSAD2 mRNA was higher (P<0.05) in the endometria of day 13 ET pregnant heifers. Expression of FABP3, PTGS2 and SLC5A1 mRNA was not different (P=0.20, P=0.97, and P=0.11, respectively) in the endometria of day 13 ET pregnant as compared to cyclic heifers.
Figure 1.
Validation of expression of selected genes by quantitative real-time RT-PCR (qPCR). Endometrial mRNA abundance was measured by real-time PCR and expressed as fold change of day 13 embryo transfer pregnant (ET) relative to cyclic control heifers. Differences are denoted (*P<0.01 and +P<0.05).
Experiments Two and Three: intrauterine infusion of prostaglandins increase classical IFN-stimulated gene (ISG) expression in the endometria of cyclic ewes
Based on the outcome of Study One, we hypothesized that the increase in endometrial ISG expression could be due to the actions of conceptus-derived PGs rather than IFNT. Using an established sheep model, ISG expression was analyzed in the endometria of cyclic ewes in which pregnancy levels of PGs or IFNT were infused into the uterine lumen from Days 10 to 14 post-estrus; indeed, the effects of IFNT and PGs on a number of candidate progesterone-regulated genes have been published recently from those experiments (Dorniak et al. 2011, Dorniak et al. 2012a). As summarized in Table 3, intrauterine infusion of a mixture of PGs (PGE2, PGF2α and PGI2), synthesized and secreted by the elongating ovine conceptus, increased (P<0.01) the abundance of ISG15 and RSAD2 mRNA in the endometrium from ewes in Experiment Two. As expected, infusion of IFNT substantially increased (P<0.01) levels of ISG15 and RSAD2 mRNAs in the endometrium of cyclic ewes. However, no interaction of IFNT and PGs relative to PGs alone was detected (P>0.10, IFNT vs IFNT+PGs). In Experiment Three, intrauterine infusion of pregnancy levels of PGF2α or PGI2 alone increased (P<0.01) ISG15 mRNA in the endometrium, whereas infusion of PGE2, PGF2α or PGI2 alone increased (P<0.01) endometrial ISG15 and RSAD2 mRNA levels.
Table 3. Effects of treatment on the abundance of mRNA for selected genes in the endometrium of cyclic sheep infused with control vehicle (CX), interferon tau (IFN) or prostaglandins (PG).
| Treatmenta | ISG15 | RSAD2 |
|---|---|---|
| Experiment Two | ||
| IFNT vs CX | 61.2* | 112.5* |
| PGs vs CX | 1.8* | 3.3* |
| IFNT+PGs vs CX | 64.2* | 116.2* |
| Experiment Three | ||
| IFNT vs CX | 47.2* | 99.0* |
| PGE2 vs CX | n.e. | 2.8* |
| PGF2α vs CX | 1.8* | 2.5* |
| PGI2 vs CX | 2.5* | 3.2* |
Data from qPCR analyses are presented as fold change (*P<0.01) for the comparisons; n.e. indicates no effect of treatment (P>0.05).
Discussion
The present study supports the hypothesis that the elongating bovine conceptus has a local regulatory effect on the endometrium that involves paracrine actions of PGs. Similarly in day 12 pregnant pigs, the ovoid conceptus induces SPP1 in the uterine luminal epithelium and STAT1 in uterine LE and subepithelial stroma via estrogens and IFNs (Johnson et al. 2009). The inability of previous studies in cattle to identify genes responsive to the small ovoid conceptus on day 12 (Bauersachs et al. 2012) or day 13 (Forde et al. 2011b) of early pregnancy is likely due to the inability to isolate and analyze endometrium immediately adjacent to the small conceptus (≤1 cm) compared to the uterine horn that is 10-15 cm in length. Thus, the experimental design of Study One ensured the presence of multiple ovoid conceptuses in the lumen apposed to the endometrium of the ipsilateral uterine horn on day 13. The loss of IVP embryos and transuterine migration of conceptuses in the present study has been observed in other studies in which multiple IVP blastocysts were transferred on day 7 (see (Berg et al. 2010)).
The lack of IFNT in the uterine flush of day 13 ET pregnant heifers is consistent with other studies in which IFNT was not detected in uterine flushings of day 12 pregnant cattle as determined by antiviral bioassay for IFNT (Short et al. 1991, Groebner et al. 2010). In cattle, IFNT mRNA and IFNT protein is first detected as the trophectoderm forms at the late morula to early blastocyst stage of development (Farin et al. 1989, Farin et al. 1990, Hernandez-Ledezma et al. 1992), but both mRNA and protein levels are very low in blastocysts (Kubisch et al. 1998, Kubisch et al. 2001). In vivo or in vitro derived hatched day 7 bovine blastocysts produce very low amounts of IFNT (∼100 to 1000 pmol per day) as measured by antiviral cell protection assay (Kubisch et al. 1998, Neira et al. 2007). Both IFNT mRNA levels in the conceptus and detectable IFNT bioactivity, determined by antiviral assay, in the uterine flush increases substantially after day 14 of pregnancy in cattle as the conceptus grows and elongates (Short et al. 1991, Ealy et al. 2001, Robinson et al. 2006, Groebner et al. 2010). Based on the sensitivity of the IFNT RIA used in the present study to measure uterine flushes with multiple ovoid conceptuses and previous studies, available data support the idea that the day 13 ovoid conceptus is not secreting detectable amounts of IFNT. One caveat is that the day 13 bovine conceptuses could be producing IFNT at levels below detection by RIA or antiviral assay. Indeed, in vitro experiments with cells and endometrial explants found that very low amounts (25 nmol and 0.1 pg) of IFNT can increase ISG15 mRNA and ISG15 protein abundance (Austin et al. 1996) (T. R. Hansen, unpublished results).
Total PG levels were approximately 3-fold higher in the uterine flush of day 13 ET pregnant as compared to cyclic heifers and likely emanate from the conceptus. The elongating conceptuses of both sheep and cattle synthesize and secrete more PG than the underlying endometrium (Lewis et al. 1982, Lewis & Waterman 1983, Lewis 1989). Thus, PG levels are much greater in the uterine lumen of pregnant as compared to cyclic or nonpregnant cattle (Bartol et al. 1981, Ulbrich et al. 2009). Ulbrich and coworkers (Ulbrich et al. 2009) found that levels of PGI2 (6-keto-PGF1α), PGF2α, PGE2, PGD2 and TXB2 were not different in day 12 cyclic and pregnant flushes, but were substantially increased in the uterine flush of day 15 pregnant as compared to cyclic heifers. Day 13 bovine conceptuses produced substantial amounts of PGs, with higher abundance of PGF2α than PGE2 (Shemesh et al. 1979, Lewis et al. 1982). Similarly, day 14 sheep conceptuses in vitro release mainly cyclooxygenase metabolites including PGF2α, 6-keto-PGF1α, a stable metabolite of PGI2, and PGE2 (Charpigny et al. 1997), and day 16 conceptuses produce substantially more of those PG than d 14 conceptuses (Lewis & Waterman 1985). Given that PG receptors are present in all cell types of the endometrium and conceptus during early pregnancy in cattle (Arosh et al. 2003, Arosh et al. 2004) and sheep (Cammas et al. 2006, Dorniak et al. 2011), PGs from the conceptus likely have paracrine, autocrine, and perhaps intracrine effects on endometrial function and conceptus development during early pregnancy (Dorniak et al. 2013a). Indeed, PGs are essential for conceptus elongation, as intrauterine infusions of meloxicam, a selective PTGS2 inhibitor, prevented conceptus elongation in early pregnant sheep (Simmons et al. 2010, Dorniak et al. 2011). Importantly, Dorniak and coworkers (Dorniak et al. 2012a) infused PGE2, PGF2α, or PGI2 at the levels produced by the day 14 conceptus into the uterus of cyclic ewes. In that study, expression of several endometrial epithelial genes (gastrin releasing peptide[GRP], insulin-like growth factor binding protein one[IGFBP1], and lectin, galactoside-binding, soluble, 15[LGALS15]) was increased by PGE2 and PGI2 in the ovine uterus. Those genes encode secreted proteins present in the uterine lumen that have biological activities to stimulate trophectoderm cell proliferation, migration and attachment, which are critical for ruminant conceptus growth and elongation (Spencer et al. 2008, Dorniak et al. 2013a). The biological effects of conceptus-derived PGs have not been explored in the endometrium of the bovine uterus. Indeed, expression of PTGS2 in biopsies of day 7 bovine blastocysts is a predictor of the successful development of that blastocyst to term and delivery of a live calf (El-Sayed et al. 2006). Thus, PG are critical regulators of conceptus elongation and implantation in sheep and likely cattle, as they are for blastocyst implantation and decidualization during pregnancy in mice, rats, hamsters, mink and likely humans (Dey et al. 2004, Wang & Dey 2006, Kennedy et al. 2007).
In Study One, a relatively small number of genes were increased in the endometria of day 13 ET pregnant as compared to cyclic heifers, and placenta-expressed transcript 1 (PLET1), also called C11ORF34, was one of the genes. A recent study found that PLET1 was one of the most abundantly up-regulated genes on days 15 and 18 of pregnancy in cattle, but not stimulated by infusion of a Type I IFN, IFNA2 (Bauersachs et al. 2012). Little is known about the biological function of PLET1, but the gene is expressed in the epithelium of the bovine uterus (Mansouri-Attia et al. 2009) and implicated in development and homeostasis (Depreter et al. 2008). Many of the other genes (BOLA, DDX58, GBP4, IFI27, IFI44L, IFI47, IFIT1, ISG15, MX1, MX2, OAS1, RSAD2, SAMD9) up-reglated in endometria of day 13 ET pregnant heifers were classical ISGs. A number of transcriptional profiling experiments conducted with human cells, ovine endometrium, bovine endometrium, and bovine peripheral blood lymphocytes have elucidated classical ISGs induced by IFNT during pregnancy (Spencer et al. 2007, Ott & Gifford 2010, Forde et al. 2011b). Comparisons of day 15 to 18 pregnant and non-pregnant or cyclic endometria revealed that most of the highly induced or up-regulated genes are classical ISGs (Bauersachs et al. 2006, Forde et al. 2009, Forde et al. 2011b, Bauersachs et al. 2012, Cerri et al. 2012, Forde & Lonergan 2012). For instance, ISG15 (ISG15 ubiquitin-like modifier) is expressed in LE of the ovine uterus on days 10 or 11 of the estrous cycle and pregnancy, but is undetectable in LE by day 12 to 13 of pregnancy (Johnson et al. 1999b). In response to IFNT from the elongating conceptus, ISG15 is induced in the stratum compactum stroma and GE by days 13 to 14, and expression extends to the stratum spongiosum stroma, deep glands, and myometrium as well as resident immune cells of the ovine uterus by days 15 to 16 of pregnancy (Johnson et al. 1999b, Johnson et al. 2000). As IFNT production by the conceptus trophectoderm declines, expression of ISGs in the stroma and GE also declines, but some remain abundant in endometrial stroma and GE on d 18 to 20 of pregnancy. Similar temporal and spatial alterations in ISG15 expression occur in the bovine uterus during early pregnancy (Johnson et al. 1999a, Austin et al. 2004). One challenge is to determine which of the large number of classical ISGs have a biological role in conceptus-endometrial interactions given that they have traditionally been associated with cellular antiviral responses, because the main function of Type I IFNs is to inhibit viral infection (Pestka 2007). ISG15 conjugates to intracellular proteins through a ubiquitin-like mechanism (Hansen et al. 1999), and deletion of Isg15 in mice results in 50% pregnancy loss manifest during early placentation (Ashley et al. 2010). In addition, MX proteins are thought to regulate secretion through an unconventional secretory pathway (Toyokawa et al. 2007). The enzymes which comprise the 2′,5′-oligoadenylate synthetase (OAS) family regulate ribonuclease L antiviral responses and may play additional roles in control of cellular growth and differentiation (Johnson et al. 2001).
Results of Study One support the hypothesis that induction of some ISGs in the endometria of day 13 ET pregnant heifers may be due to conceptus-derived PGs rather than IFNT, because IFNT was not detectable in the uterine flush of heifers containing multiple ovoid conceptuses. Therefore, we analyzed expression of two classical ISGs (ISG15 and RSAD2) in the endometrium of cyclic ewes infused with PGs at levels produced by the elongating conceptus (Dorniak et al. 2011). Indeed, ISG15 and RSAD2 mRNA abundance was increased in the endometria of cyclic ewes infused with a PG mix (Experiment Two) or individual PGs (PGE2, PGF2α, or PGI2) (Experiment Three). These studies are the first reports of PG effects on ISG expression in the ruminant uterus. Thus, classical ISGs may be initially induces locally by conceptus-derived PGs and then, once IFNT is secreted by the conceptus, their expression is maximally induced in the endometrium. Another possibility is that PGs increase the sensitivity to very low amounts of IFNT produced by the ovoid conceptus that are not detectable by RIA and antiviral activity assay. Indeed, the receptors for PGE2, PGF2α and PGI2 are expressed in the LE, GE and stroma of the ovine uterus during early pregnancy (Cammas et al. 2006, Dorniak et al. 2011), but the cellular pathways mediating the paracrine effects of conceptus PGs on endometrial function are not known in the sheep. In the ovine uterus, we recently reported that PG infusion stimulates endometrial IGFBP1 expression (Dorniak et al. 2012b). In human uterine decidua, the stimulatory effects of PGs on IGFBP1 expression are mediated by the cAMP/protein kinase A (PKA) signaling pathway (Strakova et al. 2000). In human endometrial carcinoma cells, the PKC pathway regulates IGFBP1 expression (Gong et al. 1992). Membrane receptors for PGE2 and PGI2 are coupled to adenylate cyclase and generate cAMP, whereas stimulation of membrane receptors for PGF2α results in activation of PLC and consequent elevation in calcium levels (Coleman et al. 1994, Narumiya et al. 1999). Of note, ISG15 expression is increased about 3-fold in human uterine cells treated interleukin one beta (IL1B) (Ashley et al. 2010), and IL1B actions on endometrial stromal cells involves cAMP (Strakova et al. 2000).
In summary, available studies support the idea that the day 13 bovine conceptus secretes PGs that act in a paracrine manner on the endometrium and differentially regulate gene expression and functions that are likely important for uterine receptivity and conceptus growth and development during early pregnancy. These results emphasize the importance of PGs during early pregnancy in ruminants. Indeed, PGE2 and PGI2 are critical regulators of blastocyst implantation, decidualization, and uterine angiogenesis during pregnancy in mice, rats, hamsters, mink and humans (Wang & Dey 2006, Kennedy et al. 2007, Cha et al. 2012). Studies are warranted to ascertain the cellular signaling pathways utilized by individual PGs within the various cell types in the endometrium, explore interactive effects of PGs and IFNT on endometrial gene expression and function, and determine genes and pathways that PGs regulate in the endometrium during early pregnancy in ruminants.
Supplementary Material
Acknowledgments
The authors appreciate the technical assistance of Dr. Terry Nett at Colorado State University with the measurement of IFNT by radioimmunoassay. The authors thank Derek Pouchnik and the Laboratory for Bioanalysis and Biotechnology I (LBBI) for GeneChip processing.
Funding: This project was supported, in part, by: Agriculture and Food Research Initiative Competitive Grant no. 2009-67015-01722, 2011-67015-20067 and 2012-67015-30173 from the USDA National Institute of Food and Agriculture; Grant no. 2010-38420-20397 from the USDA National Institute of Food and Agriculture, National Needs Fellows; Grant no. 1 R01 HD072898 from the National Institutes of Health; and Science Foundation Ireland under grant number 10/IN.1/B3011.
Footnotes
Declaration of interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, MacLaren LA, Fortier MA. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology. 2003;144:3076–3091. doi: 10.1210/en.2002-0088. [DOI] [PubMed] [Google Scholar]
- Arosh JA, Banu SK, Chapdelaine P, Fortier MA. Temporal and tissue-specific expression of prostaglandin receptors EP2, EP3, EP4, FP, and cyclooxygenases 1 and 2 in uterus and fetal membranes during bovine pregnancy. Endocrinology. 2004;145:407–417. doi: 10.1210/en.2003-1007. [DOI] [PubMed] [Google Scholar]
- Ashley RL, Henkes LE, Bouma GJ, Pru JK, Hansen TR. Deletion of the Isg15 gene results in up-regulation of decidual cell survival genes and down-regulation of adhesion genes: implication for regulation by IL-1beta. Endocrinology. 2010;151:4527–4536. doi: 10.1210/en.2010-0166. [DOI] [PubMed] [Google Scholar]
- Ashworth CJ, Bazer FW. Interrelationships of proteins secreted by the ovine conceptus and endometrium during the periattachment period. Anim Reprod Sci. 1989;20:117–130. [Google Scholar]
- Austin KJ, Carr AL, Pru JK, Hearne CE, George EL, Belden EL, Hansen TR. Localization of ISG15 and conjugated proteins in bovine endometrium using immunohistochemistry and electron microscopy. Endocrinology. 2004;145:967–975. doi: 10.1210/en.2003-1087. [DOI] [PubMed] [Google Scholar]
- Austin KJ, Ward SK, Teixeira MG, Dean VC, Moore DW, Hansen TR. Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol Reprod. 1996;54:600–606. doi: 10.1095/biolreprod54.3.600. [DOI] [PubMed] [Google Scholar]
- Bartol FF, Thatcher WW, Bazer FW, Kimball FA, Chenault JR, Wilcox CJ, Roberts RM. Effects of the estrous cycle and early pregnancy on bovine uterine, luteal, and follicular responses. Biol Reprod. 1981;25:759–776. doi: 10.1095/biolreprod25.4.759. [DOI] [PubMed] [Google Scholar]
- Bauersachs S, Ulbrich SE, Gross K, Schmidt SE, Meyer HH, Wenigerkind H, Vermehren M, Sinowatz F, Blum H, Wolf E. Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction. 2006;132:319–331. doi: 10.1530/rep.1.00996. [DOI] [PubMed] [Google Scholar]
- Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Buttner M, Meyer HH, Spencer TE, Minten M, Sax G, Winter G, Wolf E. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod. 2012;86:46. doi: 10.1095/biolreprod.111.094771. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Spencer TE. Methods for studying interferon tau stimulated genes. Methods Mol Med. 2006;122:367–380. doi: 10.1385/1-59259-989-3:367. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK, van Leeuwen J, Beaumont S, Berg M, Pfeffer PL. Embryo loss in cattle between Days 7 and 16 of pregnancy. Theriogenology. 2010;73:250–260. doi: 10.1016/j.theriogenology.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Betteridge KJ, Flechon JE. The anatomy and physiology of pre-attachment bovine embryos. Theriogenology. 1988;29:155–187. [Google Scholar]
- Cammas L, Reinaud P, Bordas N, Dubois O, Germain G, Charpigny G. Developmental regulation of prostacyclin synthase and prostacyclin receptors in the ovine uterus and conceptus during the peri-implantation period. Reproduction. 2006;131:917–927. doi: 10.1530/rep.1.00799. [DOI] [PubMed] [Google Scholar]
- Cerri RL, Thompson IM, Kim IH, Ealy AD, Hansen PJ, Staples CR, Li JL, Santos JE, Thatcher WW. Effects of lactation and pregnancy on gene expression of endometrium of Holstein cows at day 17 of the estrous cycle or pregnancy. J Dairy Sci. 2012;95:5657–5675. doi: 10.3168/jds.2011-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpigny G, Reinaud P, Tamby JP, Creminon C, Guillomot M. Cyclooxygenase-2 unlike cyclooxygenase-1 is highly expressed in ovine embryos during the implantation period. Biol Reprod. 1997;57:1032–1040. doi: 10.1095/biolreprod57.5.1032. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Depreter MG, Blair NF, Gaskell TL, Nowell CS, Davern K, Pagliocca A, Stenhouse FH, Farley AM, Fraser A, Vrana J, Robertson K, Morahan G, Tomlinson SR, Blackburn CC. Identification of Plet-1 as a specific marker of early thymic epithelial progenitor cells. Proc Natl Acad Sci U S A. 2008;105:961–966. doi: 10.1073/pnas.0711170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Bazer FW, Spencer TE. Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol Reprod. 2011;84:1119–1127. doi: 10.1095/biolreprod.110.089979. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Bazer FW, Wu G, Spencer TE. Conceptus-derived prostaglandins regulate endometrial function in sheep. Biol Reprod. 2012a;87:9. doi: 10.1095/biolreprod.112.100487. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Welsh TH, Jr, Bazer FW, Spencer TE. Endometrial HSD11B1 and cortisol regeneration in the ovine uterus: effects of pregnancy, interferon tau, and prostaglandins. Biol Reprod. 2012b;86:124. doi: 10.1095/biolreprod.111.097063. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Bazer FW, Spencer TE. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Biological role of interferon tau in endometrial function and conceptus elongation. J Anim Sci. 2013a;91:1627–1638. doi: 10.2527/jas.2012-5845. [DOI] [PubMed] [Google Scholar]
- Dorniak P, Welsh TH, Jr, Bazer FW, Spencer TE. Cortisol and interferon tau regulation of endometrial function and conceptus development in female sheep. Endocrinology. 2013b;154:931–941. doi: 10.1210/en.2012-1909. [DOI] [PubMed] [Google Scholar]
- Ealy AD, Larson SF, Liu L, Alexenko AP, Winkelman GL, Kubisch HM, Bixby JA, Roberts RM. Polymorphic forms of expressed bovine interferon-tau genes: relative transcript abundance during early placental development, promoter sequences of genes and biological activity of protein products. Endocrinology. 2001;142:2906–2915. doi: 10.1210/endo.142.7.8249. [DOI] [PubMed] [Google Scholar]
- El-Sayed A, Hoelker M, Rings F, Salilew D, Jennen D, Tholen E, Sirard M-A, Schellander K, Tesfaye D. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol Genomics. 2006;28:84–96. doi: 10.1152/physiolgenomics.00111.2006. [DOI] [PubMed] [Google Scholar]
- Farin CE, Imakawa K, Roberts RM. In situ localization of mRNA for the interferon, ovine trophoblast protein-1, during early embryonic development of the sheep. Mol Endocrinol. 1989;3:1099–1107. doi: 10.1210/mend-3-7-1099. [DOI] [PubMed] [Google Scholar]
- Farin CE, Imakawa K, Hansen TR, McDonnell JJ, Murphy CN, Farin PW, Roberts RM. Expression of trophoblastic interferon genes in sheep and cattle. Biol Reprod. 1990;43:210–218. doi: 10.1095/biolreprod43.2.210. [DOI] [PubMed] [Google Scholar]
- Forde N, Carter F, Fair T, Crowe MA, Evans AC, Spencer TE, Bazer FW, McBride R, Boland MP, O'Gaora P, Lonergan P, Roche JF. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod. 2009;81:784–794. doi: 10.1095/biolreprod.108.074336. [DOI] [PubMed] [Google Scholar]
- Forde N, Spencer TE, Bazer FW, Song G, Roche JF, Lonergan P. Effect of pregnancy and progesterone concentration on expression of genes encoding for transporters or secreted proteins in the bovine endometrium. Physiological genomics. 2010;41:53–62. doi: 10.1152/physiolgenomics.00162.2009. [DOI] [PubMed] [Google Scholar]
- Forde N, Beltman ME, Duffy GB, Duffy P, Mehta JP, O'Gaora P, Roche JF, Lonergan P, Crowe MA. Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol Reprod. 2011a;84:266–278. doi: 10.1095/biolreprod.110.085910. [DOI] [PubMed] [Google Scholar]
- Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O'Gaora P, Roche JF, Lonergan P. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod. 2011b;85:144–156. doi: 10.1095/biolreprod.110.090019. [DOI] [PubMed] [Google Scholar]
- Forde N, Lonergan P. Transcriptomic analysis of the bovine endometrium: what is required to establish uterine receptivity to implantation in cattle? J Reprod Dev. 2012;58:189–195. doi: 10.1262/jrd.2011-021. [DOI] [PubMed] [Google Scholar]
- Forde N, Mehta JP, Minten M, Crowe MA, Roche JF, Spencer TE, Lonergan P. Effects of low progesterone on the endometrial transcriptome in cattle. Biol Reprod. 2012;87:124. doi: 10.1095/biolreprod.112.103424. [DOI] [PubMed] [Google Scholar]
- Gong Y, Ballejo G, Alkhalaf B, Molnar P, Murphy LC, Murphy LJ. Phorbol esters differentially regulate the expression of insulin-like growth factor-binding proteins in endometrial carcinoma cells. Endocrinology. 1992;131:2747–2754. doi: 10.1210/endo.131.6.1280205. [DOI] [PubMed] [Google Scholar]
- Gray CA, Taylor KM, Ramsey WS, Hill JR, Bazer FW, Bartol FF, Spencer TE. Endometrial glands are required for conceptus elongation and survival. Biol Reprod. 2001;64:1608–1613. doi: 10.1095/biolreprod64.6.1608. [DOI] [PubMed] [Google Scholar]
- Groebner AE, Schulke K, Unterseer S, Reichenbach HD, Reichenbach M, Buttner M, Wolf E, Meyer HH, Ulbrich SE. Enhanced proapoptotic gene expression of XAF1, CASP8 and TNFSF10 in the bovine endometrium during early pregnancy is not correlated with augmented apoptosis. Placenta. 2010;31:168–177. doi: 10.1016/j.placenta.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Guillomot M, Flechon JE, Wintenberger-Torres S. Conceptus attachment in the ewe: an ultrastructural study. Placenta. 1981;2:169–182. doi: 10.1016/s0143-4004(81)80021-5. [DOI] [PubMed] [Google Scholar]
- Hansen TR, Austin KJ, Perry DJ, Pru JK, Teixeira MG, Johnson GA. Mechanism of action of interferon-tau in the uterus during early pregnancy. J Reprod Fertil. 1999;54:329–339. [PubMed] [Google Scholar]
- Hansen TR, Henkes LK, Ashley RL, Bott RC, Antoniazzi AQ, Han H. Endocrine actions of interferon-tau in ruminants. Soc Reprod Fertil Suppl. 2010;67:325–340. doi: 10.7313/upo9781907284991.026. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ledezma JJ, Sikes JD, Murphy CN, Watson AJ, Schultz GA, Roberts RM. Expression of bovine trophoblast interferon in conceptuses derived by in vitro techniques. Biol Reprod. 1992;47:374–380. doi: 10.1095/biolreprod47.3.374. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Austin KJ, Collins AM, Murdoch WJ, Hansen TR. Endometrial ISG17 mRNA and a related mRNA are induced by interferon-tau and localized to glandular epithelial and stromal cells from pregnant cows. Endocrine. 1999a;10:243–252. doi: 10.1007/BF02738623. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Hansen TR, Austin KJ, Burghardt RC, Bazer FW. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol Reprod. 1999b;61:312–318. doi: 10.1095/biolreprod61.1.312. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Spencer TE, Burghardt RC, Joyce MM, Bazer FW. Interferon-tau and progesterone regulate ubiquitin cross-reactive protein expression in the ovine uterus. Biol Reprod. 2000;62:622–627. doi: 10.1095/biolreprod62.3.622. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Stewart MD, Gray CA, Choi Y, Burghardt RC, Yu-Lee LY, Bazer FW, Spencer TE. Effects of the estrous cycle, pregnancy, and interferon tau on 2′,5′-oligoadenylate synthetase expression in the ovine uterus. Biol Reprod. 2001;64:1392–1399. doi: 10.1095/biolreprod64.5.1392. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Bazer FW, Burghardt RC, Spencer TE, Wu G, Bayless KJ. Conceptus-uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptuses. Soc Reprod Fertil Suppl. 2009;66:321–332. [PubMed] [Google Scholar]
- Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134:635–643. doi: 10.1530/REP-07-0328. [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Larson MA, Roberts RM. Relationship between age of blastocyst formation and interferon-tau secretion by in vitro-derived bovine embryos. Mol Reprod Dev. 1998;49:254–260. doi: 10.1002/(SICI)1098-2795(199803)49:3<254::AID-MRD5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Kubisch HM, Larson MA, Kiesling DO. Control of interferon-tau secretion by in vitro-derived bovine blastocysts during extended culture and outgrowth formation. Molecular reproduction and development. 2001;58:390–397. doi: 10.1002/1098-2795(20010401)58:4<390::AID-MRD6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Lewis GS. Prostaglandin secretion by the blastocyst. J Reprod Fertil Suppl. 1989;37:261–267. [PubMed] [Google Scholar]
- Lewis GS, Thatcher WW, Bazer FW, Curl JS. Metabolism of arachidonic acid in vitro by bovine blastocysts and endometrium. Biol Reprod. 1982;27:431–439. doi: 10.1095/biolreprod27.2.431. [DOI] [PubMed] [Google Scholar]
- Lewis GS, Waterman RA. Effects of endometrium on metabolism of arachidonic acid by bovine blastocysts in vitro. Prostaglandins. 1983;25:881–889. doi: 10.1016/0090-6980(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Lewis GS, Waterman RA. Metabolism of arachidonic acid in vitro by ovine conceptuses recovered during early pregnancy. Prostaglandins. 1985;30:263–283. doi: 10.1016/0090-6980(85)90190-x. [DOI] [PubMed] [Google Scholar]
- Lonergan P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology. 2011;76:1594–1601. doi: 10.1016/j.theriogenology.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Mansouri-Attia N, Aubert J, Reinaud P, Giraud-Delville C, Taghouti G, Galio L, Everts RE, Degrelle S, Richard C, Hue I, Yang X, Tian XC, Lewin HA, Renard J-P, Sandra O. Gene expression profiles of bovine caruncular and intercaruncular endometrium at implantation. Physiol Genomics. 2009;39:14–27. doi: 10.1152/physiolgenomics.90404.2008. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Neira JA, Tainturier D, L'Haridon RM, Martal J. Comparative IFN-tau secretion after hatching by bovine blastocysts derived ex vivo and completely produced in vitro. Reprod Domest Anim. 2007;42:68–75. doi: 10.1111/j.1439-0531.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- Ott TL, Gifford CA. Effects of early conceptus signals on circulating immune cells: lessons from domestic ruminants. American journal of reproductive immunology. 2010;64:245–254. doi: 10.1111/j.1600-0897.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282:20047–20051. doi: 10.1074/jbc.R700004200. [DOI] [PubMed] [Google Scholar]
- Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 2002;61:234–248. doi: 10.1002/mrd.1153. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Ezashi T, Rosenfeld CS, Ealy AD, Kubisch HM. Evolution of the interferon tau genes and their promoters, and maternal-trophoblast interactions in control of their expression. Reprod Suppl. 2003;61:239–251. [PubMed] [Google Scholar]
- Robinson RS, Fray MD, Wathes DC, Lamming GE, Mann GE. In vivo expression of interferon tau mRNA by the embryonic trophoblast and uterine concentrations of interferon tau protein during early pregnancy in the cow. Mol Reprod Dev. 2006;73:470–474. doi: 10.1002/mrd.20431. [DOI] [PubMed] [Google Scholar]
- Shemesh M, Milaguir F, Ayalon N, Hansel W. Steroidogenesis and prostaglandin synthesis by cultured bovine blastocysts. J Reprod Fertil. 1979;56:181–185. doi: 10.1530/jrf.0.0560181. [DOI] [PubMed] [Google Scholar]
- Short EC, Jr, Geisert RD, Helmer SD, Zavy MT, Fulton RW. Expression of antiviral activity and induction of 2′,5′-oligoadenylate synthetase by conceptus secretory proteins enriched in bovine trophoblast protein-1. Biol Reprod. 1991;44:261–268. doi: 10.1095/biolreprod44.2.261. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Erikson DW, Kim J, Burghardt RC, Bazer FW, Johnson GA, Spencer TE. Insulin-like growth factor binding protein one in the ruminant uterus: potential endometrial marker and regulator of conceptus elongation. Endocrinology. 2009;150:4295–4305. doi: 10.1210/en.2009-0060. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Satterfield MC, Welsh TH, Jr, Bazer FW, Spencer TE. HSD11B1, HSD11B2, PTGS2, and NR3C1 expression in the peri-implantation ovine uterus: effects of pregnancy, progesterone, and interferon tau. Biol Reprod. 2010;82:35–43. doi: 10.1095/biolreprod.109.079608. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil Suppl. 2007;64:379–396. doi: 10.5661/rdr-vi-379. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Sandra O, Wolf E. Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction. 2008;135:165–179. doi: 10.1530/REP-07-0327. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1{beta} induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000;141:4664–4670. doi: 10.1210/endo.141.12.7810. [DOI] [PubMed] [Google Scholar]
- Thatcher WW, Hansen PJ, Gross TS, Helmer SD, Plante C, Bazer FW. Antiluteolytic effects of bovine trophoblast protein-1. J Reprod Fertil Suppl. 1989;37:91–99. [PubMed] [Google Scholar]
- Toyokawa K, Leite F, Ott TL. Cellular localization and function of the antiviral protein, ovine Mx1 (oMx1): II. The oMx1 protein is a regulator of secretion in an ovine glandular epithelial cell line. American journal of reproductive immunology. 2007;57:23–33. doi: 10.1111/j.1600-0897.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Ulbrich SE, Schulke K, Groebner AE, Reichenbach HD, Angioni C, Geisslinger G, Meyer HH. Quantitative characterization of prostaglandins in the uterus of early pregnant cattle. Reproduction. 2009;138:371–382. doi: 10.1530/REP-09-0081. [DOI] [PubMed] [Google Scholar]
- Van Heeke G, Ott TL, Strauss A, Ammaturo D, Bazer FW. High yield expression and secretion of the ovine pregnancy recognition hormone interferon-tau by Pichia pastoris. J Interferon Cytokine Res. 1996;16:119–126. doi: 10.1089/jir.1996.16.119. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.