Abstract
The effects of a β-dunnione compound MB12662 on the gastric secretion and ulcers were investigated in rats. In order to assess the effects of MB12662 on the gastric secretion and acidity, rats were subjected to pylorus ligation operation, and 6 hours later, gastric fluid was collected. Treatment with MB12662 reduced the gastric fluid volume to 47.3% of control level and increased pH. In an alcohol-induced ulcer model, rats were orally administered 3 mL/kg of ethanol, and 1 hour later, the ulcer lesions ware measured under a stereomicroscope. MB12662 reduced ulcer index in a dose-dependent manner which was much stronger than a proton-pump inhibitor pantoprazole. In a stress-induced ulcer model, rats were subjected to water-immersion restraint stress, and 5 hours later, the ulcer lesions ware examined. MB12662 also attenuated the stress-induced gastric lesions, although the efficacy of MB12662 was lower than that of pantoprazole. Therefore, it is suggested that MB12662 could be a candidate compound for the prevention or treatment of gastric ulcers induced by gastric over-secretion and alcoholic hangover.
Keywords: Gastric secretion, ulcer, alcohol, stress, β-dunnione, MB12662
Peptic ulcers are caused by various factors including gastric over-secretion and retention, weakening and depleting agents of mucin layer, blood flow disturbances, and mucosal injury and inflammation [1,2]. Ulcerinducing agents include alcohols [3], stresses [2,3], non-steroidal anti-inflammatory drugs [3], and bacterial (Helicobacter pylori) infection [1,4].
For the therapy of gastric ulcers, proton-pump inhibitors that block acid secretion from parietal cells, antacids, histamine receptor (H2) antagonists, prostaglandins (PGs) that strengthen mucin layer, and antibiotics to eliminate H. pylori have been used [1]. Diverse gastric ulcers were attenuated by blockade of acid secretion, indicating that gastric secretion is a key factor for the induction of erosions and ulcers [5].
Recently, we have synthesized β-dunniones and β-lapachones, the antifungal orange-red pigments of Streptocarpus dunnii Mast. [6], and found out their potential antioxidative activity (unpublished results). A β-lapachone MB12066 decreased gastric secretion and acidity [7], and a β-dunnione MB12662 substantially alleviated cisplatin-induced intestinal necrosis (unpublished results). Thus, we assessed whether MB12662 blocks gastric secretion, and thereby improves diverse ulcers induced by alcohol and stress, the major causes of peptic ulcer in modern civilians.
A β-dunnione (2,3,3-trimethyl-2,3-dihydronaphtho [1,2-β]furan-4,5-dione; Mazence code: MB12662) was synthesized in Mazence Co. (Suwon, Korea), and stored at 4℃ until use. Male Sprague-Dawley rats (body weight 200-220 g) from Samtako Co. (Osan, Korea) were housed in a room with temperature of 23±2℃, relative humidity of 55±5%, a 12-hour light/dark cycle, and feed and water available ad libitum. All animal experiments were conducted in accordance with the Institutional Animal Care and Use Committee of the Laboratory Animal Research Center, Chungbuk National University, Korea.
For the measurement of gastric secretion and acidity, the rats (n=8/group), fasted for 48 hours, were anesthetized with ether, and their pylorus was ligated with a 4-0 silk after median incision of abdomen from xiphoid process [7]. Immediately after the operation, MB12662 or pantoprazole were injected into duodenum. Control animals were given the same volume (5 mL/kg) of vehicle (40% HP β-CD). After suture of the wounds, the rats were allowed to recover in their home cages.
Six hours after pylorus ligation, the rats were sacrificed under deep anesthesia with ether, and their stomach was removed. Gastric fluid was collected and centrifuged at 3,000 rpm for 10 min. The volume and pH of the fluid were measured. Into aliquots (500 µL) of the fluid, Töpfel reagent (1% dimethylaminoazobenzene) or 1% phenolphthalein (10 µL each) were added, and titrated with 0.1 N NaOH solution to measure free HCl and total acidity, respectively [7].
In an alcohol-induced ulcer model, the rats (n=8/group), fasted for 48 hours, were orally administered MB12662 or pantoprazole. Control animals were given the same volume (5 mL/kg) of vehicle (1% carboxymethylcellulose). Thirty minutes later, the rats were orally administered 3 mL/kg of ethanol for the induction of gastric ulcer [8,9].
One hour after alcohol administration, the rats were sacrificed, and their stomach was removed. The stomachs were inflated with 1% formalin (10 mL) and fixed in the formalin solution for 15 min. After incision along the great curvature, the gastric content was removed, and the stomach was unwrapped and fixed on a corkboard. The length of ulcer (hemorrhagic) lesions was measured under a stereomicroscope. Ulcer index was presented as the total length of the lesions [8,9].
In a stress-induced ulcer model, the rats (n=8/group), fasted for 48 hours, were orally administered MB12662 or pantoprazole. Thirty minutes later, the rats were subjected to water-immersion restraint stress (WIRS); the animals were fixed in a restraint device and immersed up to their xiphoid process in a 22℃ water bath for the induction of gastric ulcer [2].
Five hours later, the rats were sacrificed, and their stomach was removed. Ulcer index was calculated as described above.
The results were expressed as the mean±SD. Tests of significance were performed using Duncan's multiple-range test after one-way analysis of variance, with P<0.05 as a criterion of difference.
In vehicle-treated rats, during 6-hour pylorus ligation, mean 5.75 mL of gastric fluid was obtained (Table 1). The gastric secretion was inhibited by treatment with MB12662, resulting in decreases to 83.1% and 47.3% of control level at 30 and 60mg/kg, respectively, comparable to the effect of pantoprazole (30 mg/kg) at 60 mg/kg. pH of gastric fluid was significantly increased by MB12662 (30-60 mg/kg) and pantoprazole (30 mg/kg). In parallel with the increase in pH, free HCl and total acidity were also markedly decreased by MB12662, although the effects did not reach those of pantoprazole.
Table 1.
Effects of MB12662 on the gastric secretion and acidity for 6 hours
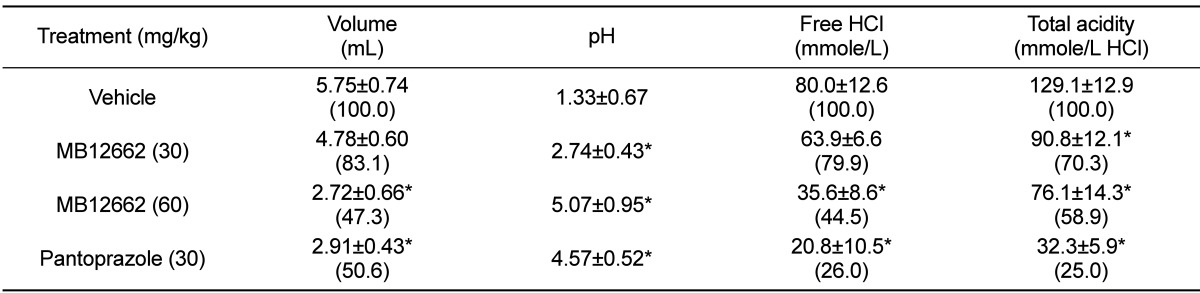
Data in parentheses are % of vehicle control. *Significantly different from vehicle control, P<0.05.
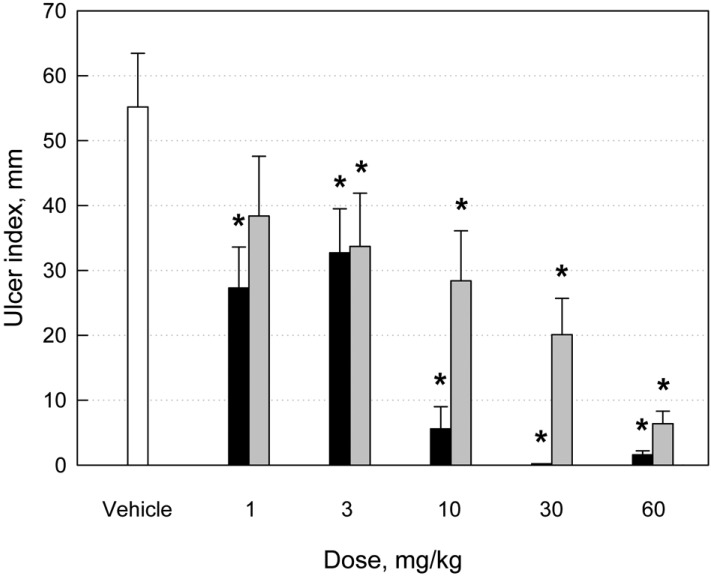
Oral administration of pure ethanol (3 mL/kg) induced severe hemorrhagic ulcers 1 hour after administration, showing mean ulcer index of 55.2 mm (Figures 1A and 2). The lesions were markedly attenuated by treatment with MB12662 in a dose-dependent manner (Figures 1B and 1C, exhibiting a full prevention at high doses (30-60 mg/kg) (Figure 2). By comparison, the effect of MB12662 was superior to pantoprazole (Figures 1D and 2). From the dose-response curve, median effective dose (ED50) value of MB12662 for the improvement of alcohol-induced ulcers was 1.2 mg/kg, which is 10-fold lower than that (ED50=11.7 mg/kg) of pantoprazole.
Figure 1.
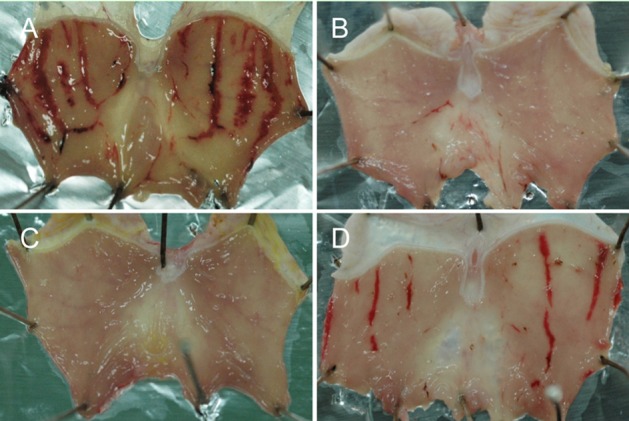
Representative findings of gastric ulcers induced by alcohol. Rats were orally administered with 3 mL/kg of pure ethanol, and sacrificed 1 hour later for the measurement of ulcer lesions. A, vehicle control; B, 10 mg/kg MB12662; C, 30 mg/kg MB12662; D, 10 mg/kg pantoprazole.
Figure 2.
Effects of MB12662 (black) and pantoprazole (gray) on the gastric ulcer index (mm) induced by ethanol (3 mL/kg). *Significantly different from vehicle control, P<0.05.
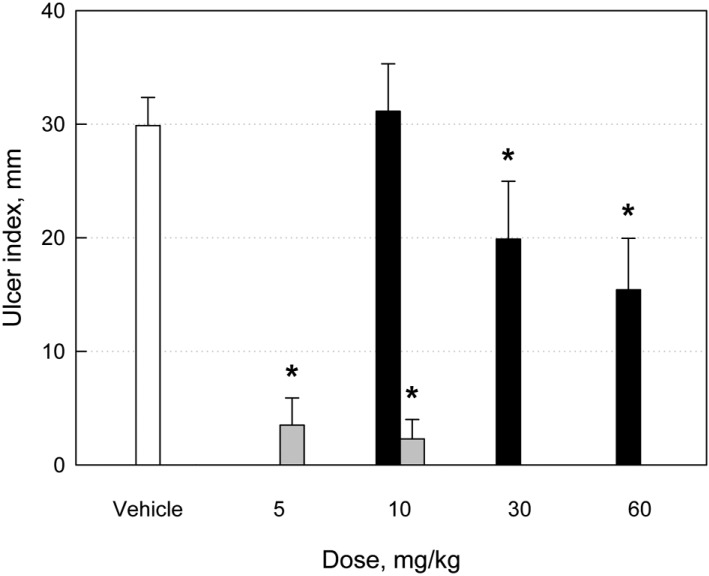
Five-hour WIRS at 22℃ induced linear type of hemorrhagic ulcers, showing mean ulcer index of 29.9 mm (Figures 3A and 4). The lesions were significantly attenuated by treatment with MB12662 (30-60 mg/kg) (Figures 3B and 4). By comparison, pantoprazole showed excellent effects (Figures 3C and 3D), markedly lowering ulcer indices even at low doses (5-10 mg/kg) (Figure 4). The efficacy of MB12662 for the improvement of stress-induced ulcers was much weaker than that of pantoprazole (ED50<5 mg/kg).
Figure 3.
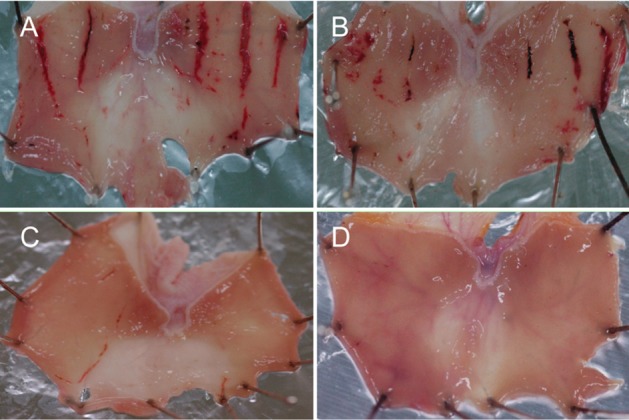
Representative findings of gastric ulcers induced by water-immersion restraint stress (WIRS). Rats were placed in a restraint device, immersed up to their xiphoid process in a 22℃ water bath, and sacrificed 5 hours later for the measurement of ulcer lesions. A, vehicle control; B, 30 mg/kg MB12662; C, 10 mg/kg pantoprazole; D, 30 mg/kg pantoprazole.
Figure 4.
Effects of MB12662 (black) and pantoprazole (gray) on the gastric ulcer index (mm) induced by water-immersion restraint stress (WIRS). *Significantly different from vehicle control, P<0.05.
Long-term pylorus ligation induces gastric wall damage, mediated by gastric acid retention following cholinergic nerve activation [1], which is blocked by proton-pump inhibitors [5]. MB12662 at 60 mg/kg exerted an inhibitory activity on gastric secretion, comparable to pantoprazole (30 mg/kg). In addition, MB12662 significantly increased pH, and decreased free HCl and acidity of the gastric fluid, indicative of an antacid activity. Mechanisms of MB12662 on the antigastric secretion, including influence on proton-pump, as well as antacid effect, remain to be clarified.
Ethanol induces not only mucosal stasis, causing hemorrhage and necrosis, but also lipid peroxidation by triggering radical reactions [10]. DNA-damaging agents such as radicals and chemotherapeutics activate a repair enzyme poly(ADP-ribose) polymerase (PARP), leading to consumption of NAD+ [11,12]. However, excessive activation of PARP depletes NAD+ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in glycolytic pathway, resulting in cell death due to ATP depletion [13]. Notably, MB12066 and MB12662 greatly increased NAD+ via NADH oxidation [14]. In addition, MB12662 exhibited membrane-preserving activity, substantially attenuating intestinal injury induced by cisplatin, a chemotherapeutic agent (unpublished results). In the present study, MB12662 exerted excellent improving effects on alcoholic ulcers. Although exact action mechanisms of MB12662 remain to be clarified, the antiulcer effect might be in part due to its NAD+-preserving potential, in addition to antioxidative and antisecretory activities.
It is well known that WIRS induces extreme mental and psychological stresses to rodents, and thereby stimulates gastric secretion related to central nervous system and pituitary-renal hormonal axis [1,2,15]. Complex neurochemical mechanisms are involved in the response to noxious stimuli like stress [15], and injection of corticosteroids induces gastric erosion [16]. The mechanisms responsible for corticosteroids-induced gastric mucosal damage include inhibition of mucus synthesis, augmented acid secretion, and suppression of PGs synthesis [17]. Therefore, it is suggested that MB12662 may attenuate the stress-induced gastric ulcer at least by decreasing gastric secretion and NAD+-preserving activities.
Taken together, it is expected that the alcohol- and stress-mediated gastric ulcers could be attenuated by MB12662, which may be attained by its substantial antisecretory, antacid, and membrane-protective actions.
Acknowledgments
This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094034).
References
- 1.Neal MJ. Medical Pharmacology at a Glance. 3rd ed. London: Blackwell Publishing Inc; 2003. pp. 30–31. [Google Scholar]
- 2.Byun SK, Lee YE, Shin SH, Jang JY, Choi BI, Park DS, Jeon JH, Nahm SS, Hwang SY, Kim YB. The role of corticosteroids in stress-induced gastric ulceration in rats. Lab Anim Res. 2007;23:127–131. [Google Scholar]
- 3.Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004;91(2-3):243–249. doi: 10.1016/j.jep.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Yang YH, Park D, Yang G, Lee SH, Bae DK, Kyung J, Kim D, Choi EK, Son JC, Hwang SY, Kim YB. Anti-Helicobacter pylori effects of IgY from egg york of immunized hens. Lab Anim Res. 2012;28(1):55–60. doi: 10.5625/lar.2012.28.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao H, Wang MW, Jia JH, Wang QG, Cheng MS. Comparison of the effects of pantoprazole enantimers on gastric mucosal lesions and gastric epithelial cells in rats. J Health Sci. 2004;50:1–8. [Google Scholar]
- 6.Khambay BP, Batty D, Jewess PJ, Bateman GL, Hollomon DW. Mode of action and pesticidal activity of the natural product dunnione and of some analogues. Pest Manag Sci. 2003;59(2):174–182. doi: 10.1002/ps.632. [DOI] [PubMed] [Google Scholar]
- 7.Park D, Cho IG, Yang YH, Kyung J, Kim D, Choi EK, Kwak TH, Yoo SK, Kim YB. Effects of β-lapachone on gastric secretion. J Biomed Res. 2011;12:141–146. [Google Scholar]
- 8.Kim JJ, Kim CH, Park D, Shin S, Jeon JH, Jang MJ, Ji HJ, O N, Song J, Lee J, Kim BY, Choi EK, Joo SS, Hwang SY, Kim YB. Effects of sweet potato fractions on alcoholic hangover and gastric ulcer. Lab Anim Res. 2008;24:209–216. [Google Scholar]
- 9.Bae DK, Park D, Lee SH, Yang G, Yang YH, Kim TK, Choi YJ, Kim JJ, Jeon JH, Jang MJ, Choi EK, Hwang SY, Kim YB. Different antiulcer activities of pantoprazole in stress, alcohol and pylorus ligation-induced ulcer models. Lab Anim Res. 2011;27(1):47–52. doi: 10.5625/lar.2011.27.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raffin RP, Colomé LM, Schapoval EE, Jornada DS, Pohlmann AR, Guterres SS. Gastro-resistant microparticles containing sodium pantoprazole: stability and in vivo anti-ulcer activity. Open Drug Deliv J. 2007;1:28–35. [Google Scholar]
- 11.Chen Y, Zhang L, Hao Q. Olaparib: a promising PARP inhibitor in ovarian cancer therapy. Arch Gynecol Obstet. 2013;288(2):367–374. doi: 10.1007/s00404-013-2856-2. [DOI] [PubMed] [Google Scholar]
- 12.Kauppinen TM, Gan L, Swanson RA. Poly(ADP-ribose) polymerase-1-induced NAD(+) depletion promotes nuclear factor-κB transcriptional activity by preventing p65 de-acetylation. Biochim Biophys Acta. 2013;1833(8):1985–1991. doi: 10.1016/j.bbamcr.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheline CT, Wang H, Cai AL, Dawson VL, Choi DW. Involvement of poly ADP ribosyl polymerase-1 in acute but not chronic zinc toxicity. Eur J Neurosci. 2003;18(6):1402–1409. doi: 10.1046/j.1460-9568.2003.02865.x. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JH, Kim DW, Jo EJ, Kim YK, Jo YS, Park JH, Yoo SK, Park MK, Kwak TH, Kho YL, Han J, Choi HS, Lee SH, Kim JM, Lee I, Kyung T, Jang C, Chung J, Kweon GR, Shong M. Pharmacological stimulation of NADH oxidation ameliorates obesity and related phenotypes in mice. Diabetes. 2009;58(4):965–974. doi: 10.2337/db08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filaretova L. The hypothalamic-pituitary-adrenocortical system: Hormonal brain-gut interaction and gastroprotection. Auton Neurosci. 2006;125(1-2):86–93. doi: 10.1016/j.autneu.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Filep JG, Hermán F, Földes-Filep E, Schneider F, Braquet P. Dexamethasone-induced gastric mucosal damage in the rat: possible role of platelet-activating factor. Br J Pharmacol. 1992;105(4):912–918. doi: 10.1111/j.1476-5381.1992.tb09077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandyopadhyay U, Biswas K, Bandyopadhyay D, Ganguly CK, Banerjee RK. Dexamethasone makes the gastric mucosa susceptible to ulceration by inhibiting prostaglandin synthetase and peroxidase--two important gastroprotective enzymes. Mol Cell Biochem. 1999;202(1-2):31–36. doi: 10.1023/a:1007018212822. [DOI] [PubMed] [Google Scholar]