Abstract
The integration of metabolism and reproduction involves complex interactions of hypothalamic neuropeptides with metabolic hormones, fuels, and sex steroids. Of these, estrogen influences food intake, body weight, and the accumulation and distribution of adipose tissue. In this study, the effects of estrogen on food intake, serum leptin levels, and leptin mRNA expression were evaluated in ovariectomized rats. Seven-week-old female Wistar-Imamichi rats were ovariectomized and divided into three treatment groups: group 1 (the control group) received sesame oil, group 2 was given 17β-estradiol benzoate, and group 3 received 17β-estradiol benzoate plus progesterone. The body weight and food consumption of each rat were determined daily. Serum leptin levels and leptin mRNA expression were measured by ELISA and quantitative RT-PCR, respectively. Food consumption in the control group was significantly higher (P<0.05) than that in groups 2 and 3, although body weight did not significantly differ among the three groups. The serum leptin concentration and leptin mRNA expression were significantly higher (P<0.05) in groups 2 and 3 than in group 1, but no significant difference existed between groups 2 and 3. In conclusion, estrogen influenced food intake via the modulation of leptin signaling pathway in ovariectomized rats.
Keywords: Estradiol, food intake, leptin, ovariectomized rats
Leptin, the obese (ob) gene product, is an adipocyte-derived hormone [1,2] that regulates body weight and metabolism [3] by influencing food intake and increasing energy expenditure [4,5]. Plasma leptin concentrations are directly related to the amount of body fat in rats [6].
Sex steroid hormones are involved in the metabolism, accumulation, and distribution of adipose tissue [7]. For example, estrogen has effects on food intake and body weight, which are similar to those of leptin: it causes a transient reduction in food intake and a moderate reduction in body weight in ovariectomized female mice [8]. Recent data indicate that women have higher levels of circulating leptin than men [9,10], suggesting that sex steroid hormones exert major effects on leptin production in humans and rats [2,11]. In an animal study, Tanaka et al. [12] indicated that the serum leptin level changed significantly during the estrous cycle and peaked at the proestrous stage. A previous report [13] showed that food intake in female rats was significantly lower at the proestrous stage, compared with other stages, whereas serum leptin levels and leptin mRNA expression were significantly higher at the proestrous stage. Fluctuations in the serum leptin level in ovariectomized rats were eliminated by estrogen replacement [7]. Given that finding, estrogen was hypothesized to be involved in the mechanism by which leptin regulates feeding behavior and adiposity in female rats.
The present study was undertaken to evaluate the effects of estrogen on food intake, body weight, serum leptin levels and leptin mRNA expression in ovariectomized rats.
Materials and Methods
Animals and housing conditions
Six-week-old female Wistar-Imamichi rats were housed in individual cages and kept at 24±2℃ in a light-controlled room (12:12 h light:dark cycle; lights on 07:00 to 19:00) and were given free access to water and laboratory chow (Oriental MF; Oriental Yeast Co., Ltd., Tokyo, Japan). At the onset of the experiment, the rats weighed 170-180 g.
All animal procedures were approved by the Institutional Animal Care and Use Committee of Nippon Veterinary and Life Science University (Tokyo, Japan) and carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All efforts were undertaken according to the "3R principles" of the directive to reduce the number of animals used in this study and to optimize the experimental protocols to acquire the maximum amount of data from each tested animal.
Experimental design
At seven weeks of age, the rats were ovariectomized under sodium pentobarbital (40 mg/kg) anesthesia. Five days after surgery, the rats were divided into three groups of six rats each. The control group (group 1) was given a daily subcutaneous injection of sesame oil (0.1 mL/rat) on days 10-13 postsurgery. The remaining groups (groups 2 and 3) were injected with 2 µg of 17β-estradiol benzoate (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.1 mL of sesame oil on days 10-12 postsurgery; half of the rats were injected with 0.5 mg of progesterone (Sigma-Aldrich) dissolved in 0.1 mL of sesame oil on day 13. Thus, the rats in group 3 (17β-estradiol benzoate+progesterone) were expected to behave like females in the proestrous stage of the estrous cycle as compared with group 2 (17β-estradiol benzoate only). Drug administration was done at 11:00 every day. All rats were anesthetized with sodium pentobarbital on day 14 postsurgery. Individual blood samples were collected from the vena cava caudalis using a syringe between 09:00 and 10:00 and centrifuged at 2,200 g for 15 min at 4℃. The serum samples were kept frozen at -35℃ until the leptin assay. Peri-uterine adipose tissue was dissected, frozen immediately in liquid nitrogen, and stored at -80℃ until RNA extraction.
Measurement of body weight and food consumption
Body weight and food consumption were monitored daily by weighting at 11:00; a visual check was made for any diet dropped on the floor of the cage. The food intake of each rat was measured by weighing the remaining chow.
Measurement of leptin
Leptin concentrations in serum (5 µL) were determined using the Rat Leptin ELISA kit (Morinaga Institute of Biological Science Inc., Yokohama, Japan). The assay sensitivity was 200 pg/mL. The inter- and intra-assay coefficients of variation were less than 10%.
Leptin mRNA expression
For the analysis of leptin mRNA expression, visceral abdominal fat was dissected, frozen immediately in liquid nitrogen, and stored at -80℃ until RNA extraction. Total RNA was isolated using an RNeasy Lipid Tissue Midi Kit (Qiagen, Gaithersburg, MD, USA) according to the manufacturer's instructions. The RNA samples were quantified spectrophotometrically (ND-1000 [V3.12], NanoDrop, Houston, TX, USA), and the integrity was confirmed using a 1.5% agarose gel containing 2.2 M formaldehyde. Total RNA from each sample was transcribed into first-strand cDNA using SuperScript™ III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) in a final volume of 20 µL; 1 µg of the RNA sample was mixed with 1 µL of 20 µM oligo dT Primer in DNase- and RNase-free water. The samples were then incubated at 65℃ for 10 min and placed on ice. Next, 4 µL of 5× first-stand buffer, 2 µL of 0.1M dithiothreitol, and 4 µL of 10 mM 2-deoxynucleoside 5-triphosphate mixed solution was added. The samples were preincubated at 42℃ for 2 min, and then 0.5 µL of SuperScript™ III Reverse Transcriptase was added. The samples were then incubated at 42℃ for 1 h, 30 µL of TE buffer (Tris-hydrochloride buffer, pH 8.0, containing 1.0 mM EDTA) was added, and the samples were heated at 65℃ for 10 min to inactivate the reverse transcriptase.
Real-time RT-PCR was performed using a leptin (Gene Bank Accession No.: NM_013076) specific forward primer (5'-GACCAGACCCTGGCAGTCTA-3') and reverse primer (5'-CTCAGCATTCAGGGCTAA GG-3') to amplify the product. The samples were normalized using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Gene Bank Accession No. : NM_017008) using a forward primer (5'-TGTCAGCAATGCATCCTGCA-3') and reverse primer (5'-CCGTTCAGCTCTGGGATGAC-3'). Amplified products' size for leptin and GAPDH cDNAs are 280 bp and 240 bp, respectively. Real-time RT-PCR was performed in a final reaction volume of 25 µL using Platinum SYBR Green qPCR Supermix-UDG (Invitrogen). Each real-time RT-PCR contained 12.5 µL of Platinum SYBR Green qPCR Supermix-UDG, 10 µM each primer, and an RNA template. Real-time RT-PCR was carried out in duplicate for each sample using an ABI 7500 Real-time RT-PCR System (Applied Biosystems, Foster City, CA, USA) with the following parameters: one cycle at 50℃ for 2 min (UDG incubation), 95℃ for 2 min, and 40 cycles at 95℃ for 15 s and 60℃ for 30 s. Melting curve analyses were performed for each series to confirm the specificity of the amplified products. To confirm the absence of a genomic DNA signal, amplification without reverse transcriptase was performed. Standard curves were prepared for the tested and housekeeping genes. All expression data were normalized by dividing the amount of GAPDH used for the target gene by the amount used for the control.
Statistical analysis
All data are expressed as the mean±SEM. Statistically significant differences were evaluated by an ANOVA and Tukey's test to examine the effects among subject variables. Differences were considered significant at P<0.05.
Results
Food intake and body weight
Data related to food intake and body weight are presented in Figures 1 and 2. Notably, the food intake of the rats did not differ among the three groups before treatment, whereas groups 2 and 3 had a significantly lower food intake than group 1 following estrogen treatment. The mean food intake in groups 1, 2, and 3 was 19.51±0.51, 16.61±0.35, and 16.83±0.34 g/day, respectively, on day 12 and 19.67±0.31, 15.86±1.13, and 16.75±1.06 g/day, respectively, on day 13. The mean food intake on day 14 in group 3 (17.93±0.40 g/day) was significantly lower (P<0.05) than that in group 1 (19.99±0.46 g/day). After estrogen treatment, the rate of food intake in groups 2 and 3 decreased by 14.86% and 13.73%, respectively, compared with group 1. The mean body weights of the rats were not significantly different among the three groups.
Figure 1.
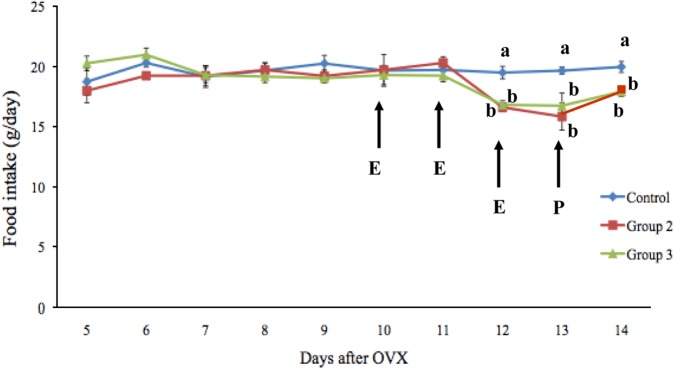
Effects of estrogen and progesterone on food intake in ovariectomized rats. Data are presented as means±SEM. *P<0.05, vs Groups 2 and 3.
Figure 2.
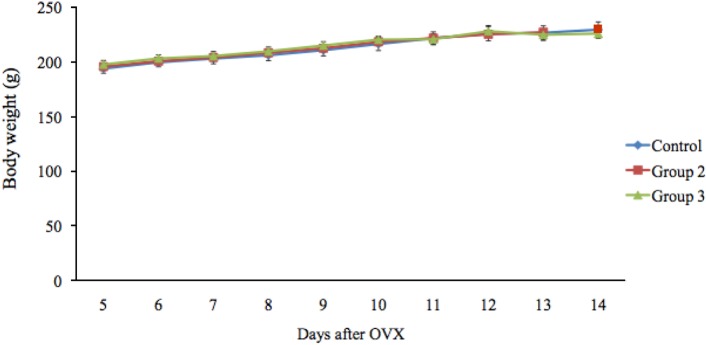
Effects of estrogen and progesterone on body weight in ovariectomized rats.
Serum leptin concentration and leptin mRNA expression
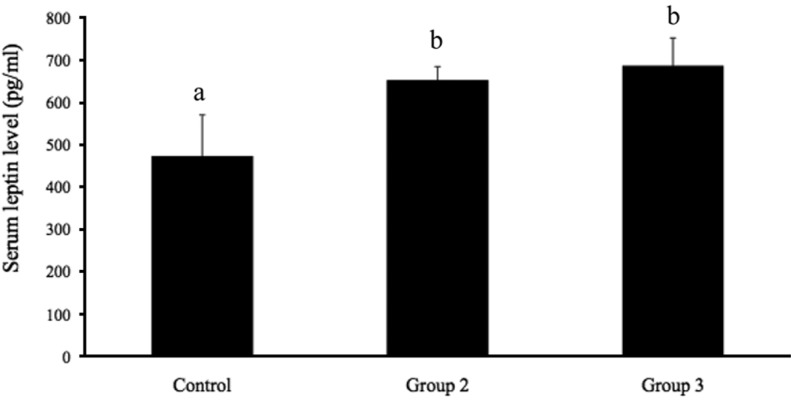
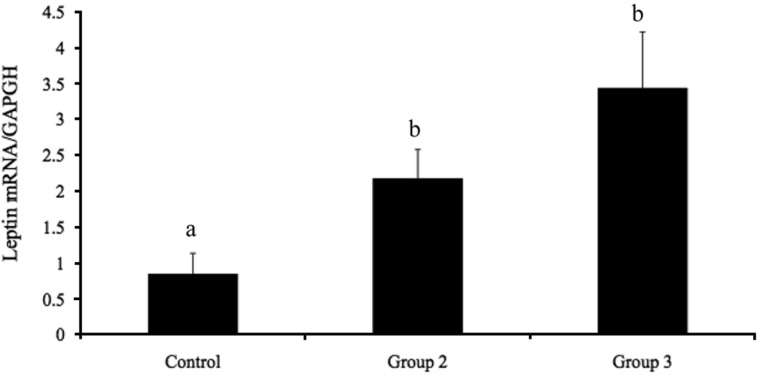
Figure 3 shows the serum leptin levels in groups 1, 2, and 3. The serum leptin levels in group 1 (473.40±97.19 pg/mL) were significantly lower (P<0.05) than those in groups 2 and 3 (653.20±31.55 and 687.20±65.18 pg/mL, respectively). No significant difference in leptin was observed between groups 2 and 3. The expression of leptin mRNA in adipose tissue is presented in Figure 4. Leptin mRNA expression was significantly lower in group 1 (0.85±0.29; P<0.05) than in groups 2 and 3 (2.17±0.40 and 3.44±0.78, respectively). No significant differences in leptin mRNA expression were detected between groups 2 and 3. These data show that estrogen stimulates leptin mRNA expression and leptin secretion from adipose tissue.
Figure 3.
Effects of estrogen (EB) and progesterone (P) on serum leptin levels in ovariectomized rats (OVX) . Data are presented as means±SEM. The different characters indicate significant differences (P<0.05).
Figure 4.
Effects of estrogen (EB) and progesterone (P) on serum leptin levels in ovariectomized rats (OVX). Data are presented as means±SEM. The different characters indicate significant differences (P<0.05).
Discussion
Sex steroid hormones are involved in the metabolism, accumulation, and distribution of adipose tissue [7]. Estrogen is a potent anorectic agent shown to reduce food intake and body weight. As expected, following the injection of estrogen and progesterone, the food intake in groups 2 and 3 decreased significantly compared with group 1, while no change in body weight was observed among the three groups. These findings indicate that estrogen administration completely reversed the high food intake in groups 2 and 3, and that progesterone administration seemed to have no effect on the anorectic effect of estrogen. The anorectic effect of estrogen was previously shown to have a delayed onset in ovariectomized rats: a decrease in food intake did not occur until 24-48 h after injection [14].
Estrogen has been demonstrated to influence feeding behavior by acting via estrogen receptor (ERα) in hypothalamic nuclei: the ventral medial nucleus, arcuate nucleus (ARC), medial preoptic area, and paraventricular nucleus are all considered to be important in feeding behavior [15,16]. Liang et al. [17] indicated that ERβ was involved in the anorectic action of estrogen, and a separate report confirmed that the activation of central estrogen receptors was necessary for the anorectic effect of the hormone [18]. Estrogen has been proposed to act directly and indirectly through orexigenic peptides, including neuropeptide Y (NPY), ghrelin, and melanin-concentrating hormone, or anorexigenic neuropeptides such as insulin, serotonin, cholecystokinin, and leptin [19,20]. Leptin receptor (Leprb) expression in the ARC is reportedly co-localized with estrogen receptor and estrogen was stated to regulate the expression of Leprb mRNA in the ARC, suggesting a closely coupled interaction between these peripheral signals in the regulation of energy homeostasis [16,21].
Several studies have reported that estrogen may also elevate the circulating leptin concentration in humans and rats [12,22], and that the reduction in leptin expression in white adipose tissue in ovariectomized rats can be reversed by estrogen administration [15,23]. Together, these results demonstrate increased serum leptin concentrations and leptin mRNA expression due to high levels of estrogen in both cyclic female rats and estrogen-treated ovariectomized rats. Furthermore, the above results are in agreement with those of previous reports [12,24-27] showing that estrogen increased the serum leptin concentration and leptin mRNA expression in adipose tissue both in vitro and in vivo. However, Clegg et al. [27] indicated that estrogen administration had no effect on serum leptin concentrations and that estrogen increased leptin sensitivity in the brain. Their results also showed that the effect of administering estrogen or estrogen plus progesterone was not significantly different in terms of the serum leptin level and leptin mRNA expression [27].
The mechanisms underlying the regulation of leptin concentrations by estrogen have been studied. O'Neil et al. [28] demonstrated that estrogen enhanced leptin promoter activity in JEG-3 cells, suggesting that estrogen regulates LEP gene expression via promoter activation, while Gambino et al. [26] showed that estrogen induced leptin expression in trophoblast cells through genomic and extragenomic actions via estrogen receptor1 and MAPK and PI3K signaling. Yi et al. [29] indicated that ERα induced leptin expression, whereas ERβ inhibited its expression, in 3T3-L1 adipocytes and that the ratio of ERα to ERβ expression in 3T3-L1 adipocytes may be an important potential regulatory factor in leptin expression. Further analysis of the regulatory role of estrogen in adipose tissue is needed to confirm our findings and to define the mechanism involved.
In conclusion, our results indicate that estrogen influences the food intake of ovariectomized rats via positive effects on leptin mRNA expression and the serum leptin concentration. Additional studies are needed to clarify the mechanism underlying the regulation of ob gene expression by estrogen.
Acknowledgments
The authors thank Dr. N. Nakao, Department of Animal Physiology, Nippon Veterinary and Life Science University, for his valuable advice. This study was supported in part by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to TRS), and the Nippon Medical School Foundation (to WF).
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Watanobe H, Suda T. A detailed study on the role of sex steroid milieu in determining plasma leptin concentrations in adult male and female rats. Biochem Biophys Res Commun. 1999;259(1):56–59. doi: 10.1006/bbrc.1999.0718. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed WF, Alsuwaigh BR, Alsuhaimi EA. Effect of gonadal sex steroid on serum leptin level of both adult male and female rat. Sci J King Faisal Univ (Basic Appl Sci) 2004;5:239–247. [Google Scholar]
- 4.Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, Mackellar W, Rosteck PR, JR, Schoner B, Smith D, Tinsley FC, Zhang XY, Heiman M. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 5.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 6.Rocha M, Grueso E, Puerta M. The anorectic effect of oestradiol does not involve changes in plasma and cerebrospinal fluid leptin concentrations in the rat. J Endocrinol. 2001;171(2):349–354. doi: 10.1677/joe.0.1710349. [DOI] [PubMed] [Google Scholar]
- 7.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5(4):197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 8.Pelleymounter MA, Baker MB, McCaleb M. Does estradiol mediate leptin's effects on adiposity and body weight? Am J Physiol. 1999;276:E955–E963. doi: 10.1152/ajpendo.1999.276.5.E955. [DOI] [PubMed] [Google Scholar]
- 9.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82(2):579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 10.Demerath EW, Towne B, Wisemandle W, Blangero J, Chumlea WC, Siervogel RM. Serum leptin concentration, body composition, and gonadal hormones during puberty. Int J Obes Relat Metab Disord. 1999;23(7):678–685. doi: 10.1038/sj.ijo.0800902. [DOI] [PubMed] [Google Scholar]
- 11.Pinilla L, Seoane LM, Gonzalez L, Carro E, Aguilar E, Casanueva FF, Dieguez C. Regulation of serum leptin levels by gonadal function in rats. Eur J Endocrinol. 1999;140(5):468–473. doi: 10.1530/eje.0.1400468. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Nakaya S, Kumai T, Watanabe M, Tateishi T, Shimizu H, Kobayashi S. Effects of estrogen on serum leptin levels and leptin mRNA expression in adipose tissue in rats. Horm Res. 2001;56(3-4):98–104. doi: 10.1159/000048099. [DOI] [PubMed] [Google Scholar]
- 13.Fungfuang W, Nakada T, Nakao N, Terada M, Yokosuka M, Gizurarson S, Hau J, Moon C, Saito TR. Serum leptin concentrations, leptin mRNA expression, and food intake during the estrous cycle in rats. Lab Anim Res. 2013;29(1):1–6. doi: 10.5625/lar.2013.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82(1):35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda N, Saito S, Kimura M, Yamada M, Iida M, Murakami T, Irahara M, Shima K, Aono T. The influence of ovariectomy on ob gene expression in rats. Horm Metab Res. 1998;30(5):263–265. doi: 10.1055/s-2007-978880. [DOI] [PubMed] [Google Scholar]
- 16.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor beta is involved in the anorectic action of estrogen. Int J Obes Relat Metab Disord. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- 18.Rivera HM, Eckel LA. Activation of central, but not peripheral, estrogen receptors is necessary for estradiol's anorexigenic effect in ovariectomized rats. Endocrinology. 2010;151(12):5680–5688. doi: 10.1210/en.2010-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122(1-3):65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto S, Shimizu M, Mizuno A, Higuchi T. Estrogens inhibit food intake in CCK-1 receptor-deficient rats. J Physiol Sci. 2010;60(4):267–271. doi: 10.1007/s12576-010-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69(6):417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu H, Shimomura Y, Nakanishi Y, Futawatari T, Ohtani K, Sato N, Mori M. Estrogen increases in vivo leptin production in rats and human subjects. J Endocrinol. 1997;154(2):285–292. doi: 10.1677/joe.0.1540285. [DOI] [PubMed] [Google Scholar]
- 23.Henson MC, Castracane VD. Leptin in pregnancy: an update. Biol Reprod. 2006;74(2):218–229. doi: 10.1095/biolreprod.105.045120. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen K, Pedersen SB, Richelsen B. Regulation of leptin by steroid hormones in rat adipose tissue. Biochem Biophys Res Commun. 1999;259(3):624–630. doi: 10.1006/bbrc.1999.0842. [DOI] [PubMed] [Google Scholar]
- 25.Alonso A, Fernández R, Moreno M, Ordóñez P, Díaz F, González C. Leptin and its receptor are controlled by 17beta-estradiol in peripheral tissues of ovariectomized rats. Exp Biol Med (Maywood) 2007;232(4):542–549. [PubMed] [Google Scholar]
- 26.Gambino YP, Maymó JL, Pérez-Pérez A, Dueñas JL, Sánchez-Margalet V, Calvo JC, Varone CL. 17Beta-estradiol enhances leptin expression in human placental cells through genomic and nongenomic actions. Biol Reprod. 2010;83(1):42–51. doi: 10.1095/biolreprod.110.083535. [DOI] [PubMed] [Google Scholar]
- 27.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 28.O'Neil JS, Burow ME, Green AE, McLachlan JA, Henson MC. Effects of estrogen on leptin gene promoter activation in MCF-7 breast cancer and JEG-3 choriocarcinoma cells: selective regulation via estrogen receptors alpha and beta. Mol Cell Endocrinol. 2001;176(1-2):67–75. doi: 10.1016/s0303-7207(01)00473-7. [DOI] [PubMed] [Google Scholar]
- 29.Yi KW, Shin JH, Seo HS, Lee JK, Oh MJ, Kim T, Saw HS, Kim SH, Hur JY. Role of estrogen receptor-alpha and -beta in regulating leptin expression in 3T3-L1 adipocytes. Obesity (Silver Spring) 2008;16(11):2393–2399. doi: 10.1038/oby.2008.389. [DOI] [PubMed] [Google Scholar]