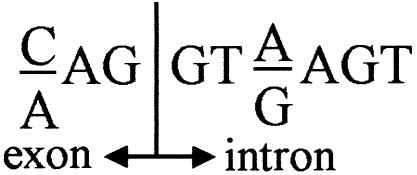Abstract
At least 40% of families affected with cerebral cavernous malformation have a mutation in Krit1. We previously identified two point mutations in Krit1 leading to changes in amino acids (D137G and Q210E) in two different families. Further RNA analysis reveals that both point mutations actually activate cryptic splice-donor sites, causing aberrant splicing and leading to a frameshift and protein truncation. To date, no simple missense mutations have been detected in Krit1.
Cerebral cavernous malformation (CCM [MIM 116860]) is a common autosomal disorder, characterized by abnormally enlarged capillary cavities in the brain without intervening normal parenchyma (Russell and Rubenstein 1989). They occur as single or multiple malformations that lead to focal neurologic signs, hemorrhagic strokes, or seizures. CCMs are found in 0.1%–0.5% of the population and represent 10%–20% of cerebral vascular lesions (Rigamonti et al. 1988). Three genetic loci have been defined: CCM1 on chromosome 7q21-q22 (Dubovsky et al. 1995; Günel et al. 1995; Marchuk et al. 1995), CCM2 on 7p13-p15, and CCM3 on 3q25.2-q27 (Craig et al. 1998). To date, only one gene has been identified: Krit1, for CCM1 (Laberge-le Couteulx et al. 1999; Sahoo et al. 1999), which is responsible for ⩾40% of CCM cases. The Krit1 protein has 736 amino acids (Zhang et al. 2000; Eerola et al. 2001; Sahoo et al. 2001) and contains three ankyrin repeats, one FERM (Band 4.1, ezrin, radixin, moesin) domain, and one NPXY (Asn-Pro-X-Tyr) motif. It has been recently demonstrated that Krit1 shows a strong interaction with the integrin cytoplasmic domain–associated protein 1 (icap1α), a protein involved with β1-dependent angiogenesis, through its NPXY motif (Zhang et al. 2001).
All Krit1 mutations, except two point mutations predicted to lead to an amino-acid change in two different families (D137G in family IFCAS-41, and Q201E in family IFCAS-28) (Davenport et al. 2001; Verlaan et al. 2002), lead to a truncated and presumably inactive protein. Both families are part of the International Familial Cavernous Angioma Study (IFCAS), which was approved by the Committee for the Protection of Human Subjects at Dartmouth College. Because of the prevalence of deleterious mutations reported in the Krit1 gene, we further investigated these two missense mutations, for effects on splicing.
Total RNA was extracted from cultured lymphocytes immortalized with the Epstein-Barr virus, for each member of the families, using a RNeasy mini kit (QIAGEN). A cDNA library was synthesized by RT-PCR, using hexanucleotides (pdN6). The cDNA sequences encompassing the mutations were PCR amplified by use of exonic primers and were electrophoresed on 2% agarose gel.
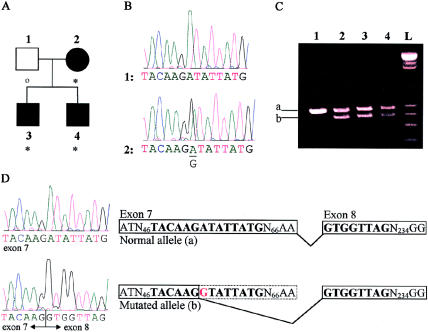
The affected members of IFCAS-41 (fig. 1A) are heterozygous for an A→G substitution (fig. 1B) in exon 7 at the nucleotide position 410 of the coding sequence. The migration pattern of the cDNA (fig. 1C) shows that affected individuals have two different-sized alleles, whereas the unaffected individual is homozygous for the larger allele. This result suggests that the substitution may lead to truncation of the transcript. Sequencing of the different cDNA alleles shows that alternative splicing is occurring in the mutated allele (fig. 1D). The A→G shift creates an alternative splice site that, when used, results in premature splicing of exon 7 and in splicing of exon 8 at the correct position but in an incorrect reading frame. This would result in a frameshift event, leading to a truncated protein of 138 amino acids that has 2 novel amino acids and contains no structural domains of Krit1.
Figure 1.
A, Pedigree of IFCAS-41. The blackened symbols denote affected individuals, and the unblackened square denotes an individual not known to be affected. Asterisks denote mutations, and the small unblackened circle denotes an absence of mutation. B, Genomic DNA sequences of unaffected (1) and affected (2) individuals. The affected individual carries an A→G substitution at nucleotide position 410 of the coding sequence. C, cDNA migration pattern of the normal (a) and mutated (b) alleles, for each member of the IFCAS family. D, cDNA sequences of the normal and mutated alleles. The mutated allele causes cryptic splicing, as is illustrated in the diagram.
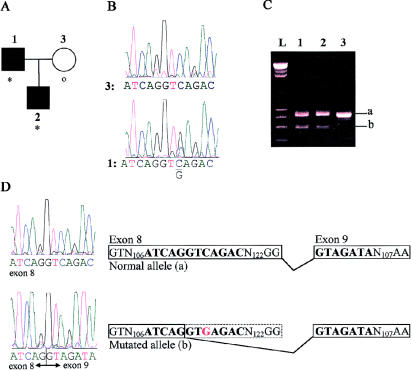
The affected members of IFCAS-28 (fig. 2A) are heterozygous for a C→G substitution (fig. 2B) in exon 8 at nucleotide position 601 of the coding sequence. Similar to IFCAS-41, the migration pattern of the cDNA (fig. 2C) of IFCAS-28 shows that affected individuals have two different-sized alleles, whereas the unaffected individual is homozygous for the larger allele. This result suggests that the substitution may truncate the transcript. Sequencing of the different cDNA alleles shows that alternative splicing is occurring in the mutated allele (fig. 2D). The C→G shift creates an alternative splice site that, when used, results in premature splicing of exon 8 and in splicing of exon 9 at the correct position but in an incorrect reading frame. This results in a frameshift, which is predicted to lead to a truncated protein of 201 amino acids that has a novel amino acid and contains only the NPXY motif.
Figure 2.
A, Pedigree of IFCAS-28. Definitions of symbols are the same as in figure 1. B, Genomic DNA sequences of a normal (3) and affected (1) individuals. The affected individual carries a C→G substitution at nucleotide position 601 of the coding sequence. C, cDNA migration pattern of the normal (a) and mutated (b) alleles, for each member of the IFCAS family. D, cDNA sequences of the normal and mutated alleles. The mutated allele causes cryptic splicing, as is illustrated in the diagram.
The fact that the RT-PCR products from the normal and mutant alleles (“a” and “b,” respectively, in figs. 1C and 2C) are of similar intensity suggests that most of the mutant allele is alternatively spliced. In the present study, we present two examples of point mutations in the coding sequence that activate a cryptic splice-donor site motif (fig. 3) that is used preferentially over the downstream authentic splice site.
Figure 3.
Splice-donor site consensus sequence. The A→G substitution in IFCAS-41 changed the first nucleotide of the intron, whereas the C→G substitution in IFCAS-28 changed the third nucleotide of the intron.
Thus, all Krit1 mutations associated with CCM that have been published to date are predicted to result in a truncated protein. This observation suggests that Krit1 protein function needs to be severely impaired for pathogenesis and that no single amino acid change results in a loss of function sufficient to cause CCM. In addition, our findings stress the importance of examining all point mutations, including silent ones, to determine whether they activate a cryptic splice-donor site motif.
Acknowledgments
We would like to thank the families and the physicians who referred the families, for participating in the IFCAS study. We would like to thank Drs. Collette Hand and André Toulouse for their insightful comments and precious aid.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CCM [MIM 116860]) [PubMed]
References
- Craig HD, Gunel M, Cepeda O, Johnson EW, Ptacek L, Steinberg GK, Ogilvy CS, Berg MJ, Crawford SC, Scott RM, Steichen-Gersdorf E, Sabroe R, Kennedy CTC, Mettler G, Beis MJ, Fryer A, Awad IA, Lifton RP (1998) Multilocus linkage identifies two new loci for a Mendelian form of stroke, cerebral cavernous malformation at 7p15-13 and 3q25.2-27. Hum Mol Genet 7:1851–1858 [DOI] [PubMed] [Google Scholar]
- Davenport WJ, Siegel AM, Dichgans J, Drigo P, Mammi I, Pereda P, Wood NW, Rouleau GA (2001) CCM1 gene mutations in families segregating cerebral cavernous malformations. Neurology 56:540–543 [DOI] [PubMed] [Google Scholar]
- Dubovsky J, Zabramski JM, Kurth J, Spetzler RF, Rick SS, Orr HT, Weber JL (1995) A gene responsible for cavernous malformations of the brain maps to chromosome 7q. Hum Mol Genet 4:453–458 [DOI] [PubMed] [Google Scholar]
- Eerola I, McIntyre B, Vikkula M (2001) Identification of eight novel 5′-exons in cerebral capillary malformation gene-1 (CCM1) encoding Krit1. Biochim Biophys Acta 1517:464–467 [DOI] [PubMed] [Google Scholar]
- Günel M, Awad IA, Anson J, Lifton RP (1995) Mapping a gene causing cerebral cavernous malformations to 7q11.2-921. Proc Natl Acad Sci USA 92:6620–6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge-le Couteulx S, Jung HH, Labauge P, Houtteville J-P, Lescoat C, Cecillon M, Marechal E, Joutel A, Bach J-F, Tournier-Lasserve E (1999) Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet 23:189–193 [DOI] [PubMed] [Google Scholar]
- Marchuk DA, Gallione CJ, Morrison LA, Clericuzio CL, Hart BL, Kosofsky BE, Louis DN, Gusella JF, Davis LE, Prenger VL (1995) A locus for cerebral cavernous malformations maps to chromosome 7q in two families. Genomics 28:311–314 [DOI] [PubMed] [Google Scholar]
- Rigamonti D, Hadley MN, Drayer BP, Johnson PC, Hoenig-Rigamonti K, Knight JT, Spetzler RF (1988) Cerebral cavernous malformations: incidence and familial occurrence. N Engl J Med 319:343–347 [DOI] [PubMed] [Google Scholar]
- Russell DS, Rubenstein LJ (1989) Pathology of tumors of the nervous system, 5th ed. Wiliams & Wilkins, Baltimore, pp 730–736 [Google Scholar]
- Sahoo T, Goenaga-Diaz E, Serebriiskii IG, Thomas JW, Kotova E, Cuellar JG, Peloquin JM, Golemis E, Beitinjaneh F, Green ED, Johnson EW, Marchuk DA (2001) Computational and experimental analyses reveal previously undetected coding exons of the Krit1 (CCM1) gene. Genomics 71:123–126 [DOI] [PubMed] [Google Scholar]
- Sahoo T, Johnson EW, Thomas JW, Kuehl PM, Jones TL, Dokken CG, Touchman JW, Gallione CJ, Lee-Lin S-Q, Kosofsky B, Kurth JH, Louis DN, Mettler G, Morrison L, Gil-Nagel A, Rich SS, Zabramski JM, Boguski MS, Green ED, Marchuk DA (1999) Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, causing cerebral cavernous malformations (CCM1). Hum Mol Genet 8:2325–2333 [DOI] [PubMed] [Google Scholar]
- Verlaan DJ, Davenport WJ, Stefan H, Sure U, Siegel AM, Rouleau GA (2002) Cerebral cavernous malformations: mutations in Krit1. Neurology 58:853–857 [DOI] [PubMed] [Google Scholar]
- Zhang J, Clatterbuck RE, Rigamonti D, Chang DD, Dietz HC (2001) Interaction between krit1 and icap1α infers perturbation of integrin β1-mediated angiogenesis in the pathogenesis of cerebral cavernous malformation. Hum Mol Genet 10:2953–2960 [DOI] [PubMed] [Google Scholar]
- Zhang J, Clatterbuck RE, Rigamonti D, Dietz HC (2000) Cloning of the murine Krit1 cDNA reveals novel mammalian 5′ coding exons. Genomics 70:392–395 [DOI] [PubMed] [Google Scholar]