Abstract
We revealed an interesting facet-dependent electrochemical behavior toward heavy metal ions (HMIs) based on their adsorption behaviors. The (111) facet of Co3O4 nanoplates has better electrochemical sensing performance than that of the (001) facet of Co3O4 nanocubes. Adsorption measurements and density-functional theory (DFT) calculations reveals that adsorption of HMIs is responsible for the difference of electrochemical properties. Our combined experimental and theoretical studies provide a solid hint to explain the mechanism of electrochemical detection of HMIs using nanoscale metal oxides. Furthermore, this study not only suggests a promising new strategy for designing high performance electrochemical sensing interface through the selective synthesis of nanoscale materials exposed with different well-defined facets, but also provides a deep understanding for a more sensitive and selective electroanalysis at nanomaterials modified electrodes.
Aiming at a more sensitive electroanalysis, a variety of nanomaterials or nanocomposites modified electrodes were very often explored for electrochemical detection of very trace levels of toxic heavy metal ions (HMIs). Very recently, nanoscale metal oxides as novel modifiers have been reported in electrochemical detection of HMIs1,2,3,4,5. Compared with traditional modifiers such as noble metals and biomolecules, the electrodes fabricated with nanoscale metal oxides are easy to synthesize with low cost1,3,5. However, as is often claimed, increased currents and increased analytical sensitivity are simply reflections of an increased microscopic surface area and not electrocatalytic activity or any other nano-effects. That is, the mechanism of using nanoscale metal oxides in electrochemical sensing of HMIs has not been proposed by considering their insulating property. Therefore, the design and implementation of new experimental approach combined with theoretical studies are extremely needed.
For a crystalline nanoscale material, different facets may have different geometric and electronic structures, and exhibit different physical and chemical properties6,7,8. Much more attention has been given to investigate the facet effects on catalysts, photocatalysts, electrocatalysts, Li-ion battery, supercapacitors and so on9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24. Take Co3O4 nanoscale materials as an example; Li et al. investigated the facet effect of Co3O4 on catalytic property for methane combustion10. Xiao et al. reported that the exposed facets of Co3O4 nanocrystals are very important for Li+ transport in Li-ion battery12. Although oriented Co3O4 nanostructures have attracted wide attention, the direct experimental detection of HMIs with controlled crystalline morphology and orientation remains a significant challenge- that is, the facet effect in electrochemical sensor especially detection of HMIs is not proposed. Further, till now nanoscale Co3O4 has not been used to examine the electrochemical sensing of HMIs. It should be also pointed out that plenary theoretical investigations are expected to help to get deeper insight into the crystal facet effect, but most reports on the crystal facet effect did not adequately combined first-principles theoretical studies at atomic level with the experimental results13,14,17,19,20,21,24.
In this work, we report the facile synthesis of Co3O4 with two different shapes. Using this non-conductive nanomaterial-modified electrode, we try to demonstrate the facet-dependent electrochemical behaviour of Co3O4 nanocrystals toward HMIs by combining the adsorption measurements and the density-functional theory (DFT) calculations. It has been recognized that the (111) facet of Co3O4 nanoplates has better electrochemical sensing performance than that of the (001) facet of Co3O4 nanocubes. Adsorption measurements and DFT calculations reveal that adsorption of Pb(II) is responsible for the difference of electrochemical properties. To the best of our knowledge, this is the first study to investigate the effect of facet on electrochemical sensing behaviour toward HMIs. Our combined experimental and theoretical studies provide a solid hint to explain the mechanism of electrochemical detection of HMIs using nanoscale metal oxides. This strategy may be extended to other electrochemical sensors based on nanoscale metal oxides.
Results
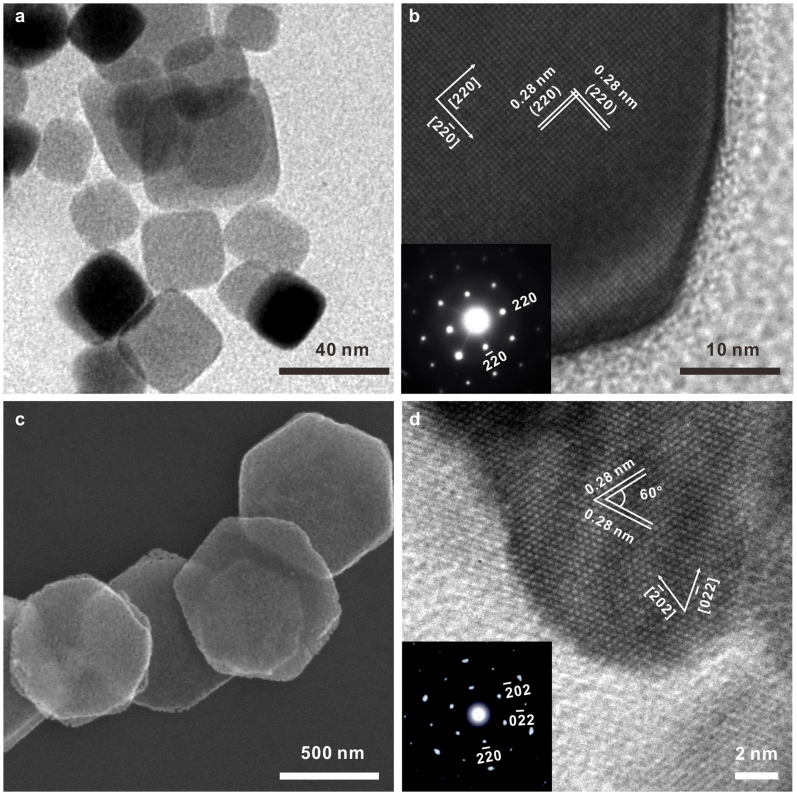
Two simple routes have been designed to fabricate Co3O4 nanocubes and nanoplates (Supporting Information, Figure S1). Co3O4 nanocubes were synthesized by a one-step hydrothermal method with Co(CH3COO)2 as the only reactant. The Co3O4 nanoplates were obtained by a solvothermal method in ethylene glycol (EG) followed by a calcination treatment in air. Figure 1 shows the representative scanning electron microscopy (SEM), transmission electron microscopy (TEM) and high resolution TEM (HRTEM) images of Co3O4 nanoplates and nanocubes. The TEM and HRTEM images of Co3O4 nanocubes are presented in Figure 1a and b, respectively. As seen, the particle size of Co3O4 nanocubes is about 20 ~ 40 nm. HRTEM (Figure 1b) and corresponding selected area electron diffraction (SAED) pattern (Inset in Figure 1b) indicate that the lattice fringe is 0.28 nm and the nanocube is exposed with six (001) facets. A typical SEM image taken for Co3O4 nanoplates is shown in Figure 1c. The low magnification SEM and TEM images in Figure S2 demonstrated the homogeneity of Co3O4 nanoplates. It is seen that most of the nanoplates display well-defined hexagonal shape. The width and the thickness of the plates is determined to be 700 ~ 900 nm and 50 ~ 60 nm, respectively. The HRTEM image in Figure 1d clearly shows that the spacing between lattice fringes with an angle of 60° is 0.28 nm, which is consistent with the 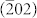 and
and 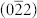 planes of cubic Co3O4. And it is also confirmed by the SAED pattern (Inset in Figure 1d) revealing a single crystal structure. Thus, the resulting dominant exposed plates of Co3O4 nanoplates are (111) facets. Powder X-ray diffraction patterns (XRD) (Supporting Information, Figure S3) of both nanocubes and nanoplates have identical peaks, which can be perfectly indexed to that of cubic spinel Co3O4 (Joint Committee on Powder Diffraction Standards (JCPDS) card no. 42-1467). No impurities have been detected, indicating the formation of pure cobalt oxides. The crystal sizes were calculated according to Sherrer equation based on the (311) diffraction peak of XRD data. The crystal size of Co3O4 nanocubes was 32.5 nm, which was consistent with the TEM observation. However, the crystal size of Co3O4 nanoplates was calculated to be 21.3 nm, which may be due to its porous structure. The specific surface areas of Co3O4 nanocubes and nanoplates have been measured by the Brunauer–Emmett–Teller (BET) method (Supporting Information, Figure S4). The measured specific surface areas for Co3O4 nanocubes and nanoplates are 20 and 13 m2 g−1, respectively.
planes of cubic Co3O4. And it is also confirmed by the SAED pattern (Inset in Figure 1d) revealing a single crystal structure. Thus, the resulting dominant exposed plates of Co3O4 nanoplates are (111) facets. Powder X-ray diffraction patterns (XRD) (Supporting Information, Figure S3) of both nanocubes and nanoplates have identical peaks, which can be perfectly indexed to that of cubic spinel Co3O4 (Joint Committee on Powder Diffraction Standards (JCPDS) card no. 42-1467). No impurities have been detected, indicating the formation of pure cobalt oxides. The crystal sizes were calculated according to Sherrer equation based on the (311) diffraction peak of XRD data. The crystal size of Co3O4 nanocubes was 32.5 nm, which was consistent with the TEM observation. However, the crystal size of Co3O4 nanoplates was calculated to be 21.3 nm, which may be due to its porous structure. The specific surface areas of Co3O4 nanocubes and nanoplates have been measured by the Brunauer–Emmett–Teller (BET) method (Supporting Information, Figure S4). The measured specific surface areas for Co3O4 nanocubes and nanoplates are 20 and 13 m2 g−1, respectively.
Figure 1. Co3O4 nanocrystals.
(a–b), TEM and HRTEM image of typical Co3O4 nanocubes. Inset in panel (b): SAED pattern of nanocubes. (c–d), SEM and HRTEM image of typical Co3O4 nanoplates. Inset in panel (d): SAED pattern of nanoplates.
In general, the obtaining of metal oxides requires a basic medium, and usually NaOH is employed for this purpose. However, in the fabrication of Co3O4 nanocubes, the hydrolysis of acetate anions from Co(CH3COO)2 becomes a good source of hydroxyl anions (Equation (1)). The O2 dissolved in the deionized water is supposed to act as the main oxidant. Partial Co(II) is transformed into Co(III) and the coexistence of Co(II) and Co(III) under the ambient environment prefers the formation of Co3O4 (Equation (2)). The low amount of hydroxyl anions in the solution may promote the formation of Co3O4 nanocubes with (001) planes. Similar result has also been reported by Xiao et al12.
From the SEM and TEM images of the Co3O4 nanoplates precursors before calcination (Data not shown), we can see that the plate-like structures of Co3O4 are inherited from these precursors. The typical XRD pattern of the precursors is shown (Supporting Information, Figure S3, black line). A strong diffraction peak around 10° in the XRD pattern is the characteristic of metal glycolates25,26,27,28. It has been assumed that EG would lose its two protons and the dianion complex with metal center25. Xia et al. has discussed the oligomerization process of metal glycolates. Longer chains of cobalt glycolate oligomers could self-assemble into ordered bundles (i.e., nanoplates) through van der Waals interactions and then precipitate out from the reaction medium25. After calcination, Co3O4 exposed mainly with (111) facets are obtained. However, the reason for the exposed (111) facets after calcination is not clear.
Co3O4 nanocubes and nanoplates modified glassy carbon electrodes (GCEs) are first electrochemically characterized by cyclic voltammetric (CV) and electrochemical impedance spectrum (EIS) (Supporting Information, Figure S5). As compared with the bare GCE, the anodic and cathodic peaks decrease at the Co3O4 modified electrode, which demonstrates that Co3O4 nanomaterials have been modified onto the surface of the GCE (Supporting Information, Figure S5a). It also indicates that the rate of electron transfer at the electrode surface is hindered with the attachment Co3O4 onto the GCE surface. In a typical Nyquist plot, the semicircle proton corresponding to the electron-transfer resistance (Ret) at higher frequency range while a linear part at lower frequency range represents the diffusion limited process3. The value corresponding to the bare GCE is about 27 Ω (Supporting Information, Figure S5b). And the value of Co3O4 nanocubes modified GCE (48 Ω) is comparable to that of Co3O4 nanoplates (50 Ω) (Supporting Information, Figure S5b). In this case, the surface area of the modified electrodes were calculated to be 0.0534 (nanoplates), 0.0519 (nanocubes), and 0.07065 (bare GCE) cm2. This may be due to the insulating property of Co3O4 nanostructures, as such, the direct contribution of microscopic surface area could be avoided, which is very helpful to understand that the difference of electrochemical behavior is indeed from the effect of crystal facet (as will be discussed in the following).
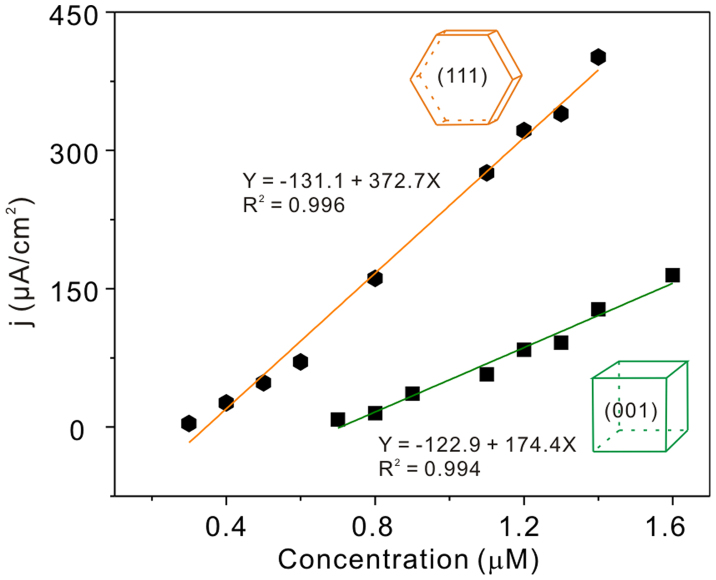
Among all of the HMIs, lead has been identified as one of the most toxic heavy metals because of its detrimental effects on the human nervous system, blood circulation system, kidneys, and reproductive system29. Subsequently, the performances of these two types of Co3O4 with different exposed facets in electrochemical detection of heavy metal ions are comprehensively investigated. Pb(II) is used as a probe heavy metal ion. We first examined the square wave stripping voltammograms (SWASV) responses to Pb(II) in 0.1 M NaAc-HAc (pH = 5.0) at Co3O4 nanoplates and nanocubes modified electrodes (Figure S6). There is almost no obvious response at bare GCE, and at the meanwhile, a weak stripping response for nanocubes/GCE is observed. However, a strong and well-defined peak at −0.584 V is clearly seen for nanoplates modified GCE. And the peak current obtained is about 8 times and 13 times that of nanocubes/GCE and bare GCE, respectively. Moreover, the stripping peak shifts toward more positive potential and become progressively less symmetrical. This is the consequence of having more ions on the electrode (this is fully consistent with that more ions are adsorbed by nanoplates and release to the bare GCE, see Discussion section), and therefore requiring a longer sweep to remove the metal from the surface entirely. Figure 2 shows that the current densities increase linearly versus the Pb(II) concentrations. For nanocubes/GCE, the linearization equation is j/(μA cm−2) = −122.9 + 174.4 c/μM. While for nanoplates/GCE, the linearization equation is j/(μA cm−2) = −131.1 + 372.7 c/μM. The results indicate that Co3O4 nanoplates with (111) facet exhibit better electrochemical detection performance than Co3O4 nanocubes with (001) facet. The sensitivity of Co3O4 nanoplates/GCE (372.7 μA cm−2/μM) is over 2 times that of Co3O4 nanocubes/GCE (174.4 μA cm−2/μM). The SWASV responses toward Pb(II) at various concentrations on Co3O4 nanoplates and nanocubes modified electrodes were seen in Figure S7. The limit of detection (LOD) as low as 0.12 nM and 0.16 nM (3σ method) for Co3O4 nanoplates and nanocubes, respectively was achieved. This meets the requirements of the World Health Organization (WHO) maximum permissible limit for lead concentration in drinking water of 10 μg L−1. The LODs are better than existing methods based on noble metal and ion-imprinted polymers, such as gold nanofilm (0.1 μM)30 and nano-sized Pb2+ imprinted polymer (0.6 nM)31, etc. The LODs are also superior to some oxides, such as tube-in-tube SnO2 (1.6 nM)32 and SnO2/graphene nanocomposites (0.18 nM)33.
Figure 2. Electrochemical properties of Co3O4 nanocrystals.
Calibration plots of Co3O4 nanoplates and Co3O4 nanocubes modified electrode toward Pb(II) at different concentrations in 0.1 M NaAc-HAc solution (pH = 5.0). Considering the weak response at nanocubes modified electrode, the initial concentration is from 0.7 μM.
Discussion
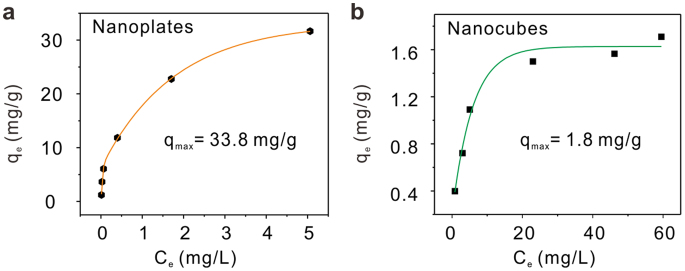
We suggest that the difference in electrochemical behavior may have a relationship with the adsorption capacities of different nanoscale Co3O4 toward Pb(II). To confirm this contribution, the adsorption measurement is conducted in 0.1 M NaAc-HAc (pH = 5.0) in order to be consistent with electrochemical sensing conditions. Co3O4 nanocubes and nanoplates are incubated in Pb(II) solutions with different initial concentrations for 24 h to reach the adsorption equilibrium. The adsorption isotherms are shown in Figure 3a and b. Both the adsorption isotherms fit the Langmuir isotherm very well (Supporting Information, Figure S8a and b). The maximum adsorption capacity (qmax) of Co3O4 nanoplates (33.8 mg g−1) is about 19 times that of Co3O4 nanocubes (1.8 mg g−1). In previous result we have shown that Co3O4 is non-conductive material (Supporting Information, Figure S5). We believe that the higher adsorption performance of Co3O4 nanoplates than that of Co3O4 nanocubes lead to its better performance in electrochemical detection. It is important to emphasize that as the adsorption experiments were done in solution with dispersed Co3O4, while the Co3O4 were just coated onto the surface of the GCE, the electrochemical sensitivities for two types of Co3O4 would be not proportional to the adsorption capacities of them.
Figure 3. Adsorption measurements.
Adsorption isotherms of Pb(II) onto (a), Co3O4 nanoplates and (b), Co3O4 nanocubes.
The above results and discussions experimentally show that Co3O4 nanocubes and nanoplates exhibit obviously different electrochemical sensing and adsorption performances. Co3O4 nanoplates with (111) planes exhibit larger sensitivity and adsorption capacity for heavy metal ions. Besides, we suggest that Pb(II) on the (111) facet are easier to diffuse onto GCE for reduction and stripping reaction than those on the (001) facet. Regarding the above surface area analysis of two kinds of Co3O4 (nanocubes > nanoplates), this further confirms that the differences in their electrochemical sensing performances are due to the facet effect.
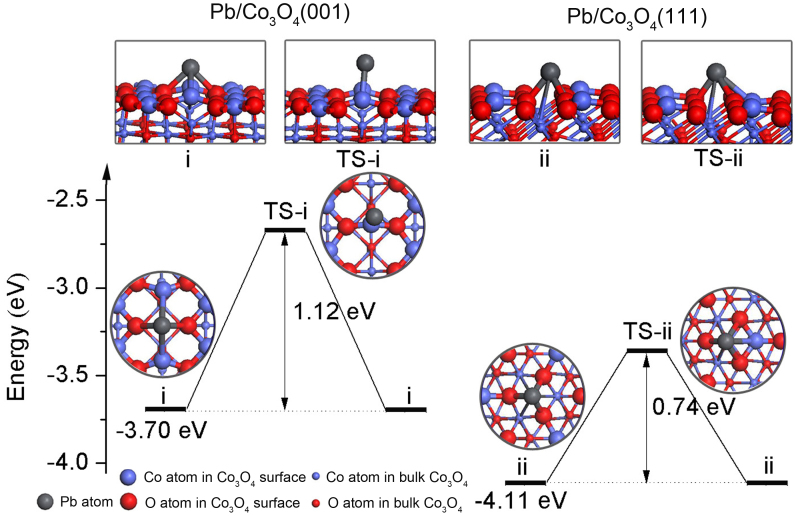
To better understand the effect of facet on electrochemical behavior of Pb(II) on Co3O4 nanoplates and nanocubes, we perform DFT calculations to explore the adsorption and diffusion behaviors of Pb on Co3O4(001) and (111) surfaces. The adopted computational models are similar to the recent report on Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer34. The adsorption configurations of Pb atom on Co3O4(001) and (111) surfaces are optimized without any symmetry constraint. In the most energetically stable configuration of Pb/Co3O4(001) system, the Pb atom is four-coordinated by two oxygen and two Co surface atoms of Co3O4(001) surface (Supporting Information, Figure S9), and the Pb-O and Pb-Co bond lengths are 2.40 and 2.27 Å, respectively. For Pb/Co3O4(111) adsorption system, the Pb atom locates at the three-fold hollow site (Supporting Information, Figure S10), and the Pb-O bond length is 2.27 Å. Two corresponding adsorption configurations of Pb atom on Co3O4(001) and (111) surfaces are illustrated in top panel of Figure 4 labeled with i and ii, respectively. The calculated adsorption energy of Pb atom on Co3O4(111) surface is −4.11 eV, which is larger than that on Co3O4(001) surface (−3.70 eV). The relative large adsorption energy for Pb/Co3O4(111) system mainly originates from the relative short Pb-O bond length. The Bader charge analysis35 (Supporting Information, Figure S11) shows that the adsorbed Pb atom carries positive +0.36 and +0.10 |e| (e, the electron charge) on Co3O4(111) and (001) surfaces, respectively, which gives the quantitative proof of the relative strong Pb-substrate interaction for Pb/Co3O4(111) system. Note that there are four equivalent stable adsorption sites in a Co3O4(001) − (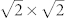 ) cell and Co3O4(111) − (1 × 1) cell (Supporting Information, Figure S9 and S10), but the area of the former cell is larger than that of the latter one. In addition, the adsorption energy for Pb atom locating at the second stable adsorption site (labeled with II′ symbol within twelve cyan circles in Supporting Information, Figure S10c) on Co3O4(111) surface is predicted to be −3.81 eV, which is also larger than that of the most stable adsorption configuration of Pb/Co3O4(001) system. On the other hand, we have examined the full-coverage of Pb on the stable adsorption sites. The adsorption energy of each Pb on Co3O4(001) and (111) surfaces is predicted to be −3.38 and −3.69 eV, respectively. It is clear that they are consistent with the calculated results of low-coverage of Pb on Co3O4 facets. Thus, the Pb atom adsorption ability on Co3O4(111) surface is significantly larger than that on Co3O4(001) surface. Moreover, the high adsorption energies and the large charge transfer suggest that the adsorbed Pb can form a strong Coulomb interaction with the Co3O4 surface.
) cell and Co3O4(111) − (1 × 1) cell (Supporting Information, Figure S9 and S10), but the area of the former cell is larger than that of the latter one. In addition, the adsorption energy for Pb atom locating at the second stable adsorption site (labeled with II′ symbol within twelve cyan circles in Supporting Information, Figure S10c) on Co3O4(111) surface is predicted to be −3.81 eV, which is also larger than that of the most stable adsorption configuration of Pb/Co3O4(001) system. On the other hand, we have examined the full-coverage of Pb on the stable adsorption sites. The adsorption energy of each Pb on Co3O4(001) and (111) surfaces is predicted to be −3.38 and −3.69 eV, respectively. It is clear that they are consistent with the calculated results of low-coverage of Pb on Co3O4 facets. Thus, the Pb atom adsorption ability on Co3O4(111) surface is significantly larger than that on Co3O4(001) surface. Moreover, the high adsorption energies and the large charge transfer suggest that the adsorbed Pb can form a strong Coulomb interaction with the Co3O4 surface.
Figure 4. DFT calculations.
Top panel: side view of the optimized stable adsorption and transition-state (TS) structures for Pb on Co3O4(001) and (111) surfaces. Bottom panel: the TS barriers of Pb on Co3O4(001) and (111) surfaces, and top view of these optimized stable adsorption and TS structures are also shown. After overcoming the TS barriers, Pb reaches the nearest neighboring stable adsorption site.
Now we turn to calculate the energy barriers of Pb diffusing on Co3O4(001) and (111) surfaces using the climbing image nudged elastic band (CI-NEB) method36. The Pb has two diffusion paths from the most stable adsorption site to its nearest neighboring stable site on Co3O4(001) surface, while there are six diffusion directions for Pb/Co3O4(111) adsorption system (Supporting Information, Figure S12). The geometric structures of the transition-states for Pb on Co3O4(001) and (111) surfaces are shown in Figure 4 labeled with TS-i and TS-ii, respectively. The calculated diffusion energy barrier of Pb on Co3O4(001) and (111) surfaces are predicted to be 1.12 and 0.74 eV, respectively. The relative low transition-state barrier results in the Pb fast diffusing on Co3O4(111) surface. These DFT results confirm that Co3O4(111) surface can capture more Pb than Co3O4(001) surface, and the adsorbed Pb diffuse more easily on the Co3O4(111) surface, consistent well with experimental observations.
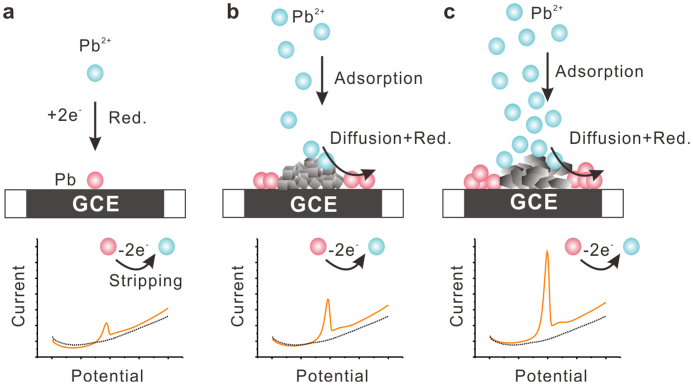
Based on the above experimental and DFT calculated results, a schematic illustration of how adsorptive nanoscale materials with different exposed crystal facets could be designed for electrochemical sensing interface is depicted in Figure 5. As for bare GCE, there are not nanoscale materials which can effectively capture HMIs and the weaker stripping peak is obtained. Large amount of HMIs could be adsorbed onto the surface of nanoscale materials and then diffuse to the GCE surface. The more target HMIs are adsorbed onto the surface of nanoscale material, the more HMIs diffuse to the GCE and the stronger the stripping peak current obtains as a result. As Co3O4 nanoplates exposed with (111) facet can adsorb more metal ions than Co3O4 nanocubes with (001) facet and the metal ions on the surface of (111) facet can diffuse more easily onto the GCE, Co3O4 nanoplates modified GCE obtain larger stripping peak current. In contrast to previous studies37,38,39,40,41 focusing on elevating the specific surface area of nanoscale materials in electrochemical sensing; our findings demonstrate that the construction of nanoscale materials onto electrode with well-defined exposed facets is crucial when considering the design of high-performance electrochemical sensor.
Figure 5. Schematics of how adsorptive nanoscale materials exposed with different crystal facets could be designed to enhance the performance of electrochemical sensing.
(a), Bare GCE. (b), Co3O4 nanocubes modified GCE. (c), Co3O4 nanoplates modified GCE. Facet-dependent electrochemical properties of Co3O4 nanocrystals toward heavy metal ions (e.g. Pb2+) are investigated. The (111)-bound Co3O4 nanoplates are superior to (001)-bound Co3O4 nanocubes. Adsorption measurements reveal that Co3O4 (111) facet can adsorb more metal ions than the (001) facet. DFT calculations demonstrate that Pb on Co3O4(111) facet exhibits larger adsorption energies, more adsorption sites, and faster diffusion than on (001) facet.
In summary, Co3O4 nanocubes and nanoplates have been successfully synthesized by facile methods by using different solvents. The predominantly exposed facets are (001) in the Co3O4 nanocubes and (111) in the Co3O4 nanoplates, respectively. Studies of their sensing properties revealed that the as-prepared Co3O4 nanocrystals exhibit interesting facet-dependent electrochemical behaviors toward HMIs based on their adsorption behaviors. The (111)-bound Co3O4 nanoplates are superior to (001)-bound Co3O4 nanocubes. Adsorption measurements revealed that Co3O4 (111) facets can adsorb more metal ions than the (001) facets. DFT calculations suggest that Co3O4(111) facets exhibit a relative larger adsorption energy, more adsorption sites, and a relative lower transition-state barrier than Co3O4(001) facets, which may be the predominant reason accounting for the facet-dependent electrochemical sensing behavior toward HMIs. Both adsorption experiments and DFT results agree well with the electrochemical sensing results. This study not only suggests a promising new strategy for designing high performance electrochemical sensing interface through the selective synthesis of nanoscale materials exposed with different well-defined facets, but also provides a deep understanding for a more sensitive and selective electroanalysis at nanomaterials modified electrodes.
Methods
Materials
All reagents were commercially available from Sinopharm Chemical Reagent Co., Ltd (China) with analytical grade. Stock solution used in electrochemical measurement of Pb(II) was prepared by dissolving Pb(NO3)2 in deionized water. 0.1 M acetate buffer (NaAc-HAc) solution of pH = 5.0 was prepared with NaAc and HAc. Ultrapure fresh water obtained from a Millipore water purification system (MilliQ, specific resistivity > 18 MΩ cm, S.A., Molsheim, France) are used in all runs.
Synthesis of Co3O4 nanocubes
0.25 g of Co(CH3COO)2·4H2O was loaded into a 23 mL poly(tetrafluoroethylene) (PTFE)-lined stainless steel autoclave, which was then filled with 18 mL water. The autoclave was sealed and maintained at 200°C for 12 h, and then cooled down to room temperature. The final products were centrifuged, rinsed with distilled water and ethanol for several times to remove any impurities.
Synthesis of Co3O4 nanoplates
0.25 g of Co(CH3COO)2·4H2O was loaded into a 23 mL poly(tetrafluoroethylene) (PTFE)-lined stainless steel autoclave, which was then filled with 18 mL ethylene glycol. The autoclave was sealed and maintained at 200°C for 12 h, and then cooled down to room temperature. The final products were centrifuged, rinsed with distilled water and ethanol several times to remove any impurities. The as-prepared precursors were finally calcined at 350°C in air for 3 h.
Characterization
The SEM images were taken by a FEI Quanta 200 FEG field emission scanning electron microscope. The TEM and HRTEM images analyses were carried out on a JEM-2010 microscope. XRD was performed on a D/MaxIIIA X-ray diffractometer (Rigaku Co., Japan), using Cu Kα (λKα1 = 1.5418 Å) as the radiation source. The nitrogen adsorption and desorption isotherms at 77 K were measured with a Micromeritics ASAP 2020 M analyzer. The Brunauer, Emmett, and Teller (BET) equation was used to obtain the specific surface areas. The Pb(II) concentrations were determined in the liquid phase using inductively coupled plasma atomic emission spectrometry (ICP-AES, Jarrell-Ash model ICAP 9000). Electrochemical experiments were recorded using a CHI 660D computer-controlled potentiostat (ChenHua Instruments Co., Shanghai, China). A conventional three-electrode system consisted of a glassy carbon working electrode (GCE, 3 mm diameter), an Ag/AgCl as the reference electrode and a platinum wire as the counter electrode.
Fabrication of modified electrode
2 mg of the as-prepared Co3O4 nanocubes and nanoplates were dispersed in 4 mL water with ultrasonic agitation to give a homogeneous solution. Prior to each modification, the bare GCE was sequentially polished with 1.0 μm and 0.05 μm alumina power slurries to a mirror shiny surface, and then sonicated with 1:1 HNO3 solution, absolute ethanol and deionized water. The construction of Co3O4 on the surface of GCE was performed as follows: 5.0 μL of Co3O4 solution dripped onto the surface of a freshly polished GCE and then evaporating it at room temperature in the air.
Electrochemical measurements
The electrochemical measurement was carried out in SWASV mode for Pb(II) detection in 0.1 M NaAc-HAc solution. A deposition potential of −1.2 V was applied for 180 s to the working electrode under stirring. The SWASV responses were recorded between −1.0 to −0.2 V with step potential of 5 mV, amplitude of 20 mV, and frequency of 25 Hz. A desorption potential of 0 V for 210 s was performed to remove the residual metals under stirring condition.
Adsorption experiments
Experiments were carried out at 298 K in 10 mL polyethylene centrifuge tubes containing 1 g·L−1 adsorbent and various concentrations of Pb(II) for 24 h. The pH values of all these Pb(II) solutions 5.0 ± 0.2. After adsorption equilibrium, the adsorbent was separated by centrifugation. The Pb(II) concentrations remaining in the solution were analyzed. The amount of metal adsorbed (qe) was calculated according to the following equation:
where 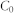 and
and 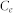 represent the initial and equilibrium Pb(II) concentrations (mg L−1), respectively, V is the volume of the solutions (mL), and m is the amount (mg) of adsorbent. A Langmuir isotherms model was used to analyze the experimental data. The mathematical expressions of the Langmuir isotherm is
represent the initial and equilibrium Pb(II) concentrations (mg L−1), respectively, V is the volume of the solutions (mL), and m is the amount (mg) of adsorbent. A Langmuir isotherms model was used to analyze the experimental data. The mathematical expressions of the Langmuir isotherm is
where 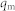 and
and 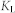 are Langmuir constants, representing the maximum adsorption capacity of adsorbents (mg/g) and the energy of adsorption, respectively. The values of
are Langmuir constants, representing the maximum adsorption capacity of adsorbents (mg/g) and the energy of adsorption, respectively. The values of 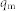 and
and 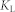 can be calculated from the slope and intercept of plots of
can be calculated from the slope and intercept of plots of 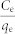 versus
versus 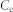 .
.
Computational methods
The DFT calculations were performed by using the Vienna ab-initio simulation package (VASP)42,43 with the Perdew-Burke-Ernzerh parameterization of the generalized gradient approximation (GGA) adopted for the exchange correlation potential44. An energy cut of 400 eV were consistently used in our calculations. The atomic positions are fully relaxed with the conjugate gradient procedure until the residual forces vanished within 0.02 eV/Å. A 2 × 2 × 1 Monkhorst-Pack k-point was used to sample the surface Brillouin zone. Because Co3O4 is a strongly correlated antiferromagnet and Pb ion is one of HMIs, we also performed test DFT + U45,46 (U = 3.3 eV and J = 0 eV according to previous reports47) calculations as well as PBE calculations with relativistic corrections48 to Pb. As shown in Table S1, we can confirm that our PBE results are qualitatively correct. The transition states were obtained by using the climbing image nudged elastic band (CI-NEB) method36.
Computational models
The optimized lattice constant (a0) of the bulk Co3O4 is 8.12 Å, which is close to the experimental value (8.08 Å)47. The bulk Co3O4 shows antiferromagnetic properties. The 1 × 1 × 10 and 1 × 1 × 11 supercells were used to model the Co3O4 (001) and (111) surfaces, respectively, in which a 12 Å of vacuum is adopted. During the structural optimizations, we allowed the atoms in the top four layers in the supercells to relax fully, and fixed all other atoms in the bulk configuration. The side and top views of the optimized Co3O4 (001) and (111) surfaces are shown in Figures S9 and S10, respectively. These optimized geometric structures agree well with the previous reports on the surface atoms arrangement of the Co3O4 nanocrystals with different crystal planes10,47,49.
Adsorption energy
To compare the Pb adsorption ability on two different crystal planes, we define the Pb adsorption energy as Eads = EPb/surface − (Esurface + EPb). Here, Esurface and EPb/surface are the total energies of the surface and the Pb atom adsorbing on surface, respectively, and EPb is the atomic energy of single isolated Pb atom. Under this definition, the more negative value stands for the more energetically stable adsorption.
Bader charge analysis and diffusion paths
To address the Pb-substrate interaction, we conduct the Bader charge analysis for Pb atom adsorbing on Co3O4 (111) and (001) surfaces. The corresponding results are presented in Figure S11. For clarity, the diffusion directions of Pb on Co3O4 (111) and (001) surfaces are illustrated in Figure S12.
Author Contributions
X.J.H. conceived the electrochemical studies of nanocrystals with different facets. Q.X.L. designed the calculations. X.Y.Y. carried out all the experiments and Q.Q.M. performed calculation and contributed equally to this work. X.Y.Y. and Q.Q.M. co-wrote the paper. T.L., Y.J., B.S., and J.H.L. contributed data analysis and interpretation equally.
Supplementary Material
Supplmentary Information
Acknowledgments
This work was supported by the National Basic Research Program of China (2011CB933700, 2011CB921404, and 2014CB921101), the Natural Science Foundation of China (21073197, 21103198, 11205204, 11074235, and 11034006), and the China Postdoctoral Science Foundation (20110490386 and 2011M501073). X.J. H. acknowledges the One Hundred Person Project of the Chinese Academy of Sciences, China, for financial support.
References
- Wu Z. C. et al. Synthesis of mesoporous NiO nanosheet and its application on mercury(II) sensor. J. Solid State Electrochem. 16, 3171–3177 (2012). [Google Scholar]
- Wu Z. et al. Synthesis of folding flake-like CuO sub-microstructure and its application on mercury(II) sensor. J. Mater. Sci.: Mater. Electron. 23, 858–864 (2011). [Google Scholar]
- Wei Y. et al. High adsorptive γ-AlOOH(boehmite)@SiO2/Fe3O4 porous magnetic microspheres for detection of toxic metal ions in drinking water. Chem. Commun. 47, 11062–11064 (2011). [DOI] [PubMed] [Google Scholar]
- Yin Z. J., Wu J. J. & Yang Z. S. A sensitive mercury(II) sensor based on CuO nanoshuttles/poly(thionine) modified glassy carbon electrode. Microchim. Acta 170, 307–312 (2010). [Google Scholar]
- Wei Y. et al. Stripping voltammetry study of ultra-trace toxic metal ions on highly selectively adsorptive porous magnesium oxide nanoflowers. Analyst 137, 2183–2191 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y. et al. Syntheses and properties of micro/nanostructured crystallites with high-energy surfaces. Adv. Funct. Mater. 20, 3634–3645 (2010). [Google Scholar]
- Tao A. R., Habas S. & Yang P. D. Shape control of colloidal metal nanocrystals. Small 4, 310–325 (2008). [Google Scholar]
- Jiang L. et al. Synthesis of fivefold stellate polyhedral gold nanoparticles with {110}-facets via a seed-mediated growth method. Small 9, 705–710 (2013). [DOI] [PubMed] [Google Scholar]
- Zhou K. B. & Li Y. D. Catalysis based on nanocrystals with well-defined facets. Angew. Chem. Int. Ed. 51, 602–613 (2012). [DOI] [PubMed] [Google Scholar]
- Hu L. H., Peng Q. & Li Y. D. Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J. Am. Chem. Soc. 130, 16136–16137 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Controllable synthesis of Co3O4 from nanosize to microsize with large-scale exposure of active crystal planes and their excellent rate capability in supercapacitors based on the crystal plane effect. Nano Res. 4, 695–704 (2011). [Google Scholar]
- Xiao X. et al. Facile shape control of Co3O4 and the effect of the crystal plane on electrochemical performance. Adv. Mater. 24, 5762–5766 (2012). [DOI] [PubMed] [Google Scholar]
- Huang W. C. et al. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 134, 1261–1267 (2012). [DOI] [PubMed] [Google Scholar]
- Jin M. S. et al. Shape-controlled synthesis of copper nanocrystals in an aqueous solution with glucose as a reducing agent and hexadecylamine as a capping agent. Angew. Chem. Int. Ed. 50, 10560–10564 (2011). [DOI] [PubMed] [Google Scholar]
- Jin M. S. et al. Palladium concave nanocubes with high-index facets and their enhanced catalytic properties. Angew. Chem. Int. Ed. 50, 7850–7854 (2011). [DOI] [PubMed] [Google Scholar]
- Jun Y. W., Choi J. S. & Cheon J. Shape control of semiconductor and metal oxide nanocrystals through nonhydrolytic colloidal routes. Angew. Chem. Int. Ed. 45, 3414–3439 (2006). [DOI] [PubMed] [Google Scholar]
- Kim D. et al. Convex polyhedral Au@Pd core-shell nanocrystals with high-index facets. Angew. Chem. Int. Ed. 51, 159–163 (2012). [DOI] [PubMed] [Google Scholar]
- Kuo C. H. et al. Facet-dependent and Au nanocrystal-enhanced electrical and photocatalytic properties of Au-Cu2O core-shell heterostructures. J. Am. Chem. Soc. 133, 1052–1057 (2011). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Well shaped Mn3O4 nano-octahedra with anomalous magnetic behavior and enhanced photodecomposition properties. Small 7, 475–483 (2011). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Facet-dependent photocatalytic properties of AgBr nanocrystals. Small 8, 2802–2806 (2012). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Controlled synthesis and enhanced catalytic and gas-sensing properties of tin dioxide nanoparticles with exposed high-energy facets. Chem.- Eur. J. 18, 2283–2289 (2012). [DOI] [PubMed] [Google Scholar]
- Wu X. et al. Nanosized anatase TiO2 single crystals with tunable exposed (001) facets for enhanced energy conversion efficiency of dye-sensitized solar cells. Adv. Funct. Mater. 21, 4167–4172 (2011). [Google Scholar]
- Zheng Z. K. et al. Hierarchical TiO2 microspheres: Synergetic effect of {001} and {101} facets for enhanced photocatalytic activity. Chem.- Eur. J. 17, 15032–15038 (2011). [DOI] [PubMed] [Google Scholar]
- Zhou X. M. et al. Facet-mediated photodegradation of organic dye over hematite architectures by visible light. Angew. Chem. Int. Ed. 51, 178–182 (2012). [DOI] [PubMed] [Google Scholar]
- Jiang X. C. et al. Ethylene glycol-mediated synthesis of metal oxide nanowires. J. Mater. Chem. 14, 695–703 (2004). [Google Scholar]
- Zhong L. S. et al. 3D flowerlike ceria micro/nanocomposite structure and its application for water treatment and CO removal. Chem. Mater. 19, 1648–1655 (2007). [Google Scholar]
- Chakroune N. et al. Synthesis, characterization and magnetic properties of disk-shaped particles of a cobalt alkoxide: CoII(C2H4O2). New J. Chem. 29, 355–361 (2005). [Google Scholar]
- Larcher D. et al. Some insights on the use of polyols-based metal alkoxides powders as precursors for tailored metal-oxides particles. Chem. Mater. 15, 3543–3551 (2003). [Google Scholar]
- Yu X. Y. et al. Adsorption of lead(II) on O2-plasma-oxidized multiwalled carbon nanotubes: Thermodynamics, kinetics, and desorption. ACS Appl. Mater. Interfaces 3, 2585–2593 (2011). [DOI] [PubMed] [Google Scholar]
- Zhao W. et al. Catalytic deposition of Pb on regenerated gold nanofilm surface and its application in selective determination of Pb2+. Langmuir 23, 8597–8601 (2007). [DOI] [PubMed] [Google Scholar]
- Alizadeh T. & Amjadi S. Preparation of nano-sized Pb2+ imprinted polymer and its application as the chemical interface of an electrochemical sensor for toxic lead determination in different real samples. J. Hazard. Mater. 190, 451–459 (2011). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. SnO2 tube-in-tube nanostructures: Cu@C nanocable templated synthesis and their mutual interferences between heavy metal ions revealed by stripping voltammetry. Small 9, 2233–2239 (2013). [DOI] [PubMed] [Google Scholar]
- Wei Y. et al. SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II): An interesting favorable mutual interference. J. Phys. Chem. C 116, 1034–1041 (2012). [Google Scholar]
- Tang Q., Zhou Z. & Shen P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 134, 16909–16916 (2012). [DOI] [PubMed] [Google Scholar]
- Sanville E. et al. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 28, 899–908 (2007). [DOI] [PubMed] [Google Scholar]
- Henkelman G., Uberuaga B. P. & Jonsson H. J. Chem. Phys. 113, 9901 (2000). [Google Scholar]
- Hou C. et al. Metal-organic framework templated synthesis of Co3O4 nanoparticles for direct glucose and H2O2 detection. Analyst 137, 5803–5808 (2012). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. Facile synthesis of hierarchically porous Co3O4 nanowire arrays with enhanced electrochemical catalysis. Electrochem. Commun. 25, 119–123 (2012). [Google Scholar]
- Li J. et al. High-sensitivity determination of lead and cadmium based on the Nafion-graphene composite film. Anal. Chim. Acta 649, 196–201 (2009). [DOI] [PubMed] [Google Scholar]
- Ye D. X. et al. Fabrication of Co3O4 nanoparticles-decorated graphene composite for determination of L-tryptophan. Analyst 137, 2840–2845 (2012). [DOI] [PubMed] [Google Scholar]
- Li Y. G., Tan B. & Wu Y. Y. Freestanding mesoporous quasi-single-crystalline Co3O4 nanowire arrays. J. Am. Chem. Soc. 128, 14258–14259 (2006). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993). [DOI] [PubMed] [Google Scholar]
- Blochl P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- Anisimov V. V., Zaanen J. & Andersen O. K. Band theory and Mott insulators: Hubbard U instead of Stoner I. Phys. Rev. B 44, 943–954 (1991). [DOI] [PubMed] [Google Scholar]
- Anisimov V. I., Aryasetiawan F. & Lichtenstein A. I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: the LDA + U method. J. Phys.: Condens. Matter 9, 767–808 (1997). [Google Scholar]
- Montoya A. & Haynes B. S. Periodic density functional study of Co3O4 surfaces. Chem. Phys. Lett. 502, 63–68 (2011). [Google Scholar]
- Grabowski B., Hickel T. & Neugebauer J. Ab initio study of the thermodynamic properties of nonmagnetic elementary fcc metals: Exchange-correlation-related error bars and chemical trends. Phys. Rev. B 76 (2007). [Google Scholar]
- Xu X.-L. et al. Bulk and surface properties of spinel Co3O4 by density functional calculations. Surf. Sci. 603, 653–658 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplmentary Information