Summary
Prefrontal cortex has been proposed to show highly adaptive information coding, with neurons dynamically allocated to processing task-relevant information. To track this dynamic allocation in monkey prefrontal cortex, we used time-resolved measures of neural population activity in a simple case of competition between target (behaviorally critical) and nontarget objects in opposite visual hemifields. Early in processing, there were parallel responses to competing inputs, with neurons in each hemisphere dominated by the contralateral stimulus. Later, the nontarget lost control of neural activity, with emerging global control by the behaviorally critical target. The speed of transition reflected the competitive weights of different display elements, occurring most rapidly when relative behavioral significance was well established by training history. In line with adaptive coding, the results show widespread reallocation of prefrontal processing resources as an attentional focus is established.
Highlights
-
•
Prefrontal neurons show classic properties of a limited attentional resource
-
•
Division of processing resources impairs accuracy of neural discriminations
-
•
As processing unfolds, neurons are reallocated to the most important stimuli
-
•
The speed and degree of reallocation depend on relative attentional weights
Like a flexible processing resource, prefrontal cortex can respond to many kinds of information. Kadohisa et al. show how neurons in monkey prefrontal cortex are dynamically reallocated to construct a coherent attentional focus as a visual display is processed.
Introduction
Attention has widespread effects on brain activity. From thalamus and colliculus to many regions of cortex, for example, responses to visual input are enhanced when this input is relevant to behavior (O’Connor et al., 2002; Ignashchenkova et al., 2004; Roelfsema et al., 1998; Moran and Desimone, 1985). Often, attentional modulations grow over time from stimulus onset as the appropriate attentional focus is established (Roelfsema et al., 1998; Chelazzi et al., 1998; Schall et al., 1995). In some cases, inputs appear to compete for control of neuronal activity, for example, when two stimuli fall within the receptive field of a visual cell (Chelazzi et al., 1998; Reynolds et al., 1999; Bundesen et al., 2005). In such cases, directing attention to one or the other stimulus determines how closely neural activity resembles the response to that stimulus presented alone (Moran and Desimone, 1985; Reynolds et al., 1999; see also Reynolds and Heeger, 2009). Such competition for control of neural activity resembles classic attentional models, in which concurrent stimuli or cognitive events compete for processing resources (e.g., Broadbent, 1958; Kahneman, 1973).
This form of competition is best established in early visual areas, where it is predominantly local. When two stimuli fall within a cell’s spatial receptive field, moving attention from one to the other determines which of the two drives activity. Competition and attentional modulation are much weaker when stimuli are widely separated (Moran and Desimone, 1985; Lee and Maunsell, 2010). In behavior, however, there are global limits on attentional capacity, such that even very dissimilar tasks can be hard to carry out together (Bourke et al., 1996; Broadbent, 1958; Kahneman, 1973). Neurophysiologically, attentional modulations are strong in prefrontal cortex (Rainer et al., 1998; Lennert and Martinez-Trujillo, 2011), even with stimuli in opposite visual hemifields (Everling et al., 2002), and it is commonly proposed that prefrontal cortex plays a central role in attentional competition and control (Norman and Shallice, 1980; Dehaene et al., 1998; Botvinick et al., 2001; Miller and Cohen, 2001; Duncan 2001). According to adaptive coding proposals (Duncan, 2001; Duncan and Miller, 2002), prefrontal neurons have highly flexible response properties, allocated to coding different information in different task contexts. Functional brain imaging shows that similar regions of prefrontal cortex are active during many different kinds of cognitive activity (Duncan and Owen, 2000; Miller and Cohen, 2001), providing a plausible basis for global limits on attentional capacity (e.g., Dehaene et al., 1998; Marois and Ivanoff, 2005; Bourke et al., 1996). On such a view, processing activity in prefrontal cortex would be flexible but limited, allocated to a currently attended stimulus or task, and providing a critical prefrontal mechanism for attentional competition and its resolution.
Here we examined the dynamics of attentional allocation in prefrontal cortex with widely separated visual stimuli. In the behaving monkey, we used time-resolved measures of neural population activity (e.g., Buschman et al., 2012; Kaping et al., 2011; Stokes et al., 2013) to track development of the attentional focus under varying levels of attentional competition. Attentional competition was manipulated using a simple form of visual search, in which the animal detected and later responded to a cued target object (T). In some trials, T was presented alone, while in others, competition was introduced by an additional nontarget (N) in the opposite visual hemifield. It is well known from human search experiments that processing conflict in such a task is determined by training history, with strong competition from a nontarget that has often previously been experienced as a target (inconsistent nontarget or NI), but much less from a nontarget that can always be ignored (consistent nontarget or NC) (Schneider and Shiffrin, 1977; Schneider and Fisk, 1982). Analogous effects of training history have been shown in the frontal eye field, with a relative enhancement of response to stimuli previously trained as targets (Bichot et al., 1996; Bichot and Schall, 1999). In our task (Figure 1A), each trial began with a central cue indicating this trial’s target object. Based on preexperimental training, two different cues were paired with two alternative targets. After a brief delay, there followed a choice display containing either a single object, to left or right of fixation, or one object to either side. For single-object displays, the choice stimulus could be either the cued target (T), the stimulus associated with the other cue and thus serving as a target on other trials (NI), or a fixed nontarget object never serving as a target (NC). In the two-object case, a target T could be accompanied by either NI or NC, or NI and NC could appear together. Following the 500 ms choice display and a subsequent brief delay, the monkey was rewarded for a saccade to the T location, or if no T had been presented, maintained fixation (no-go response) for later reward (see Experimental Procedures).
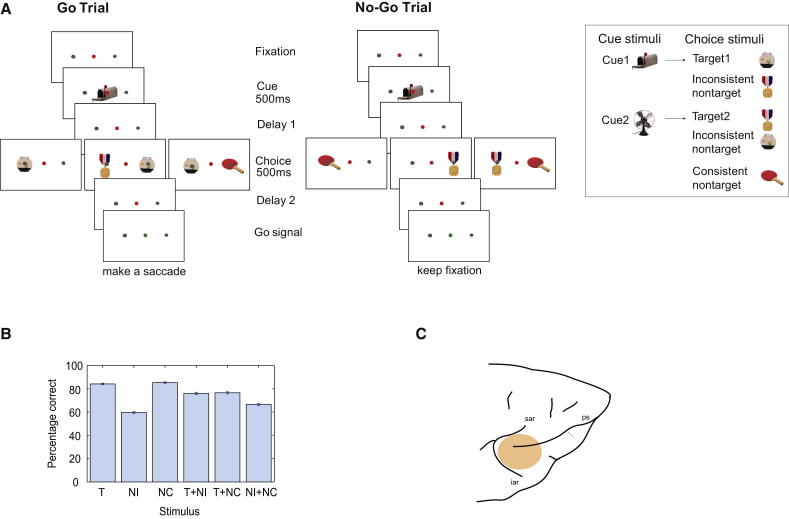
Figure 1.
Task, Behavior, and Recording Locations
(A) Task. Following fixation on a central red dot (held until response interval; see below), each trial began with a cue stimulus indicating the current target. For each animal, two alternative cues were associated with two alternative targets based on preexperimental training (illustrated by stimuli for one animal in inset). After a first delay, the animal saw a choice display consisting of a single object to left or right of fixation or two objects, one to each side. Possible display objects included the cued target (T), the object associated with the alternative cue (inconsistent nontarget, NI), and a third object never serving as a target (consistent nontarget, NC). After a second delay, a change of fixation point to green cued the animal to indicate his behavioral decision. At this point, for go trials (T present in choice display), the monkey was rewarded with a drop of liquid for a saccade to the T location. For no-go trials (T absent), reward was contingent on holding fixation until the response interval finished (see Experimental Procedures).
(B) Mean response accuracies for each type of choice display.
(C) Approximate recording locations. ps, principal sulcus; sar, superior arcuate sulcus; iar, inferior arcuate sulcus. For further details, see Figure S1.
In line with the proposal of adaptive coding, our results show how, in a prefrontal cell population, activity resembles the limited processing resource of classic attentional models. As previously described (e.g., Freedman et al., 2001; Kusunoki et al., 2010), many cells were devoted to making task-relevant stimulus discriminations. With attentional competition, processing capacity was initially divided between competing display objects, with different neurons responding to different objects. Specifically, neural events in each hemisphere were initially dominated by response to the display item in the contralateral visual field, whether T or N. The result was that neurons failed to make critical stimulus discriminations in the ipsilateral visual field, resembling poor information coding when processing resources are diverted in classic attentional models (Broadbent, 1958; Kahneman, 1973). Subsequently, this initial, incoherent state was replaced by transition to a global focus on the behaviorally critical target, with this target now controlling neural activity in both hemispheres. The speed and extent of transition between these states reflected the strength of attentional competition, being more rapid and complete for T + NC than for T + NI displays. The results track dynamic allocation of processing resources in prefrontal cortex, with gradual establishment of a coherent and global attentional focus.
Results
Behavioral data (Figure 1B) showed high accuracy for singly presented T and NC stimuli. The comparative difficulty of ignoring NI was confirmed by much reduced accuracy for this stimulus presented alone, with the majority of errors (72.2%) being saccades to the stimulus location at the time of the go signal. Saccades to the NI location were also common (59.2% of errors) for T + NI displays, while for T + NC displays, the most common form of error (79.1%) was a no-go response.
During performance of the task, responses of 461 single neurons were recorded on the lateral frontal surface of three hemispheres, two in monkey A (n = 192 right, 113 left) and one in monkey B (n = 156, right). Pooled results are presented here, as similar patterns were seen in all three recorded hemispheres. Recording locations (Figure 1C; Figure S1 available online) were located in dorsal and ventral regions of the posterior lateral prefrontal cortex, including the posterior third of the principal sulcus.
Coding of Single Choice Stimuli
To ask how prefrontal cortex represents task events, we examined responses to the 12 possible single-object displays (3 stimulus categories [T, NI, NC] × 2 visual fields [contralateral or ipsilateral to recording location] × 2 cues). For each neuron, data were examined by ANOVA with factors stimulus category, visual field, and cue. Analyses were separately conducted on firing rates from early (50–250 ms from display onset) and late (300–500 ms) response periods.
In both analysis periods, many cells (79/461 early, 118/461 late) showed significant (p < 0.05) main effects of stimulus category. Two examples are shown on the left of Figure 2A. In the first cell (top row), responses were strongest to T and weakest to NC, this pattern arising much earlier for contralateral stimuli. A complementary pattern is illustrated by the second cell (Figure 2A left, bottom row), with a late, selective response to nontargets, especially NC. In both analysis periods, there were also many cells (155/461 early, 114/461 late) showing significant main effects of visual field. An early preference for contralateral stimuli and a late preference for ipsilateral stimuli are illustrated by the two cells on the right of Figure 2A. Main effects of cue were less common (47/461 cells early, 43/461 late). Numbers of cells showing different patterns of interaction are listed in Table S1. As shown in Figure S1, both category- and location-selective cells were broadly distributed across recording locations, including dorsolateral and ventrolateral surfaces, as well as the posterior recording area lying between arcuate and principal sulci.
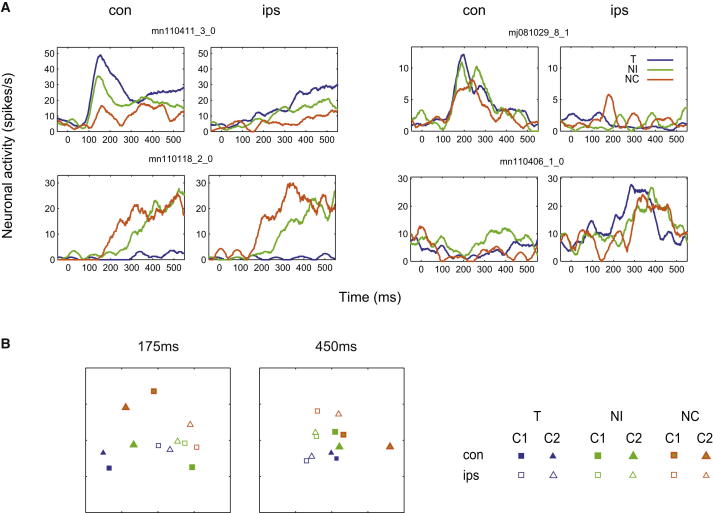
Figure 2.
Response to Single-Object Displays
(A) Responses of four example cells to single T, NI, and NC stimuli in contralateral and ipsilateral hemifields. con, stimulus contralateral to recoding location; ips, stimulus ipsilateral to recording location.
(B) Similarity space of population activity patterns for single-object displays at two times from stimulus onset. For details, see text. C1, cue 1; C2, cue 2.
Though these results suggest many cells coding the behavioral category of stimuli, the data suggested little direct role in the saccadic response. Of 58 cells showing an interaction of stimulus category and visual field in the late period (see Table S1), there were 33 with a strong, sustained response for targets in one location (Figure S2). Though such a pattern might plausibly reflect oculomotor preparation, even these cells showed little evidence of activity linked to the saccadic response (Figure S2). These results match prior findings from similar tasks, suggesting prefrontal activity linked largely to behavioral categorization rather than motor output (Everling et al., 2002; Kusunoki et al., 2009).
To examine stimulus coding across the whole cell population, neural activity at each time point from stimulus onset (see Experimental Procedures) was represented as a vector of firing rates across the full sample of 461 recorded cells. Twelve such vectors were obtained for each of the 12 separate single-object displays, and to measure separation of population activity for any two displays, we used Euclidean distance between their activity vectors. A two-dimensional representation of the resulting similarity space, derived using multidimensional scaling (MDS), is shown in Figure 2B. Separate similarity spaces are shown for early (175 ms from stimulus onset) and late (450 ms) stages of processing. Even early in processing, the prefrontal representation already showed discrimination of both stimulus category and hemifield. In particular there was clear separation of T, NI, and NC categories, especially in the contralateral hemifield. Representations of a given behavioral category were similar for the two cues, despite actual stimuli exchanging roles as T or NI. Coding of both stimulus category and visual hemifield persisted into the later processing stage. It is noteworthy that hemifield coding was strong for both targets and nontargets, though for the latter it had no behavioral significance. At both stages of processing, neural representations for NI were intermediate between those for T and NC, in agreement with previous data (Kusunoki et al., 2010) and the example cells on the left side of Figure 2A.
As usual in lateral prefrontal cortex, these data show many cells coding current task events, with strong but not exclusive emphasis on behaviorally relevant stimulus categorizations.
Impact of Competition on Critical Stimulus Discriminations
To examine attentional competition, we turned to two-object displays and the dynamics of information coding as the choice display is processed. On a resource model of prefrontal activity, a plausible hypothesis is that, just as attentional competition impairs behavioral accuracy, it might impair neural discrimination. Such results would match classic attentional models, in which division of attentional resource reduces processing efficiency (Broadbent, 1958; Kahneman, 1973).
To address this question, we used Euclidean distance to measure discrimination between critical stimulus pairs, T versus NI and T versus NC. In each case, we measured discrimination either for the critical stimuli presented alone (no-competition case), or in the presence of an additional nontarget in the opposite hemifield (attentional competition). The design of our displays (Figure 1) allowed us to examine four such cases (Figure 3): discrimination of T versus NI in the hemifield contralateral to the recording location (Tcon versus NIcon), either presented alone or with a concurrent stimulus (NC) in the ipsilateral hemifield; discrimination of Tcon versus NCcon, either presented alone or acompanied by NIips; and similarly for discrimination of Tips/NIips, presented alone or with NCcon, and discrimination of Tips/NCips, presented alone or with NIcon. In each case, we selected for analysis a population of cells that were most relevant to the critical discrimination (e.g., Tcon versus NIcon), ensuring that this selection was unbiased for comparison of no-competition and competition cases (see Experimental Procedures). We predicted that critical T/N discriminations might be impaired when an additional stimulus is added in the opposite hemifield.
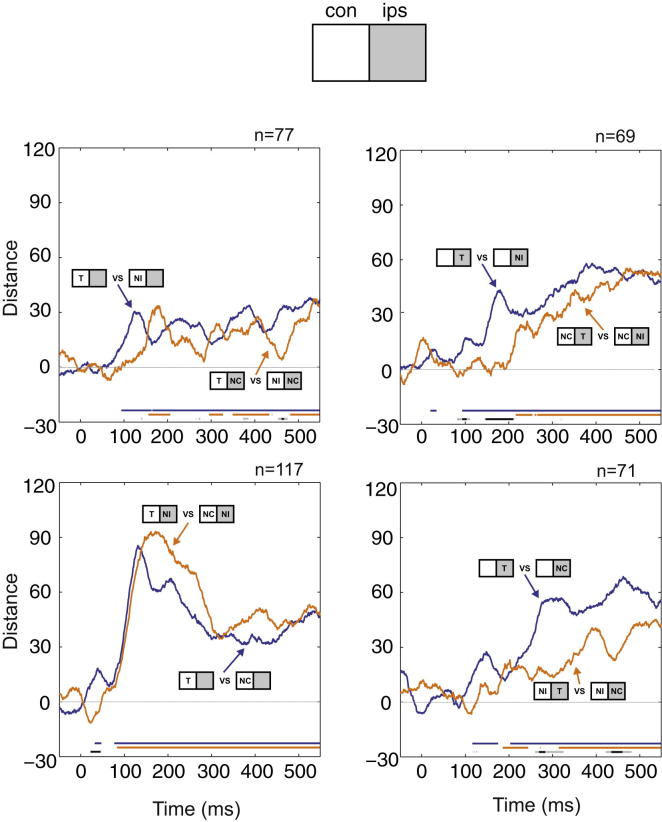
Figure 3.
Impairment of Neural Discrimination by Attentional Competition
Neural discrimination between critical T/N pairs presented either alone (blue) or accompanied by a nontarget in the opposite hemifield (orange), plotted as a function of time from choice stimulus onset. Discrimination is measured by Euclidean distance between activity vectors in a selected cell population, after subtraction of distance expected by chance (permutation correction; see Experimental Procedures). Discrimination significantly greater than zero (p < 0.05, blue and orange) and significant differences between single- and two-object displays (p < 0.05, light gray; p < 0.01, dark gray) are shown by lines at bottom. Top left: discrimination of Tcon versus NIcon, presented alone (blue) or accompanied by NCips (orange). Bottom left: discrimination of Tcon versus NCcon, presented alone (blue) or accompanied by NIips (orange). Top right: discrimination of Tips versus NIips, presented alone (blue) or accompanied by NCcon (orange). Bottom right: discrimination of Tips versus NCips, presented alone (blue) or accompanied by NIcon (orange). n indicates number of cells in each analysis.
The pattern of results was strikingly different for contralateral and ipsilateral discriminations (Figure 3). For single stimuli in the contralateral hemifield, T/N discrimination became significant at around 100 ms from onset and remained throughout the duration of the choice stimulus (Figure 3, left panels; cf. T/N separation in MDS plots in Figure 2B). Data for two-object displays closely tracked those for single objects, suggesting little change in contralateral T/N discrimination with attentional competition.
Ipsilateral discriminations, in contrast, showed evidence of impairment by competing contralateral stimuli (Figure 3, right panels). Again, T/N discrimination began at around 100 ms for single-object displays. With addition of NCcon, discrimination of Tips from NIips remained close to zero until around 200 ms and then rapidly increased toward single-object values (Figure 3, top right). This delay led to a period of strongly significant difference between discrimination strength in one- and two-object displays (Figure 3, top right, dark gray bars). A larger and more extended impairment in discriminating Tips from NCips was created by addition of NIcon, lasting throughout most of the stimulus duration (Figure 3, bottom right).
The results confirm that critical neural discriminations can be impaired by competing stimuli, especially early in stimulus processing, and when training history makes a competing stimulus (NI) hard to ignore. The impairment, however, takes an intriguing form—impairment of ipsilateral discrimination by a contralateral competitor, but not vice versa.
Responses to Separate Components of a Competition Display
How might the idea of flexible resource allocation, in particular competition of inputs to drive neural responses, explain these discrimination data? The results suggest a critical role of visual field, with a tendency at the start of processing the choice display to ignore the ipsilateral field. Anatomical connections from visual cortex to frontal lobe are much stronger within than between hemispheres (Ungerleider et al., 1989), and if competing stimuli are presented in opposite hemifields, neural activity in inferotemporal cortex is dominated by the contralateral input (Chelazzi et al., 1998). At least early in processing a choice display, prefrontal activity may show a similar contralateral dominance, meaning that in each hemisphere, there is little information concerning a competing ipsilateral event. When a target is present, however, an accompanying nontarget has no relevance to behavior. We considered the hypothesis that, across time of processing a choice display, activity evolves to a coherent attentional state, with responses in both hemispheres controlled by the behaviorally critical target. A neural mechanism of this sort would be analogous to progressive focus of processing resources in classical attention modes (Kahneman, 1973).
On this hypothesis, responses to a T + N display should be predictable from the separate responses produced by the component T and N presented alone. For Tcon + Nips, activity should follow response to Tcon alone throughout choice stimulus processing. Early in processing, Tcon dominates because it is contralateral, while late in processing, the same stimulus dominates because it is the target, but in either case, response to Tcon + Nips follows response to Tcon alone. For Tips + Ncon, however, very different dynamics are predicted. Now, an early response to Ncon should be replaced by later activity based on Tips.
These dynamics are illustrated by single cell examples in Figure 4A. The top left panel shows responses to Tcon + Nips displays and their component single stimuli for a cell with very different Tcon and Nips responses. Throughout choice stimulus processing, response to two-object displays was closely similar to the Tcon response alone. The bottom left panel shows a complementary pattern, where again, response to the two-object display resembled the suppression produced by Tcon alone. The right panels show very different dynamics for Tips + Ncon displays. In the top panel is a cell showing a strong, sustained response to Tips alone. In the two-object display, this strong Tips response was initially suppressed by the accompanying Ncon. Later, suppression tended to be released but earlier and more rapidly when the suppressing stimulus was NC. The bottom right shows a complementary example, with strong response to a single Ncon but little response to Tips. In the two-object display, again, the early response resembled that to Ncon alone, but, especially when the nontarget was NC, later activity was dominated by Tips.
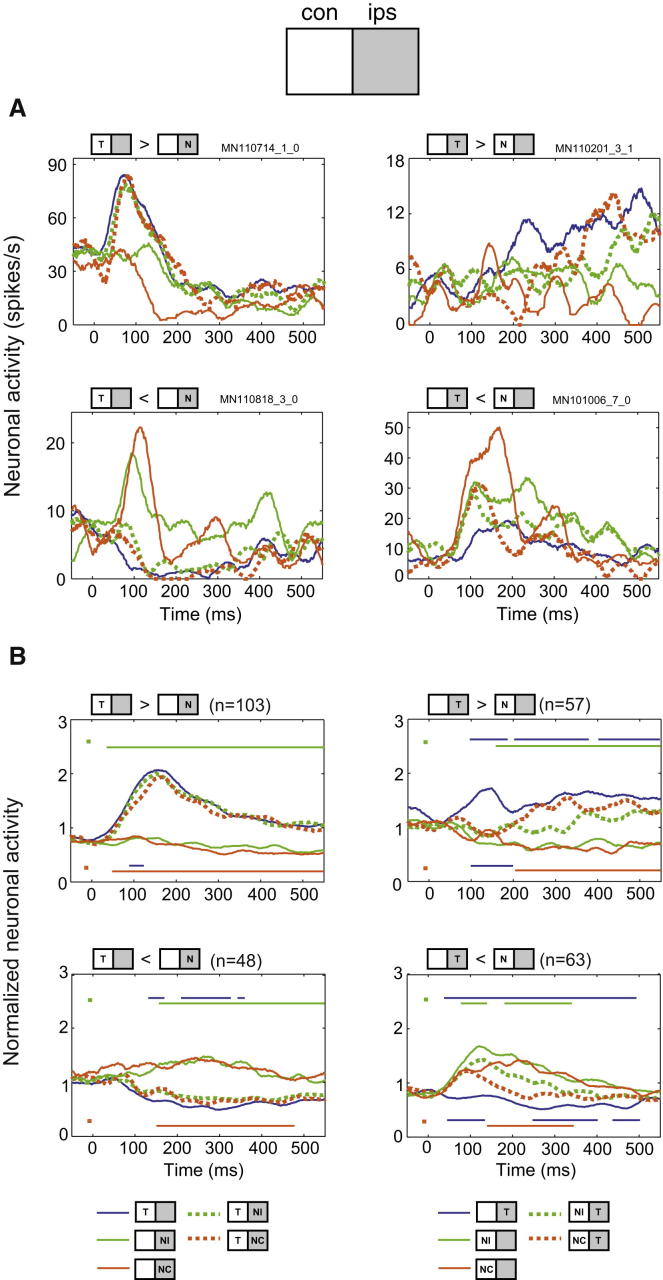
Figure 4.
Responses to Selected Two-Object Displays and Their Component Single Objects
(A) Single cell examples.
(B) Mean normalized firing rates for selected cell groups. Top left: cells (n = 103) with significant difference Tcon > Nips. Bottom left: cells (n = 48) with significant difference Tcon < Nips. Top right: cells (n = 57) with significant difference Tips > Ncon. Bottom right: cells (n = 63) with significant difference Tips < Ncon. Across each group of cells, timelines above each plot (marked by green dot) show significant differences (repeated-measures ANOVA with factors stimulus × cue × cell) between the T + NI display and its component single stimuli (T [blue], NI [green]). Timelines below each plot (orange dot) show equivalent comparisons for T + NC versus T (blue), NC (orange).
To confirm this pattern at the population level, we undertook three kinds of analysis. In the first, we examined mean responses in selected cell groups (Figure 4B) with clear differences in response to the two component stimuli of a two-object display. To examine responses to Tcon + Nips displays, we selected all cells with significantly different responses to Tcon versus Nips (see Experimental Procedures). For Tcon > Nips cells (Figure 4B, top left), strong responses to Tcon alone were matched by closely similar responses to both Tcon + NIips and Tcon + NCips displays. A corresponding result was seen for Tcon < Nips cells, with similar suppression to Tcon presented alone or with an accompanying nontarget (Figure 4B, bottom left). A contrasting pattern was seen for Tips > Ncon cells responding to the Tips + Ncon display (Figure 4B, top right). Now a strong early response to Tips was eliminated by an accompanying contralateral nontarget. Early responses to the Tips + Ncon display resembled the suppression produced by Ncon alone, then, beginning before 200 ms, departed to approach Tips responses. For Tips + NCcon, activity rapidly approached the response to Tips alone, while for Tips + NIcon, some suppression compared to Tips alone remained throughout stimulus processing. A corresponding pattern of results was seen for Tips < Ncon cells (Figure 4B, bottom right).
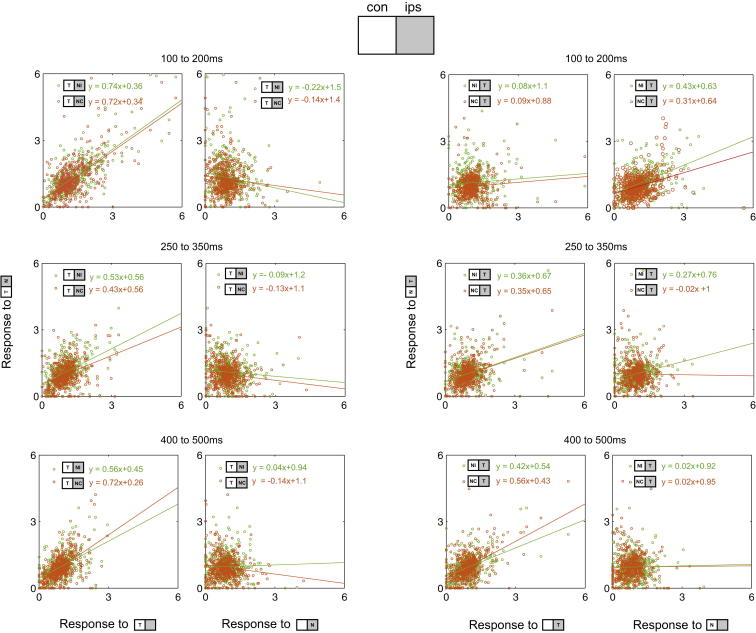
In a second analysis, we turned to the whole recorded cell population and asked how well single neuron responses to each two-object display were predicted by responses to the two component objects individually. Results for three different analysis windows—early (100–200 ms from choice stimulus onset), middle (250–350 ms) and late (400–500 ms)—are shown in Figure 5. Responses of each neuron to the different stimulus displays were first normalized (division by mean response to all displays; see Experimental Procedures), and responses to two-object displays were then plotted against responses to each of the two component single stimuli. For Tcon + Nips displays, results were similar at all time points from stimulus onset. Across neurons, there was a strong tendency for response to the two-object display to match the response given by Tcon rather than Nips. For Tips + Ncon displays dynamics were more complex. In the early window, response to the two-object display tended to follow response to Ncon alone, with response to Tips unpredictive. In the middle window, the Ncon response retained some influence for the case of NI, though this influence had already disappeared for NC. In contrast, response to the two-object display was increasingly predicted by response to Tips. By the late window, results resembled those for Tcon + Nips, with target activity dominant.
Figure 5.
Response to Two-Object Displays as a Function of Response to Component Single Stimuli across the Whole Cell Population
Each point represents activity of a single neuron. Top row: early analysis window (100–200 ms from choice stimulus onset). Middle row: middle window (250–350 ms). Bottom row: late window (400–500 ms). Left two columns: response to Tcon + Nips (green dots, NI; orange dots NC) versus component single stimuli. Right two columns: response to Tips + Ncon (green dots, NI; orange dots NC) versus component single stimuli.
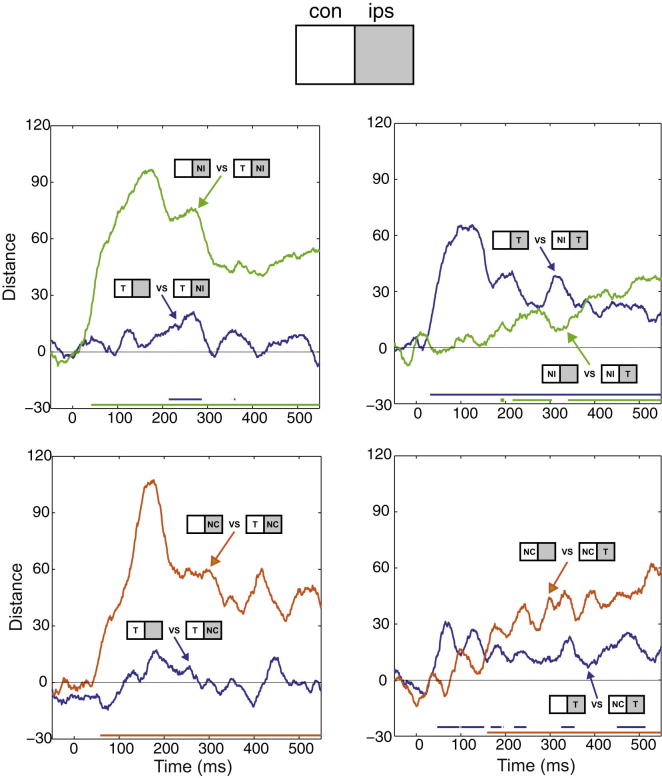
Finally, to ask how closely population responses to a two-object display approached those to component single stimuli, we again used a Euclidean distance measure calculated across the whole recorded sample of 461 cells. Across the time course of choice stimulus processing, we measured population discrimination of each two-object display (e.g., Tcon + NIips; Figure 6, top left) from its component single stimuli (Tcon, NIips). A simple pattern of results emerged when T was contralateral. Throughout stimulus processing, the population response to the two-object display was barely discriminable from response to the contralateral T presented alone (Figure 6, left panels). Beginning <100 ms from stimulus onset, in contrast, response to the two-object display diverged rapidly from response to the component ipsilateral N (Figure 6, left panels). When T was ipsilateral, however, events followed a more complex time course, in particular for the highest-competition case (Tips + NIcon; Figure 6, top right). In the first phase of processing, the two-object display was strongly discriminated from Tips presented alone, but not from NIcon. Only toward the end of the stimulus presentation did the curves cross, indicating a response to the two-object display that more closely resembled response to its component target. For the lower-competition case (Tips + NCcon), strong discrimination from the component target was short lived, with a correspondingly rapid increase in discrimination from the component nontarget (Figure 6, bottom right).
Figure 6.
Population Discrimination between Two-Object Displays and Their Component Single Stimuli
Discrimination (Euclidean distance of activity vectors in whole cell population) is plotted as a function of time from choice stimulus onset. Bars at bottom show periods of significant discrimination (permutation test). Top left: TconNIips compared to Tcon alone (blue) and NIips alone (green). Bottom left: TconNCips compared to Tcon alone (blue) and NCips alone (orange). Top right: TipsNIcon compared to Tips alone (blue) and NIcon alone (green). Bottom right: TipsNCcon compared to Tips alone (blue) and NCcon alone (orange).
These results show how, as prefrontal processing evolves, there is large-scale reallocation of processing resources. Early in choice stimulus processing, neural activity in each hemisphere is dominated by response to the contralateral stimulus. In different groups of neurons (Figure 4), this response can be either an increase or decrease from baseline; in either case, the response to a competition display resembles response to the contralateral stimulus alone. Later, this separation between hemispheres resolves to a coherent state of activity based on the critical T stimulus, especially when the accompanying stimulus is NC. Across both hemispheres, the final, global state is close to the state produced by the T stimulus alone.
Generality
In further analyses, we examined the generality of attentional reallocation across recording locations and cell types. To produce an index of reallocation for each cell, we defined dT as the absolute difference between firing rates for a Tips + Ncon display and for Tips alone and, similarly, dN as the absolute difference between firing rates for the Tips + Ncon display and its component Ncon alone. In an early analysis window (100–200 ms from stimulus onset), we defined a dominance index as
Following the results shown in Figure 6, this early index was generally positive, reflecting response to Tips + Ncon that was closer to Ncon than to Tips alone. The same dominance index calculated for a late analysis window (400–500 ms) was generally negative, indicating response to Tips + Ncon that was closer to Tips than to Ncon alone. Subtracting the late index from the early index gave us a final reallocation index, separately calculated for each cell and for Tips + NIcon and Tips + NCcon displays.
Across the whole recorded cell population, the mean reallocation index for Tips + NIcon displays was 0.147. By t test, this value was significantly greater than zero (p < 0.001). For Tips + NCcon displays, the mean reallocation index was 0.124, again significantly greater than zero (p < 0.001).
To examine generality across anatomical regions, we divided the full recorded cell sample into three groups, a smaller group (n = 42) recorded in a posterior region between the principle and arcuate sulci (see Figure S1) and larger groups recorded in more anterior dorsolateral (n = 145) and ventrolateral (n = 274) regions, divided by the fundus of the principal sulcus. Both for Tips + NIcon and Tips + NCcon displays, the mean reallocation index was positive for all three cell groups. ANOVA showed no difference between cell groups, for Tips + NIcon F(2, 456) = 1.17, for Tips + NCcon F(2, 455) = 1.29 (missing data for cases in which dT = dN = 0).
In a second analysis, we examined generality across cell types, defined by coding of stimulus category and/or location in single-stimulus displays. To examine category-selective cells, we took all those cells (n = 162) with a main effect of stimulus category in either early- or late-period ANOVAs on single-stimulus activity (see earlier section, Coding of Single Choice Stimuli). To examine location-selective cells, we took all cells (n = 210) with a main effect of location in either analysis period. Again, the mean reallocation index was significantly positive in both groups (category-selective cells: mean index = 0.222, p < 0.005 for Tips + NIcon, mean index = 0.226, p < 0.001 for Tips + NCcon; location-selective cells: mean index = 0.233, p < 0.001 for Tips + NIcon, mean index = 0.096, p < 0.06 for Tips + NCcon).
These results show substantial generality in the overall pattern of attentional reallocation for Tips + Ncon displays. For all regions in our recording area, and whatever stimulus feature a cell coded, early response was determined largely by the contralateral N, while later response was determined largely by T.
Error Trials
Finally, we found no evidence for attentional reallocation on error trials. Combining data for all cells, and for both major error types (saccade to wrong location, no-go), mean reallocation index on error trials was −0.006, t test against zero p = 0.56 for Tips + NIcon displays, and mean reallocation index 0.014, p = 0.49 for Tips + NCcon displays. Only correct trials, evidently, were associated with reallocation of prefrontal processing resources from contralateral nontarget to ipsilateral target.
Discussion
When stimuli or other cognitive events compete for attention, processing resources must be allocated to the most important (Broadbent, 1958; Kahneman, 1973). Our results track development of an attentional focus in the population activity of prefrontal cortex.
In line with the proposal of adaptive prefrontal coding of task-relevant information, we found that many prefrontal cells discriminated task-critical stimulus categories and locations. When two stimuli were present in the display, attentional competition resolved through widespread reallocation of neural resources. Early processing lacked attentional coherence, with different neurons responding to different items in the display. Specifically, neural activity in each hemisphere was dominated by the contralateral display item—a pattern (Figure 2) coding both hemifield and behavioral category of that stimulus. Accordingly, critical stimulus discriminations within one visual field were impaired in the ipsilateral hemisphere, matching the classical proposal of reduced processing efficiency when processing resources are withheld (Broadbent, 1958; Kahneman, 1973). Later, neural activity in both hemispheres was dominated by the behaviorally critical target. Construction of this global attentional focus resembles the classical proposal that processing resources are allocated to the most important cognitive events (Broadbent, 1958; Kahneman, 1973).
We found that the time course of transition depends on the attentional weight of nontargets. For NC, a stimulus never serving as a target, control of the contralateral hemisphere was released quickly and easily. For NI, a target stimulus on other trials, release was slow and incomplete. Again, these results match comparable findings from human studies, showing how processing resources are rapidly allocated to targets when nontargets have been extensively practiced as task irrelevant (Schneider and Shiffrin, 1977; Schneider and Fisk, 1982).
It is commonly proposed that, in early visual areas, stimuli within or close to the receptive field of a cell compete to control its activity (Moran and Desimone, 1985; Reynolds et al., 1999; Reynolds and Heeger, 2009). Accordingly, moving attention from one stimulus to another can have large effects when the two are close together; with widely separated stimuli, the effect is much smaller, with only modest enhancement of response to the attended input (Moran and Desimone, 1985; Lee and Maunsell, 2010). In prefrontal cortex, instead, we found widespread target dominance by the end of display processing, reflecting global allocation of processing resources to the behaviorally critical stimulus. Global division of prefrontal processing capacity is a plausible neural basis for many cases of attentional competition, including interference between widely separated visual stimuli and even between very dissimilar tasks (Marois and Ivanoff, 2005; Dehaene et al., 1998; Bourke et al., 1996). In prefrontal cortex, global processing competition, and its dynamic resolution, probably reflect the breadth of inputs from other brain regions (Pandya and Yeterian, 1996) and the strong interconnectivity between one prefrontal region and another (Pucak et al., 1996).
Our finding of early activity dominated by the contralateral visual field resembles results previously reported for inferotemporal cortex (Chelazzi et al., 1998). In that study, pairs of stimuli were presented either within one hemifield or one to each hemifield. As in the current study, animals searched for a prespecified target, responding with an immediate saccade or lever release. When both stimuli fell in the same hemifield, neural activity was dominated by the target, but with stimuli in opposite hemifields, activity was dominated by the contralateral stimulus, whether target or nontarget. Because responses in that study were immediate, data were only available for the brief period before the response was made. It is not known whether, with a longer stimulus presentation, global dominance by the attended target might develop in inferotemporal cortex, as we have shown here for prefrontal cortex.
The neural mechanisms of visual search have also been examined in the frontal eye field, with some similarities to the current results. In the frontal eye field, as in early visual areas, there is response enhancement when the stimulus within the receptive field is the current target (Schall et al., 1995). Enhancement reflects training history, with earlier enhancement after long practice in searching for a given target (Bichot et al., 1996) and enhancement for stimuli that share features with a previous target (Bichot and Schall, 1999). As in early visual areas, however, such target enhancements are far from the widespread reallocation of processing activity we observed in prefrontal cortex. Even when the target is well outside the receptive field, the nontarget within the receptive field drives strong activity up to the time of the response (Schall et al., 1995; Thompson et al., 1997).
For coherence to be established in the current task, a likely mechanism is communication between the two frontal lobes (Tomita et al., 1999), allowing processing on both sides to be dominated by the same, critical stimulus event. On this model, target information from one hemisphere displaces nontarget information in the other; this happens rapidly and relatively completely when nontarget status is fixed throughout training but only more slowly and partially when the nontarget to be displaced is a target on other trials. Evidently, the competitive mechanisms allowing nontarget displacement must be influenced both by current behavioral status—allowing T to dominate even NI—but also by long-term learning. On the current trial, some context signal initiated by the cue (Stokes et al., 2013) must determine which stimulus is T and which is NI, thus directing the outcome of competition for control of population activity. Across learning, in contrast, NC is always irrelevant, resulting in long-term reduction of competitive weight.
An even stronger separation of competitive weights may be obtained with spatial cues, directly indicating which visual field should be attended in a subsequent visual display. With advance spatial cueing, information from the attended field may dominate some cells even from the outset of visual processing (Everling et al., 2002), though even in this case, there is some response to the unattended side (Everling et al., 2006). Again, the strength of such attentional modulations reflects the variable strength of spatial cues (Lennert and Martinez-Trujillo, 2011).
An enduring debate in the search literature rests on the distinction between serial and parallel processing. Behavioral (Egeth and Dagenbach, 1991; Kyllingsbaek and Bundesen, 2007) and neurophysiological (Buschman and Miller, 2009) arguments can be assembled on both sides of this debate and, in the present case, data are not easily explained by a simple serial model. Instead, processing begins with parallel coding of both display inputs, one dominating each hemisphere, then resolves over several hundred milliseconds (Figures 5 and 6) to a global state of target dominance. It is an open question how this conclusion relates to other kinds of task and search display, e.g., displays containing larger numbers of stimuli (Buschman and Miller, 2009).
A second enduring question in the cognitive literature is the extent to which the two hemispheres act as separate pools of processing capacity (Pashler and O’Brien, 1993; Alvarez and Cavanagh, 2005; see also Buschman et al., 2011). Our data suggest that the answer may be dynamic, evolving with construction of the attentional focus. Early in processing a two-object display, we indeed found that the two hemispheres were focused on different stimuli, like parallel processing pools. Later, we found coherence, with both hemispheres focused on the same, behaviorally critical stimulus.
Though here we examined attentional competition between visual fields, more generally, similar processing principles may apply to many different cases of processing competition. In many such cases, prefrontal activity may move from an early, unfocused state to attentional coherence. Attentional coherence is critical to organized cognition, as multiple brain systems must converge to process the stimuli, responses, reward, etc. of current behavior. Through feedback to multiple brain systems (Dehaene et al., 1998; Miller and Cohen, 2001; Desimone and Duncan, 1995; Moore and Armstrong, 2003), the construction of globally consistent prefrontal activity patterns may be critical in assembly of distributed yet coherent attentional episodes.
Experimental Procedures
Subjects
Subjects were two male rhesus monkeys (Macaca mulatta) weighing 11 (monkey A) and 10 (monkey B) kg. All experimental procedures were approved by the UK Home Office and were in compliance with the guidelines of the European Community for the care and use of laboratory animals (EUVD, European Union directive 86/609/EEC).
Task
Task events were controlled by a Pentium PC running CORTEX software, with displays presented on a 19 inch LED screen placed in front of the monkey’s chair. Trial events are illustrated in Figure 1A. Each trial began with onset of a red dot at screen center, which the animal was required to fixate (window 5° × 5° for monkey A, 4° × 4° for monkey B) until the final saccadic response at the end of the trial. A premature saccade away from screen center immediately terminated the trial without reward (trials discarded from all data analyses). Once fixation had been held for 1,000 ms, a central cue stimulus (500 ms) indicated the target for the current trial. Based on preexperimental training, each of two alternative cue stimuli was associated with a different target (see Figure 1A inset for cue-target pairs for monkey A; different cue, target, and nontarget images were used for monkey B). A randomly varying delay of 400–600 (monkey A) or 400–800 (monkey B) ms was followed by a 500 ms choice display. The display contained either a single object, centered on the horizontal meridian randomly 6° to left or right of fixation, or two objects, one to either side. For single-object displays, the stimulus object was either the cued target T, the object associated with the alternative cue (inconsistent nontarget, NI), or a third object never used as a target (consistent nontarget, NC). For two-object displays, major trial types were T + NI, T + NC, and NI + NC (in randomly varying left-right or right-left configuration), though in some sessions, small numbers of NI + NI trials were also included (data not shown). To avoid response biases, we adjusted frequencies of individual trial types to ensure that T was present in half of all single-object and half of all two-object displays; otherwise, frequencies of all major trial types were the same.
Following choice stimulus offset, there was a further random delay of 100–150 (monkey A) or 300–500 (monkey B) ms, after which the fixation point turned green to indicate the monkey’s response interval. For go trials (T present in choice display), the monkey was immediately rewarded with a drop of liquid for a saccade to the remembered T location (target window 6° × 6° for monkey A, 3.5° × 3.5° for monkey B). For no-go trials (T absent), the monkey was required to hold fixation for the whole 1,000 ms response interval and was then either given immediate reward (monkey B) or rewarded for a further saccadic response (monkey A).
For monkey A, some sessions had cues randomly varying between trials, while others had alternating brief (15–20 trials) blocks of fixed cues. Physiological data were very similar in the two cases and were combined. For monkey B, cues always varied randomly between trials.
Recordings
Each monkey was implanted with a custom-designed titanium head holder and recording chamber(s) (Max Planck Institute for Biological Cybernetics), fixed on the skull with stainless steel screws. Chambers were placed over the lateral prefrontal cortex of the left (AP = 25.3, ML = 20.0; AP, anterior-posterior; ML, medio-lateral) and right (AP = 31.5, ML = 22.5) hemispheres for monkey A and the right hemisphere (AP = 30.0, ML = 24.0) for monkey B. Recording locations for each animal are shown in Figure S1. A craniotomy was made under each chamber for physiological recording. All surgical procedures were aseptic and carried out under general anesthesia.
Data were recorded over a total of 140 daily sessions. We used arrays of tungsten microelectrodes (FHC) mounted on a grid (Crist Instrument) with 1 mm spacing between adjacent locations inside the recording chamber. The electrodes were independently controlled by a hydraulic, digitally controlled microdrive (Electrodes Drive, NAN for monkey A; Multidrive 8 Channel System, FHC for monkey B). Neural activity was amplified, filtered, and stored for offline cluster separation and analysis with the Plexon MAP system (Plexon). Eye position was sampled using an infrared eye tracking system (120 Hz, ASL for monkey A; 60 Hz, Iscan for monkey B) and stored for offline analysis. We did not preselect neurons for task-related responses; instead, we advanced microelectrodes until we could isolate neuronal activity before starting the task.
At the end of the experiments, animals were deeply anaesthetized with barbiturate and then perfused through the heart with heparinized saline followed by 10% formaldehyde in saline. The brains were removed for histology and recording locations confirmed on dorsal and ventral frontal convexities and within the principal sulcus.
Data and Analysis
Physiological data were analyzed just from successfully completed trials, on average including 17 repetitions for each combination of cue, choice stimulus type, and hemifield/spatial arrangement. All statistical analyses were done using MATLAB (MathWorks). For all analyses, spike data were smoothed with a Gaussian kernel of SD 20 ms, cutoffs ±1.5 SD.
To measure neural discrimination between any two choice stimuli X and Y, we used Euclidean distance between activity vectors in the selected cell population. For each cell in the population, we measured the difference in absolute firing rate for X and Y. As is standard, Euclidean distance was defined as the square root of the sum of these squared differences. For MDS (Figure 2B), we used raw Euclidean distances. For quantitative analysis (Figures 3 and 6), we used a correction for the fact that Euclidean distance must always be positive and scales with absolute firing rate. For each distance measure, we calculated the expected chance value by randomly permuting X, Y labels across trials, then subtracted the median permuted value (across 1,000 permutations) from the obtained raw value. Distances plotted in Figures 3 and 6 are raw values minus median permuted values. Significance bars are based on 95% confidence intervals of the permuted distribution. Analyses were repeated at each 1 ms time point from −100 ms to 600 ms from choice stimulus onset.
To measure discrimination between two-object displays and their component single stimuli (Figure 6), we used activity vectors based on the full cell sample (n = 461). To compare discrimination of the same stimulus pairs (X, Y) in single- versus two-object displays (Figure 3), we selected just those cells most sensitive to the critical discrimination, giving equal weight to single- and two-object data. For this purpose we used ANOVA on activity of each cell over the full (0–500 ms) period of choice stimulus presentation, with factors critical stimulus (X, Y) × accompanying stimulus (absent, present) × cue, and selected just those cells with a significant (p < 0.05) effect of critical stimulus. Permutation testing was used to compare distances in single- and two-object cases (Figure 3). On each permutation, for each cell we randomly maintained or switched single- and two-object labels; when labels were switched, they were switched for all of that cell’s data. After this permutation of labels, we repeated the entire calculation of single- and two-object distances. The true difference in distances (Figure 3) was compared with the distribution of permuted values across 1,000 permutations.
For comparison of mean neural activities for two-object displays and their component single stimuli (Figure 4B), we selected all cells with a significant difference between responses to singly presented targets on one side and nontargets on the other. Significance (p < 0.05) was again determined by ANOVA on activity across the full period of choice stimulus presentation, with factors stimulus (e.g., Tcon, Nips) × cue. Significant cells were divided into four groups (Tcon > Nips; Tcon < Nips; Tips > Ncon; Tips < Ncon) as shown in Figure 4B. For calculation of mean activity across cells (Figure 4B, Figure S2), responses of each cell were first normalized by dividing by mean firing rate across all choice displays, calculated across the full 0–500 ms display period. The same normalization was used to create scatterplots of response to each two-object display as a function of response to component single stimuli (Figure 5).
Acknowledgments
This work was supported by MRC intramural programme MC-A060-5PQ14 and research award 220020081 from the James S. McDonnell Foundation. The work of M.S. is supported by an MRC Career Development Fellowship, the National Institute for Health Research (NIHR), and Oxford Biomedical Research Centre based at Oxford University Hospitals Trust, Oxford University. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Published: September 12, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes two figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2013.07.041.
Contributor Information
Mikiko Kadohisa, Email: miki.kadohisa@psy.ox.ac.uk.
John Duncan, Email: john.duncan@mrc-cbu.cam.ac.uk.
Supplemental Information
References
- Alvarez G.A., Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol. Sci. 2005;16:637–643. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- Bichot N.P., Schall J.D. Effects of similarity and history on neural mechanisms of visual selection. Nat. Neurosci. 1999;2:549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- Bichot N.P., Schall J.D., Thompson K.G. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 1996;381:697–699. doi: 10.1038/381697a0. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bourke P.A., Duncan J., Nimmo-Smith I. A general factor involved in dual task performance decrement. Q. J. Exp. Psychol. 1996;49A:525–545. [Google Scholar]
- Broadbent D.E. Pergamon; London: 1958. Perception and Communication. [Google Scholar]
- Bundesen C., Habekost T., Kyllingsbaek S. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol. Rev. 2005;112:291–328. doi: 10.1037/0033-295X.112.2.291. [DOI] [PubMed] [Google Scholar]
- Buschman T.J., Miller E.K. Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron. 2009;63:386–396. doi: 10.1016/j.neuron.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman T.J., Siegel M., Roy J.E., Miller E.K. Neural substrates of cognitive capacity limitations. Proc. Natl. Acad. Sci. USA. 2011;108:11252–11255. doi: 10.1073/pnas.1104666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman T.J., Denovellis E.L., Diogo C., Bullock D., Miller E.K. Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron. 2012;76:838–846. doi: 10.1016/j.neuron.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L., Duncan J., Miller E.K., Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J. Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Kerszberg M., Changeux J.P. A neuronal model of a global workspace in effortful cognitive tasks. Proc. Natl. Acad. Sci. USA. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Duncan J., Miller E.K. Cognitive focus through adaptive neural coding in the primate prefrontal cortex. In: Stuss D.T., Knight R.T., editors. Principles of Frontal Lobe Function. Oxford University Press; Oxford: 2002. pp. 278–291. [Google Scholar]
- Duncan J., Owen A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Egeth H., Dagenbach D. Parallel versus serial processing in visual search: further evidence from subadditive effects of visual quality. J. Exp. Psychol. Hum. Percept. Perform. 1991;17:551–560. doi: 10.1037//0096-1523.17.2.551. [DOI] [PubMed] [Google Scholar]
- Everling S., Tinsley C.J., Gaffan D., Duncan J. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat. Neurosci. 2002;5:671–676. doi: 10.1038/nn874. [DOI] [PubMed] [Google Scholar]
- Everling S., Tinsley C.J., Gaffan D., Duncan J. Selective representation of task-relevant objects and locations in the monkey prefrontal cortex. Eur. J. Neurosci. 2006;23:2197–2214. doi: 10.1111/j.1460-9568.2006.04736.x. [DOI] [PubMed] [Google Scholar]
- Freedman D.J., Riesenhuber M., Poggio T., Miller E.K. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A., Dicke P.W., Haarmeier T., Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat. Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Prentice-Hall; Englewood Cliffs: 1973. Attention and Effort. [Google Scholar]
- Kaping D., Vinck M., Hutchison R.M., Everling S., Womelsdorf T. Specific contributions of ventromedial, anterior cingulate, and lateral prefrontal cortex for attentional selection and stimulus valuation. PLoS Biol. 2011;9:e1001224. doi: 10.1371/journal.pbio.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki M., Sigala N., Gaffan D., Duncan J. Detection of fixed and variable targets in the monkey prefrontal cortex. Cereb. Cortex. 2009;19:2522–2534. doi: 10.1093/cercor/bhp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki M., Sigala N., Nili H., Gaffan D., Duncan J. Target detection by opponent coding in monkey prefrontal cortex. J. Cogn. Neurosci. 2010;22:751–760. doi: 10.1162/jocn.2009.21216. [DOI] [PubMed] [Google Scholar]
- Kyllingsbaek S., Bundesen C. Parallel processing in a multifeature whole-report paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2007;33:64–82. doi: 10.1037/0096-1523.33.1.64. [DOI] [PubMed] [Google Scholar]
- Lee J., Maunsell J.H. Attentional modulation of MT neurons with single or multiple stimuli in their receptive fields. J. Neurosci. 2010;30:3058–3066. doi: 10.1523/JNEUROSCI.3766-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennert T., Martinez-Trujillo J. Strength of response suppression to distracter stimuli determines attentional-filtering performance in primate prefrontal neurons. Neuron. 2011;70:141–152. doi: 10.1016/j.neuron.2011.02.041. [DOI] [PubMed] [Google Scholar]
- Marois R., Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn. Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moore T., Armstrong K.M. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moran J., Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Norman D., Shallice T. ERIC Clearinghouse; Washington, DC: 1980. Attention to Action: Willed and Automatic Control of Behavior, Technical Report No. 8006. [Google Scholar]
- O’Connor D.H., Fukui M.M., Pinsk M.A., Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat. Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Pandya D.N., Yeterian E.H. Comparison of prefrontal architecture and connections. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Pashler H., O’Brien S. Dual-task interference and the cerebral hemispheres. J. Exp. Psychol. Hum. Percept. Perform. 1993;19:315–330. [PubMed] [Google Scholar]
- Pucak M.L., Levitt J.B., Lund J.S., Lewis D.A. Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. J. Comp. Neurol. 1996;376:614–630. doi: 10.1002/(SICI)1096-9861(19961223)376:4<614::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rainer G., Asaad W.F., Miller E.K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Reynolds J.H., Heeger D.J. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J.H., Chelazzi L., Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J. Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema P.R., Lamme V.A.F., Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- Schall J.D., Hanes D.P., Thompson K.G., King D.J. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J. Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Fisk A.D. Degree of consistent training: improvements in search performance and automatic process development. Percept. Psychophys. 1982;31:160–168. doi: 10.3758/bf03206216. [DOI] [PubMed] [Google Scholar]
- Schneider W., Shiffrin R.M. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol. Rev. 1977;84:1–66. [Google Scholar]
- Stokes M.G., Kusunoki M., Sigala N., Nili H., Gaffan D., Duncan J. Dynamic coding for cognitive control in prefrontal cortex. Neuron. 2013;78:364–375. doi: 10.1016/j.neuron.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.G., Bichot N.P., Schall J.D. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J. Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Tomita H., Ohbayashi M., Nakahara K., Hasegawa I., Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G., Gaffan D., Pelak V.S. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp. Brain Res. 1989;76:473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.