Abstract
Introduction:
Molecular testing of fine-needle aspiration (FNA) results helps diagnose thyroid cancer, although the additional cost of this adjunct has not been studied. We hypothesized that FNA molecular testing of two indeterminate categories (follicular lesion of undetermined significance and follicular/Hürthle cell neoplasm) can be cost saving.
Methods:
For a hypothetical group of euthyroid patients with a 1-cm or larger solitary thyroid nodule, a decision-tree model was constructed to compare the estimated costs of initial evaluation according to the current American Thyroid Association guidelines, either with molecular testing (MT) or without [standard of care (StC)]. Model endpoints were either benign FNA results or definitive histological diagnosis.
Results:
Molecular testing added $104 per patient to the overall cost of nodule evaluation (StC $578 vs. MT $682). In this distributed cost model, MT was associated with a decrease in the number of diagnostic lobectomies (9.7% vs. StC 11.6%), whereas initial total thyroidectomy was more frequent (18.2% vs. StC 16.1%). Although MT use added a diagnostic cost of $5031 to each additional indicated total thyroidectomy ($11,383), the cumulative cost was still less than the comparable cost of performing lobectomy ($7684) followed by completion thyroidectomy ($11,954) in the StC pathway, when indicated by histological results. In sensitivity analysis, savings were demonstrated if molecular testing cost was less than $870.
Conclusions:
Molecular testing of cytologically indeterminate FNA results is cost saving predominantly because of reduction in two-stage thyroidectomy. Appropriate use of emerging molecular testing techniques may thus help optimize patient care, improve resource use, and avoid unnecessary operation.
Thyroid nodules are a frequently encountered clinical problem. With the routine use of high-resolution ultrasound, the detection of thyroid nodules has increased, and the incidence of thyroid nodules in adults is now 20–75% (1). Approximately 5% of nodules are malignant, and evaluation to exclude thyroid cancer is important, especially because the incidence is rising and thyroid cancer is now the fifth most common cancer diagnosed in women (2). Systematic and accurate algorithms to evaluate thyroid nodules are therefore becoming increasingly relevant. Commonly used evidence-based guidelines have been developed through the American Thyroid Association (ATA) and American Association of Clinical Endocrinologists (AACE) (3, 4). Neck ultrasound is the best initial diagnostic imaging modality for thyroid nodule characterization, and modern algorithms rely on fine-needle aspiration (FNA) cytology results to guide subsequent management.
FNA is highly sensitive for thyroid cancer, but specificity is low. Cytology results are considered indeterminate in 20–30% of cases and include results in the follicular lesion of undetermined significance (FLUS), follicular or Hürthle cell neoplasm (FN), or suspicious for malignancy (SUSP) categories. The risk of cancer in these indeterminate FNA categories can range widely, from 5–75% (5) thus diagnostic thyroidectomy is often necessary. In the absence of clinical risk factors for thyroid malignancy, an initial thyroid lobectomy is a common surgical option for patients with a solitary thyroid nodule and FNA results in the cytology categories considered low-risk for malignancy (FN or repeatedly FLUS); however, those patients diagnosed with thyroid cancer after lobectomy often require a second operation for completion thyroidectomy, which incurs additional costs and risks. Improving preoperative risk stratification to better discriminate which thyroid nodules are malignant will facilitate initial definitive surgery and could reduce the costs of care.
Testing of FNA specimens for gene mutations and rearrangements associated with thyroid cancer is a recent adjunct that can increase the diagnostic utility of FNA (6, 7). Studies have shown that detection of any one of the nonoverlapping genetic alterations such as BRAF, RAS, RET/PTC, or PAX8/PPARg is 85–99% predictive of differentiated thyroid cancer, regardless of cytological category (8–10) and can prompt initial definitive total thyroidectomy (11, 12). Consideration for molecular testing to help clinical management of indeterminate FNA results is recommended by the latest version of the ATA guidelines (3), but its routine use has not been evaluated at many centers.
Although molecular testing does add cost to the preoperative evaluation, we hypothesized that this cost will be offset by the savings associated with avoiding two-stage thyroidectomy. We used a decision-tree analysis to examine the cost of adding molecular testing of FNA results in the FLUS and FN categories to the management of a hypothetical group of thyroid nodule patients as directed by the current ATA guidelines (3).
Patients and Methods
A decision-tree model using TreeAge Software (TreeAge Inc., Williamstown, MA) was constructed to analyze the estimated cost of testing in a hypothetical group of patients evaluated for a thyroid nodule according to the current ATA guidelines with the addition of molecular marker testing. This management strategy was compared with the cost of thyroid nodule evaluation using current ATA guidelines but without molecular marker testing (Fig. 1). In the decision tree, a hypothetical population of adults presenting with a new 1-cm or larger solitary thyroid nodule and a normal baseline TSH level progressed through the model according to chance outcomes determined by test specificities, test sensitivities, and clinical probabilities. The mean age at presentation was taken to be 49 yr (2). The mean natural life expectancy of 78 yr was obtained from U.S. census data (13). All patients were assumed to be surgical candidates, to consent to surgery, and to have no previous history of neck surgery or other confounding medical conditions. All patients were evaluated using the current ATA guidelines, which employ routine diagnostic ultrasound and ultrasound guidance for FNA (3) and which use the Bethesda 2007 Thyroid Cytology Classification system (5). According to the Bethesda classification scheme, the six cytological categories for thyroid FNA results are defined as: FLUS, FN, SUSP, malignant, benign, and nondiagnostic. In both arms of our model, the endpoints of care were either a benign FNA result or definitive thyroid surgery.
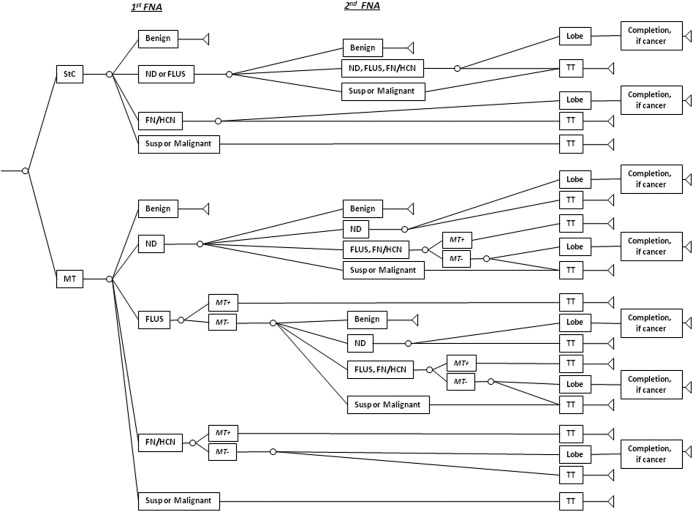
Fig. 1.
Decision-tree structure for the clinical course of patients referred with a thyroid nodule. HCN, Hürthle cell neoplasm; Lobe, lobectomy; ND, nondiagnostic; Susp, suspicious.
In the decision tree, patients were separated into two groups. The first group was assigned to the standard of care (StC) arm, and treatment was based solely on FNA results. Patients with one nondiagnostic or FLUS FNA result received a repeat FNA and proceeded to surgery if the second biopsy was again nondiagnostic or FLUS (5, 14, 15). Patients with a FN, SUSP, or malignant FNA result proceeded to initial thyroid lobectomy or total thyroidectomy (TT) (3). Neither routine frozen section during lobectomy nor central lymphadenectomy during TT were included in the model, because these adjuncts represent ongoing areas of controversy. The second group [molecular testing (MR) group] additionally had mutation marker panel testing of all FNA results that were in the FLUS and FN categories. We did not include molecular testing for benign FNA results as cytology alone is 98–99% accurate, and further immediate evaluation is not recommended (3). Similarly, molecular testing for the nondiagnostic category is not included because such samples are often inadequate for further analysis (10). We also did not include routine molecular testing for FNA in the SUSP or malignant categories because according to the ATA guidelines, these results should lead to definitive initial TT, and molecular testing would therefore not alter the initial extent of thyroidectomy (3).
Because of available cost, sensitivity, and specificity data, the molecular marker panel for the model was composed of testing for BRAF V600E and K601E, NRAS codon 61, HRAS codon 61, and KRAS codon12 and13 point mutations, and RET/PTC1, RET/PTC3, and PAX8/PPARγ rearrangements (8–10). In the model, patients in the MT group with positive molecular testing for any marker proceeded directly to TT, whereas patients with negative testing had repeat FNA, lobectomy, or TT according to the ATA guidelines (3).
Estimates of the prevalence, sensitivity, and specificity values used in analysis were based on a comprehensive Medline literature search and were limited to those studies that used the Bethesda 2007 Thyroid Cytology Classification system (Table 1) (8–10, 15–24). A true positive was defined as a nodule that was classified in the respective cytology category (nondiagnostic, FLUS, FN, or SUSP/malignant), and malignancy was diagnosed on histology. A true negative had both a benign cytology result and benign histology. A false positive was defined as a nodule classified in the specified FNA cytology category but was benign on histology. A false negative was defined as a nodule with benign cytology, but had malignant histology. In the nondiagnostic category, the sensitivity and specificity of FNA for thyroid cancer based on literature review were taken as 50 and 84%, respectively; in the FLUS category, the values were 73 and 50%; in the FN category, the values were 84 and 60%; and in the SUSP/malignant category, the respective sensitivity and specificity values were 97 and 89%. The sensitivity and specificity of molecular marker testing to diagnose thyroid cancer in FNA results from the FLUS and FN categories were 63 and 98%, respectively (8–10).
Table 1.
Base-case clinical and test probability estimates
| Probability [mean (range)] | Refs. | |
|---|---|---|
| Initial FNA result | ||
| Benign | 0.65 (0.60–0.70) | 15–20 |
| Nondiagnostic | 0.10 (0.02–0.20) | |
| FLUS | 0.045 (0.03–0.06) | |
| FN | 0.09 (0.06–0.12) | |
| SUSP/malignant | 0.115 (0.07–0.16) | |
| Repeat FNA result after initial nondiagnostic biopsy | 18–20 | |
| Benign | 0.50 (0.42–0.57) | |
| Nondiagnostic | 0.33 (0.17–0.48) | |
| FLUS | 0.04 (0.02–0.05) | |
| FN | 0.07a (0.05–0.085) | |
| SUSP/malignant | 0.06 (0.035–0.085) | |
| Repeat FNA result after initial FLUS biopsy | 15, 20 | |
| Benign | 0.52 (0.49–0.54) | |
| Nondiagnostic | 0.03a (0.00–0.07) | |
| FLUS | 0.25 (0.19–0.31) | |
| FN | 0.12 (0.06–0.17) | |
| SUSP/malignant | 0.08a (0.02–0.15) | |
| Probability of positive molecular marker result | ||
| FLUS | 0.10 (0.10–0.14) | 8–10 |
| FN | 0.20 (0.18–0.39) | |
| Sensitivity/specificity of FNA results to predict thyroid cancer (range)b | 9, 15, 17 | |
| Inadequate | 0.5 (0.25–0.75)b/0.84 (0.81–0.85) | |
| FLUS | 0.68 (0.38–0.81)/0.5 (0.28–0.84) | |
| FN | 0.81 (0.71–0.86)/0.6 (0.52–0.79) | |
| SUSP/malignant | 0.96 (0.84–0.98)/0.89 (0.78–0.95) | |
| Sensitivity/specificity of molecular markers to predict thyroid cancer | 0.63 (0.59–0.86)/0.98 (0.97–1.0) | 8–10 |
| Probability of initial lobectomy if surgery indicated | 0.76 (0–100)b | 22 |
| Complication rates after lobectomy/total | 22–24, 37 | |
| Hematoma | 0.004/0.016 | |
| Recurrent laryngeal nerve injury | 0.006/0.013 | |
| Hypoparathyroidism | 0.0007/0.022 | |
| Hypothyroidism | 0.21/1.0 |
Mean changed ± 0.03 while keeping the value within the reported range to maintain the sum probability of the parameter set equal to 1.0.
Range of values used for sensitivity analysis.
Estimates of inpatient hospital costs were obtained from the Healthcare Costs and Utilization Project (25). After literature review, we adjusted hospital costs to include outpatient or same-day discharge for 20% of thyroidectomies with an associated 30% reduction in hospital costs (26–28). Cost estimates for physician fees were based on median 2010 Medicare reimbursement rates for the corresponding common procedural terminology code for each type of thyroid operation (29). Costs of treatment included anesthesia, hospital, surgeon fees, and management of complications inclusive of the costs of daily medications, follow-up visits, and annual laboratory studies (Table 2). Costs related to prolonged hospitalization or readmission due to surgical complications or medical comorbidities were not included. Costs that were common to the two treatment strategies or costs from outside the healthcare system including lost workplace productivity were not included in the analysis. The cost estimate for the molecular marker panel was $650 and was obtained from current institutional Molecular Anatomic Pathology laboratory cost estimation and reimbursement data.
Table 2.
| Cost ($) | |
|---|---|
| FNA biopsy | 505.16 |
| Molecular marker panel | 650.00 |
| Lobectomy (mean hospital cost/se) | 6549.00/237.00 |
| Lobectomy (physician fee) | 751.84 |
| TT (mean hospital cost/se) | 7907.00/312.00 |
| TT (physician fee) | 953.53 |
| Hypocalcemia per year | 101.26 |
| Medialization laryngoplasty | 4511.80 |
| Hematoma | 5754.24 |
| Hypothyroidism per year | 110.83 |
| Completion thyroidectomy (physician fee) | 1075.95 |
Sensitivity analyses were also performed to assess effects on the model conclusions after varying the values assigned to prevalence of malignancy, FNA sensitivity and specificity, molecular testing sensitivity and specificity, likelihood of initial thyroid lobectomy, and cost estimates for FNA biopsy and molecular testing. The base-case variables were varied according to the ranges identified during the literature review or if this was not available, the inputs were varied by a ±50% magnitude from the base-case variable (Table 1).
Results
In the base-case analysis, the average per-patient cost of StC for evaluation and treatment of a thyroid nodule was $578, which includes all testing and procedure-related costs. Use of molecular testing increased the average per-patient cost of nodule evaluation by $104, i.e. to $682. In the model, fewer thyroid lobectomies were performed in the MT arm (StC 11.6% vs. MT 9.7%). The added cost in the MT arm associated with each avoided lobectomy was $5679, which was lower than the total cost in the StC arm of performing diagnostic lobectomy including hospital and physician fees and complication costs ($7684). Conversely, initial TT was more often indicated in the MT pathway than the StC group (18.2 vs. 16.1%). The added cost that molecular testing incurred for each additional TT performed was $5031. Even with the added cost of molecular testing, the total cost of each additional TT ($16,414) was less than the cost in the StC pathway for performing a lobectomy plus completion thyroidectomy when indicated by final histological results ($19,638). Thus, use of molecular testing produced a distributed cost savings.
Relative to StC, diagnostic sensitivity in the MT arm increased from 97.7 to 98.2% at a cost of $22,720 for each additional true positive (i.e. each additional thyroid cancer) detected. Use of molecular testing also improved the diagnostic specificity of FNA from 76.6 to 77.5% at a cost of $11,278 for each additional true negative (i.e. each additional benign nodule) detected. Given an estimated cancer prevalence of 5%, if a hypothetical population of 10,000 patients were diagnosed via StC procedures and compared with 10,000 diagnosed via the MT algorithm, the number of true-positive cases would increase slightly from 489 in the StC arm to 491 in the MT arm, and the number of false-positive cases would decrease from 2246 (StC) to 2139 (MT).
Univariate sensitivity analysis was performed by varying key model parameters to verify the robustness of model conclusion with respect to costs associated with lobectomies avoided. The cost of performing a lobectomy ($7684) was set as the willingness-to-pay threshold. No changes to the model conclusion was seen after varying the clinical probabilities within the ranges shown in Table 1, by changing the prevalence of cancer from 0–10%, or by varying the cost of FNA from 50 to 150% of base-case values (Table 2). The model conclusion also remained the same after two-way sensitivity analysis allowing FNA and molecular testing sensitivity estimates to be varied simultaneously with specificity estimates and within the ranges shown in Table 1. With molecular testing, the cost of avoiding lobectomy exceeded the actual cost of the procedure if the cost of the marker panel was more than $870 (Fig. 2). The probability of undergoing an initial lobectomy was varied in a one-way sensitivity analysis from 0–100%, and as long as the probability was greater than 57%, the cumulative diagnostic costs associated with each avoided lobectomy was less than the cost of the surgery.
Fig. 2.
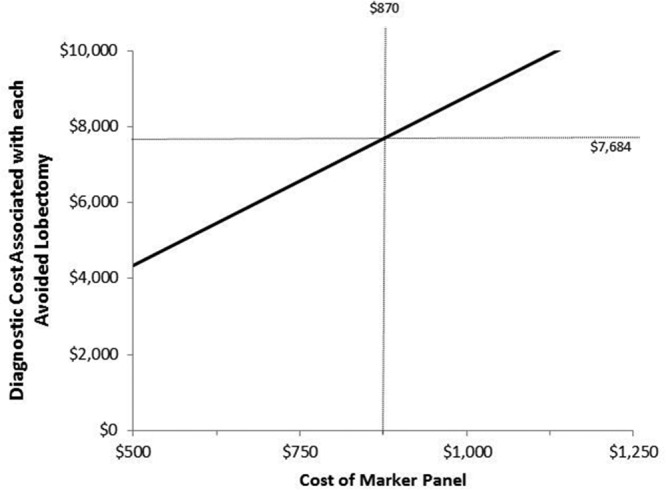
One-way sensitivity analysis showing that the cost of the marker panel must be greater than $870 before the total diagnostic costs associated with each avoided lobectomy increases above the willingness-to-pay threshold ($7684).
The threshold at which the cumulative diagnostic costs associated with each additional TT performed in the MT pathway would exceed the total costs of a two-stage thyroidectomy ($19,638) was $7800, and this threshold was used in sensitivity analysis to verify the model conclusion with respect to costs associated with each additional TT. Again, no change in the conclusion was seen after one-way sensitivity analysis varying the key parameters including clinical probabilities, cancer prevalence, and FNA cost. There was also no change in the model conclusion after two-way sensitivity analysis that varied the FNA and molecular testing sensitivity and specificity estimates within the ranges shown in Table 1. The cost of each additional TT in the MT arm exceeded the $7800 willingness-to-pay threshold if the cost of the marker panel was over $1000 (Fig. 3). As long as the likelihood of an initial lobectomy remained above 46%, the cost associated with each additional initial TT remained less than the willingness-to-pay threshold.
Fig. 3.
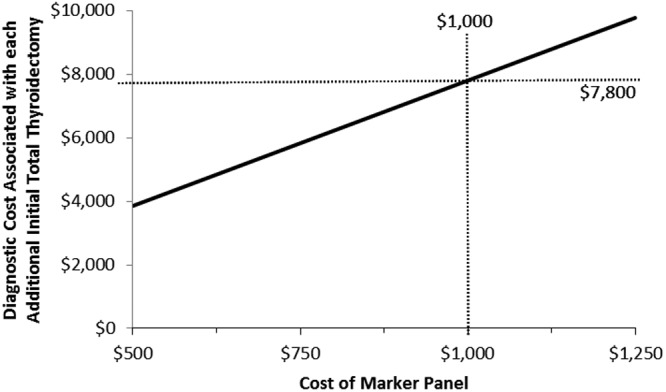
One-way sensitivity analysis showing that the cost of the marker panel must be greater than $1000 before the total diagnostic costs associated with each additional TT in the MT pathway increases beyond the willingness-to-pay threshold ($7800).
Discussion
Although FNA is a well-validated and sensitive diagnostic test, 20–30% of results fall into an indeterminate category, which leads to diagnostic thyroid surgery. Unfortunately, many diagnostic lobectomies are required for what is determined to be benign disease, whereas for other patients, a second definitive surgery is needed when thyroid cancer is diagnosed after lobectomy. As we and others have previously shown, improved preoperative risk stratification has been achieved by use of molecular markers identified to be commonly associated with thyroid cancer (7–11); by using a variety of testing techniques and markers, we now know that preoperative detection of an associated genetic alteration is 85–99% predictive of thyroid cancer. However, with the implementation of any new and potentially costly testing, analysis to determine comparative efficacy is important to demonstrate.
Our study found that although the use of the described molecular panel added a distributed cost of $104 per patient, use of the molecular marker panel resulted in an approximately 20% reduction in diagnostic lobectomy use (MT 9.7 vs. 11.6%) and a corresponding increase in initial TT use (MT 18.2 vs. 16.1%). Furthermore, improvements in diagnostic sensitivity and specificity were also observed with use of molecular testing hypothetically resulting in an increase in the number of true-positive cases (two of 10,000) and a decrease in the number of false positives (107 of 10,000). We believe that the observed sensitivity and specificity changes were small precisely because the model used the current standards of care under the ATA guidelines, instead of using a potentially amended care algorithm. In our current clinical practice, patients with FLUS and FN still undergo diagnostic surgery even with negative molecular testing results (10). In short, this hypothetical model demonstrates that the diagnosis of thyroid cancer in patients with FLUS and FN results is facilitated by molecular testing, with cost savings in part due to small improvements in test performance but predominantly due to reduction in the number of unnecessary operations.
Sensitivity analysis also predicted that the cost savings is seen only because of a low set cost for performing the molecular marker panel. In 2007, this cost was determined to be $650 at our facility (10) based on the reagents and personnel costs of our clinical molecular laboratory and the patterns of reimbursement in our region. Although the cost of molecular testing may fluctuate at different institutions and various commercial testing facilities, we have observed that it represents a reasonable and economically sustainable cost for an established laboratory. Importantly, analysis also indicates that if the cost of molecular testing were to increase to more than $870, the associated savings would no longer be observed in this model. Although limiting the molecular testing to only one or two of the genetic alterations would reduce cost, there would also be an associated decrease in the diagnostic sensitivity and specificity (10).
Our model was based on certain assumptions because we were interested in capturing the cost savings associated with using molecular testing during the initial diagnostic testing of a thyroid nodule. We set the endpoints of both the StC and MT diagnostic algorithms to be identical, including either the diagnosis of a benign nodule by FNA or pathology or the diagnosis of thyroid cancer after histological evaluation. Therefore, costs of long-term nonoperative follow-up or of thyroid cancer treatment such as radioactive iodine ablation should be equivalent in both algorithms and were not included in analysis. Similarly, the addition of molecular testing to current standard care nodule evaluation should not result in a significant decrease to the long-term quality of life in the decision-tree model that we used, because the diagnostic outcome is identical in both the StC and MT algorithms. In at least one respect, our model was designed to favor the null hypothesis (to favor no cost benefit for MT testing); e.g. one of the factors that could potentially diminish the benefits associated with MT is if the complication rate for initial TT is significantly higher than the complication rate for two-stage thyroidectomy. Although there is a decreased quality of life associated with permanent vocal cord paralysis and permanent hypoparathyroidism, these risks occur less frequently at high volume centers (30) and, moreover, are usually reported to be higher (not lower) with two-stage reoperative thyroidectomy than with initial TT; however, the model set these risks as equal. The impact on long-term quality of life is unclear, because quality of life estimates have been shown to be equivalent in patients who receive lobectomy compared with TT (31–33).
A potential limitation of the model is the assumption that indeterminate FNA results with negative molecular testing have the same risk of malignancy as without molecular testing. Studies have suggested that molecular marker-negative FNA results may actually have a lower risk of malignancy. In Ohori et al. (34), which evaluated the utility of molecular marker testing for a small series of FLUS results, the overall probability of cancer was 17%, but molecular marker testing was able to further risk stratify the FLUS category: all (100%) molecular marker-positive FNA were cancer, whereas only 8% of molecular marker-negative FNA were cancer, and in our recent large prospective series, the cancer risk was 5.9% in this group (10). However, the risk of malignancy is certainly not eliminated by molecular marker-negative FNA results, and to date, diagnostic surgery is still standard care in this setting. As we better understand the correlation between genotype and thyroid cancer phenotype, we may yet be able to identify a subset of nodules with low-risk cytology and negative molecular marker results that could potentially be followed with close interval imaging surveillance instead of requiring surgical treatment, possibly further reducing costs while augmenting the diagnostic utility of molecular marker testing. We strongly support further study to clarify the natural history of nodules with negative molecular testing and indeterminate cytology results before surgical treatment may be routinely deferred.
Another limitation is the model assumption that a solitary 1-cm or larger dominant nodule is identified at patient presentation. Multinodular disease is a common clinical scenario, and we did not include this consideration in the model because the prevalence values of such a scenario, its cancer risk, and its management practice are less straightforward (3). Therefore, the costs and clinical implications of molecular testing for patients with multiple thyroid nodules are not yet known.
The conclusion that molecular testing of FNA results can be cost saving was also very recently described by Li et al. (35) in a hypothetical analysis that used an alternate molecular technique to classify indeterminate FNA results based on the expression profile of 142 genes. In diagnosing thyroid cancer, the sensitivity and specificity of their testing technique applied to indeterminate FNA findings (inclusive of results in the FLUS, FN, and SUSP categories) was 91 and 75%, respectively. Several other model design features differed importantly from ours, including the nonstandard use of nonoperative management for nodules with indeterminate FNA results and employment of higher cost figures for surgery because no adjustment for outpatient thyroid procedures was incorporated that would favor a perceived cost benefit. Furthermore, with a truncated follow-up of 5 yr, the natural history of a Hürthle cell neoplasm, for example, that is not surgically excised will likely not be apparent. The cumulative effects and costs of ongoing surveillance, which is a more likely clinical scenario in this setting, may well exceed any initial cost benefit of nonoperative management. Appropriate risk stratification of thyroid nodules with indeterminate FNA results remains an important area of study, but until longitudinal studies support the theory that molecular-negative nodules with indeterminate FNA results carry an acceptably low risk of malignancy and malignant transformation, surgery currently remains the standard of care.
Although one documented benefit of routine molecular testing for thyroid nodules is certainly to improve the diagnostic sensitivity of FNA, molecular results have prognostic implications as well, which over time are being better defined. BRAF V600E, for example, has been shown in a number of studies to be associated with papillary thyroid cancer with aggressive characteristics such as extrathyroidal extension, lymph node metastasis, and higher risk of locoregional recurrence (36). Whether extensive initial surgery or adjuvant treatment will improve outcomes for patients with BRAF V600E positive thyroid cancer remains to be seen. Because the data are somewhat controversial, we did not design our model to consider the prognostic significance of molecular results, although we anticipate that as investigators obtain more information there will be important clinical implications allowing future changes to both tailor thyroid cancer management algorithms and improve use of healthcare resources.
In summary, using the standard treatment algorithms currently recommended by the ATA and a long-term decision-tree analysis ending in definitive care, we demonstrate a distributed cost savings with molecular testing of FNA results in two indeterminate cytological categories: FLUS and FN. Cost saving was due both to better preoperative stratification of thyroid nodule malignancy risk and to improved ability to guide initial definitive thyroidectomy. In this era of rising healthcare costs and increasing thyroid nodule detection, appropriate and efficacious use of modern molecular testing techniques can facilitate personalized medical care to optimize clinical outcomes and management algorithms.
Acknowledgments
Y.E.N. was supported by National Institutes of Health.
Disclosure Summary: L.Y., C.F., A.S.K., S.P.H., M.N.N., K.L.M., M.T.S., K.J.S., and Y.E.N. have nothing to disclose. S.E.C. is Section Editor for Endocrine Surgery at UpToDate Inc.
Footnotes
- FLUS
- Follicular lesion of undetermined significance
- FN
- follicular or Hürthle cell neoplasm
- FNA
- fine-needle aspiration
- MT
- molecular testing
- StC
- standard of care
- SUSP
- suspicious for malignancy
- TT
- total thyroidectomy.
References
- 1. Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2. Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK. (eds) 2010. SEER Cancer Statistics Review, 1975–2007. http://seer.cancer.gov/csr/1975_2007/ Bethesda, MD: National Cancer Institute [Google Scholar]
- 3. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 4. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, Vitti P. 2010. AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest 33(5 Suppl):51–56 [PubMed] [Google Scholar]
- 5. Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, Vielh P, DeMay RM, Sidawy MK, Frable WJ. 2008. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: A synopsis of the national cancer institute thyroid fine-needle aspiration state of the science conference. Diagn Cytopathol 36:425–437 [DOI] [PubMed] [Google Scholar]
- 6. Nikiforova MN, Nikiforov YE. 2009. Molecular diagnostics and predictors in thyroid cancer. Thyroid 19:1351–1361 [DOI] [PubMed] [Google Scholar]
- 7. Moses W, Weng J, Sansano I, Peng M, Khanafshar E, Ljung BM, Duh QY, Clark OH, Kebebew E. 2010. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg 34:2589–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, Di Santo A, Caruso G, Carli AF, Brilli L, Montanaro A, Pacini F. 2010. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab 95:1365–1369 [DOI] [PubMed] [Google Scholar]
- 9. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. 2009. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 10. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT, Coyne C, Johnson JT, Stewart AF, Nikiforova MN. 2011. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 96:3390–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yip L, Nikiforova MN, Carty SE, Yim JH, Stang MT, Tublin MJ, Lebeau SO, Hodak SP, Ogilvie JB, Nikiforov YE. 2009. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery 146:1215–1223 [DOI] [PubMed] [Google Scholar]
- 12. O'Neill CJ, Bullock M, Chou A, Sidhu SB, Delbridge LW, Robinson BG, Gill AJ, Learoyd DL, Clifton-Bligh R, Sywak MS. 2010. BRAF(V600E) mutation is associated with an increased risk of nodal recurrence requiring reoperative surgery in patients with papillary thyroid cancer. Surgery 148:1139–1145; discussion 1145–1146 [DOI] [PubMed] [Google Scholar]
- 13. Arias E. 2010. United States life tables, 2006. Natl Vital Stat Rep 58:1–40 [PubMed] [Google Scholar]
- 14. Faquin WC, Baloch ZW. 2010. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol 38:731–73920049964 [Google Scholar]
- 15. Nayar R, Ivanovic M. 2009. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer 117:195–202 [DOI] [PubMed] [Google Scholar]
- 16. Cibas ES, Ali SZ; NCI Thyroid FNA 2009. State of the Science Conference. The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol 132:658–665 [DOI] [PubMed] [Google Scholar]
- 17. Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. 2009. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid 19:1215–1223 [DOI] [PubMed] [Google Scholar]
- 18. Jo VY, Stelow EB, Dustin SM, Hanley KZ. 2010. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol 134:450–456 [DOI] [PubMed] [Google Scholar]
- 19. Yang J, Schnadig V, Logrono R, Wasserman PG. 2007. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 111:306–315 [DOI] [PubMed] [Google Scholar]
- 20. Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, Moore FD, Jr, Kim BW, Nosé V, Marqusee E, Larsen PR, Alexander EK. 2007. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 111:508–516 [DOI] [PubMed] [Google Scholar]
- 21. Asari R, Niederle BE, Scheuba C, Riss P, Koperek O, Kaserer K, Niederle B. 2010. Indeterminate thyroid nodules: a challenge for the surgical strategy. Surgery 148:516–525 [DOI] [PubMed] [Google Scholar]
- 22. Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, Pelizzo MR, Pezzullo L. 2004. Complications of thyroid surgery: analysis of multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg 28:271–276 [DOI] [PubMed] [Google Scholar]
- 23. Stoll SJ, Pitt SC, Liu J, Schaefer S, Sippel RS, Chen H. 2009. Thyroid hormone replacement after thyroid lobectomy. Surgery 146:554–558; discussion 558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McHenry CR, Slusarczyk SJ. 2000. Hypothyroidism following hyemithyroidectomy: incidence, risk factors, and management. Surgery 128:994–998 [DOI] [PubMed] [Google Scholar]
- 25. Agency for Healthcare and Research Quality, US Department of Health and Human Services 2010. Welcome to HCUPnet (Healthcare Cost and Utilization Project). http://hcupnet.ahrq.gov/ Accessed December 2010
- 26. McHenry CR. 1997. “Same-day” thyroid surgery: an analysis of safety, cost savings, and outcome. Am Surg 63:586–589; discussion 589–590 [PubMed] [Google Scholar]
- 27. Mowschenson PM, Hodin RA. 1995. Outpatient thyroid and parathyroid surgery: a prospective study of feasibility, safety and costs. Surgery 118:1051–1053; discussion 1053–1054 [DOI] [PubMed] [Google Scholar]
- 28. Tuggle CT, Roman S, Udelsman R, Sosa JA. 2011. Same-day thyroidectomy: a review of practice patterns and outcomes for 1,168 procedures in New York state. Ann Surg Oncol 18:1035–1040 [DOI] [PubMed] [Google Scholar]
- 29. Centers for Medicare and Medicaid Services, US Department of Health and Human Services 2010. Physician Fee Schedule Search. http://www.cms.gov/apps/physician-fee-schedule/ (accessed December 2010)
- 30. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. 1998. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg 228:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vidal-Trecan GM, Stahl JE, Durand-Zaleski I. 2002. Managing toxic thyroid adenoma: a cost-effectiveness analysis. Eur J Endocrinol 146:283–294 [DOI] [PubMed] [Google Scholar]
- 32. Gold MR. 1996. Cost-effectiveness in health and medicine. New York: Oxford University Press [Google Scholar]
- 33. Shah MD, Witterick IJ, Eski SJ, Pinto R, Freeman JL. 2006. Quality of life in patients undergoing thyroid surgery. J Otolaryngol 35:209–215 [DOI] [PubMed] [Google Scholar]
- 34. Ohori NP, Nikiforova MN, Schoedel KE, LeBeau SO, Hodak SP, Seethala RR, Carty SE, Ogilvie JB, Yip L, Nikiforov YE. 2010. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.” Cancer Cytopathol 118:17–23 [DOI] [PubMed] [Google Scholar]
- 35. Li H, Robinson KA, Anton B, Saldanha IJ, Ladenson PW. 2011. Cost-effectiveness of a novel molecular test for cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab 96:E1719–E1726 [DOI] [PubMed] [Google Scholar]
- 36. Melck AL, Yip L, Carty SE. 2010. The utility of BRAF testing in the management of papillary thyroid cancer. Oncologist 15:1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shrime MG, Goldstein DP, Seaberg RM, Sawka AM, Rotstein L, Freeman JL, Gullane PJ. 2007. Cost-effective management of low-risk papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 133:1245–1253 [DOI] [PubMed] [Google Scholar]