Abstract
Repeated semen specimens from healthy men were analyzed by sperm fluorescence in situ hybridization (FISH), to identify men who consistently produced elevated frequencies of aneuploid sperm and to determine whether men who were identified as stable variants of sperm aneuploidy also exhibited higher frequencies of aneuploidy in their peripheral blood lymphocytes. Seven semen specimens were provided by each of 15 men over a 2-year period and were evaluated by the X-Y-8 multicolor sperm FISH method (i.e., ∼1,050,000 sperm were analyzed from 105 specimens). Three men were identified as stable aneuploidy variants producing significantly higher frequencies of XY, disomy X, disomy Y, disomy 8, and/or diploid sperm over time. In addition, one man and three men were identified as sperm-morphology and sperm-motility variants, respectively. Strong correlations were found between the frequencies of sperm with autosomal and sex-chromosome aneuploidies and between the two types of meiosis II diploidy; but not between sperm aneuploidy and semen quality. A significant association was found between the frequencies of sex-chromosome aneuploidies in sperm and lymphocytes in a subset of 10 men (r2=0.67, P=.004), especially between XY sperm and sex-chromosome aneuploidy in lymphocytes (r2=0.70, P=.003). These findings suggest that certain apparently healthy men can produce significantly higher frequencies of both aneuploid sperm and lymphocytes. Serious long-term somatic and reproductive health consequences may include increased risks of aneuploidy-related somatic diseases and of having children with paternally transmitted aneuploidies, such as Klinefelter, Turner, triple-X, and XYY syndromes.
Introduction
It is well known that aneuploidy can have major detrimental health consequences when it occurs in either germinal or somatic cells (reviewed by Hassold and Hunt 2001). Germinal aneuploidies, a major cause of pregnancy loss, aneuploid births, and developmental defects (Wyrobek et al. 2000), are thought to arise de novo, through meiotic errors in the germ cells of either parent, or mitotically, shortly after fertilization. The resulting embryos typically die early during development, at times that generally correspond to the specific chromosome affected. Only a few aneuploidies survive to birth (i.e., trisomy 21, trisomy 13, trisomy 18, triple X, XO, XXY, and XYY). Although advanced maternal age is known to be a primary risk factor for transmitted aneuploidy, less is known about paternal risk factors (Wyrobek et al. 1996). Several paternal risk factors for sperm aneuploidy have been described, including advanced age (Griffin et al. 1995; Lowe et al. 1995; Martin et al. 1995; Robbins et al. 1995; Kinakin et al. 1997; Lowe et al. 2001), cancer chemotherapy (Robbins 1996, 1997a; De Mas et al. 2001; Marchetti et al. 2001), suicide attempts by use of high-dose diazepam (Baumgartner et al. 2001), cigarette smoking (Robbins et al. 1997b; Rubes et al. 1998; Harkonen et al. 1999; Shi et al. 2001), and exposure to air pollution (Robbins et al. 1999).
By use of the hamster-egg method and FISH cytogenetics, it has been shown that healthy individuals generally exhibit low baseline frequencies of aneuploidy in their sperm (e.g., see Brandriff and Gordon 1990; Martin and Rademaker 1990; Robbins et al. 1995). Despite numerous reports showing (1) that there is interchromosomal and interindividual variability in the baseline frequencies of specific sperm aneuploidies and (2) that some men may have significantly higher frequencies for some aneuploidies than for others (reviewed by Sloter et al. 2000; Shi and Martin 2000a), very few studies have actually evaluated multiple semen samples from the same individuals to determine whether the observed variations are due to technical factors or sporadic events in individuals or are time-stable characteristics of the individuals. The paucity of data on repeated semen samples has made it difficult to estimate the prevalence of men who consistently exhibit increased frequencies of sperm aneuploidy—that is, who are “stable” variants. For example, Robbins et al. (1995) identified 1 variant man among 10 with increased disomy Y, on the basis of three specimens. Thus, the first objective of our study was to determine whether repeated specimens from healthy normospermic young men could be used to identify stable variants of sperm aneuploidy.
Somatic aneuploidy has also been associated with serious health consequences. For example, aneuploidy is considered to be a critical event in the latter stages of the progression of certain cancers (Duesberg et al. 1999; Duesberg and Rasnick 2000; Sen 2000). However, it is not known whether there are constitutive genetic susceptibilities for increased frequencies of aneuploidy and whether such susceptibilities affect both germ cells and somatic cells. Therefore, the second objective of our study was to determine whether men with consistently elevated frequencies of sperm aneuploidy also exhibit an increased incidence of malsegregation in their somatic cells—specifically, in their lymphocytes. Accordingly, a multicolor FISH procedure that employs the same probes for chromosomes X, Y, and 8 was applied to both peripheral lymphocytes and sperm, so that potential correlations between sex-chromosome and autosomal aneuploidy frequencies could be examined in both tissue types.
Patients, Material, and Methods
Semen Collection
A male reproductive-health study was conducted during 1995–97 that involved collection of serial semen samples from 37 men. These men were 20 or 21 years of age at the start of the study and were a subset of men originally recruited for a study of the effect of air pollution, sponsored by the Czech Ministry of the Environment and the U.S. Environmental Protection Agency. All the donors provided their written informed consent and completed a questionnaire concerning their health, medication, lifestyle (e.g., smoking and alcohol and coffee consumption), and exposure to potential reproductive toxicants (e.g., solvents, pesticides, and metals), with emphasis on the 3 mo prior to providing their specimens. The study protocol was reviewed and approved by the institutional review boards of the Czech Academy of Sciences and the University of California's Lawrence Livermore National Laboratory. The donors lived in the same community and provided samples in the same windows of time. Of the 37 men who started the longitudinal study, 22 gave seven semen samples each. Seven of these men were eliminated because of heavy smoking (⩾20 cigarettes/day), or because they had undergone some drug treatment during the two years of the follow-up. The remaining 15 men were healthy when examined on entry into the study, did not report drug use or occupational exposure, and declared themselves to be either nonsmokers (n=7) or light-to-moderate smokers (<1 pack/day; n=8). Semen specimens were provided by each man in September 1995; in January, February, March, and September 1996; and in February and September 1997.
Semen Processing
Semen specimens were provided by masturbation into clean glass containers (Kavalier) in a private room located at the Regional Institute of Hygiene. The semen was allowed to liquefy at room temperature and then was analyzed within 1 h of collection, according to standard procedures (World Health Organization [WHO] 1992; Selevan et al. 2000). Semen volume, sperm concentration, and total sperm count, as well as percentages of motile and morphologically normal sperm, were assessed. The remaining semen was loaded into straws, was snap frozen on dry ice, and was stored frozen at −80°C without any cryoprotectives. For the FISH assay, straws were thawed at room temperature, and semen was smeared onto clean microscopic slides and was allowed to air dry. Sperm nuclei were decondensed as described by Robbins et al. (1993) and were hybridized immediately.
Sperm FISH
A multicolor FISH assay was performed by use of chromosome-specific α-satellite DNA probes, for chromosomes X and 8, and satellite III DNA probe, for chromosome Y (Vysis), as adapted from our previous method (Rubes et al. 1998). This method allows for an accurate distinction between disomic and diploid sperm nuclei, nullisomic, and nonhybridizing spermatozoa, and meiosis I and meiosis II errors in sex-chromosome aneuploidy and diploidy. The probe for chromosome X was labeled with Spectrum green and that for chromosome Y was labeled with Spectrum orange. A 1:1 mixture of probes for chromosome 8 labeled with Spectrum orange and Spectrum green was used to obtain a yellow signal when examined with a dual red-green or triple red-green-blue band-pass filters. The slides were denatured in 70% formamide/2× saline sodium citrate (SSC) (pH 7.2) at 72°C for 4 min, were dehydrated in an ethanol series, and were air-dried. The hybridization mixture containing centromeric enumeration probe hybridization buffer (Vysis) and the probes were denatured at 72°C for 5 min and were immediately chilled on ice. A 10-ml sample of the mixture was applied onto the slide, covered with a 24×24 mm2 cover slip, and sealed with rubber cement. The slides were incubated in a humidified chamber at 37°C overnight. After hybridization, the slides were washed for 10 min in 50% formamide/2× SSC (pH 7.2) at 45°C, for 10 min in 2× SSC at 45°C, and for 10 min in 2× SSC at room temperature, and the preparations were mounted by use of the antifade solution (Vector Laboratories) containing 0.1 mg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Sigma).
Sperm Scoring under the Microscope
All scoring was performed on coded, randomly ordered slides. At least 10,000 spermatozoa were scored per sample (∼70,000 spermatozoa per donor). The slides were examined using an Olympus BX60 fluorescence microscope equipped with a fluorescein isothiocyanate/propidium iodide (FITC/PI) dual and DAPI/FITC/Texas Red triple–band-pass filters, and phase-contrast optics. It is generally accepted that strict scoring criteria are important for the valid determination of sperm aneuploidy (Robbins et al. 1993; Martin and Rademaker 1995). In our study, we evaluated a minimum of 10,000 sperm per semen specimen, using strict scoring criteria that we previously validated against human-sperm/hamster-egg data (Robbins et al. 1993). According to our criteria, sperm nuclei were scored only if they were intact, were not overlapped, were not overdecondensed, and were in a well-hybridized area of the slide. The sperm was identified as disomic when the two fluorescence domains of the same color comparable in size and intensity were separated by a distance of at least one domain, were not connected by fluorescent threads, and were similar (in size and intensity) to those in neighboring cells. Because of the larger size of the Y domain, two Y domains in cells being scored as disomic had to be separated by a distance corresponding to the absolute separations between domains in X-X and 8-8 cells on the same slide. Diploid spermatozoa were discriminated from somatic cells or two overlapping spermatozoa by use of phase-contrast optics under which sperm tails and cell borders could be easily recognized.
Blood Collection and Processing
Ten donors were selected for lymphocyte analyses: four sperm variants (i.e., those with the four highest statistically significant elevations in frequencies of aneuploid or diploid sperm) plus six men with consistently lower (and more typical) levels of sperm aneuploidy. A single blood specimen was drawn from each of these 10 men on the same day that their final semen sample was provided. One milliliter of blood was added to 10 ml of the culture medium RPMI 1640 (Sevapharma) supplemented with 20% fetal calf serum, phytohemagglutinin (Murex Biotech) and L-glutamine (Sevac). The cultures were harvested after 48 h of incubation. Colchicine (Fluka Chemie GmbH) was added to a final concentration of 0.5 mg/ml for 1 h before the end of the incubation. Slides were prepared using the air-dry method (Verma and Babu 1989) and were stored at −20°C for FISH.
Lymphocyte FISH
The multicolor three-chromosome FISH protocol, including the DNA probes and scoring criteria, was the same as that used for sperm, except that the decondensation step was omitted for lymphocytes. All slides were coded and randomized prior to analysis. The scoring was done by the same person who scored the sperm slide.
For each donor, ∼5,000 interphase lymphocytes and 200 metaphases were examined. The findings in metaphase and interphase cells were pooled to calculate the frequency of aneuploid lymphocytes for each donor.
Statistical Analysis
All statistical analyses were performed by SPSS version 10.1 for Windows software package (SPSS) and the statistical package STATA (Hamilton 1998). The frequencies of aneuploidy and diploidy for the chromosomal outcomes were generally not normally distributed and required square-root transformations for all parametric tests. Repeated measures analysis was conducted to ascertain whether any of these outcomes changed in association with collection cycle (1–7) or season of sampling, since our previous study found an association between exposure to seasonal air pollution and increased YY disomy (Robbins et al. 1999). No significant associations were found. Analysis of variance was then applied to assess differences in the mean frequency of aneuploid sperm category and interindividual variability. The Kendall’s tau b correlation coefficient was used to determine whether there were associations between categories of sperm disomy and diploidy. Simple linear regression was used to evaluate within-donor relationships between the frequencies of lymphocytes carrying somatic or sex-chromosome trisomy versus the frequency of sperm carrying somatic or sex-chromosome aneuploidy.
Results
Seven semen specimens that were provided by each of 15 healthy men over a 2-year period (105 total specimens) were analyzed for semen quality by conventional parameters (semen volume, sperm concentration, total sperm count, motility, and morphology) and for sperm aneuploidy by multicolor FISH (tables 1 and 2). Semen quality among the 15 men was generally within the WHO reference ranges for normospermic men (WHO 1999): average sperm concentrations ranged from 62 × 106 to 177 × 106 per ml, total count from 161 to 724 × 106 per specimen, and all but two men averaged >50% motile sperm (table 1). When strict microscopic scoring criteria for sperm-head morphology were used (WHO 1992), the study group showed an average (± SD) of 27.1 ± 7.7% sperm with normal sperm-head morphology, which was very similar to the overall 27.3 ± 7.4% established for entire group of the 37 young healthy men who started the longitudinal study (Rubes et al., unpublished data). All men were 20 or 21 years old at the beginning of the study. Of the men, 10 were of untested fertility, and 5 were reported to have fathered at least one child. For each semen-quality parameter, we calculated the study mean, its overall coefficient of variation (CV), and estimated among- and within-donor contributions to variation. The CVs of the semen-quality parameters ranged from ∼27% to ∼69%, and there was little difference between among- and within-donor variation. One sperm-morphology outlier (donor 14) and three sperm-motility outliers (donors 8, 9, and 15) were identified as variants, defined as in the legend to figure 1.
Table 1.
Demographics and Semen Parameters of the Study Group
|
Semen Parametersc |
|||||||
| Donor | Agea(years/mo) | SmokingStatusb | Sperm Concentration(ml × 106 ± SD) | Total Sperm Count(per sample× 106± SD) | Motility(% motile ± SD) | MorphologicallyNormal Heads(% ± SD) | FertilityStatusd |
| 1 | 21/7 | 16 (300) | 98 ± 30 | 445 ± 147 | 75.8 ± 9.4 | 27.2 ± 5.0 | SA |
| 2 | 21/8 | 0 | 91 ± 30 | 411 ± 197 | 84.2 ± 7.0 | 27.1 ± 3.3 | UT |
| 3 | 21/0 | 19 (347) | 126 ± 57 | 161 ± 73 | 66.9 ± 8.9 | 27.4 ± 4.9 | UT |
| 4 | 21/4 | 5 (92) | 140 ± 70 | 245 ± 114 | 69.0 ± 5.6 | 30.7 ± 3.2 | 1 |
| 5 | 21/1 | 5 (91) | 65 ± 36 | 240 ± 175 | 68.9 ± 10.9 | 20.5 ± 6.2 | UT |
| 6 | 21/9 | 0 | 100 ± 34 | 331 ± 103 | 71.6 ± 13.3 | 37.1 ± 11.0 | IAe/1 |
| 7 | 20/6 | 0 | 62 ± 41 | 205 ± 157 | 68.8 ± 15.6 | 30.8 ± 4.1 | UT |
| 8 | 20/8 | 0 | 117 ± 33 | 271 ± 115 | 36.5 ± 25.5 | 24.1 ± 5.5 | 1 |
| 9 | 20/6 | 1 (18) | 77 ± 44 | 270 ± 191 | 41.8 ± 17.2 | 29.5 ± 3.5 | UT |
| 10 | 20/7 | 0 | 84 ± 29 | 724 ± 388 | 67.2 ± 12.0 | 26.0 ± 3.9 | UT |
| 11 | 20/2 | 4 (74) | 84 ± 35 | 219 ± 126 | 52.2 ± 11.0 | 19.8 ± 5.2 | SA/1 |
| 12 | 20/6 | 8 (147) | 91 ± 43 | 197 ± 102 | 65.3 ± 15.4 | 35.5 ± 10.2 | SA/SA/1 |
| 13 | 20/4 | 0 | 79 ± 29 | 197 ± 100 | 69.1 ± 13.1 | 25.1 ± 3.8 | UT |
| 14 | 20/0 | 0 | 177 ± 83 | 593 ± 180 | 65.6 ± 15.8 | 16.3 ± 7.0 | UT |
| 15 | 20/2 | 15 (274) | 168 ± 55 | 514 ± 223 | 83.1 ± 6.3 | 29.3 ± 4.0 | UT |
At first specimen.
The values shown are zero for nonsmokers and the number of smoked cigarettes per day for current smokers; numbers in parenthesis are the total estimated number of packs consumed during the smoker's lifetime.
Values represent the mean of seven repeated samples.
Reproductive outcome in pregnancy order: UT = untested fertility; numerical values are the number of children; SA = spontaneous abortion; and IA = induced abortion.
Trisomy 21.
Table 2.
Mean Frequencies of Aneuploid and Diploid Sperm for the Group of 15 Healthy Donors
|
Frequency ± SD per 10,000 Sperm |
||||||||||||
| Specific Aneuploidies |
Specific Diploidies |
|||||||||||
| Donor | SpermScored | Total Numerical | Total Aneuploidy | Total Sex Aneuploidy | Total Diploidy | Disomy Y | Disomy X | XY | Disomy 8 | X-Y-8-8 (MI)a | X-X-8-8 (MII)b | Y-Y-8-8 (MII)b |
| 1 | 70,581 | 17.1 ± 8.3 | 7.9 ± 3.6 | 6.1 ± 3.4 | 9.2 ± 6.5 | 0.7 ± 1.1 | 0.4 ± 0.5 | 5.0 ± 3.0 | 1.8 ± 0.9 | 4.7 ± 3.3 | 0.6 ± 0.8 | 4.0 ± 3.2 |
| 2 | 70,374 | 18.2 ± 5.6 | 10.9 ± 2.3 | 8.2 ± 2.5 | 7.2 ± 4.3 | 2.0 ± 0.8 | 1.6 ± 0.8 | 4.7 ± 3.1 | 2.7 ± 2.1 | 6.2 ± 3.1 | 1.0 ± 1.4 | 0.0 ± 0.0 |
| 3 | 70,031 | 11.7 ± 2.1 | 7.9 ± 1.9 | 6.4 ± 1.7 | 3.9 ± 2.0 | 0.7 ± 0.5 | 0.1 ± 0.4 | 5.6 ± 1.7 | 1.4 ± 0.8 | 3.4 ± 2.0 | 0.1 ± 0.4 | 0.3 ± 0.5 |
| 4 | 70,585 | 23.2 ± 5.4 | 14.7 ± 4.4 | 12.3 ± 4.4 | 8.5 ± 3.1 | 6.2 ± 4.6 | 0.8 ± 1.1 | 5.2 ± 3.9 | 2.4 ± 1.4 | 7.7 ± 3.0 | 0.3 ± 0.5 | 0.6 ± 0.8 |
| 5 | 71,083 | 35.7 ± 5.9 | 25.3 ± 3.7 | 20.5 ± 3.3 | 10.4 ± 4.4 | 5.8 ± 2.5 | 0.7 ± 0.7 | 14.1 ± 1.3 | 4.8 ± 3.6 | 6.9 ± 3.2 | 2.1 ± 1.2 | 1.4 ± 1.0 |
| 6 | 70,298 | 16.8 ± 4.9 | 9.0 ± 2.7 | 7.4 ± 2.1 | 7.8 ± 3.9 | 4.1 ± 1.8 | 1.4 ± 1.6 | 1.8 ± 1.2 | 1.6 ± 1.1 | 6.4 ± 4.0 | 0.4 ± 0.5 | 1.0 ± 1.0 |
| 7 | 70,333 | 19.2 ± 5.3 | 13.5 ± 5.9 | 11.8 ± 5.9 | 5.7 ± 2.5 | 5.7 ± 4.9 | 0.7 ± 1.3 | 5.4 ± 3.7 | 1.7 ± 1.6 | 3.0 ± 1.7 | 1.3 ± 1.2 | 1.4 ± 1.0 |
| 8 | 70,629 | 9.8 ± 2.5 | 7.5 ± 2.6 | 6.4 ± 2.0 | 2.3 ± 1.4 | 2.3 ± 0.9 | 0.6 ± 0.8 | 3.5 ± 1.6 | 1.1 ± 0.7 | 2.3 ± 1.4 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 9 | 70,356 | 12.1 ± 3.3 | 9.4 ± 3.1 | 9.1 ± 2.7 | 2.7 ± 0.8 | 5.8 ± 1.7 | 1.0 ± 1.2 | 2.3 ± 0.7 | 0.3 ± 0.8 | 1.9 ± 1.1 | 0.4 ± 0.8 | 0.4 ± 0.5 |
| 10 | 70,314 | 8.7 ± 4.7 | 5.3 ± 3.7 | 4.8 ± 3.3 | 3.4 ± 1.8 | 3.0 ± 1.6 | 0.7 ± 1.1 | 1.1 ± 1.5 | 0.4 ± 0.8 | 3.1 ± 1.9 | 0.0 ± 0.0 | 0.3 ± 0.5 |
| 11 | 70,258 | 20.2 ± 4.6 | 6.5 ± 2.4 | 5.1 ± 2.2 | 13.7 ± 4.4 | 3.8 ± 2.5 | 0.6 ± 0.8 | 0.7 ± 0.8 | 1.4 ± 1.0 | 12.8 ± 5.0 | 0.7 ± 0.8 | 0.1 ± 0.4 |
| 12 | 70,593 | 34.9 ± 8.7 | 18.0 ± 5.3 | 14.3 ± 4.5 | 16.9 ± 7.5 | 3.7 ± 3.9 | 3.1 ± 1.7 | 7.5 ± 4.0 | 3.7 ± 1.5 | 12.9 ± 5.8 | 1.6 ± 1.5 | 2.4 ± 1.5 |
| 13 | 70,642 | 50.8 ± 12.8 | 20.7 ± 5.3 | 14.0 ± 5.2 | 30.1 ± 11.0 | 9.8 ± 4.7 | 2.0 ± 1.0 | 2.3 ± 1.1 | 5.4 ± 3.0 | 6.8 ± 4.0 | 14.6 ± 5.3 | 8.8 ± 3.3 |
| 14 | 70,330 | 23.9 ± 9.3 | 9.4 ± 4.1 | 7.7 ± 3.1 | 14.5 ± 6.9 | 4.1 ± 2.6 | 1.0 ± 0.8 | 2.6 ± 1.3 | 1.7 ± 1.2 | 12.2 ± 5.1 | 1.4 ± 2.1 | 0.9 ± 1.1 |
| 15 | 70,221 | 23.2 ± 6.7 | 17.8 ± 7.0 | 16.7 ± 6.9 | 5.4 ± 2.6 | 5.7 ± 2.1 | 1.0 ± 0.8 | 10.0 ± 6.1 | 1.1 ± 1.2 | 3.3 ± 2.9 | 1.1 ± 1.1 | 1.0 ± 0.6 |
“MI” refers to a possible error in meiosis I cytokinesis.
“MII” refers to a possible error in meiosis II cytokinesis.
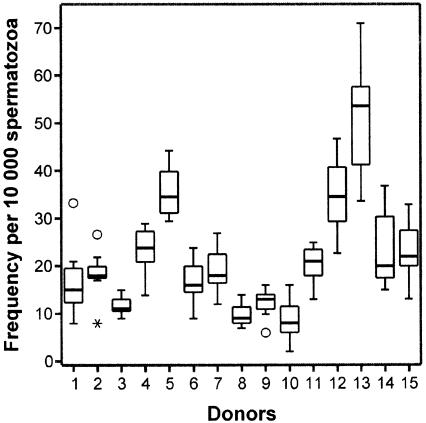
Figure 1.
Box plots for comparing the within-person distributions of aggregate frequencies of sperm carrying any of the numerical abnormalities detected by the X-Y-8 sperm method FISH among the 15 men of the study, on the basis of seven samples per donor collected over a 2-year period. The aggregate frequency is the sum of the four aneuploidy and three diploidy categories detected by the X-Y-8 FISH sperm method (see “Patients, Materials, and Methods” section). The vertical height of each box represents the 25%–75% data range, the horizontal line within each box represents the median value, and the upper and lower extensions represent the largest and smallest values that were determined to not be outlier specimen among the seven specimens for each donor. Among the 105 specimens in this study, 4 were considered to be either of two types of outliers: unblackened circles (○) denote simple outliers, individual sperm specimens whose frequency of abnormal sperm fell >1.5 box lengths from the 25th percentile of the distribution for the seven specimens evaluated per donor, whereas an asterisk (*) denotes an extreme outlier, an individual specimen whose frequency of abnormal sperm fell >3 box lengths from the 25th percentile of the distribution for the seven specimens evaluated per donor.
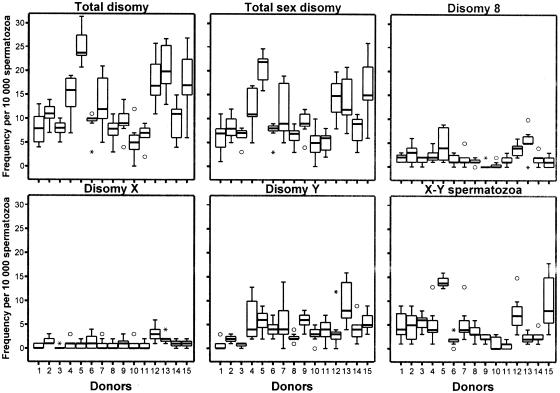
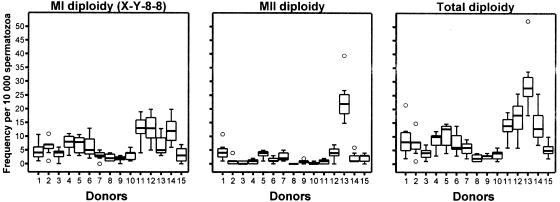
Each of the 105 semen specimens was evaluated for the frequencies of sperm carrying abnormalities in chromosome number (table 2) using the multicolor X-Y-8 sperm FISH method, which detects four categories of sperm aneuploidies (XY sperm plus sperm that are disomic for chromosomes X, Y, and 8) and three categories of sperm diploidies (two categories of meiosis II [MII] diploidy plus one category of meiosis I [MI] diploidy) (table 2). Comparisons of the distributions of aggregate frequencies of numerically abnormal sperm (fig. 1) showed significant approximately six-fold range of variation among the 15 men, from ∼9 to ∼51 abnormal cells per 10,000 cells (table 2); donors 5, 12, and 13 showed the highest aggregate frequencies of abnormal cells. There were significant among-donor variations (P<.001) for each category of aneuploid sperm (fig. 2) and diploid sperm (fig. 3). This remained true when individual outlier males were removed for each category except disomy X, in which among-donor variation changed to nonsignificant when the outlier (donor 12) was removed.
Figure 2.
Within-donor distributions of frequencies of various categories of aneuploid sperm that were detected by the X-Y-8 sperm FISH method (see the legend to fig. 1). Total disomy is the sum of the frequencies of sperm carrying disomy X, Y, and 8 and the frequency of XY sperm. Total sex disomy is the sum of the frequencies of disomy X, disomy Y, and XY sperm.
Figure 3.
Within-donor distributions of frequencies for the three categories of diploid sperm detected by the X-Y-8 sperm FISH method. Meiosis I diploidy refers to the frequency of sperm with the genotype X-Y-8-8, which is expected to arise as errors in the first meiotic division (MI). Meiosis II diploidies refer to the frequencies of sperm with the genotypes X-X-8-8 and Y-Y-8-8, both of which are expected to arise as errors in the second meiotic division (MII). Total diploidy is the sum of the frequencies of MI and MII sperm diploidies.
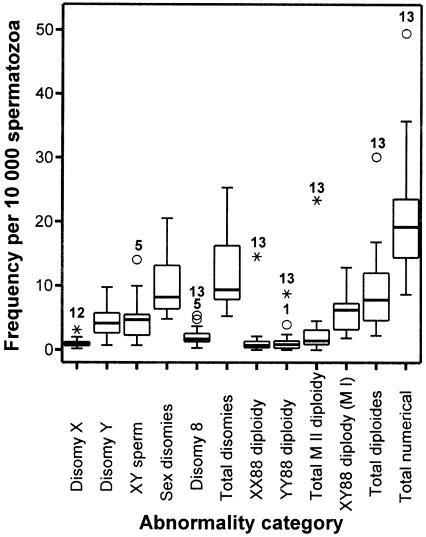
For each category of sperm chromosomal abnormality, we calculated the mean frequency across all study specimens, its CV, estimated the between-donor and within-donor contributions, and used these to identify males who were consistent outliers (fig. 4). For the sperm disomies 8, X, and Y and for XY sperm, group means ranged from ∼1 to 5 per 10,000 sperm, and CVs ranged from ∼47% to 75%. For the sperm diploidies MI and MII, group means ranged from ∼1 to 6 per 10,000 sperm, and CVs ranged from ∼61% to 211%. “Stable variants” were defined as men who consistently produced significantly higher frequencies of aneuploid or diploid sperm over a 2-year period, in contrast to the rest of the men in the study. Two statistical methods were used: Z score and outliers on a box-plot analysis (fig. 4). Three donors were identified as stable variants of sperm aneuploidy: donor 13 for disomy 8, donor 12 for disomy X, and donor 5 for XY sperm. Donor 13 was identified as a stable variant for all three categories of sperm diploidy. Donor 1 was identified as a borderline variant for YY88 diploidy (fig. 4).
Figure 4.
Distributions of frequencies of aneuploid and diploid sperm among donors. The numbered data points represent the identity code for the category-specific outliers (i.e., stable variants). See the legend to figure 1.
Associations among Sperm Endpoints
Correlation analyses were performed to determine whether there might be possible common underlying mechanisms among the different sperm chromosomal aneuploidies. As shown in table 3, we found several significant associations. The frequencies of sperm carrying disomy 8 were correlated (P=.01) with the frequency of sperm carrying any sex-chromosome aneuploidy (sum of disomy X, disomy Y, and XY sperm). Disomy 8 was also highly correlated (P=.001) with total diploidy (sum of XX88, YY88, and XY88) as well as with all three subcategories of sperm diploidy (P=.005, P=.005, and P=.009, respectively) In addition, there was a significant correlation between the two categories of meiosis II diploidies (P=.006), but there was a trend only between disomy X and Y (P=.06), which were also meiosis II errors. No evidence was found for associations between disomy 8 and the individual sex-chromosome aneuploidies, nor between meiosis I and II diploidies, nor between sex-chromosome aneuploidies and diploidies. Also, no stable trends for associations were noted between aneuploidy and smoking status or between any of the aneuploidy outcomes and alcohol or caffeine across the seven collection cycles. No consistent associations were found between the frequency of chromosomally abnormal sperm (aggregate frequency, individual category of aneuploidies, and individual categories of sperm diploidies) and sperm quality (sperm concentration, motility, and morphology) with one exception. The numbers of sperm (both concentration and total) were significantly correlated with the frequency of sperm with disomy 8 (data not shown; P<.05).
Table 3.
Correlations among Categories of Sperm Disomy and Diploidy
|
Correlation |
||||
| With Outliers |
Without Outliers |
|||
| Category | Kendall’s tau bCorrelation Coefficient | P | Kendall’s tau bCorrelation Coefficient | P |
| Disomies: | ||||
| Disomy 8 vs. total sex aneuploidya | .49 | .012 | .44 | .038 |
| Disomy X vs. Yb | .35 | .067 | −.13 | .542 |
| Diploidies: | ||||
| Between two MII diploidiesc | .53 | .006 | .46 | .024 |
| Diploidy vs. disomy: | ||||
| Total sex aneuploidy vs. total diploidyd | .26 | .181 | .25 | .208 |
| Disomy 8 vs. total diploidye | .62 | .001 | .56 | .007 |
X88 versus sum(XX8+YY8+XY8).
XX8 versus YY8.
XX88 versus YY88.
Sum(XY8+XX8+YY8) versus sum(XY88+YY88+XX88).
X88 versus sum(XY88+YY88+XX88).
Sperm and Lymphocyte Comparison Study of a Subset of 10 Men
A nested study of a subset of 10 men was undertaken to determine whether there were associations between the frequencies of numerical chromosomal abnormalities in sperm and peripheral blood lymphocytes. The 10 donors were selected on the basis of their initial sperm FISH analyses (table 2): 6 donors with sperm abnormality values within the normal ranges and 4 variants (donor 5 for XY sperm, donor 12 for disomy X, donor 1 for YY88 diploidy, and donor 13 with generalized increase in several categories). Conventional karyotypic analyses of blood lymphocytes confirmed that all the donors were 46,XY. Since the number of metaphases examined in routine chromosomal analyses of peripheral lymphocytes was too low for the detection of low-level aneuploidies, this analysis was expanded to include ∼5,000 interphase cells per donor (table 4). Significant interdonor variations were observed in the frequencies of lymphocytes carrying disomy Y (range 0%–0.25%), disomy X (range 0%–0.13%), and trisomy 8 (range 0%–0.10%). The overall frequency of triploid or tetraploid lymphocytes was 0.011%, which is significantly lower (P<.01) than the proportion of diploid sperm (0.094%).
Table 4.
Chromosome 8, X, and Y Aneuploidies in Peripheral Lymphocytes of 10 Male Donors
|
Frequency per 1,000 Cells |
||||
| Donor | Cells Scored | Disomy Y | Disomy X | Trisomy 8 |
| 1 | 5,252 | .38 | .00 | .19 |
| 2 | 5,039 | 1.59 | .40 | .99 |
| 5a | 5,463 | 1.28 | 1.28 | .18 |
| 6 | 5,367 | .37 | .56 | .00 |
| 8 | 5,187 | .58 | .19 | .39 |
| 9 | 5,371 | .19 | .19 | .19 |
| 10 | 5,281 | .00 | .19 | .00 |
| 12a | 5,562 | 1.26 | .54 | .72 |
| 13a | 5,567 | .72 | .36 | .36 |
| 15 | 5,210 | 2.50 | .38 | .58 |
Identifies sperm variants.
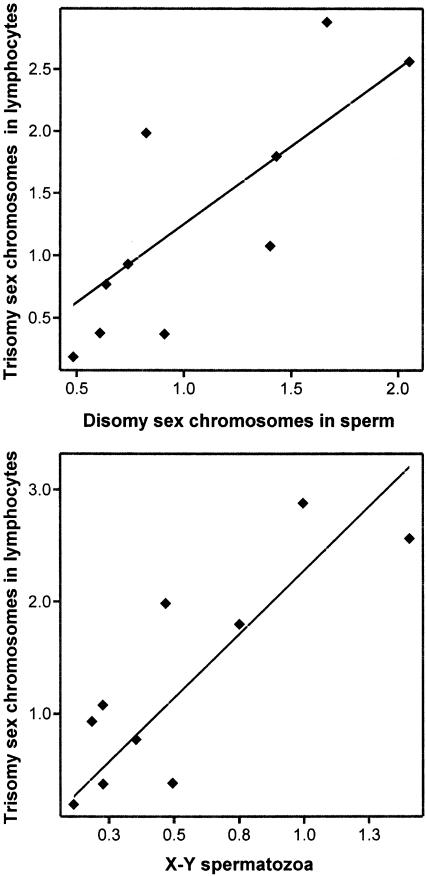
There was a significant correlation between the aggregate frequency of aneuploidy in sperm and lymphocytes (r2=0.50, P=.02). On inspection, this correlation was not due to an association between disomy 8 in sperm and trisomy 8 in lymphocytes. Rather, it was due to a highly significant correlation for sex-chromosome aneuploidies in sperm and lymphocytes (top, fig. 5) (r2=0.67, P=.004). Post hoc analyses of the individual sex-chromosome categories showed no evidence of a correlation between sperm disomy X or Y versus sex-chromosome aneuploidy in lymphocytes, suggesting that mechanisms related to meiosis II errors were not involved. However, there was a significant correlation between XY sperm and sex-chromosome aneuploidy in lymphocytes (bottom, fig. 5) (r2=0.70, P=.003), suggesting that mechanism(s) underlying somatic and germinal malsegregation may be associated.
Figure 5.
Relationships between the frequencies of aneuploid somatic and germinal cells. Top, Within-donor relationship between the frequencies of lymphocytes carrying sex-chromosome trisomy versus the frequency of sperm carrying sex-chromosome aneuploidyaneuploidy for ten donors (r2=0.665, P=.004). The intercept and the slope of the regression line are −0.3 (95% CI −1.3–0.7) and 1.5 (95% CI 0.6–2.3), respectively. Bottom, Within-donor relationship between the frequencies of lymphocytes carrying sex-chromosome trisomy and the frequency of XY sperm for ten donors (r2=0.697, P=.003). The intercept and the slope of the regression line are 0.3 (95% CI −0.4–1) and 1.9 (95% CI 0.9–3), respectively. All the data points fall well within the 95% CI for the regression lines. Frequencies are per 1,000 cells.
Discussion
Analysis of repeated semen specimens from 15 healthy donors over a 2-year period (7 specimens each) identified at least 3 men who were stable variants (outliers) for sperm aneuploidies and/or diploidies. A significant association was found between the frequencies of sex-chromosome aneuploidy in sperm (primarily MI errors) and in lymphocytes. These findings suggest that there are certain men with constitutive factors that can elevate the frequencies of both somatic and germinal aneuploidy, even for individuals who are otherwise apparently healthy and of normal fertility status.
The baseline frequencies of sperm aneuploidy among the group of 15 men compares favorably with the literature for normal healthy men. Shi and Martin (2000a) surveyed prior studies covering millions of sperm from ∼500 normal men and calculated frequencies of sperm with autosomal and sex-chromosome disomies of 0.15% and 0.26%, respectively. However, it is well known that there is substantial technical variation among labs (different methods, scoring criteria, probes, etc.), which may undermine the relevance of frequency estimates based on cross-laboratory data (Wyrobek et al. 2000; Schmid et al. 2001). Sperm FISH scoring criteria used in the present study were calibrated by use of sperm cytogenetic data with the human-sperm/hamster-egg cytogenetic technique (Brandriff and Gordon 1990; Robbins et al. 1993, 1995). The calibrated frequencies are typically lower than those reported by other sperm-FISH laboratories. Thus, the 0.02% frequency of sperm with disomy 8 (0.003%–0.05%) is consistent with our previous reports that used these calibrated scoring criteria (Robbins et al. 1995; Van Hummelen et al. 1996; Rubes et al. 1998). Also, the 0.1% frequency of sperm with sex disomies (0.05 to 0.2) is consistent with prior reports from our laboratory (Robbins et al. 1995; Van Hummelen et al. 1997) and from others (Griffin et al. 1995; Abruzzo et al. 1996; Downie et al. 1997; Baumgartner et al. 1999; Pang et al. 1999).
The use of repeated semen specimens yielded the statistical power to detect significant variation among normal men and to identify stable variants of sperm aneuploidy and diploidy. Although prior studies have also reported interindividual variations in the frequency of sperm aneuploidies (Martin et al. 1994; Spriggs et al. 1995; Morel et al. 1998; Schultz et al. 2000; Shi and Martin 2000b), they were typically limited to the analyses of a single or very few specimens per donor and, therefore, did not address stability and persistence over time. Our study is the first major effort to identify men who have persistent elevations in sperm aneuploidy.
The causes underlying the stable elevations in sperm aneuploidy and diploidy are uncertain. The young men were all about the same age, lived in the same locality in the Czech Republic, and had similar lifestyles with no obvious reasons for variation. Examination of the detailed questionnaire data for each donor did not identify simple explanations for the elevated frequencies among stable variants. Data were examined for relationships between semen quality and sperm aneuploidy, because prior studies had found associations between poor semen quality and increased frequencies of sperm aneuploidies (Colombero et al. 1999; Pfeffer et al. 1999; Vegetti et al. 2000). However, our donors were normospermic, according to WHO criteria (WHO 1992, 1999), and in general, semen-quality measures were not correlated with sperm aneuploidies, diploidies, or subgroupings of these. The exception was a significant association between disomy 8 and sperm concentration (as well as total sperm count per sample); further studies will be needed to determine whether this finding was spurious or can be replicated among other healthy men.
The significant associations found between aneuploidy in sperm and lymphocytes appeared limited to sex-chromosome aneuploidies and were not detected for chromosome 8. These findings are consistent with those of Gazvani et al. (2000a), who reported increased somatic aneuploidy among infertile oligospermic men and found a correlation between sex-chromosome aneuploidies in peripheral lymphocytes and spermatozoa in a group of fertile and infertile men, on the basis of single semen and blood analyses (Gazvani et al. 2000b). These authors also observed a correlation between disomy 21 in sperm and trisomy 21 in lymphocytes. Taken together, these studies suggest that common mechanisms may lead to increased frequencies of somatic and germinal aneuploidies in certain normal and infertile men and that chromosomes may be differentially affected. Several mechanisms can be considered:
First, there may be constitutive biochemical mechanism(s) that increase the risk of segregation errors of certain chromosomes affecting the fidelity of both mitotic and meiotic segregation. Gazvani et al. (2000b) hypothesized that mitotic instability might lead to aneuploidy in somatic and germinal cells undergoing mitotic division, including type A and B spermatogonia, and thereby could increase the incidence of aneuploidy in spermatozoa. Evidence is accruing that cells containing extra sex chromosomes are capable of undergoing meiotic divisions (Lim et al. 1999a, 1999b; Bielanska et al. 2000; Morel et al. 2000; Rives et al. 2000; Shi and Martin 2000a; Wang et al. 2000).
Second, variant individuals may carry mutations or genetic variants (i.e., polymorphisms) for genes that control common aspects of mitotic and meiotic chromosomal segregation or in cell-cycle checkpoints affecting mitotic or meiotic chromosomal stability. The existence of such dysmorphic individuals who have severe cases of aneuploidy in their lymphocytes has been described (Tolmie et al. 1988; Papi et al. 1989; Nash et al. 1997; Gazvani et al. 2000a).
Third, variants of sperm aneuploidy may be chronically exposed to toxicants that increase the frequencies of sex-chromosome aneuploidies in both somatic and germinal cells. Rubes et al. (1998) showed that smoking lifestyle showed significant increases only in disomy Y, suggesting chromosome-limited effects of certain chemical exposures. Sperm studies of men who received cancer chemotherapeutic agents suggest that the aneugenic effects of these drugs seem to be democratic across all chromosomes tested (e.g., Robbins et al. 1997a).
Fourth, variants of sperm aneuploidy may be individuals who have increased somatic and germinal susceptibility to some common exposure(s) that do not affect the majority of the population. Further studies will be needed to identify prevalence, underlying genes and pathways, and possible exposures responsible for the higher frequencies of somatic and germinal aneuploidy in the variants of sperm aneuploidy.
A constitutive elevation in the frequency of aneuploid somatic cells of the stable sperm-aneuploidy variants may have important implications for their risk of somatic diseases that involve aneuploidy. Increased age has been shown to lead to increased sex-chromosome loss in lymphocytes (Galloway and Buckton 1978; Catalan et al. 1995). Sex-chromosome aneuploidy has been associated with oligospermia (Gazvani et al. 2000a) and with premature ovarian failure (Devi et al. 1998). Sex-chromosome aneuploidies have also been observed in several cancers, associated with genomic instability in tumor cells, and several tumor-suppressor genes have been linked to the X chromosomes (Amalfitano et al. 2000; Dasari et al. 2001; Yamaki et al. 2001). Aneuploidy is a common finding in human patients with cancer and, according to the aneuploidy-cancer hypothesis, is regarded as the causative factor of cancer (Duesberg et al. 1999; Duesberg and Rasnick 2000; Sen 2000). There is little information of whether cancer patients have inherently higher frequencies of aneuploid cells. Most of the studies of frequency of aneuploidies in sperm of cancer patients have been performed after chemotherapy or radiation therapy. Recently, Fait et al. (2001) reported increased frequency of sex-chromosome disomies, in sperm of patients with Hodgkin disease, before their therapy. This was consistent with earlier findings of elevated disomy 8 in patients, with Hodgkin disease, before treatment (Robbins et al. 1997a). A larger study will be needed that includes probes for multiple autosomes to determine whether different autosomes are affected differently in sperm, lymphocytes, and other somatic tissue.
It is also of critical concern whether stable sperm-aneuploidy variants are at increased risk of fathering aneuploid pregnancies. Several sperm studies have been conducted in fathers of children with aneuploidy syndromes of proven paternal origin. An analysis of four fathers of daughters with Turner syndrome has shown an increase in the incidence of XY sperm in all the four subjects (Martínez-Pasarell et al. 1999). These authors suggested that the four men had an increased frequency of nondisjunctional errors in MI, resulting in the production of increased proportions of XY sperm and sperm lacking a sex chromosome. An increase in frequencies of disomy 13, 21, and 22 was detected in semen of two fathers who had children with Down syndrome (Blanco et al. 1998; Soares at al. 2001). One of these fathers also had an increased frequency of XY sperm. A recent study of sperm aneuploidy in the fathers of boys with Klinefelter syndrome (Eskenazi et al. 2002) showed that the fathers who contribute the extra X chromosome to their sons with Klinefelter syndrome also produced significantly higher frequencies of XY sperm. In general, these data are consistent with the hypothesis that offspring with paternally transmitted aneuploidies can result from three possible categories of fathers: (a) fathers who produced the rare aneuploid sperm that make up the low baseline frequencies among male humans; (b) fathers who have been exposed to aneugenic agents that act in the short term, such as those exposed to certain cancer-chemotherapeutic agents; or (c) fathers who consistently produce unusually high frequencies of aneuploid sperm, such as the stable aneuploidy variants described in this study.
Our findings suggest that there are normospermic men who produce significantly elevated frequencies of aneuploid sperm and lymphocytes. Our small study of 15 men identified at least three variant men, providing a crude estimate of 20% variants. A better estimate is needed of the prevalence of variants for designing epidemiology studies to identify potential paternal risk factors for sperm aneuploidy (genetics, age, diet, drug consumption, occupational exposures, environmental exposures, etc.). Further studies are also needed of the somatic and reproductive health risks faced by constitutive aneuploidy variants.
Acknowledgments
This work was supported by grant VaV340/1/1997 from the Czech Ministry of the Environment and grant 98-NCERQA-C1 from the U.S. Environmental Protection Agency. Part of this work was also performed under the auspices of the U.S. Department of Energy and the University of California's Lawrence Livermore National Laboratories, under contract W-7405-ENG-48, with support from the National Institute of Environmental Health Sciences Superfund Project number P4ZES04705. We thank Dr. Sherry G. Selevan, for assistance with database transfer and epidemiologic insights, and Zdena Zudova, M.D., for medical examinations. Disclaimer: the information in this document has been funded in part by the U.S. Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Effects Research laboratory and has been approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Abruzzo MA, Griffin DK, Millie EA, Sheean LA, Hassold TJ (1996) The effect of y-chromosome alpha-satellite array length on the rate of sex chromosome disomy in human sperm. Hum Genet 97:819–823 [DOI] [PubMed] [Google Scholar]
- Amalfitano G, Chatel M, Paquis P, Michiels JF (2000) Fluorescence in situ hybridization study of aneuploidy of chromosomes 7, 10, X, and Y in primary and secondary glioblastomas. Cancer Genet Cytogenet 116:6–9 [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Schmid TE, Schuetz CG, Adler ID (2001) Detection of aneuploidy in rodent and human sperm by multicolor FISH after chronic exposure to diazepam. Mutat Res 490:11–19 [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Van Hummelen P, Lowe XR, Adler ID, Wyrobek AJ (1999) Numerical and structural chromosomal abnormalities detected in human sperm with a combination of multicolor FISH assays. Environ Mol Mutagen 33:49–58 [DOI] [PubMed] [Google Scholar]
- Bielanska M, Tan SL, Ao A (2000) Fluorescence in-situ hybridization of sex chromosomes in spermatozoa and spare preimplantation embryos of a Klinefelter 46,XY/47,XXY male. Hum Reprod 15:440–444 [DOI] [PubMed] [Google Scholar]
- Blanco J, Gabau E, Gómez D, Baena N, Guitart M, Egozcue J, Vidal F (1998) Chromosome 21 disomy in the spermatozoa of the fathers of children with trisomy 21 in a population with a high prevalence of Down’s syndrome: increased incidence in cases of paternal origin. Am J Hum Genet 63:1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriff B, Gordon L (1990) Human sperm cytogenetics and the one-cell zygote. In: Allen JW, Bridges BA, Lyon MF, Moses MJ, Russell LB (eds) Biology of mammalian germ cell mutagenesis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 183–194 [Google Scholar]
- Catalan J, Autio K, Wessman M, Lindholm C, Knuutila S, Sorsa M, Norppa H (1995) Age associated micronuclei containing centromeres and the X chromosome in lymphocytes of women. Cytogenet Cell Genet 68:11–33 [DOI] [PubMed] [Google Scholar]
- Colombero LT, Hariprashad JJ, Tsai MC, Rosenwaks Z, Palermo GD (1999) Incidence of sperm aneuploidy in relation to semen characteristics and assisted reproductive outcome. Fertil Steril 72:90–96 [DOI] [PubMed] [Google Scholar]
- Dasari VK, Goharderakhshan RZ, Perinchery G, Li LC, Tanaka Y, Alonzo J, Dahiya R (2001) Expression analysis of Y chromosome genes in human prostate cancer. J Urol 165:1335–1341 [PubMed] [Google Scholar]
- De Mas P, Daudin M, Vincent MC, Bourrouillou G, Calvas P, Mieusset R, Bujan L (2001) Increased aneuploidy in spermatozoa from testicular tumour patients after chemotherapy with cisplatin, etoposide and bleomycin. Hum Reprod 16:1204–1208 [DOI] [PubMed] [Google Scholar]
- Devi AS, Metzger DA, Luciano AA, Benn PA (1998) 45, X/46, XX mosaicism in patients with idiopathic premature ovarian failure. Fertil Steril 70:89–93 [DOI] [PubMed] [Google Scholar]
- Downie SE, Flaherty SP, Swann NJ, Matthews CD (1997) Estimation of aneuploidy for chromosomes 3, 7, 16, X and Y in spermatozoa from 10 normospermic men using fluorescence in-situ hybridization. Mol Hum Reprod 3:815–819 [DOI] [PubMed] [Google Scholar]
- Duesberg P, Rasnick D (2000) Aneuploidy, the somatic mutation that makes cancer a species of its own. Cell Motil Cytoskeleton 47:81–107 [DOI] [PubMed] [Google Scholar]
- Duesberg P, Rasnick D, Li RH, Winters L, Rausch C, Hehlmann R (1999) How aneuploidy may cause cancer and genetic instability. Anticancer Res 19:4887–4906 [PubMed] [Google Scholar]
- Eskenazi B, Wyrobek AJ, Kidd SA, Lowe X, Moore DII, Weisinger, K, Aylstock M (2002) Sperm aneuploidy in fathers of children with paternally and maternally inherited Klinefelter syndrome. Hum Reprod 17:576–583 [DOI] [PubMed] [Google Scholar]
- Fait G, Yogev L, Botchan A, Paz G, Lessing JB, Yavetz H (2001) Sex chromosome aneuploidy in sperm cells obtained from Hodkin’s lymphoma patients before therapy. Fertil Steril 75:828–829 [DOI] [PubMed] [Google Scholar]
- Galloway SM, Buckton KE (1978) Aneuploidy and ageing: chromosome studies on a random sample of the population using G-banding. Cytogenet Cell Genet 20:78–95 [DOI] [PubMed] [Google Scholar]
- Gazvani MR, Wilson EDA, Richmond DH, Howard PJ, Kingsland CR, Lewis-Jones DI (2000a) Evaluation of the role of mitotic instability in karyotypically normal men with oligozoospermia. Fertil Steril 73:51–55 [DOI] [PubMed] [Google Scholar]
- ——— (2000b) Role of mitotic control in spermatogenesis. Fertil Steril 74:251–256 [DOI] [PubMed] [Google Scholar]
- Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ (1995) Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Genet 4:2227–2232 [DOI] [PubMed] [Google Scholar]
- Hamilton LC (1998) Statistics with STATA 5. Brooks/Cole Publishing, Pacific Grove, CA [Google Scholar]
- Harkonen K, Viitanen T, Larsen SB, Bonde JP, Lahdetie J (1999) Aneuploidy in sperm and exposure to fungicides and lifestyle factors. Environ Mol Mutagen 34:39–46 [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280–291 [DOI] [PubMed] [Google Scholar]
- Kinakin B, Rademaker A, Martin R (1997) Paternal age effect of YY aneuploidy in human sperm, as by fluorescence in situ hybridization. Cytogenet Cell Genet 78:116–119 [DOI] [PubMed] [Google Scholar]
- Lim AST, Fong Y, Yu SL (1999a) Analysis of the sex chromosome constitution of sperm in men with a 47,XYY mosaic karyotype by fluorescence in situ hybridization. Fertil Steril 72:121–123 [DOI] [PubMed] [Google Scholar]
- ——— (1999b) Estimates of sperm sex chromosome disomy and diploidy rates in a 47,XXY/46,XY mosaic Klinefelter patient. Hum Genet 104:405–409 [DOI] [PubMed] [Google Scholar]
- Lowe X, Collins B, Allen J, Titenko Holland N, Breneman J, van Beek M, Bishop J, Wyrobek AJ (1995) Aneuploidies and micronuclei in the germ cells of male mice of advanced age. Mutat Res 338:59–76 [DOI] [PubMed] [Google Scholar]
- Lowe X, Eskenazi B, Nelson DO, Kidd S, Alme A, Wyrobek AJ (2001) Frequency of XY sperm increases with age in fathers of boys with Klinefelter syndrome. Am J Hum Genet 69:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti F, Bishop JB, Lowe X, Generoso WM, Hozier J, Wyrobek AJ (2001) Etoposide induces heritable chromosomal aberrations and aneuploidy during male meiosis in the mouse. Proc Natl Acad Sci USA 98:3952–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RH, Chan K, Ko E, Rademaker AW (1994) Detection of aneuploidy in human sperm by fluorescence in situ hybridization (FISH): different frequencies in fresh and stored sperm nuclei. Cytogenet Cell Genet 65:95–96 [DOI] [PubMed] [Google Scholar]
- Martin RH, Rademaker A (1990) The frequency of aneuploidy among individual chromosomes in 6,821 human sperm chromosome complements. Cytogenet Cell Genet 53:103–107 [DOI] [PubMed] [Google Scholar]
- ——— (1995) Reliability of aneuploidy estimates in human sperm: results of fluorescence in situ hybridization studies using two different scoring criteria. Mol Reprod Dev 42:89–93 [DOI] [PubMed] [Google Scholar]
- Martin RH, Spriggs E, Ko E, Rademaker AW (1995) The relationship between paternal age, sex ratios, and aneuploidy frequencies in human sperm, as assessed by multicolor FISH. Am J Hum Genet 57:1395–1399 [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pasarell O, Nogués C, Bosch M, Egoscue J, Templado C (1999) Analysis of sex chromosome aneuploidy in sperm from fathers of Turner syndrome patients. Hum Genet 104:345–349 [DOI] [PubMed] [Google Scholar]
- Morel F, Mercier S, Roux C, Elmrini T, Clavequin MC, Bresson JL (1998) Interindividual variations in the disomy frequencies of human spermatozoa and their correlation with nuclear maturity as evaluated by aniline blue staining. Fertil Steril 69:1122–1127 [DOI] [PubMed] [Google Scholar]
- Morel F, Roux C, Bresson JL (2000) Segregation of sex chromosomes in spermatozoa of 46,XY/47,XXY men by multicolour fluorescence in-situ hybridization. Mol Hum Reprod 6:566–570 [DOI] [PubMed] [Google Scholar]
- Nash RN, Willalt LR, Andrew T, Green AJ (1997) Recurrent multiple aneuploidies: a family with autosomal recessive failure of mitotic control. J Med Genet Suppl 34:63 [Google Scholar]
- Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, Kearns WG (1999) Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod 14:1266–1273 [DOI] [PubMed] [Google Scholar]
- Papi L, Montali E, Marconi G, Guazzelli R, Bigozzi U, Maraschio P, Zuffardi O (1989) Evidence for a human mitotic mutant with pleiotropic effect. Ann Hum Genet 53:243–248 [DOI] [PubMed] [Google Scholar]
- Pfeffer J, Pang MG, Hoegerman SF, Osgood CJ, Stacey MW, Mayer J, Oehninger S, Kearns WG (1999) Aneuploidy frequencies in semen fractions from ten oligoasthenoteratozoospermic patients donating sperm for intracytoplasmic sperm injection. Fertil Steril 72:472–478 [DOI] [PubMed] [Google Scholar]
- Rives N, Joly G, Machy A, Simeon N, Leclerc P, Mace B (2000) Assessment of sex chromosome aneuploidy in sperm nuclei from 47,XXY and 46,XY/47,XXY males: comparison with fertile and infertile males with normal karyotype. Mol Hum Reprod 6:107–112 [DOI] [PubMed] [Google Scholar]
- Robbins WA (1996) Cytogenetic damage measured in human sperm following cancer chemotherapy. Mutat Res 355:235–252 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Baulch JE, Moore D, Weier HU, Blakey D, Wyrobek AJ (1995) Three-probe fluorescence in situ hybridization to assess chromosome X, Y, and 8 aneuploidy in sperm of 14 men from two healthy groups: evidence for a paternal age effect on sperm aneuploidy. Reprod Fertil Dev 7:799–809 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Meistrich ML, Moore D, Hagemeister FB, Weier HU, Cassel MJ, Wilson G, Eskenazi B, Wyrobek AJ (1997a) Chemotherapy induces transient sex chromosomal and autosomal aneuploidy in human sperm. Nat Genet 16:74–78 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Rubes J, Selevan, SG, Perreault, SD (1999) Air pollution and sperm aneuploidy in healthy young men. Environ Epidemiol Toxicol 1:125–131 [Google Scholar]
- Robbins WA, Segraves R, Pinkel D, Wyrobek AJ (1993) Detection of aneuploid human sperm by fluorescence in situ hybridization—evidence for a donor difference in frequency of sperm disomic for chromosomes 1 and Y. Am J Hum Genet 52:799–807 [PMC free article] [PubMed] [Google Scholar]
- Robbins WA, Vine MF, Truong KY, Everson RB (1997b) Use of fluorescence in situ hybridization (FISH) to assess effects of smoking, caffeine, and alcohol on aneuploidy load in sperm of healthy men. Environ Mol Mutagen 30:175–183 [DOI] [PubMed] [Google Scholar]
- Rubes J, Lowe X, Moore D, Perreault S, Slott V, Evenson D, Selevan SG, Wyrobek AJ (1998) Smoking cigarettes is associated with increased sperm disomy in teenage men. Fertil Steril 70:715–723 [DOI] [PubMed] [Google Scholar]
- Schmid TE, Lowe X, Marchetti F, Bishop J, Haseman J, Wyrobek AJ (2001) Evaluation of inter-scorer and inter-laboratory reliability of the mouse epididymal sperm aneuploidy (m-ESA) assay. Mutagenesis 16:189–195 [DOI] [PubMed] [Google Scholar]
- Schultz H, Mennicke K, Schlieker H, Alhasani S, Balspratsch M, Diedrich K, Schwinger E (2000) Comparative study of disomy and diploidy rates in spermatozoa of fertile and infertile men: a donor-adapted protocol for multi-colour fluorescence in situ hybridization (FISH). Int J Androl 23:300–308 [DOI] [PubMed] [Google Scholar]
- Selevan SG, Borkovec L, Slott VL, Zudová Z, Rubes J, Evenson DP, Perreault SD (2000) Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect 108:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S (2000) Aneuploidy and cancer. Curr Opin Oncol 12:82–88 [DOI] [PubMed] [Google Scholar]
- Shi QH, Ko E, Barclay L, Hoang T, Rademaker A, Martin R (2001) Cigarette smoking and aneuploidy in human sperm. Mol Reprod Dev 59:417–421 [DOI] [PubMed] [Google Scholar]
- Shi Q, Martin RH (2000a) Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet Cell Genet 90:219–226 [DOI] [PubMed] [Google Scholar]
- ——— (2000b) Spontaneous frequencies of aneuploid and diploid sperm in 10 normal Chinese men: assessed by multicolor fluorescence in situ hybridization. Cytogenet Cell Genet 90:79–83 [DOI] [PubMed] [Google Scholar]
- Sloter ED, Lowe X, Moore DH, Nath J, Wyrobek AJ (2000) Multicolor FISH analysis of chromosomal breaks, duplications, deletions, and numerical abnormalities in the sperm of healthy men. Am J Hum Genet 67:862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares SR, Templado C, Blanco J, Egozcue J, Vidal F (2001) Numerical chromosome abnormalities in the spermatozoa of the fathers of children with trisomy 21 of paternal origin: generalised tendency to meiotic non-disjunction. Hum Genet 108:134–139 [DOI] [PubMed] [Google Scholar]
- Spriggs EL, Rademaker AW, Martin RH (1995) Aneuploidy in human sperm: results of two- and three-color fluorescence in situ hybridization using centromeric probes for chromosomes 1, 12, 15, 18, X, and Y. Cytogenet Cell Genet 71:47–53 [DOI] [PubMed] [Google Scholar]
- Tolmie JL, Boyd E, Batstare P, Fergusson-Smith ME, Al Roomi L, Connor JM (1988) Siblings with chromosome mosaicism, microcephaly and growth retardation: the phenotypic expression of a human mitotic mutant? Hum Genet 80:197–200 [DOI] [PubMed] [Google Scholar]
- Van Hummelen P, Lowe XR, Wyrobek AJ (1996) Simultaneous detection of structural and numerical chromosome abnormalities in sperm of healthy men by multicolor fluorescence in situ hybridization. Hum Genet 98:608–615 [DOI] [PubMed] [Google Scholar]
- Van Hummelen P, Manchester D, Lowe X, Wyrobek AJ (1997) Meiotic segregation, recombination, and gamete aneuploidy assessed in a t(1;10)(p22.1;q22.3) reciprocal translocation carrier by three- and four-probe multicolor FISH in sperm. Am J Hum Genet 61:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegetti W, Vanassche E, Frias A, Verheyen G, Bianchi MM, Bonduelle M, Liebaers I, Vansteirteghem A (2000) Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod 15:351–365 [DOI] [PubMed] [Google Scholar]
- Verma RS, Babu A (1989) Human chromosomes: manual of basic techniques, Pergamon Press, New York, pp 4–9 [Google Scholar]
- Wang JY, Samura O, Zhen DK, Cowan JM, Cardone V, Summers M, Bianchi DW (2000) Fluorescence in-situ hybridization analysis of chromosomal constitution in spermatozoa from a mosaic 47, XYY/46,XY male. Mol Hum Reprod 6:665–668 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1992) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, Cambridge [Google Scholar]
- World Health Organization (1999) WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. Cambridge University Press, Cambridge [Google Scholar]
- Wyrobek AJ, Aardema M, Eichenlaubritter U, Ferguson L, Marchetti F (1996) Mechanisms and targets involved in maternal and paternal age effects on numerical aneuploidy. Environ Mol Mutagen 28:254–264 [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Marchetti F, Sloter E, Bishop J (2000) Chromosomally defective sperm and their developmental consequences. In: Anderson D, Karakaya AE, Srám RJ (eds) Human monitoring after environmental and occupational exposure to chemical and physical agents. IOS Press, Amsterdam, pp 134–150 [Google Scholar]
- Yamaki H, Sasano H, Ohashi Y, Shizawa S, Shineha R, Satomi S, Nagura H (2001) Alteration of X and Y chromosomes in human esophageal squamous cell carcinoma. Anticancer Res 21:985–990 [PubMed] [Google Scholar]