Abstract
Deleterious sustained inflammation mediated by activated microglia is common to most of neurologic disorders. Here, we identified sirtuin 2 (SIRT2), an abundant deacetylase in the brain, as a major inhibitor of microglia-mediated inflammation and neurotoxicity. SIRT2-deficient mice (SIRT2−/−) showed morphological changes in microglia and an increase in pro-inflammatory cytokines upon intracortical injection of lipopolysaccharide (LPS). This response was associated with increased nitrotyrosination and neuronal cell death. Interestingly, manipulation of SIRT2 levels in microglia determined the response to Toll-like receptor (TLR) activation. SIRT2 overexpression inhibited microglia activation in a process dependent on serine 331 (S331) phosphorylation. Conversely, reduction of SIRT2 in microglia dramatically increased the expression of inflammatory markers, the production of free radicals, and neurotoxicity. Consistent with increased NF-κB-dependent transcription of inflammatory genes, NF-κB was found hyperacetylated in the absence of SIRT2, and became hypoacetylated in the presence of S331A mutant SIRT2. This finding indicates that SIRT2 functions as a ‘gatekeeper’, preventing excessive microglial activation through NF-κB deacetylation. Our data uncover a novel role for SIRT2 opening new perspectives for therapeutic intervention in neuroinflammatory disorders.
Keywords: brain, inflammation, microglia
Introduction
Microglia, the innate immune cells in the CNS, are adapted to sense and immediately react to pathogens, misfolded proteins, or molecules released by damaged cells. It is now known that microglia may adopt different activated phenotypes in response to external stimuli. Whether activated microglia is beneficial or noxious to the CNS is determined by several variables, including the balance between cytotoxic and neurotrophic molecules generated by activated microglia, and the intensity and timing of microglial activation (reviewed in Lucin and Wyss-Coray, 2009; Perry et al, 2010; and Saijo and Glass, 2011). A deleterious sustained inflammatory response mediated by microglia has been associated to several neurologic conditions, including CNS infections (Garden, 2002; Dellacasa-Lindberg et al, 2011), ischemic stroke (Yrjanheikki et al, 1998), and neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s disease (McGeer et al, 1988; Sulzer, 2007; Tai et al, 2007). In these diseases, secretion of inflammatory mediators by microglia, such as pro-inflammatory cytokines (i.e., tumour necrosis factor (TNF), IL-6, and IL-1β), metalloproteases, reactive oxygen species (ROS), nitric oxide (NO), and glutamate, are thought to contribute to neuronal cell death (Block et al, 2007). Therefore, dissecting the mechanisms that selectively shut off deleterious activation pathways might play an important role in controlling neurologic diseases. In fact, deregulation of microglial receptors CD200R and TREM2 signalling-mediated pathways, which were shown to prevent toxicity of microglia, is associated to increased inflammation and neurodegeneration in humans (Piccio et al, 2008; Walker et al, 2009; Guerreiro et al, 2012).
SIRT2 is one of the seven known mammalian SIRTs, a family of NAD+-dependent deacetylases. SIRT1 and SIRT6 emerged recently as regulators of several age-related pathologies, including neurodegenerative diseases and peripheral inflammation (Galli et al, 2011). SIRT2 is expressed as two isoforms, resulting from alternative splicing. The shorter isoform lacks the first 37 amino acids (North et al, 2003), is preferentially expressed in the adult nervous system, and is 20 times more expressed in the hippocampus than other sirtuins (Pandithage et al, 2008). SIRT2 is predominantly cytosolic but the shuttling between the cytoplasm and nucleus explains that both α-tubulin (North et al, 2003) and histones (Vaquero et al, 2006) are deacetylated by this sirtuin. Several studies indicate that SIRT2 plays an important role in brain function. SIRT2 is required for myelination through deacetylation of the polarity protein Par-3, as recently demonstrated by Schwann cell-specific ablation of SIRT2 in mice (Beirowski et al, 2011). SIRT2 activity is inhibited by cyclin-dependent kinase-2 (Cdk2) and Cdk5-dependent phosphorylation at residue S331. Accordingly, the S331A SIRT2 mutant, which cannot be phosphorylated at this specific residue, reduces neurite length and tubulin acetylation to a higher extent than the wild-type (wt) protein (Pandithage et al, 2008). Also, SIRT2 has been implicated in neuronal degeneration in cellular and animal models of Parkinson’s disease (PD). Chemical inhibition of SIRT2 rescued neuronal cells from alpha-synuclein-induced toxicity (Outeiro et al, 2007).
Other SIRT2 substrates were recently identified, including the p65 subunit of NF-κB, a major transcription regulator of inflammation. Fibroblasts from SIRT2−/− mice show hyperacetylation of p65 concomitantly with increased expression of NF-κB-dependent genes induced by TNF (Rothgiesser et al, 2010a). The receptor-interacting protein 1 (RIP-1), which is also involved in inflammatory signalling pathways, was also found to be a SIRT2 substrate. The deacetylation of RIP-1 by SIRT2 stabilizes the RIP-1–RIP-3 protein complex required for TNF-induced cell death in fibroblasts (Narayan et al, 2012). Altogether, these results provide evidence that SIRT2 deacetylates important regulators of inflammation such as NF-κB and RIP-1.
Stimulation of Toll-like receptors (TLRs) in microglia triggers CNS inflammation mainly through NF-κB-dependent transcriptional activation. TLRs recognize pathogen-associated molecular patterns (PAMPs) during infection whereas in non-infectious-mediated CNS injury, TLRs bind to endogeneous damage-associated molecular patterns (DAMPs) released by dead cells (Hanamsagar et al, 2012). TLR4 is a receptor for the Gram-negative bacterial cell wall component, lipopolysaccharide (LPS) (Poltorak et al, 1998). Microglial activation by LPS occurs in bacterial meningitis caused by Escherichia coli (Kim, 2003) and Citrobacter koseri (Liu and Kielian, 2009) or during Staphylococcus aureus-induced brain abscess (Stenzel et al, 2008). Moreover, peripheral infection by Gram-negative bacteria is also known to activate the innate immune response in the CNS through TLR4 (Chakravarty and Herkenham, 2005), which may also contribute to the pathology of chronic neurodegenerative diseases (Cunningham, 2013). Also, several endogeneous TLR4 ligands have been identified such as High mobility group box 1 protein (HMGB1) (Yang et al, 2010), heme (Figueiredo et al, 2007), fibrinogen (Smiley et al, 2001), beta-amyloid (Reed-Geaghan et al, 2009), and alpha-synuclein (Fellner et al, 2013). Therefore, microglial activation by TLR4 and by other TLRs may have implications in CNS infection, stroke, and neurodegenerative diseases, which makes TLR signalling pivotal in neuroinflammatory conditions.
Here, we addressed the biological significance of SIRT2 in the inflammatory response in the brain, where it is highly expressed, and in a context of TLR activation. We investigated SIRT2 as a potential regulator of brain inflammatory responses mediated by microglia. Our findings provide the first direct evidence for a key inhibitory role of SIRT2 in microglia-mediated inflammation and neurotoxicity. This indicates that SIRT2 might be explored as a target to prevent deleterious inflammatory responses, which may ultimately bear major implications in CNS inflammatory diseases.
Results
SIRT2 regulates the inflammatory response to LPS in vivo
First, we examined whether SIRT2 was expressed by microglia since, to our knowledge, this had not been previously reported. Immunohistochemical analysis of the mouse brain showed SIRT2 expression in cells co-stained for Iba-1, a specific microglial cell marker that is upregulated in activated cells (Figure 1A) (Ito et al, 1998; Harting and Knoll, 2010). We also found that both full-length (LSIRT2) and the shorter truncated (sSIRT2) alternative splice isoforms (Dryden et al, 2003) were expressed in primary cultures of microglia, in the N9 microglial cell line, as well as in other brain cells as previously described (Harting and Knoll, 2010). Interestingly, the adult brain is particularly enriched in the shorter isoform in comparison with other organs (Supplementary Figure S1A).
Figure 1.
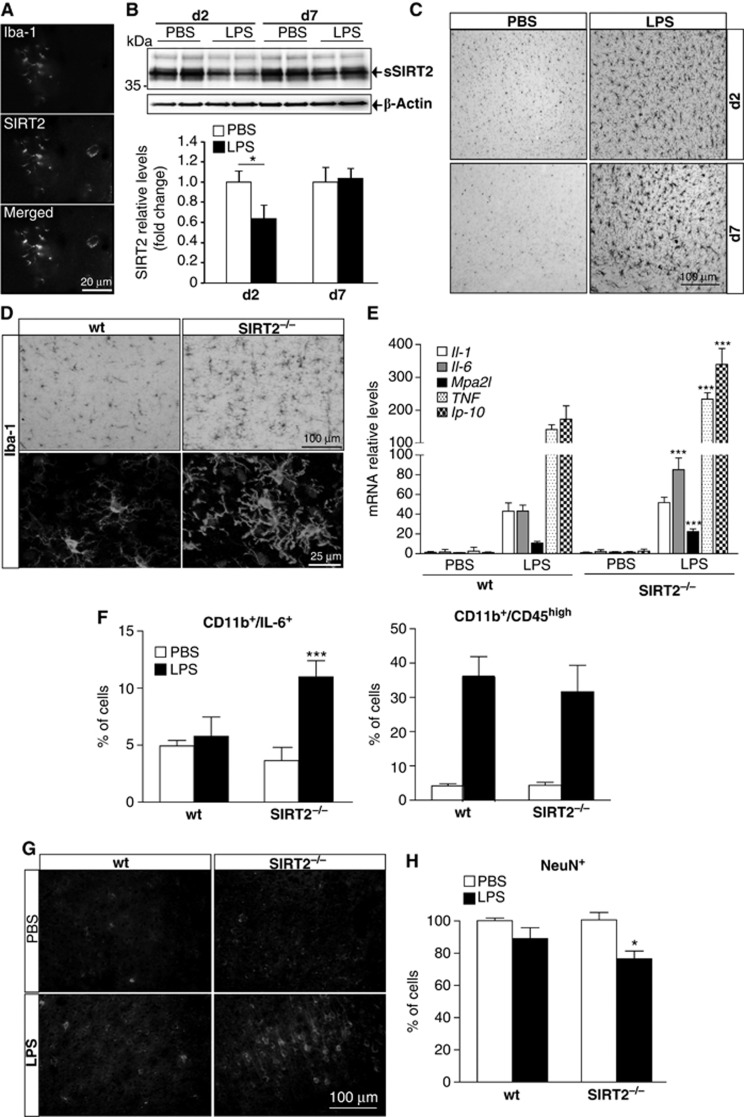
SIRT2 regulates the brain response to LPS. (A) SIRT2 expression by microglial cells. A representative image of co-localization of SIRT2 with Iba-1 was obtained from brain sections stained for Iba-1 (red) and SIRT2 (green). (B) SIRT2 protein levels in mice injected intracortically with PBS or LPS (0.2 μg). Cortical extracts from mice injected with PBS or LPS were analysed by western blotting and stained for SIRT2. Quantification was done by densitometry analysis of the protein bands and normalized to β-actin levels (mean±s.d.; n=3, PBS; n=5, LPS; t-test, *P<0.05). (C) Microglial activation induced by intracortical injection of LPS. Representative images of brain sections of mice injected with PBS and LPS at day 2 and 7 post injection and stained for Iba-1. (D) Microglial activation in wild-type versus SIRT2−/− mice. SIRT2−/−mice show increased microglial activation induced by LPS injection on bright-field and confocal (below) microscopy images of mice brain sections stained for Iba-1. Confocal images reveal thicker microglial processes in SIRT2−/−mice. (E) mRNA levels of IL-1, IL-6, Mpa2l, TNF, and Ip-10 inflammatory genes were determined by qPCR, normalized to β-actin expression levels. IL-6, Mpa2I, TNF, and lp-10 expression is increased after 6 h in LPS-injected SIRT2−/−mice compared to LPS-injected WT mice (mean±s.d., n=4; two-way ANOVA, ***P<0.001). (F) Flow cytometry analysis of cortical cells isolated from injected mice and stained for CD11b, CD45 and IL-6 (mean±s.d.; n=3, PBS; n=4, LPS; two-way ANOVA, test, ***P<0.001). (G) Induction of free radicals by LPS. Representative images of wt and SIRT2−/− mouse brain sections stained for nitro tyrosine 5 days post injection with PBS or LPS (5 μg). (H) Neuronal cell loss induced by LPS. Mice brain sections were stained for NeuN and the number of positive cells counted and normalized to the numbers of cells in the cortice of wt mice 5 days after PBS or LPS (5 μg) injection (mean±s.d., n=3; two-way ANOVA, *P<0.05).
To investigate whether SIRT2 plays a direct role in microglia activation, mice were injected with 0.2 μg of LPS intracortically. LPS is a general pro-inflammatory stimulus that binds to TLR4 exclusively expressed by microglia in the mouse brain (Lehnardt et al, 2002), and very relevant in the context of bacterial meningitis (Kim, 2003). This is also a well-established model of direct activation of innate immunity in the brain, which activates NF-κB-dependent pathways in microglia (Milatovic et al, 2003). Surprisingly, the levels of SIRT2, but not SIRT1 (Supplementary Figure S1B), were significantly reduced in the cortex of mice 2 days after injection of LPS (Figure 1B). At this time point, there was a clear increase in microglial Iba-1 immunoreactivity that was still observed at day 7 post injection (Figure 1C). To elucidate the role of SIRT2 in microglia activation in vivo, we used SIRT2−/− mice. The number of Iba-1-positive cells in the cortex of SIRT2−/− adult mice is not significantly different from wt mice, suggesting that SIRT2 is not crucial for microglia differentiation (Supplementary Figure S2A). Notably, 2 days after LPS injection, microglial cells displayed enhanced Iba-1 immunoreactivity and thicker processes around the injection site in SIRT2−/− mice, a morphological change associated with microglial activation (Figure 1D). Furthermore, the mRNA levels of pro-inflammatory factors such as interleukin-6 (IL-6), macrophage activating 2 like (Mpa2l), interferon gamma-induced protein 10 (Ip 10) and TNF were significantly upregulated (∼2-fold) in the cortex of SIRT2−/−mice (Figure 1E). Flow cytometry analysis of cortical cells stained intracellularly for IL-6 supported the induction of this cytokine (∼2-fold) within the CD11b-positive (microglia/macrophages) population isolated from SIRT2−/− mice (Figure 1F; Supplementary Figure S2B). There was no significant difference if cells isolated from LPS-injected mice were intracellular stained with an isotype control antibody (wt, 2.9±0.5 versus 3.9±0.7 in SIRT2−/− mice). The expression of CD45, a marker of microglia/macrophage activation (Kettenmann et al, 2011), was increased 48 h after LPS injection and was not significantly different between wt and SIRT2−/− mice (Figure 1F; Supplementary Figure S2B). These results clearly show, for the first time, that in the absence of SIRT2 there is an increase in pro-inflammatory cytokine response by microglia in vivo. Moreover, the response to LPS in SIRT2−/− mice was associated with a clear induction of free radicals, namely peroxynitrite, as measured by the increased staining for nitrotyrosine in cortical cells 5 days after injection of 5 μg of LPS (Figure 1G). We used a higher dose of LPS in these experiments based on the previous studies reporting LPS-induced neurodegeneration (de Pablos et al, 2006). Thus, to further test whether increased inflammation and oxidative stress could lead to neuronal cell loss, we stained the brain sections for NeuN, a neuron-specific protein (Mullen et al, 1992). We found a small but a significant decrease in NeuN-positive cells in SIRT2/− mice injected with LPS compared with wt mice 5 days after LPS injection (Figure 1H; Supplementary Figure S2C). Altogether, our results clearly indicate that loss of SIRT2 renders microglia more prone to activation by pro-inflammatory stimuli in vivo, accompanied by higher levels of oxidative stress and neuronal cell loss.
SIRT2 inhibits microglial activation in a phosphorylation-dependent manner
To clarify the molecular mechanisms involved in SIRT2-mediated microglia activation, we next used the well-characterized N9 microglial cell line (Stansley et al, 2012) (Figure 2A). The sSIRT2 form appeared as a doublet in non-activated N9 cells. The slower migrating band disappeared after N9 cell extracts were treated with λPP1 phosphatase (Supplementary Figure S3A), indicating that this band corresponds to a phosphorylated form of SIRT2 (psSIRT2). Interestingly, microglial stimulation with LPS and TNF (LPS+TNF) significantly decreased sSIRT2 phosphorylation compared with non-stimulated cells (CTR) (Figure 2B).
Figure 2.
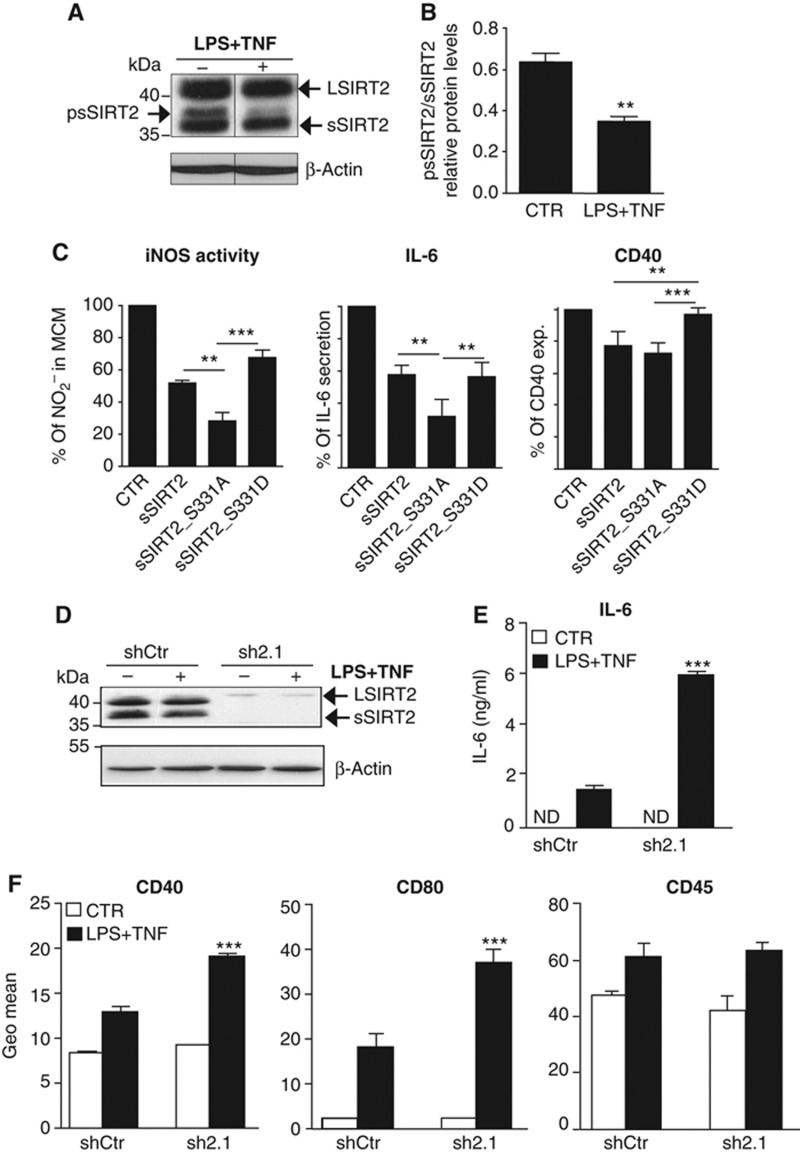
SIRT2 inhibition of microglial activation depends on S331 phosphorylation. (A) Western blot analysis of microglia activated with LPS and TNF (LPS+TNF) shows clear reduction in a slower migrating protein band (higher MW) corresponding to psSIRT2. Arrows on the right indicate the full (LSIRT2) and shorter (sSIRT2) SIRT2 splicing variants. (B) Microglial activation with LPS+TNF leads to a significant decrease in the level of phosphorylated sSIRT2 (mean±s.d. of three independent experiments, t-test, **P<0.01). (C) Viral-transduced microglial cells were untreated or activated with LPS+TNF and supernatants analysed for nitrites and IL-6, and cells immunostained against CD40 for flow cytometry. The quantification of iNOS activity, IL-6 and CD40 expression results is presented as a relative percentage of the levels quantified in cells viral-transduced with empty vector (CTR) and stimulated with LPS+TNF (mean±s.d., n=4 independent experiments; one-way ANOVA, Tukey’s multiple comparison test, P<0.01). ** and *** are defined by the 95% confidence interval (CI) of difference between means. (D) Western blot analysis showing reduction of SIRT2 protein levels in non-activated (−) or activated with LPS+TNF (+) N9 cells viral-transduced with shRNA specific for SIRT2 (sh2.1) versus control shRNA (shCtr). (E) IL-6 secretion in SIRT2 KD cells. KD or shCtr-transduced cells were left untreated (CTR, white squares) or activated with LPS+TNF (black squares). IL-6 levels were determined by ELISA. (F) Expression of membrane activation markers in SIRT2 KD cells. Quantification of CD40, CD80, and CD45 expression was done by flow cytometry analysis and represented by the Geo Mean of the histograms obtained for the fluorescence intensity of stained cells. In (E) and (F), data are presented as mean±s.d. and representative of three independent experiments done in triplicate, two-way ANOVA, ***P<0.001. ND, not detectable.
Source data for this figure is available on the online supplementary information page.
We next hypothesized that SIRT2 may regulate microglial responses in a phosphorylation-dependent manner since SIRT2 phosphorylation at S331 is known to inhibit its deacetylase activity (Pandithage et al, 2008). To address this, we tested whether major pathways associated with microglial responses were affected by overexpression of wt sSIRT2 or SIRT2 phospho-mutants (S331A, phospho-resistant; and S331D, phospho-mimetic). The S331D mutant form migrated at a slight higher MW, as expected from the negative charge of the aspartate (Supplementary Figure S3B). N9 microglial cells were stimulated with LPS+TNF 48 h after viral transduction with the different sSIRT2 forms. Wild-type sSIRT2 reduced significantly the NO (assayed as nitrite) generated by the inducible isoform of nitric oxide synthase (iNOS) (∼50%), secretion of the pro-inflammatory IL-6 cytokine (∼40%), and expression of the membrane protein and activation marker CD40 (Kettenmann et al, 2011) (∼20%) in N9 cells stimulated with LPS+TNF (Figure 2C). The phospho-resistant mutant (sSIRT2_S331A) was significantly more effective than the phospho-mimetic mutant (sSIRT2_S331D) in reducing iNOS activity (72 versus 32%), IL-6 secretion (68 versus 44%), and CD40 expression (21 versus 8%). In addition, while the sSIRT2_S331A mutant induced a significant decrease in iNOS activity and IL-6 secretion, the S331D mutant was less effective in reducing CD40 expression in comparison with wt sSIRT2.
In order to examine the effect of decreased levels of SIRT2 in microglia, N9 cells were transduced with lentiviral particles producing different short hairpin RNAs (shRNAs) specific for SIRT2 (sh2.1) or a non-coding sequence (scrambled) as a control (shCtr). shRNA transduction significantly reduced the expression levels of SIRT2 (∼90%) (Figure 2D). Microglia activation phenotype was then studied in SIRT2 knock-down (KD) N9 cells. Contrary to SIRT2 overexpression, decrease in SIRT2 protein levels significantly (P<0.05) enhanced the secretion of IL-6 (Figure 2E) and CD40 expression (Figure 2F) induced by LPS+TNF. CD80, another microglia/macrophage activation marker (Kettenmann et al, 2011), was also increased in SIRT2 KD cells while the levels of CD45, a protein tyrosine phosphatase, did not differ between control and KD activated cells (Figure 2E).
Additionally, we did not detect differences in MAPK signalling pathways, assessed by ERK1/2 and p38 phosphorylation levels, phagocytic activity, glutamate production or induction of IL-1β mRNA levels (Supplementary Figure S4).
Taken together, the overexpression and KD of SIRT2 in microglia confirmed that SIRT2 is an inhibitor of microglial inflammatory responses, and support our observations in SIRT2−/−mice. On the basis of our results, SIRT2 is probably acting downstream of activation signalling cascades triggered by inflammatory stimuli, and its effect on microglia is modulated by phosphorylation at S331.
SIRT2 modulates microglia sensing by TLR2, 3 and 4
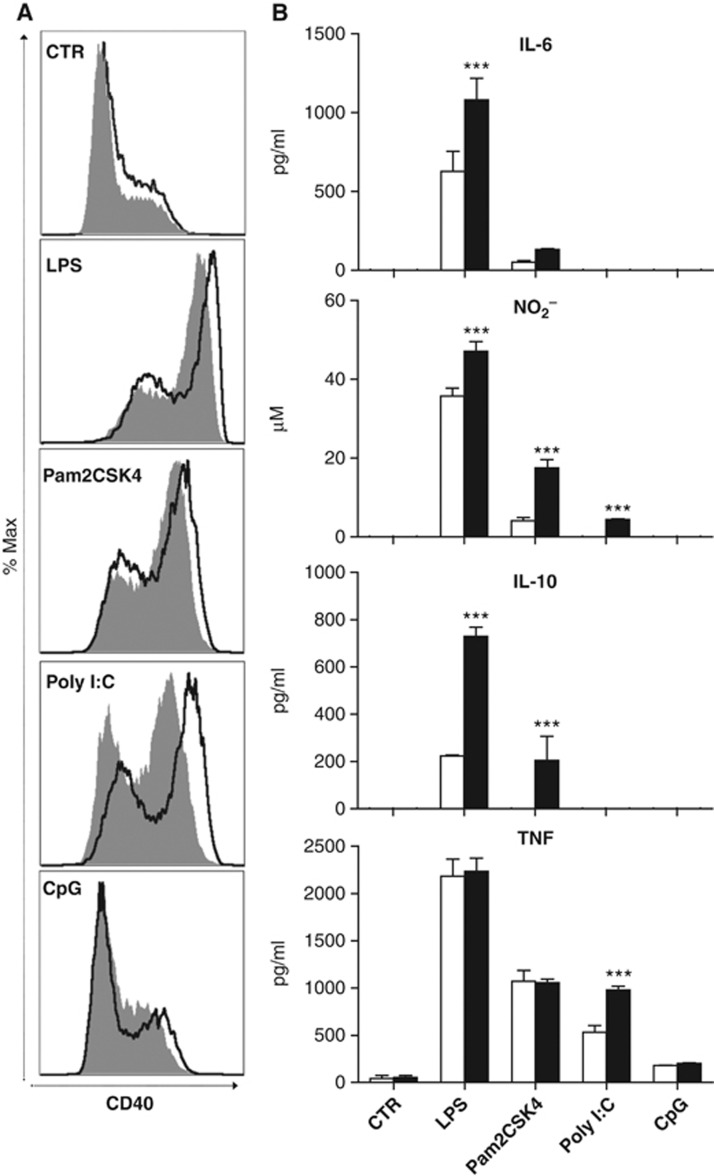
We then tested whether SIRT2 regulates the microglial response to other TLRs using specific ligands for TLR2 (Pam3CSK4), TLR3 (Poly I:C), TLR4 (LPS), and TLR9 (CpG). We analysed several established activation markers, including IL-10 production, which is induced following TLR ligation in microglia (Jack et al, 2005). IL-10 is also part of a negative-feedback mechanism to restrain inflammatory response in LPS-activated macrophages (Chang et al, 2007). SIRT2 KD in N9 cells increased CD40 expression, IL-6 and IL-10 secretion, and iNOS activity induced by TLR4 and TLR2 activation. We did not observe major alterations in response to TLR9 activation (Figure 3). Interestingly, SIRT2 KD in microglia only affected TNF induction significantly upon TLR3 activation.
Figure 3.
SIRT2 regulates microglial response through TLR2, 3 and 4. (A) Representative histograms of the fluorescence intensity for CD40 showing the overlays of shCtr (filled) and sh2.1 (solid line) viral-transduced N9 cells that were stimulated with different TLR ligands. (B) Induction of IL-10, IL-6, TNF, and nitrites (NO2−) by TLR ligands in control (white bars) and SIRT2 KD (black bars) N9 cells. LPS (50 ng/ml), Pam2CSK4 (0.05 μg/ml), Poly I:C (10 μg/ml), and CpG (10 μg/ml) are ligands for TLR4, 2, 3, and 9, respectively. Data shown are presented as mean±s.d. and representative of three independent experiments done in triplicate, two-way ANOVA, ***P<0.001.
Together, these results show that SIRT2 is able to regulate microglial activation induced by several stimuli and therefore widens the spectrum of conditions where SIRT2 modulation could be relevant to CNS injury.
SIRT2 suppresses ROS/RNS production, activation-induced cell death in microglia, and microglial-induced neurotoxicity
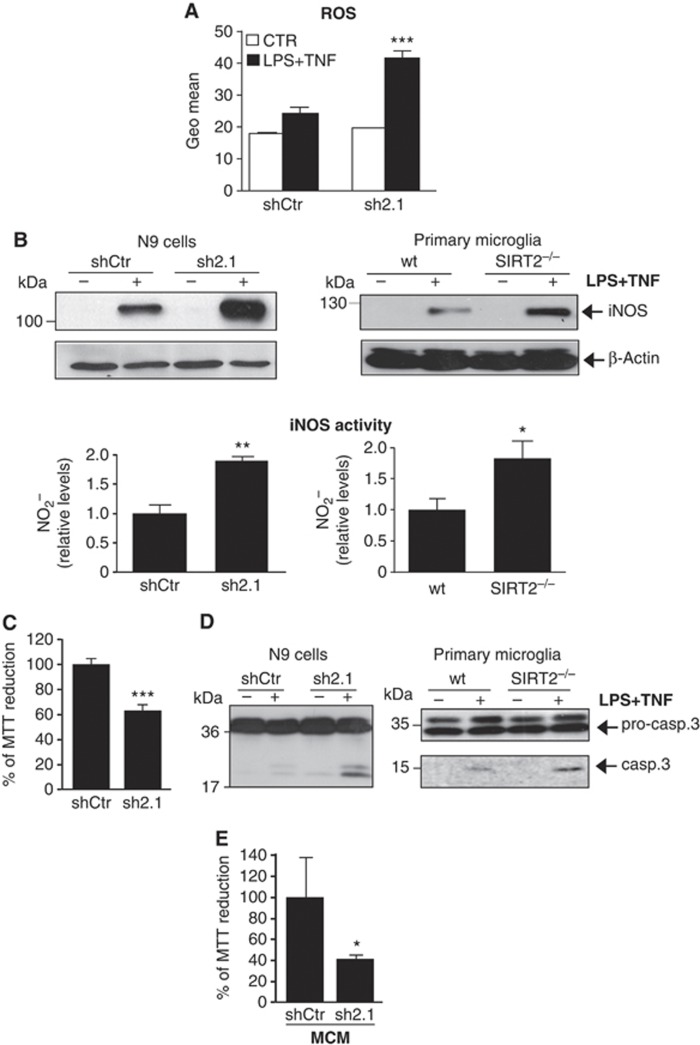
Microglial cells contribute to the oxidative damage in neurodegenerative diseases through the generation of ROS and reactive nitrogen species (RNS) (Reynolds et al, 2007). Moreover, the simultaneous activation of NADPH oxidase and iNOS produces peroxynitrite, a highly toxic oxidant that crosses cellular membranes and causes neuronal and oligodendroglial cell death (Mander and Brown, 2005). Interestingly, reduced levels of SIRT2 enhanced both intracellular ROS formation (Figure 4A) and iNOS protein levels both in activated N9 cells and in primary microglia (Figure 4B). The upregulation of iNOS protein levels was accompanied by a significant increase in its activity, measured by the levels of nitrites in the supernatants of activated cells (Figure 4B). The induction of iNOS in activated SIRT2 KD N9 cells was also observed at the mRNA level (Supplementary Figure S5D). These data demonstrate that the loss of SIRT2 increases microglial pro-inflammatory responses and production of ROS and RNS during microglial activation, which is in agreement with our observation in SIRT2−/− mice (Figure 1).
Figure 4.
SIRT2 suppresses the neurotoxic response of microglia. (A) Quantification of intracellular ROS by flow cytometry analysis is represented by the Geo Mean of the histograms obtained for the fluorescence intensity of the probe. Data shown are presented as mean±s.d. and representative of three independent experiments done in triplicate, two-way ANOVA, ***P<0.001. (B) Western blot analysis of iNOS expression in activated shCtr-transduced and SIRT2 KD cells and in primary microglial cultures prepared from wt and SIRT2−/− newborn mice. Quantification of RNS by assessing iNOS activity through measurement of nitrites accumulation in the culture supernatants. Results are presented as fold induction values compared to control activated microglia. (C) Effect of SIRT2 KD on microglial cell death. Cell viability was measured by reduction of MTT to formazan in activated control or SIRT2-KD N9 cells. t-test ***P<0.005. (D) The caspase-3 activity was analysed by detection of cleaved caspase-3 levels by western blotting in SIRT2 KD N9 cells and SIRT2−/− primary microglia. (E) Effect of microglial conditioned media (MCM) on neuronal survival. HT22 neurons were incubated with medium obtained from viral-transduced microglia non-treated or treated with LPS+TNF and cell viability measured by MTT assay. Data are presented as mean±s.d. of three independent experiments or representative of three independent experiments. t-test *P<0.05; **P<0.01.
Source data for this figure is available on the online supplementary information page.
We also investigated microglial activation in microglial cells knock-down for SIRT1, a sirtuin previously linked to inflammation (Schug et al, 2010). Although IL-6 was also increased in activated microglia upon a 50% decrease in SIRT1 protein levels (Supplementary Figure S5B), none of these pathways were affected (Supplementary Figure S5C and D). Therefore, this is consistent with the fact that SIRT1 and SIRT2 target different substrates.
We next asked whether increased production of ROS and RNS in the absence of SIRT2 was associated with enhanced microglia and neuronal cell death. Activated SIRT2 KD cells showed an ∼1.6-fold increase in apoptotic cells (Annexin+/PI−) (Supplementary Figure S6A) together with a significant decrease in cell viability (Figure 4C). Microglial cell death was concomitant with a 1.7-fold significant increase in caspase-3 activity in SIRT2 KD cells when compared to control activated microglia (Supplementary Figure S6B). Accordingly, the levels of the active cleaved form of caspase-3 were increased in the SIRT2 KD cell line and both in purified primary microglial (Figure 4D) and in mixed cell cultures derived from SIRT2−/−mice (Supplementary Figure S6C). Furthermore, we found that microglial-conditioned medium (MCM) from activated SIRT2 KD cells induced an ∼60% decrease in cell viability in HT22 hippocampal mouse cells compared to shCtr-activated cells (Figure 4E). We tested whether N-acetyl cysteine (NAC), an antioxidant and L-N6-(1-iminoethyl)-L-lysine (L-NIL), an inhibitor of iNOS activity, could prevent toxicity associated with decreased levels of SIRT2. Both NAC and L-NIL reduced ROS production and iNOS activity (Supplementary Figure S7A) in SIRT2 KD cells. The inhibitory effect of NAC and L-NIL on both ROS and RNS production pathways was expected since they have been shown to be connected to each other (Rota et al, 2002; Bents et al, 2012). This effect was even enhanced when the two drugs were added together. NAC and L-NIL were not toxic for microglia and did not rescue activation-induced cell death in microglia (Supplementary Figure S7B). CD40 expression was also not markedly affected by drug treatment (Supplementary Figure S7C). However, neuronal cell loss induced by MCM was prevented if microglia was treated with NAC and L-NIL during activation (Supplementary Figure S7D), suggesting that ROS/RNS production by microglia is required for neurotoxicity induced by LPS and TNF when SIRT2 levels are reduced. In summary, our results show that SIRT2 regulates microglial survival pathways upon cell activation and microglia-induced neurotoxicity, both of them being important processes in the context of most, if not all, neurodegenerative disorders.
SIRT2 deacetylates NF-κB and inhibits NF-κB-dependent transcription in microglia
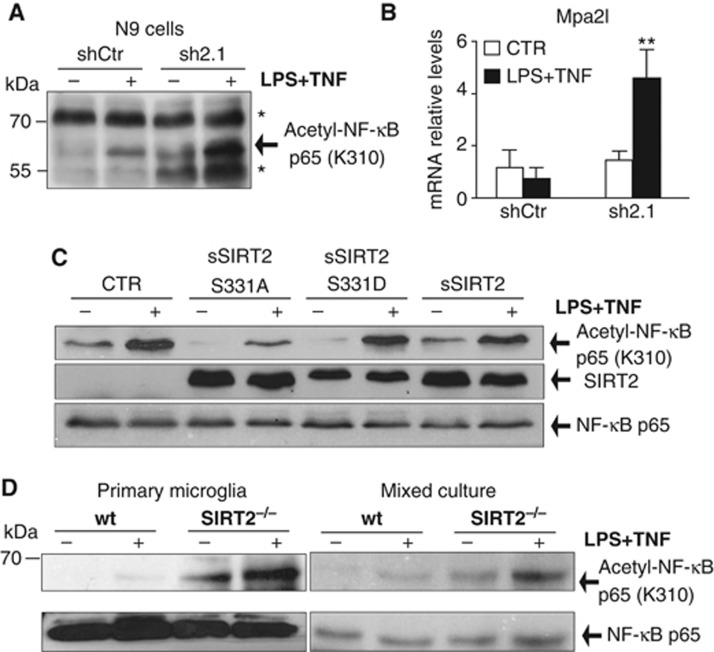
NF-κβ activation plays a major role in transcriptional regulation of inflammation and is associated with several chronic inflammatory diseases (Lawrence, 2009). Hyperacetylation of Lys310 of endogeneous p65 subunit of NF-κB in fibroblasts lacking SIRT2 has been linked to increased NF-κB-dependent transcription (Rothgiesser et al, 2010a). In particular, mpa2l gene expression was shown to be specifically regulated by acetylation of p65 at Lys310 (Rothgiesser et al, 2010b). To gain further insight into the molecular mechanisms underlying the effects of SIRT2 on microglial pathways, we investigated whether the effects observed were associated with NF-κB acetylation levels. The loss of SIRT2 in microglial cells increased the basal and inducible levels of acetylated p65 at Lys310 upon stimulation (Figure 5A). The increase in p65 acetylation was concomitant with increased levels of Mpa21 mRNA in stimulated cells (Figure 5B).
Figure 5.
SIRT2 deacetylates NF-κB p65 subunit at Lys310 in microglia. (A) Western blot analysis of endogeneous NF-κB p65 acetylation at Lys310 in CTR and SIRT2 KD N9 cells. * Indicates a non-specific band previously described for this antibody (Rothgiesser et al, 2010a). (B) Quantification of mpa2l mRNA levels showed increased transcription in activated microglia lacking SIRT2. Data shown are presented as mean±s.d. and representative of three independent experiments done in triplicate, two-way ANOVA, **P<0.01. (C) Levels of NF-κB p65 acetylation at Lys310 in SIRT2 KD cells viral-transduced cells with CTR, SIRT2, and SIRT2 S331 phosphorylation mutants (S331A and S331D) analysed by western blotting. The blot is representative of three independent experiments. (D) Acetylation of NF-κB p65 at Lys310 in microglia and mixed primary cultures analysed by western blotting.
Source data for this figure is available on the online supplementary information page.
Overexpression of the SIRT2 phospho-resistant mutant (SIRT2_S331A) clearly reduced both basal and inducible levels of NF-κB acetylation in SIRT2 KD cells (Figure 5C). This result is in accordance to published results that show a higher reduction in acetylated α-tubulin, another SIRT2 substrate, induced by this mutated form compared to SIRT2 (Pandithage et al, 2008).
Hyperacetylated NF-κB was also detected both in primary microglia and in mixed brain cell cultures obtained from newborn SIRT2−/−mice (Figure 5D). We assessed other parameters associated with NF-κB activation such as the kinetics of NF-κB p65 subunit migration to the cell nucleus (Supplementary Figure S8A), of IκB-alpha degradation (Supplementary Figure S8B), and of NF-κB p65 subunit phosphorylation at S536 (Supplementary Figure S8C) (Hayden and Ghosh, 2012). None of them was affected in SIRT2 KD N9 cells. Altogether, our data support that, in microglia, SIRT2 targets Lys310 on the p65 subunit of NF-κB affecting pro-inflammatory gene transcription.
Discussion
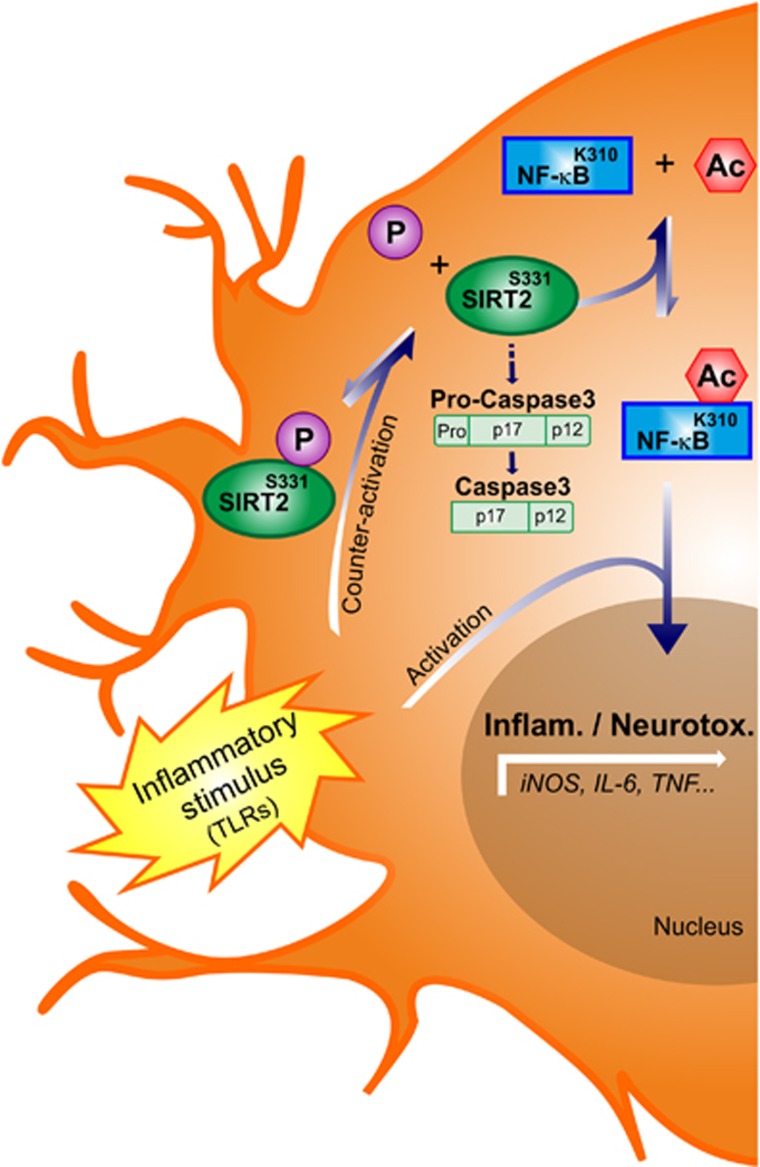
In the present study, we demonstrate a hitherto unknown regulatory function of SIRT2 in microglia-mediated inflammatory processes. We report, for the first time, that SIRT2 is expressed in microglia and that LPS-induced inflammation reduces SIRT2 levels in the brain. Importantly, we show that the absence of SIRT2 in vivo as well as in cell cultures results in enhanced microglia activation associated with a pro-inflammatory phenotype. In particular, microglial cells knock-down for SIRT2 display enhanced production of IL-6, CD40, CD80, ROS and NO upon LPS+TNF stimulation. We also observe induction of IL-10, an anti-inflammatory cytokine, though this can be a feedback mechanism in response to higher activation in microglia cells that do not express SIRT2 rather than a direct effect on IL-10 transcription. In contrast, SIRT2 overexpression prevents microglial cell activation pathways, and this effect is dependent on phosphorylation at S331. Moreover, we demonstrate that SIRT2 modulates activation-induced microglial cell death and neurotoxicity. Importantly, we also show that SIRT2 regulates the microglial response to different stimuli that act through TLR family. Therefore, SIRT2 can regulate microglia responses under exposure to both endogeneous and pathogen-derived danger signals known to activate TLRs. Finally, we observe that in microglial cells SIRT2 targets NF-κB, whose deacetylation seems to prevent overactivation of microglia upon pro-inflammatory stimulation (Figure 6).
Figure 6.
Model for SIRT2-mediated regulation of microglial activation. Pro-inflammatory stimuli such as TLR ligands derived from CNS injury lead to increased NF-κB-dependent gene transcription in microglia. To counteract microglial overactivation, SIRT2 is dephosphorylated, which enhances its deacetylase activity. This increases NF-κB deacetylation and inhibition by SIRT2 preventing excessive transcription of pro-inflammatory genes. Loss of this endogeneous regulatory mechanism by deletion of SIRT2 in mice and in microglial cell cultures results in excessive microglial activation, caspase-3 activity, and cell death.
Our data show, for the first time, an SIRT2-dependent mechanism to restrain the deleterious effects of excessive microglia activation (Figure 6). The non-phosphorylatable S331A SIRT2 mutant that we show to deacetylate NF-κB more efficiently is a stronger inhibitor of microglial pro-inflammatory response than the phospho-mimetic mutant. Our findings bring additional insight into the mechanisms by which SIRT2 controls inflammation. Thus, the dephosphorylation of SIRT2 observed upon microglia stimulation may increase the SIRT2 activity, thereby counteracting deleterious microglia overactivation (Figure 6). Cdk1 and phosphatase CDC14B were shown to regulate SIRT2 phosphorylation at S331 in a cell cycle-dependent manner. p35/Cdk5, which also phosphorylates this residue, is highly expressed in the brain and regulates inflammation by still poorly elucidated pathways (Contreras-Vallejos et al, 2012). We favour p35/Cdk5 as a candidate to regulate SIRT2 phosphorylation in microglia but this awaits further studies.
In microglia, the simultaneous activation of NADPH oxidase and iNOS produces peroxynitrite, a highly toxic oxidant species that crosses cellular membranes, induces protein nitrotyrosination, and causes neuronal and oligodendroglial cell death (Li et al, 2005; Mander and Brown, 2005). We show that protein nitrotyrosination is increased in the brain of SIRT2−/−-deficient mice in response to a pro-inflammatory stimulus together with increased neuronal cell loss. Moreover, SIRT2 inhibits both the production of neurotoxic ROS and NO in microglial cells. These results are in line with a previous study, showing that SIRT2 reduces ROS in fibroblasts. The mechanism proposed by the authors is likely due to SIRT2-mediated deacetylation of FOXO3a transcription factor leading to increased expression of the FOXO3a-dependent manganese superoxide dismutase gene (Wang et al, 2007). Taken together, SIRT2 could be a very attractive target for dampening microglial-mediated neurotoxicity due to its role in modulating the production of ROS and NO. This would have major implications in neurodegenerative diseases where it has been shown that microglia mediates oxidative damage (Reynolds et al, 2007).
Loss of microglial cells due to microglial overactivation may play a dual role in the context of neurodegenerative diseases. On one hand, removal of activated toxic microglia could be beneficial, because it would decrease the production of neurotoxic factors. On the other hand, loss of microglia could also hinder neuronal recovery due to the potential neuroprotective function of microglial cells. Our results show that SIRT2 deficiency enhances activation-induced microglial cell death but by a pathway independent of ROS/RNS. The increased caspase-3 activity and apoptosis in SIRT2-deficient cells contrast other findings obtained in fibroblasts where reduction in SIRT2 levels or activity was protective towards TNF-induced cell death (Rothgiesser et al, 2010a; Narayan et al, 2012). Curiously, the SIRT2 inhibitor AGK2 was recently shown to induce apoptosis in another microglial cell line, BV2 (Li et al, 2013). On the contrary, chemical inhibition of SIRT2 was shown to protect neurons from the toxicity induced by alpha-synuclein (Outeiro et al, 2007) and huntingtin (Rotili et al, 2010; Chopra et al, 2012). As SIRT2 downregulation has also been shown to reduce cell survival in cancer cells (Li et al, 2011; He et al, 2012) and in neuronal PC12 cells (Nie et al, 2011), it is likely that SIRT2 regulation of cell survival depends on external stimuli, cell cycle, and cell type. On the basis of published results, SIRT2 may contribute to neuronal autonomous cell death but our data point to a crucial role in preventing non-cell autonomous microglia-mediated neurotoxicity. Indeed, we observe an increase in neuronal loss in response to inflammation in SIRT2−/− mice though we cannot exclude that this is also due to the absence of SIRT2 in other brain cells. To confirm this hypothesis, cell-specific knock-out mice for SIRT2 will be required.
Finally, we identified deacetylation of NF-κB by SIRT2 as a contributing mechanism to control overactivation of microglia upon stimulation with a pro-inflammatory stimulus, namely through TLR activation (Figure 6). Nevertheless, we cannot exclude that other SIRT2 substrates, such as FOXO3a and RIP-1, are also involved in the phenotype described. Indeed, TNF production was only induced by TLR3 activation in SIRT2 KD cells. This could be because NF-κB activation through TLR3, contrary to the other TLRs, is dependent on RIP-1 (Meylan et al, 2004), another recently identified target of SIRT2 (Narayan et al, 2012). In addition, not all of NF-κB-targeted genes were regulated by SIRT2. For instance, we did not observe upregulation of IL-1β in SIRT2−/− mice or SIRT2 KD microglia upon challenge with LPS, though this gene is activated by NF-κB p65. This result suggests that NF-κB-dependent genes are not equally affected by NF-κB acetylation levels, which is in agreement with the existence of several mechanisms of NF-κB regulation (Hayden and Ghosh, 2012). Although, we clearly show that SIRT2 regulates the inflammatory response in the CNS, namely by microglial cells, this regulation may be extended to astrocyte-mediated inflammation.
Microglia monitor the CNS for danger signals and react by releasing molecules that can be either protective or toxic for neighbour cells. Tight and rapid regulation of microglial toxicity may be sufficient to diminish the pathogenic role of microglia in several neurodegenerative conditions. SIRTs, as enzymes and mediators of post-translational modifications, are very attractive targets for pharmacological manipulation.
We demonstrate that SIRT2 is a central inhibitor of microglial inflammatory and neurotoxic responses. Our findings bear major implications in the context of several inflammatory conditions of the CNS where microglia are known to mediate oxidative damage. However, previous data showing that SIRT2 inhibition is neuroptotective suggest that manipulation of SIRT2 activity should be regarded with caution in the context of neurodegenerative diseases, and further investigations will be required for the rational use of SIRT2 as a therapeutic target.
Materials and methods
Ethics statement
Animals experiments were performed according to institutional and national guidelines (license number: 33,9-42502-04-12/0733).
Animal studies
Two-month-old C57Bl/6N male mice received LPS or vehicle (PBS, pH 7.4) injection under deep anaesthesia (80 mg/kg ketamine hydrochloride, 5 mg/kg xylasine hydrochloride). Two microlitres of LPS (Escherichia coli 055:B5, 0.1 μg/μl, Sigma) or PBS was injected into the cortex (stereotactic coordinates: AP: 0.86; L: 1; DV: 0.5/0.75 from dura) using glass microcaplillary with a flow rate of 250 nl/min. At day 2 or day 7 post injection, brains were extracted and processed for flow cytometry, immunohistochemistry, and immunoblotting.
C57Bl/6N and SIRT2−/− mice (NKI, The Netherlands) were injected intracortically with either LPS or PBS and sacrificed 6 h or 2 days later for quantitative reverse transcription PCR (qPCR) and immunohistochemistry, respectively. qPCR was performed using specific primers (Rothgiesser et al, 2010a; Zarruk et al, 2012) on a MX3000P qPCR machine (Stratagene).
To study the effect of LPS on oxidative stress and neurodegeneration, mice were injected with 5 μg of LPS and brain slices from mice 5 days post injection, analysed for oxidative stress and neuronal cell loss.
Immunohistochemistry
Mice were transcardially perfused with 4% paraformaldehyde in PBS (pH 7.6). Brains were removed and post-fixed for 2 h at 4°C and cryoprotected in Tris-phosphate-buffered solution (TBS, pH 7.6) containing 30% sucrose (w/v) overnight at 4°C. In all, 30 μm coronal free floating sections were stained as reported previously (Szego et al, 2006). Briefly, sections were incubated with anti-Iba-1 rabbit polyclonal (Sigma), anti-NeuN mouse monoclonal (Millipore), or anti-nitro tyrosin (Abcam) for 48 h at 4°C. Sections stained for Iba-1 and NeuN were then treated with biotinylated anti-rabbit IgG (Vector Laboratories) followed by avidin–biotin–horseradish peroxidase (HRP) complex (Vector Standard Elite Kit, 1:500). Peroxidase labelling was visualized by diaminobenzidine tetrahydrochloride. Anti-nitro tyrosin was detected with anti-mouse AlexaFluor 555 (Invitrogen). For fluorescent double-labelling, sections were treated with anti-Iba-1 goat polyclonal and anti-SIRT2 (Sigma) antibodies, then incubated with AlexaFluor-488 conjugated donkey anti-goat and AlexaFluor-555 donkey anti-rabbit antibodies. Omission of primary or secondary antibodies resulted in a complete absence of immunoreactivity. Images were taken using a Zeiss AxioImager microscope or an Olympus IX81 fluorescence microscope. Serial images from sections stained for Iba1 were acquired on a Leica TCS SP2 confocal microscope.
Numbers of NeuN-positive neurons or Iba1-positive microglia were counted by using the optical fractionator, an unbiased stereological technique of cell counting (StereoInvestigator; MBF Bioscience). Numbers of NeuN-positive neurons or Iba1-positive microglia in the cortex were estimated with the optical dissector method (Szego et al, 2013) (× 100 objective, AxioImager M2; Zeiss; counting frame: 100 × 100 μm, grid size: 300 × 300 μm; every third section was analysed). Counts were performed manually and blinded for experimental grouping.
Western blot analysis
Cells or homogenized cortical tissue samples were prepared in NP-40 lysis buffer (150 mM NaCl, 50 mM Tris–HCl, 1% NP-40 for cell culture; 50 mM Tris, 1 mM EDTA, 5 mM MgCl2, 1% Triton X-100 for brain tissue) containing protease and phosphatase inhibitors (Roche Diagnostics GmbH) and resolved in 10 or 12% SDS gels. Proteins were transferred onto nitrocellulose membranes (Bio-Rad) and incubated with primary antibodies against: SIRT2 (Sigma), SIRT1 (Millipore), lamin B (Santa Cruz), iNOS (BD Biosciences), caspase-3 (Cell Signaling), NF-κB p65 (Santa Cruz), β-actin (Applied Biosystems) or NF-κB p65 (acetyl K310) (Abcam) followed by incubation with horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). The immunoreactivity was detected with an Immobilon Western Chemiluminescent HRP Substrate (Millipore).
For λ-phosphatase treatments, cell lysates were left untreated or treated with 200 U of λ-phosphatase (New England Biolabs) at 30°C for 30 min. The enzymatic reaction was stopped by addition of Laemmli buffer and proteins analysed as described above.
NF-κB acetylation at Lys310 in CTR, SIRT2 KD N9 cells, and SIRT2 KD N9 cells overexpressing the different forms of SIRT2 was analysed as previously described (Rothgiesser et al, 2010a). Cells were pre-treated with the HDAC inhibitors (HDACi) trichostatin (TSA) (100 nM) and nicotinamide (400 μM) for 30 min and then stimulated with LPS and TNF for 1 h. In primary cell cultures, NF-κB acetylation at Lys310 was detected after cell stimulation for 20 h and in the absence of HDACi.
Mutagenesis
Mouse SIRT2 short isoform was amplified from brain cDNA. An oligonucleotide starting at the second ATG, 3′-ATGGACTTCCTGAGGAATTTATTCA-5′ was used as a forward primer and 3′-TTACTGCTGTTCCTCTTTCTCTT-5′ as a reverse primer. The resulting PCR product was cloned into a lentiviral vector containing IRES-Emerald (eGFP) under the control of the CMV promoter (pHR-CMV-eGFP) (Ikeda et al, 2002). Site-directed mutagenesis to introduce mutations at Serine residue 331 (S331) in SIRT2 gene was performed with the QuikChange site-directed mutagenesis kit (Stratagene). S331 was mutated into a non-phosphorylatable alanine residue (pHR-CMV-sSIRT2_S331A-eGFP) or into a phosphorylation-mimetic aspartate residue (pHR-CMV-sSIRT2_S331D-eGFP). The resulting constructs were verified by sequencing.
Viral transduction
Lentiviral particles were produced according to the RNAi Consortium shRNA Library protocols from the Broad Institute, MIT, Harvard Medical School. Briefly, HEK293T cells were transfected with the two packaging plasmids (pCMV-dR8.91 and pCMV-VSV-G) and the constructs containing shRNAs or SIRT2. The cell medium was harvested 48 h after transfection, filtered through 0.45 μm filters. To knock down SIRT1 and SIRT2, N9 cells were incubated with supernatants containing the viral particles and polybrene (8 μg/ml) for 4 h, which was then replaced by fresh medium. After 48 h, shRNA viral-transduced cells were selected for 4 days in medium containing 5 μg/ml puromycin.
To overexpress the different forms of SIRT2, the supernatants containing the viral particles were first concentrated in ultrafiltration spin columns (100 kDa MWCO, Sartorius). N9 cells were then incubated with the concentrated supernatant containing polybrene for 48 h. The percentage of SIRT2-transduced N9 cells was within 50–60% as determined by flow cytometry.
Microglial stimulation
The mouse microglial cell line N9 was cultured in RPMI medium containing Glutamax (Invitrogen) and supplemented with 10% FBS (Endotoxin levels lower than 10 EU/ml). N9 cells lentiviral-transduced with shRNA for SIRT1 or SIRT2 KD or with scrambled control shRNA were plated in six-well plates (5 × 105/well) and cultured overnight. The cells were then stimulated with TLR ligands: LPS (5–100 ng/ml) (TLR4 ligand), Pam2CSK4 (0.01–2 μg/ml) (TLR2 ligand), Poly I:C (5–10 μg/ml) (TLR3 ligand), CpG (5–10 μg/ml) (TLR9 ligand) (Invivogen) or LPS (100 ng/ml) together with TNF (10 ng/ml) (Peprotech) for 20 h.
To study the effect of SIRT2 overexpression on N9 cell activation, cells were plated in six-well plates (5 × 104/well) and lentiviral-transduced with virus containing SIRT2 constructs. After 48 h, the cell culture medium was replaced for new medium containing the stimulus (LPS+TNF) and incubated for 20 h.
Primary microglial cell cultures were prepared from newborn wt and SIRT2−/−mice as described before (Pais et al, 2008). Briefly, after removal of the meninges, brains were mechanically disrupted in cold Hank’s balanced salt solution (HBSS). Cells were cultured in high-glucose Dulbecco’s Modified Eagle Medium (DMEM) containing Glutamax (Invitrogen) and supplemented with 10% FBS (Endotoxin <10 EU/ml), 5 μg/ml insulin (Sigma), 2.0 mg/ml L-glucose (Sigma), 0.25 ng/ml Granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech) and 1% Penicillin-Streptomycin 50 mM (Invitrogen) for 10 days. Medium was changed after 3 days and at day 6, an equal amount of medium was added to the culture. The confluent mixed glial cell cultures were shaken for 2 h at 250 r.p.m. and microglial cells obtained from the supernatant after centrifugation. The remaining adherent and mixed cell cultures were enriched in astrocytes and contained 12% (wt cells) and 7% (SIRT2 −/− cells) of microglial cells by FACS analysis of CD11b-positive cells.
Flow cytometry
Isolation of brain cells for intracellular staining and FACS analysis was performed as previously described (Pais and Chatterjee, 2005) with few modifications. Mice were perfused with 30 ml of PBS and the brains were removed and homogenized in HBSS containing collagenase VIII (0.2 mg/ml) (Sigma). After incubation for 45 min at 37°C, the digested tissue was minced, strained trough a nylon cell strainer of 100 μm (BD Falconk) and centrifuged. The pellet was resuspended in 30% Percoll (Sigma) in PBS and centrifuged at 515 g for 30 min at room temperature. Cells were collected and washed with PBS. Before staining against CD45 and CD11b, the cells were incubated with anti-Fc receptor in FACS buffer (PBS containing 2% FCS and 0.01% NaN3) for 30 min at 4°C to block irrelevant binding of the antibodies to the cells. After cell-surface staining, cells were fixed with 2% paraformaldehyde for 20′ at RT and then washed and permeabilized by incubation in PBS containing 0.5% BSA, 0.01% NaN3, and 0.5% saponin for 10′. The cells were then stained with anti-IL-6 or isotype control antibody (eBioscience) for 30′ at RT.
N9 cells were detached with PBS and incubated with anti-FcγR in FACS buffer (PBS containing 2% fetal calf serum and 0.01% NaN3) for 30 min at 4°C before staining. Cells were then incubated with antibodies against CD11b (M1/70), CD40 (3/23), and CD45 (30-F11) (BD Biosciences) followed by incubation with streptavidin-allophycocyanin (APC) to detect biotinylated antibodies. Cells were acquired using the BD LSRFortessa™ cell analyser. Post-acquisition, analysis was done with the FlowJo™ software. CD40 expression was quantified within eGFP-positive cell population of viral-transduced cells and represented as Geo Mean of the histograms.
Analysis of IL-6, ROS, and nitrites (NO2 −)
IL-6 was quantified in microglial supernatants by ELISA performed by following the manufacturer’s instructions (OptEIA™, BD Biosciences).
ROS was detected by flow cytometry after microglia incubation with 10 μm of 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) (Invitrogen) for 20 min. Quantification of ROS was represented by the Geo Mean of the histograms obtained for the fluorescence intensity.
The production of NO by iNOS was measured indirectly by assaying nitrites in the culture supernatant using the Griess reaction (Green et al, 1982). Briefly, 100 μl of supernatants was incubated with equal amount of Griess reagent (1% sulphanilamide, 0.1% naphthylethylenediamine in 2% phosphoric acid solution) and the absorbance read at 550 nm after 20′ of incubation at RT.
In lentiviral-transduced cells, the quantification of iNOS activity and IL-6 levels was obtained as a relative percentage of the levels quantified in cells viral-transduced with empty vector (CTR) and stimulated with LPS+TNF.
Cell death assays
MCM was obtained from non-stimulated N9 cells or from cells activated with LPS and TNF for 20 h. HT22 hippocampal cells were plated in 24-well plates at a density of 5 × 104 cells/well. On the day after, MCM was filtered through 0.22 μm filters and added to neurons at a final concentration of 1:20 for 14 h.
Microglial and neuronal cell viability were studied by assaying mitochondrial-dependent reduction of methylthiazol tetrazolium (MTT) to formazan (Zingarelli et al, 1996). Briefly, neurons were incubated with MTT (50 μg/ml) for 40 min at 37°C, and lysed with DMSO. The extent of reduction of MTT to formazan was determined by measuring the OD at 550 nm. Neurotoxicity was calculated by the difference between the viability of neurons incubated with non-activated MCM and neurons treated with MCM from stimulated microglia. This difference was normalized to the MCM obtained from shCtr-transduced microglia.
To determine the caspase-3 activity, N9 or HT22 cells were seeded on 96-well plates (2 × 104 cells/well) and stimulated with LPS and TNF or incubated with MCM (1:20) respectively on the following day. After 14 h, cells were lysed and assayed for caspase-3 activity (Sigma) following the manufacturer’s instructions. Caspase-3 activity was further confirmed by assessing cleaved caspase-3 (Cell Signaling) by western blot.
Alternatively, cell death was assessed by annexin V staining as previously described (Pais and Appelberg, 2000). Briefly, N9 cells were detached with PBS and stained with 2.5 μl of annexin V-FITC (BD Pharmingen) in binding buffer for 20 min at 4°C.
Statistical analysis
Data are expressed as mean±s.d. and was analysed for significance using ANOVA, when different genotypes/cells and treatments were compared at the same time, or Student’s t-test when comparing control versus treatment or KD/KO cells versus control/wt cells. The statistical test used is mentioned in the figure legends.
Supplementary Material
Acknowledgments
We thank Dr Luís Moita for providing the SIRT1 and SIRT2 shRNA constructs. N9 and HT22 cells were a kind gift from Dr Castagnoli and Dr Pocock, respectively. The antibody against Fcγ receptor (FcγR, clone 2.4G2) was prepared from hybridoma culture supernatants and provided by Instituto Gulbenkian de Ciência. Protein extracts from neuronal cell cultures were provided by Dr Joana Ferreira. We are grateful to Drs Hugo Vicente Miranda, Ângelo Chora, and Federico Herrera for critically reading the manuscript. We thank HVM for assistance with figure preparation. SIRT2−/−mice, from Prof. Maarten van Lohuizen, were used through an MTA with NKI, The Netherlands. TFO was supported by a Marie Curie International Reintegration Grant (Neurofold) and by an EMBO Installation Grant. EMS and this work were also supported by Deutsche Forschungsgemeinschaft (DFG) through the DFG Center for Nanoscale Microscopy and Molecular Physiology of the Brain. TFP (Ciência 2007), OM (SFRH/BD/44446/2008), LMF (SFRH/BD/36065/2007), PA (SFRH/BD/79337/2011), PG (SFRH/BD/61495/2009), and RMO (SFRH/BPD/41416/2007) were supported by Fundação para a Ciência e Tecnologia (FCT, Portugal).
Author contributions: TFP conceived the project, designed and performed most of the experiments and wrote the manuscript with the input of co-authors. ÉMS performed the animal studies and the other authors participated in the other in vitro experiments. All authors contributed to the discussions and writing. TFO directed the project and wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Beirowski B, Gustin J, Armour SM, Yamamoto H, Viader A, North BJ, Michan S, Baloh RH, Golden JP, Schmidt RE, Sinclair DA, Auwerx J, Milbrandt J (2011) Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc Natl Acad Sci USA 108: E952–E961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz M, Zaouter C, Shi Q, Fahmi H, Moldovan F, Fernandes JC, Benderdour M (2012) Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J Cell Biochem 113: 2256–2267 [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8: 57–69 [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M (2005) Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 25: 1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EY, Guo B, Doyle SE, Cheng G (2007) Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J Immunol 178: 6705–6709 [DOI] [PubMed] [Google Scholar]
- Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, Ferrante RJ, Hersch S, Kazantsev AG (2012) The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell Rep 2: 1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Vallejos E, Utreras E, Gonzalez-Billault C (2012) Going out of the brain: non-nervous system physiological and pathological functions of Cdk5. Cell Signal 24: 44–52 [DOI] [PubMed] [Google Scholar]
- Cunningham C (2013) Microglia and neurodegeneration: The role of systemic inflammation. Glia 61: 71–90 [DOI] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A (2006) Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci 26: 5709–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellacasa-Lindberg I, Fuks JM, Arrighi RB, Lambert H, Wallin RP, Chambers BJ, Barragan A (2011) Migratory activation of primary cortical microglia upon infection with Toxoplasma gondii. Infect Immun 79: 3046–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA (2003) Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol 23: 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N (2013) Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 61: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT (2007) Characterization of heme as activator of Toll-like receptor 4. J Biol Chem 282: 20221–20229 [DOI] [PubMed] [Google Scholar]
- Galli M, Van Gool F, Leo O (2011) Sirtuins and inflammation: Friends or foes? Biochem Pharmacol 81: 569–576 [DOI] [PubMed] [Google Scholar]
- Garden GA (2002) Microglia in human immunodeficiency virus-associated neurodegeneration. Glia 40: 240–251 [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138 [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K et al. (2012) TREM2 variants in Alzheimer’s disease. N Engl J Med 368: 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Hanke ML, Kielian T (2012) Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol 33: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting K, Knoll B (2010) SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol 89: 262–269 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2012) NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26: 203–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Nie H, Hong Y, Sheng C, Xia W, Ying W (2012) SIRT2 activity is required for the survival of C6 glioma cells. Biochem Biophys Res Commun 417: 468–472 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Collins MK, Radcliffe PA, Mitrophanous KA, Takeuchi Y (2002) Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther 9: 932–938 [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57: 1–9 [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP (2005) TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 175: 4320–4330 [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91: 461–553 [DOI] [PubMed] [Google Scholar]
- Kim KS (2003) Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci 4: 376–385 [DOI] [PubMed] [Google Scholar]
- Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22: 2478–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA (2005) Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA 102: 9936–9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Matsumori H, Nakayama Y, Osaki M, Kojima H, Kurimasa A, Ito H, Mori S, Katoh M, Oshimura M, Inoue T (2011) SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells 16: 34–45 [DOI] [PubMed] [Google Scholar]
- Li Y, Nie H, Wu D, Zhang J, Wei X, Ying W (2013) Poly(ADP-ribose) polymerase mediates both cell death and ATP decreases in SIRT2 inhibitor AGK2-treated microglial BV2 cells. Neurosci Lett 544: 36–40 [DOI] [PubMed] [Google Scholar]
- Liu S, Kielian T (2009) Microglial activation by Citrobacter koseri is mediated by TLR4- and MyD88-dependent pathways. J Immunol 183: 5537–5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64: 110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander P, Brown GC (2005) Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J Neuroinflammation 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38: 1285–1291 [DOI] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J (2004) RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 5: 503–507 [DOI] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Montine KS, Horner PJ, Montine TJ (2003) Pharmacologic suppression of neuronal oxidative damage and dendritic degeneration following direct activation of glial innate immunity in mouse cerebrum. J Neurochem 87: 1518–1526 [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116: 201–211 [DOI] [PubMed] [Google Scholar]
- Narayan N, Lee IH, Borenstein R, Sun J, Wong R, Tong G, Fergusson MM, Liu J, Rovira II, Cheng HL, Wang G, Gucek M, Lombard D, Alt FW, Sack MN, Murphy E, Cao L, Finkel T (2012) The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 492: 199–204 [DOI] [PubMed] [Google Scholar]
- Nie H, Chen H, Han J, Hong Y, Ma Y, Xia W, Ying W (2011) Silencing of SIRT2 induces cell death and a decrease in the intracellular ATP level of PC12 cells. Int J Physiol Pathophysiol Pharmacol 3: 65–70 [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11: 437–444 [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317: 516–519 [DOI] [PubMed] [Google Scholar]
- Pais TF, Appelberg R (2000) Macrophage control of mycobacterial growth induced by picolinic acid is dependent on host cell apoptosis. J Immunol 164: 389–397 [DOI] [PubMed] [Google Scholar]
- Pais TF, Chatterjee S (2005) Brain macrophage activation in murine cerebral malaria precedes accumulation of leukocytes and CD8+ T cell proliferation. J Neuroimmunol 163: 73–83 [DOI] [PubMed] [Google Scholar]
- Pais TF, Figueiredo C, Peixoto R, Braz MH, Chatterjee S (2008) Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J Neuroinflammation 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Luscher-Firzlaff J, Vervoorts J, Lasonder E, Kremmer E, Knoll B, Luscher B (2008) The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol 180: 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6: 193–201 [DOI] [PubMed] [Google Scholar]
- Piccio L, Buonsanti C, Cella M, Tassi I, Schmidt RE, Fenoglio C, Rinker J 2nd, Naismith RT, Panina-Bordignon P, Passini N, Galimberti D, Scarpini E, Colonna M, Cross AH (2008) Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 131: 3081–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088 [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE (2009) CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci 29: 11982–11992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A, Laurie C, Mosley RL, Gendelman HE (2007) Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol 82: 297–325 [DOI] [PubMed] [Google Scholar]
- Rota C, Bergamini S, Daneri F, Tomasi A, Virgili F, Iannone A (2002) N-Acetylcysteine negatively modulates nitric oxide production in endotoxin-treated rats through inhibition of NF-kappaB activation. Antioxid Redox Signal 4: 221–226 [DOI] [PubMed] [Google Scholar]
- Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010a) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123: 4251–4258 [DOI] [PubMed] [Google Scholar]
- Rothgiesser KM, Fey M, Hottiger MO (2010b) Acetylation of p65 at lysine 314 is important for late NF-kappaB-dependent gene expression. BMC Genomics 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotili D, Tarantino D, Carafa V, Lara E, Meade S, Botta G, Nebbioso A, Schemies J, Jung M, Kazantsev AG, Esteller M, Fraga MF, Altucci L, Mai A (2010) Identification of tri- and tetracyclic pyrimidinediones as sirtuin inhibitors. ChemMedChem 5: 674–677 [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK (2011) Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11: 775–787 [DOI] [PubMed] [Google Scholar]
- Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X (2010) Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol 30: 4712–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley ST, King JA, Hancock WW (2001) Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167: 2887–2894 [DOI] [PubMed] [Google Scholar]
- Stansley B, Post J, Hensley K (2012) A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J Neuroinflammation 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schluter D, Deckert M (2008) Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am J Pathol 172: 132–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci 30: 244–250 [DOI] [PubMed] [Google Scholar]
- Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM (2006) Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci 26: 4104–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego EM, Outeiro TF, Kermer P, Schulz JB (2013) Impairment of the septal cholinergic neurons in MPTP-treated A30P alpha-synuclein mice. Neurobiol Aging 34: 589–601 [DOI] [PubMed] [Google Scholar]
- Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, Piccini P (2007) Imaging microglial activation in Huntington’s disease. Brain Res Bull 72: 148–151 [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D (2006) SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 20: 1256–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF (2009) Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol 215: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nguyen M, Qin FX, Tong Q (2007) SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6: 505–514 [DOI] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107: 11942–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95: 15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, Torres M, Burguete MC, Manzanares J, Lizasoain I, Moro MA (2012) Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke 43: 211–219 [DOI] [PubMed] [Google Scholar]
- Zingarelli B, O’Connor M, Wong H, Salzman AL, Szabo C (1996) Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol 156: 350–358 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.