Abstract
The CD4 (T4) antigen is a cell-surface glycoprotein that is expressed predominantly on the surface of helper T cells and has been implicated in the regulation of T-cell activation and in the associative recognition of class II antigens of the major histocompatibility complex. In addition, the CD4 antigen appears to serve as a receptor for the human immunodeficiency virus (HIV). An important question has been whether the CD4 receptor is linked to an intracellular mediator that could regulate the activation of the CD4+ subset. In this paper, we provide preliminary evidence that the CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (PTK) of 55–60 kDa, which is expressed specifically in T cells. The PTK is the human analogue of the murine pp56LSTRA (pp56lck) and has significant homology with c-src, c-yes, and other members of the src family. The identification of the PTK associated with CD4 receptor was made by use of an antiserum to a synthetic peptide that was deduced from the DNA sequence of PTK. Two-dimensional nonequilibrium pH gradient gel electrophoresis/NaDodSO4/PAGE revealed the kinase to focus as a heterogeneous collection of spots in the pH range of 4.0–5.0. Furthermore, in vitro phosphorylation revealed the phosphorylation of two additional polypeptides at 40 and 80 kDa, in addition to the autophosphorylation of the PTK at 55–60 kDa. The potential importance of the association between the CD4 receptor and the PTK of T cells is discussed in relation to T-cell activation and HIV infectivity.
The CD4 (T4) antigen is a polypeptide of 55 kDa that is expressed predominately on the surface of helper T cells and has been implicated in association with the T3 (CD3)–Ti complex in the recognition of class II antigens of the major histocompatibility complex (1, 2). The CD4 antigen also appears to serve as a receptor for the human immunodeficiency virus (HIV) [the acquired immunodeficiency syndrome (AIDS) virus] (3-5). Structurally, the CD4 antigen is a member of the immunoglobulin superfamily (6) and, as such, appears capable of regulating the proliferation of the CD4 subset of T cells (7-11). Monoclonal antibodies to the CD4 antigen were initially reported to inhibit specific T-cell function by reducing the strength of interaction of the T cell with its target (1, 2, 7). However, it also appears that monoclonal antibodies to the CD4 receptor can inhibit or potentiate the activation of T lymphocytes in a manner independent of the recognition of HLA-D region antigens (8-11). The inhibitory effect of antibodies to CD4 appears to preferentially influence the T4+ 2H4+ subset within the CD4 population, a subset defined by the differential expression of the L-C/T200 (CD45) antigens (12, 13). These observations have implied that the CD4 receptor may regulate the activation of T cells, either singularly or in conjunction with the antigen receptor [T3 (CD3)–Ti] on the surface of T cells.
Protein-tyrosine kinases (PTKs) have been found to play crucial roles in the activation and transformation of mammalian cells. Oncogenic transformation by Rous sarcoma virus was first shown to be mediated by pp60src and its ability to phosphorylate was at a tyrosine residue (14, 15). The immune complexes containing pp60src have been found to possess phosphotransferase activity capable of transferring the γ-phosphate of ATP to an exogenous substrate (16). Tyrosine phosphorylation is thought to be a relatively rare modification to proteins; however, the past few years have seen a rapidly expanding number of retroviral oncogenes and cellular receptors within the src family. These include the retroviral oncogenes such as v-abl, v-erb, v-fes/fps, v-fgr, v-src, and v-yes, as well as the cellular receptors for epidermal growth factor, platelet-derived growth factor, and insulin (17, 18). Surprisingly, little is known regarding the role of PTKs in lymphoid cells. Murine T cells possess relatively high levels of PTK activity, and distinct patterns of tyrosine phosphorylation have been noted in T and B cells (19-25). In particular, a protein kinase termed pp56LSTRA/lck has been identified in murine T cells (26, 27) and, recently, in human T lymphocytes (28-30). The rather dramatic decrease of pp56lck within T cells after activation is suggestive of an importance of this enzyme in the activation process (31). One putative substrate of PTKs is the p21 subunit of the Ti–T3 complex (32, 33). However, the identity and role of PTKs in the activation of T cells and their relationship to receptors on the cell surface remain elusive.
In this paper, we provide preliminary evidence that the T4 (CD4) receptor is complexed in detergent lysates from T lymphocytes to a T-cell-specific PTK of 55–60 kDa with homology to c-src and c-yes. The PTK was identified by use of an antiserum against a synthetic peptide that was deduced from the DNA sequence of the kinase. A number of polypeptides of 40 and 80 kDa were detected in an in vitro kinase assay in addition to the autophosphorylated band of PTK at 55–60 kDa. The identity of this PTK and its association with the T4 (CD4) receptor may provide a crucial link in understanding the mechanism by which T cells are activated and can be infected with the AIDS virus (HIV).
MATERIALS AND METHODS
Monoclonal Antibodies and Antisera
The production and characterization of the monoclonal antibodies to the T4 (CD4) antigen, 19thy5D7 (IgG2), 18T3A9 (IgGl), and 12D11T4 (IgGl) have been described elsewhere (1). The antibodies W6/32 (a gift from W. F. Bodmer, Imperial Cancer Research Fund, London) against HLA-A, -B, -C antigens has also been described (34). The antibody 1F7 is directed against a 120-kDa polypeptide on T cells (unpublished data). Lastly, the anti-PTK antiserum was generated against a synthetic peptide of the sequence Cys-Lys-GIu-Arg-Pro-Glu-Asp-Arg-Pro-Thr-Phe-Asp-Tyr-Leu-Arg-Ser-Val-Leu-Glu-Asp-Phe-Phe-Thr-Ala-Thr-Glu-Gly-Gln-Tyr-Gtn-Pro-Gln-Pro, as described (29). The peptide was coupled to bovine serum albumin through the N-terminal cysteine at position 33 (Cys-Pro) by using 3-(2-pyridyldithio)proprionic acid N-hydroxysuccinimide ester as described (35).
Cells
Peripheral blood cells were isolated by Iso-Hypaque centrifugation and either used as resting cells or stimulated with the mitogen Con A (5 μg/ml; Sigma) for 24–36 hr. These cells, as well as the transformed T-cell lines HPB-MLT, REX, the B-lymphoblastoid cell line Raji, and the myeloid cell line U937, were cultured in RPMI1640 medium with 10% (vol/vol) fetal calf serum and 1% (wt/vol) penicillin/streptomycin at 37°C in an atmosphere of 5% CO2/95% air.
Immunoprecipitation and Kinase Assay
Cells at 25 × 106 per ml were solubilized in Nonidet NP-40 lysis buffer [1% (vol/vol) or 3% (vol/vol), Nonidet P-40 in 20 mM Tris·HCl buffer, pH 8.0/150 mM NaCl 1 mM phenylmethylsulfonyl fluoride] for 30 min at 4°C as described (36). The cells were initially lysed by 1% (vol/vol) Nonidet P-40; however, in later experiments, 3% (vol/vol) detergent was found to be a more efficient method of extraction of the kinase. The lysate was centrifuged at 12,000 × g and precleared for 30 min with 50 μl of 10% (wt/vol) Staphylococcus Cowan strain I. The lysate was then incubated for 1–2 hr at 4°C with either 5 μl of ascites fluid and 50 μl of 10% (wt/vol) protein A-Sepharose or 50 μl of 10% (wt/vol) protein A-Sepharose that had been crosslinked to antibody according to Schneider et al. (37). The immunoprecipitates were then washed three times with Nonidet P-40 lysis buffer prior to incubation with 30 μl of 25 mM Hepes containing 0.1% (vol/vol) Nonidet P-40, 10 μM ATP, and 1–10 μCi of [γ-32P]ATP (1 Ci = 37 GBq; ICN Chemicals). After an incubation of 15–30 min at 25°C, the reaction mixture was subjected to NaDodSO4/PAGE and autoradiography (38). For the analysis of reprecipitated antigen, the reaction mixture was supplemented to 1.0–2.0% (wt/vol) NaDodSO4, boiled for 5 min, and then diluted to 0.1% NaDodSO4 with a 1:10 or 1:20 dilution of lysis buffer. Two-dimensional nonequilibrium pH gradient gel electrophoresis (NEPHGE) NaDodSO4/PAGE was conducted with ampholines of a pH range between 2 and 11 as described (36, 39).
Analysis or Phosphorylated Amino Acids
Proteins were eluted from fixed polyacrylamide gels and precipitated with trichloroacetic acid as described (20, 40). The precipitate was washed in acetone and hydrolyzed in 6 M HCI at 100°C for 2 hr. The individual phosphorylated amino acids were separated by electrophoresis (2500 V, 30 min) at pH 3.5 in pyridine/acetic acid/water (1:10:189: vol/vol). Nonradioactive standards were detected with ninhydrin, while radiolabeled phosphorylated amino acids were observed by autoradiography.
RESULTS AND DISCUSSION
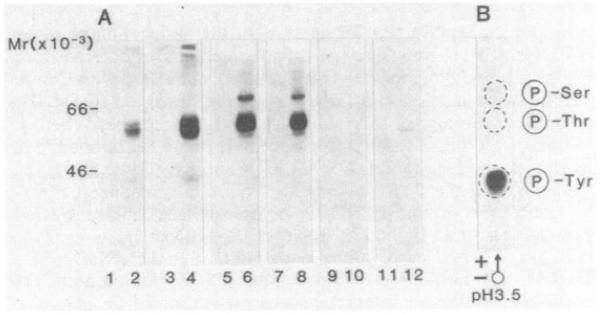
In an attempt to ascertain whether the CD4 antigen was associated with an endogenous protein kinase, a monoclonal antibody against the CD4 antigen (19thy5D7) was used to precipitate the antigen from unlabeled peripheral blood lymphocytes and a number of transformed cell lines. The immunoprecipitates were then assessed for their ability to phosphorylate polypeptides during an incubation with [γ-32P]ATP. As shown in Fig. 1A, the anti-CD4 antibody precipitated several polypeptides at 38 and 55–60 kDa from both resting (lane 2) and Con A-activated cells (lane 4), which were phosphorylated in the presence of [γ-32P]ATP. The bands at 55/60 kDa were found to be more intense on a per cell basis from Con A-activated cells than from resting lymphocytes. The anti-CD4 antibody was also found to precipitate a similar pattern of bands within the 55- to 60-kDa range from the human T-lymphoblastoid cell lines REX (lane 6) and HPB-MLT (lane 8). However, in this case, the presence of the polypeptide at 40 kDa was somewhat variable. In addition, an extra band at ≈80 kDa was often observed. Neither the myeloid cell U937 (lane 10) nor the B-lymphoblastoid cell Raji (lane 12), which have been reported to express the CD4 antigen (41), were found to precipitate significant amounts of material labeled by [γ-32P]ATP. A faint amount of material at 50 kDa was occasionally observed from Raji cells (lane 12); however, the position of this polypeptide was different from that observed from the T lymphocytes. As a negative control, the monoclonal antibody W6/32, which reacts with class I antigens of the major histocompatibility complex at 45 and 12 kDa, was unable to precipitate material capable of being labeled from the various cells (lanes 1, 3, 5, 7, and 9). Other monoclonal antibodies against antigens on T cells such as the T3 (CD3) complex, the Til (CD2) antigen, HLA-D region antigens, and fibronectin receptor/VLA antigens (4B4) also failed to precipitate material capable of being labeled under this regime (data not shown).
Fig. 1. Phosphorylation by [γ-32P]ATP of the CD4 antigen from peripheral blood lymphocytes and various cell lines.
A) Lanes; 1, 3, 5, 7, 9, and 11, anti-W6/32 antibody; 2, 4, 6, 8, 10, and 12, anti-CD4 antibody (19thy5D7). Resting cells (lanes 1 and 2); Con A-activated (12 hr) cells (lanes 3 and 4). REX (lanes 5 and 6), HPB-MLT (lanes 7 and 8), U937 (lanes 9 and 10), and Raji (lanes 11 and 12). (B) Phospho amino acid analysis of the individual 55/60-kDa bands precipitated by anli-CD4 antibody.
Analysis of phosphorylated amino acids was then carried out to investigate the nature of amino acid residues that were phosphorylated with [γ-32P]ATP. Fig. 1B reveals that the bands at 55/60 kDa, which were precipitated by the anti-CD4 antibody, were heavily phosphorylated at tyrosine residue(s) and to a much lesser extent at serine residue(s). The presence of a phosphoserine residue on the CD4 antigen has been detected by in vivo labeling techniques (42) and is consistent with the presence of a serine in the cytoplasmic tail of the antigen (6). However, in contrast, the cytoplasmic tail of CD4 lacks a site for tyrosine phosphorylation as well as a tyrosine kinase domain, as based on its DNA sequence. These data argued that the 55- to 60-kDa band was unlikely to correspond to the CD4 receptor, and, instead, it suggested that the 55- to 60-kDa band was a PTK and/or a substrate for the protein kinase that coprecipitated with the CD4 antigen.
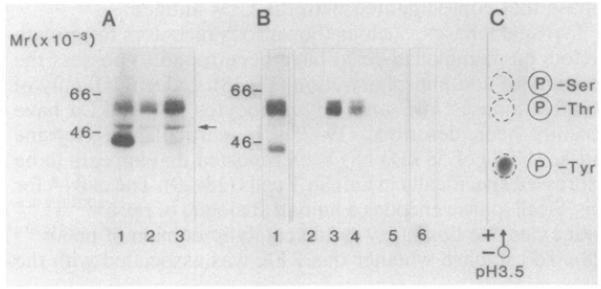
Tyrosine kinases, such as the various receptors for growth factors on mammalian cells, have been found to possess the property of autophosphorylation (17, 18). Indeed, a family of putative kinases in B and T lymphocytes at ≈55 kDa have recently been described (19-25). In particular, a tyrosine kinase (PTK) of 58 kDa has been reported that appears to be expressed specifically in human T cells (28, 29). The cDNA for this T-cell kinase encodes a human analogue of pp56LSRTA/lck with extensive homology to the catalytic domain of pp60c-src (28). To establish whether this PTK was associated with the CD4 antigen, an antiserum that had been raised against a synthetic peptide (Cys-Lys-Glu-Arg-Pro-Glu-Asp-Arg-Pro-Thr-Phe-Asp-Tyr-Leu-Arg-Ser-Val-Leu-Glu-Asp-Phe-Phe-Thr-Ala-Thr-Glu-Gly-Gln-Tyr-Gln-Pro-Gln-Pro), corresponding to the C-terminus of the tyrosine kinase, was used in immunoprecipitation analysis and compared with precipitates formed by a monoclonal antibody against the CD4 antigen. Fig. 2A shows a composite of patterns of the polypeptides phosphorylated in immunoprecipitates of the anti-PTK antibody (lane 1) and in immunoprecipitates formed by two monoclonal antibodies to the CD4 antigen, termed 12T4D11 (lane 2) and 19Thy5D7 (lane 3). In each case, the exposure of immunoprecipitates produced a similar spectrum of phosphorylated bands with molecular sizes of about 40, 55, and 60 kDa. However, differences were noted in the relative intensities of the bands in the pattern. The anti-CD4 immunoprecipitates showed greater amounts of phosphorylation of the 55- and 60-kDa bands relative to the 40-kDa band, while the anti-PTK immunoprecipitates showed greater amounts of phosphorylation of the 40-kDa than the 55- and 60-kDa bands. The anti-PTK antibody was unable to precipitate material from B cells (data not shown). As an internal control for tyrosine phosphorylation, each of the immunoprecipitates was found to label enolase, which was added as substrate during the labeling procedure (arrow). Thus, the similarity of patterns suggested that a common spectrum of polypeptides was associated with the anti-CD4 and the PTK immunoprecipitates.
Fig. 2. NaDodSO4/PAGE analysis of the association between the CD4 and PTK antigens.
(A) Immunoprecipitates derived from peripheral blood lymphocytes that had been stimulated with Con A for 12 hr were labeled in a kinase assay (as described in Fig. 1). Enolase (1–2 μg; Sigma) was added as substrate to the beads before addition of the reaction mixture. Lanes: 1, anti-PTK antibody; 2, anti-CD4 antibody (12T4D11); 3, anti-CD4 antibody (19thy5D7). (B) Precipitation of the PTK polypeptide from CD4 immunoprecipitation. Anti-CD4 or anti-PTK precipitations were labeled by [γ-32P]ATP, denatured by boiling in either 1% NaDodSO4 (lanes 2, 3, 5, and 6) or 2% NaDodSO4 (lane 4), and diluted to 0.1% NaDodSO4 by a 1:10 or 1:20 dilution of lysis buffer, The denatured anti-CD4 immunoprecipitates were reprecipitated with the anti-PTK antibody (lanes 3 and 4). Conversely, the denatured anti-PTK immunoprecipitates were reprecipitated with a cocktail of anti-CD4 antibodies (12T4D11, 18T3A9, 19thy5D7) (lane 6). Lanes: 1, anti-CD4 pattern (19thy5D7) prior to denaturation; 2 and 5, anti-lF7 antibody; 3 and 4, anti-PTK antiserum; 6, anti-CD4 antibodies. (C) Phospho amino acid analysis of the polypeptides reprecipitated by the anti-PTK antiserum from immunoprecipitates formed by the anti-CD4 antibody. Polypeptides were eluted and subjected to phospho amino acid analysis as described (Fig. 1).
A direct demonstration of the association was shown by denaturing the phosphorylated immunoprecipitates in the presence of NaDodSO4 and then attempting to reprecipitate with the reciprocal antibody. Fig. 2B shows a typical pattern in which an immunoprecipitate formed by an anti-CD4 antibody was subjected to the in vitro phosphorylation with [32P]ATP (lane 1). The anti-CD4 immunoprecipitation was then denatured in either 1% (wt/vol) or 2% (wt/vol) NaDodSO4 and reprecipitated with the anti-PTK antibody (lanes 3 and 4, respectively). Importantly, the anti-PTK antibody recognized the labeled 55- to 60-kDa band from the anti-CD4 immunoprecipitate. Conversely, a cocktail of anti-CD4 antibodies (12T4D11, 18T3A9, 19thy5D7) was found to reprecipitate a very faint band from the phosphorylated precipitates formed by the anti-PTK antiserum (lane 6). In neither case was the control antibody 1F7 found to reprecipitate antigen (lanes 2 and 5). The specificity in the recognition by the antibody of the polypeptides was shown by the ability of the synthetic peptide to block the precipitation by anti-PTK antiserum, but not by the anti-CD4 antibody (data not shown). Subsequent phospho amino acid analysis of the reprecipitated PTK antigen by the anti-PTK antiserum detected radiolabel at a tyrosine residue (Fig. 2C). These data demonstrated that the 55- to 60-kDa bands corresponded primarily to the autophosphorylation of the T-cell-specific PTK, which coprecipitated with the CD4 receptor.
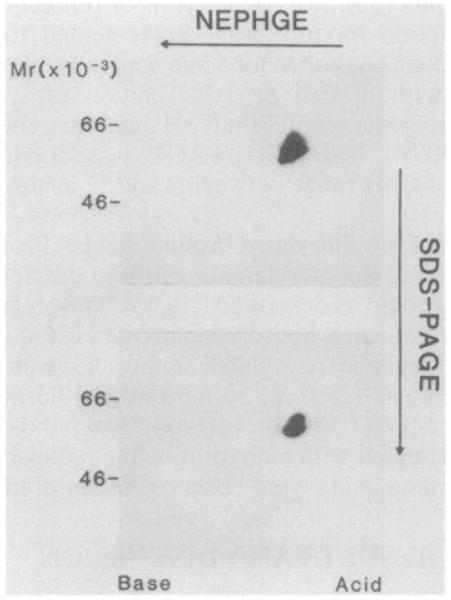
Lastly, two-dimensional NEPHGE/NaDodSO4/PAGE was conducted to confirm the identity in structure of the PTK associated with the CD4 antigen with that recognized directly by the anti-PTK antiserum in cells (Fig. 3). The polypeptides reprecipitated by the anti-PTK antiserum from a denatured anti-CD4 immunoprecipitate focused as two separate series of spots of slightly different molecular sizes and isoelectric positions over a pH range of 4.0–5.0 (Lower). This pattern was similar to that observed when the anti-PTK antiserum was used to reprecipitate PTK from denatured anti-PTK immunoprecipitates (Upper). The only detectable difference in the two patterns was that the pattern derived from the anti-CD4 precipitate appeared to extend over a slightly smaller pH range than that recognized by the anti-PTK antiserum. These data confirm that the PTK associated with the T4 (CD4) receptor is a member of the series of spots recognized by the anti-PTK antiserum.
Fig. 3. Two-dimensional NEPHGE/NaDodSO4/PAGE of reprecipitated PTK polypeptides.
The anti-PTK antiserum and the anti-CD4 antibody were used to precipitate antigen from Con A-stimulated peripheral blood lymphocytes. Immunoprecipitates were then subjected to labeling with [32P]ATP, as described in Fig. 1. The labeled polypeptides were eluted from protein A-Sepharose beads by boiling in the presence of 1% (wt/vol) NaDodSO4 for 5 min and diluting 1:10 in Nonidet P-40 lysis buffer. The anti-PTK antiserum was then used to precipitate antigen from these preparations followed by three washes in lysis buffer and two-dimensional NEPHGE as described (36, 39). (Upper) Anti-PTK precipitated from an anti-PTK immunoprecipitate; (Lower) anti-PTK precipitated from an anti-CD4 immunoprecipitate.
An association of the T-cell-specific PTK with the CD4 receptor is potentially of major importance to understanding the mechanism of T-cell activation. Tyrosine phosphorylation has been shown to play a key role in the activation of cells by surface receptors (epidermal growth factor, insulin, and platelet-derived growth factor receptors) and in the transformation of cells by various retroviral oncogenes (v-abl, v-erb, v-src, v-yes, etc.) (17, 18). In fact, the amino acid sequence of the T-cell protein kinase identified in this study shares some 70–80% similarity with the oncogenes c-src and v-yes in the region of the catalytic domain at the C terminus (29, 30). The overall sequence is homologous to the human YT16 gene (30) and appears to be the human analogue of the mouse pp56LSTRA/lck (26, 27). The PTK thus belongs to a family of PTKs that generally comprise a portion of the cytoplasmic tail of the epidermal growth factor, insulin, and platelet-derived growth factor receptors. In this case, however, the PTK lacks a recognizable transmembrane domain, and, instead, may function by means of an association with the T4 (CD4) antigen. Interestingly, the NEPHGE/NaDodSO4/PAGE pattern revealed a heterogeneity of spots, a result consistent with either differences in the degree of phosphorylation of an individual PTK and/or the presence of a family of related PTKs. In either case, this association appears to represent the only known case of an association between a receptor on the surface of T cells and a member of a family of intracellular mediators with an established ability to activate and transform cells.
A crucial remaining question is whether the association between CD4 and the PTK also exists in the microenvironment of the T cell. It appears unlikely that the association is an artifact of detergent lysis since immunoprecipitates of numerous other T-cell antigens, including the T3 (CD3) subunits, the Til (CD2) antigen, the T200/L-C (CD45) antigens, and the fibronectin receptor, failed to possess detectable kinase activity. The presence of alkylating agents inhibits the activity of the kinase; however, this inhibition appears unrelated to disulfide bonding in solution (data not shown). Thus, an association between the T4 (CD4) receptor and the PTK within the cell would introduce a specific pathway by which T lymphocytes become activated. The T4 (CD4)-associated kinase could act to phosphorylate various intracellular substrates. An obvious and important candidate would be the subunits of the T3–Ti antigen–receptor complex. Of particular interest is the observation that a member of the murine T3 (CD3) complex (termed p21) has been found to be phosphorylated at a tyrosine residue in a major histocompatibility complex class II-restricted response to soluble antigen (32, 33). Several studies have provided evidence in support of an association between the T3 (CD3)–Ti complex and T4 (CD4), although this has yet to be demonstrated by biochemical approaches (43, 44). An interaction via the PTK could act to regulate the cooperative interaction between the T3–Ti complex and the CD4 antigen. Another potentially important role is suggested by the ability of the CD4 antigen to act as receptor for the AIDS virus (HIV) (3-5). A linkage of CD4 with the kinase could regulate the ability of the AIDS virus (HIV) to enter and/or replicate within the CD4+ subset of T cells.
Acknowledgments
We thank Drs. John Lambert, David Kaplan, Gordin Mclntyre, Judith Swack, and Morris White for helpful discussions, and Monica Reed and Barry Millsap for secretarial assistance. C.E.R. is a recipient of a postdoctoral fellowship from the Cancer Research Institute. This work was supported by National Institutes of Health Grants AI 12069 and R23 AI 23392.
Abbreviations
- PTK
protein-tyrosine kinase
- HIV
human immunodeficiency virus
- AIDS
acquired immunodeficiency syndrome
- NEPHGE
nonequilibrium pH gradient gel electrophoresis
Footnotes
Communicated by Herman N. Eisen
References
- 1.Reinherz EL, Meuer SC, Schlossman SF. Immunol. Rev. 1983;74:83–112. doi: 10.1111/j.1600-065x.1983.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 2.Swain SL. Immunol. Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 3.Dalgleish A, Beverley PCL, Clapham PR, Crawford DH, Greaves MF, Weiss RA. Nature (London) 1984;312:763–766. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 4.Klatzman D, Champayne E, Chamaret S, Gruest J, Guefard D, Hercend T, Gluckman JC, Montagnier L. Nature (London) 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 5.Maddon P, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 6.Maddon P, Littman DR, Godfrey M, Maddon DE, Chess L, Axel R. Cell. 1985;42:93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- 7.Bank I, Chess L. J. Exp. Med. 1985;162:1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekoff M, Kakiuchi T, Grey HM. J. Immunol. 1985;134:1337–1342. [PubMed] [Google Scholar]
- 9.Owen T, Fazekas de St. Groth B. J. Immunol. 1987;138:2402–2409. [PubMed] [Google Scholar]
- 10.Eichmann K, Jonsson J-I, Falk I, Emmrich F. Eur. J. Immunol. 1987;17:643–650. doi: 10.1002/eji.1830170510. [DOI] [PubMed] [Google Scholar]
- 11.Anderson P, Blue M-L, Morimoto C, Schlossman SF. J. Immunol. 1987;139:678–682. [PubMed] [Google Scholar]
- 12.Takeuchi T, Schlossman SF, Morimoto C. J. Immunol. 1987;139:665–671. [PubMed] [Google Scholar]
- 13.Rudd CE, Morimoto C, Wong LL, Schlossman SF. J. Exp. Med. 1987;166:1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brugge JS, Erickson RL. Nature (London) 1977;269:346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- 15.Hunter T, Sefton B. Proc. Natl. Acad. Sci. USA. 1980;77:1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collett MS, Erickson RL. Proc. Natl. Acad. Sci. USA. 1978;75:2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter T, Cooper JA. Annu. Rev. Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- 18.Bishop JM. Annu. Rev. Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- 19.Earp HS, Austin KS, Buessow SC, Dy R, Gillespie GY. Proc. Natl. Acad. Sci. USA. 1984;81:2347–2351. doi: 10.1073/pnas.81.8.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swarup G, Dasgupta JD, Garbers DL. J. Biol. Chem. 1983;258:10341–10347. [PubMed] [Google Scholar]
- 21.Wassmer P, Chan C, Logdberg L, Shevach EM. J. Immunol. 1985;135:2237–2242. [PubMed] [Google Scholar]
- 22.Hoxre IA, Matthews DM, Callahan KJ, Cassel DL, Cooper RA. J. Immunol. 1986;137:1194–1201. [PubMed] [Google Scholar]
- 23.Casnellie JE, Harrison ML, Pike LJ, Hellstrom KE, Krebs EG. Proc. Natl. Acad. Sci. USA. 1982;79:282–286. doi: 10.1073/pnas.79.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison ML, Low PS, Geahlen RL. J. Biol. Chem. 1984;259:9348–9350. [PubMed] [Google Scholar]
- 25.Piga A, Reza Taheri M, Yaxley JC, Gitendra Wickremasinghe R, Victor Hoffbrand A. Biochem. Biophys. Res. Commun. 1984;124:766–773. doi: 10.1016/0006-291x(84)91024-6. [DOI] [PubMed] [Google Scholar]
- 26.Marth JD, Peet R, Krebs EG, Perlmutter RM. Cell. 1985;43:393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- 27.Voronova AF, Sefton BM. Nature (London) 1986;319:682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- 28.Trevillyan JM, Lin Y, Chen SJ, Phillips CA, Canna C, Linna TJ. Biochim. Biophys. Acta. 1986;888:286–295. doi: 10.1016/0167-4889(86)90228-4. [DOI] [PubMed] [Google Scholar]
- 29.Trevillyan JM, Canna C, Maley D, Linna TJ, Phillips CA. Biochem. Biophys. Res. Commun. 1986;140:392–398. doi: 10.1016/0006-291x(86)91103-4. [DOI] [PubMed] [Google Scholar]
- 30.Koga Y, Caccia N, Toyonaga B, Spolski R, Yanagi Y, Yahikai Y, Mak TW. Eur. J. Immunol. 1986;16:1643–1646. doi: 10.1002/eji.1830161229. [DOI] [PubMed] [Google Scholar]
- 31.Marth JD, Lewis DB, Wilson CB, Gearn ME, Krebs EG, Perlmutter RM. EMBO J. 1987;6:2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samelson LE, Patel MD, Weismann AM, Harford JB, Klausner RD. Cell. 1986;46:1083–1090. doi: 10.1016/0092-8674(86)90708-7. [DOI] [PubMed] [Google Scholar]
- 33.Samelson LE, Harford J, Schwartz RH, Klausner RD. Proc. Natl. Acad. Sci. USA. 1985;82:1969–1973. doi: 10.1073/pnas.82.7.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parham P, Bodmer WF. Nature (London) 1978;276:397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson J, Drevin H, Axen R. Biochem. J. 1978;173:723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudd CE, Bodmer JG, Bodmer WF, Crumpton MJ. J. Biol. Chem. 1985;260:1927–1936. [PubMed] [Google Scholar]
- 37.Schneider C, Newman RA, Sutherland DR, Asser V, Greaves MF. J. Biol. Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- 38.Laemmli UK. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.O’Farrell PZ, Goodman HM, O’Farrell PH. Cell. 1977;12:1133–1142. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 40.Cooper JA, Sefton BM, Hunter T. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- 41.Stewart SJ, Fujimoto J, Levy R. J. Immunol. 1986;136:3773–3778. [PubMed] [Google Scholar]
- 42.Acres RB, Conlon PJ, Mochizuki DY, Gallis B. J. Biol. Chem. 1986;261:16210–16214. [PubMed] [Google Scholar]
- 43.Saizawa K, Rojo J, Janeway CA. Nature (London) 1987;328:260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- 44.Brenner MB, Trowbridge IS, Strominger JL. Cell. 1985;40:183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]