Abstract
Primary open-angle glaucoma (POAG) is an optic neuropathy that has a high worldwide prevalence and that shows strong evidence of complex inheritance. The myocilin (MYOC) gene is the only one that has thus far been shown to have mutations in patients with POAG. Apolipoprotein E (APOE) plays an essential role in lipid metabolism, and the APOE gene has been involved in neuronal degeneration that occurs in Alzheimer disease (AD). Here, we report that two APOE-promoter single-nucleotide polymorphisms (SNPs) previously associated with AD also modify the POAG phenotype. APOE(−219G) is associated with increased optic nerve damage, as reflected by increased cup:disk ratio and visual field alteration. In addition, APOE(−491T), interacting at a highly significant level with an SNP in the MYOC promoter, MYOC(−1000G), is associated with increased intraocular pressure (IOP) and with limited effectiveness of IOP-lowering treatments in patients with POAG. Together, these findings establish APOE as a potent modifier for POAG, which could explain the linkage to chromosome 19q previously observed by use of a genome scan for this condition and an increased frequency of glaucoma in patients with AD. The findings also shed new light on potential mechanisms of optic nerve damage and of IOP regulation in POAG.
Primary open-angle glaucoma (POAG [MIM 137760]) is an optic neuropathy that affects 70 million people worldwide and is defined by cupping of the optic nerve head and irreversible loss of retinal ganglion cells (Quigley 1993). It progresses insidiously and may lead to severe visual impairment in some individuals. Elevation of intraocular pressure (IOP) is recognized as a major risk factor for damage to the optic nerve and for visual field loss in POAG (Anderson 1989). The identification of the TIGR (trabecular meshwork–inducible glucocorticoid response) protein, also called “myocilin” (MYOC [MIM 601652]), has provided the first and, until now, the sole genetic basis for study of the molecular mechanisms that underlie IOP elevation (Adam et al. 1997; Polansky et al. 1997; Stone et al. 1997; Nguyen et al. 1998). TIGR protein and MYOC gene expression are also abundantly up-regulated in the trabecular meshwork in response to steroids and oxidative stress (Polansky et al. 1997, 2000; Tamm et al. 1999). Infusion of recombinant MYOC protein in the anterior chamber of human eyes in organ culture increases outflow resistance and IOP (Fautsch et al. 2000). The factors regulating the protein expression, in addition to genetic alteration in its structure, may therefore be important for understanding the molecular mechanisms related to the increased outflow resistance in the trabecular meshwork that accounts for IOP elevation.
It is well recognized that several still-to-be-determined genes are likely to play substantial roles, alone or through gene interactions, in both IOP elevation and susceptibility to visual field loss. Among the unknown factors are those that influence the physiological and the pathological processes at the posterior segment of the eye (Drance 1997; Martinez-Bello et al. 2000). In particular, genes influencing the retinal ganglion cell layer or the optic nerve head are of interest because of their potential ability to modulate predisposition to and progression of POAG. The molecular nature of factors that, alone and together, may modulate the glaucoma phenotype are of considerable research and clinical interest.
The findings reported here implicate apolipoprotein E (APOE [MIM 107741]), an essential protein in lipid transport (notably in the metabolism of neuronal membrane [Mahley and Huang 1999]), as influencing the glaucoma phenotype. APOE is up-regulated in response to oxidative stress and is endowed with antioxidant properties (Miyata and Smith 1996). Allelic forms of APOE, including APOE protein alleles and promoter single-nucleotide polymorphisms (SNPs), have been associated with elevated plasma levels of cholesterol, increased risk of myocardial infarction (Mahley and Huang 1999; Lambert et al. 2000), and predisposition to Alzheimer disease (Roses 1996; Bullido et al. 1998; Lambert et al. 1998). Although the mechanisms by which APOE participates in different pathogenic processes remain to be elucidated, APOE seems to be key to neuronal degeneration and, beyond this, appears to be involved in stress-induced injury that could affect many tissues and organs.
To investigate a possible association of APOE polymorphisms with POAG, we identified a group of 191 unrelated white patients with POAG through retrospective chart review, according to a protocol described elsewhere (Colomb et al. 2001). POAG was defined by the conjunction of a characteristic cupping of the optic disk, an open iridocorneal angle (grade III or IV gonioscopy), and an alteration of the visual field, which was tested by automated perimetry (with Humphrey’s perimeter or Octopus). IOP was measured by applanation tonometry. Cup:disk ratios were assessed by individual clinicians. Patients with cataract or media opacities and those with factors causally associated with secondary glaucoma (including exfoliation, pigment dispersion, history of trauma, surgery, and glucocorticoid exposure) were excluded. Final POAG diagnosis was made at the time of inclusion, after review of inclusion and exclusion criteria. Diagnosis and other classifications were made substantially before the genotyping was conducted. All patients had been checked for the absence of the MYOC coding region mutations—including G246R, Q368X, P370L, I477S, N480K, I499F, and R272G—that have been described elsewhere as being present in the French population (Adam et al. 1997). The presence of a mutation or of an unusual variant in patients carrying the MYOC.mt1 marker (Colomb et al. 2001) was also ruled out by direct sequencing of MYOC exon III, in which the great majority of POAG-causing mutations were mapped.
Three informative SNPs in the APOE promoter, at positions −491, −427, and −219, were investigated, along with the APOE protein alleles, ε2, ε3, and ε4 (Lai et al. 1998). As shown in table 1, the genotype frequencies of the three SNPs were similar in patients with POAG and in 102 control subjects, who were unaffected spouses from families with MYOC-linked glaucoma. Likewise, protein allele frequencies were similar in both groups (data not shown). These frequencies were also similar to those reported in a larger French control group (Lambert et al. 2000).
Table 1.
Frequencies of APOE-Promoter Genotypes
| APOE-Promoter SNP | No. (%) of Patients(n = 191) | No. (%) of Control Subjects(n = 102) |
| −491: | ||
| AA | 129 (67.5) | 70 (68.6) |
| AT | 58 (30.4) | 28 (27.4) |
| TT | 4 (2.1) | 4 (3.9) |
| −427: | ||
| CC | 165 (86.4) | 84 (82.4) |
| TC | 25 (13.1) | 17 (16.7) |
| TT | 1 (.5) | 1 (.98) |
| −219: | ||
| GG | 57 (29.9) | 38 (37.2) |
| GT | 105 (54.9) | 45 (44.1) |
| TT | 29 (15.2) | 19 (18.6) |
We then evaluated a potential effect of these APOE polymorphisms on the principal clinical parameters of POAG. IOP measurements at diagnosis and at the time of inclusion in the study were considered; cup:disk ratios and visual field scores were used as indices of optic nerve alteration. Values for the IOPs, the age at diagnosis, the age at inclusion, and the length of observation (period between age at inclusion and age at diagnosis), were normally distributed, as verified with the D statistic of Kolmogorov-Smirnov and with Shapiro-Wilk’s W test. The means of these values in the various genotypic groups were compared using Student t test or one-way analysis of variance (ANOVA). If variance heterogeneity was detected through the use of Levene’s test, then the Student t test was performed after a separate estimate of variances. To combine data from different quantitative measurements of visual fields, we employed a semiquantitative five-point scale, as described elsewhere (Brézin et al. 1997). Visual field evaluations and assignments were made prior to genotyping. Visual field scores and cup:disk ratios were compared using nonparametric procedures. Only two-sided P values <.05 were reported.
As seen in table 2, the APOE(−219) SNP influenced both the visual field score (P=.003) and the cup:disk ratio (P=.04). The means of these two variables were similar in patients carrying one or two G alleles and were higher than those in TT homozygotes. When GG and GT patients were grouped, the fit of the data (P=.0012 for visual field score and P=.015 for cup:disk ratio) improved. The TT genotype was reported elsewhere to be associated with decreased plasma levels of APOE and with an increased risk of myocardial infarction (Lambert et al. 2000). To our knowledge, the present association suggests, for the first time, that visual field and cup:disk ratio—two parameters that are commonly monitored to assess optic nerve damage in patients with POAG—might be specifically controlled, independently of IOP, by a genetic factor. It is relevant to these observations that APOE is known to be expressed in Müller cells, whose presence separates neural tissue from retinal vasculature in the posterior part of the eye (Shanmugaratnam et al. 1997; Klaver et al. 1998). Müller cell processes envelop axons and ganglion cell bodies and are thought to play an essential role in the maintenance of the microenvironment of optic nerve fibers. At the level of the optic nerve head, an essential anatomical site in POAG pathogenesis, Müller cells are replaced by astrocytes, a cell type in which expression of APOE has been well documented (Poirier 1994; Baskin et al. 1997). Of additional interest to pathological processes at the back of the eye, the E4 allele of APOE protein has been associated with an increased risk of age-related macular degeneration (Klaver et al. 1998; Souied et al. 1998).
Table 2.
Association of APOE-Promoter SNPs with POAG Phenotypes
| APOE(−219) |
APOE(−491) |
||||
| Variable | GG | GT | TT | AA | AT or TT |
| Age at inclusion (years) | 59.4 ± 15.6 (56)a | 57.2 ± 15.8 (105) | 58.8 ± 14.8 (28) | 56.9 ± 15.6 (127) | 60.6 ± 15.2 (62) |
| Age at diagnosis (years) | 48.1 ± 12.6 (54) | 44.5 ± 15.4 (100) | 45.4 ± 14.7 (27) | 45 ± 13.6 (123) | 47.2 ± 16.5 (58) |
| Length of observation (years)b | 12.9 ± 7.2 (53) | 12.8 ± 8.4 (100) | 13.4 ± 11.1 (27) | 12.6 ± 8.2 (122) | 13.6 ± 9 (58) |
| IOP at diagnosis (mmHg) | 30.9 ± 7.5 (48) | 33.3 ± 10.4 (86) | 33 ± 9.6 (21) | 31.3 ± 9 (112) | 35.6 ± 10.1 (43)c |
| IOP at inclusion (mmHg) | 19.9 ± 5.8 (56) | 19.3 ± 5.9 (97) | 20.9 ± 5.1 (28) | 19.6 ± 5.4 (125) | 20 ± 6.6 (56) |
| Visual field scored | 3.2 ± .8 (52) | 3.3 ± 1.1 (93) | 2.6 ± .9 (24)e | 3.2 ± 1 (118) | 3.2 ± 1 (51) |
| Cup:disk ratio (×10) | 7.6 ± 1.8 (51) | 7.3 ± 2.1 (86) | 6.6 ± 1.7 (26)f | 7.3 ± 2.1 (112) | 7.3 ± 1.7 (51) |
Mean ± SD (number of patients).
Time between diagnosis and inclusion in the study.
P=.01 with Student t test.
Data obtained with different perimeters were combined using a five-point scale, defined as follows : 1 = no alteration; 2 = early defect; 3 = arcuate defect; 4 = advanced scotoma; 5 = light perception only or no vision (Brézin et al. 1997).
P=.003 for the comparison of the three genotypic groups by use of Kruskal-Wallis ANOVA; P=.0012 for the comparison of GG + GT versus TT for Mann-Whitney U test.
P=.04 for the comparison of the three genotypic groups by use of Kruskal-Wallis ANOVA; P=.015 for the comparison of GG + GT versus TT by use of the Mann-Whitney U test.
The APOE(−427) SNP and the ε2/ε3/ε4 allele system did not affect the clinical parameters listed in table 2 (data not shown), despite linkage disequilibrium (Fullerton et al. 2000; Martin et al. 2000; Nickerson et al. 2000). However, the APOE(−491T) allele was associated with a higher IOP at diagnosis (P=.01) (table 2). This appeared noteworthy, because we have reported elsewhere that an allele of an SNP in the promoter of the MYOC gene (MYOC −1000G, also designated as MYOC.mt1) was associated with higher IOP at inclusion in the study and with a lack of IOP lowering between diagnosis and the time of the study (Colomb et al. 2001). We therefore reevaluated the effect of MYOC.mt1 on individual changes in IOP during the period from diagnosis to inclusion in the study, also taking into account the influence of APOE(−491T). This analysis was performed with a three-factor ANOVA, including a repeated-measures factor (“within-subject” variation of IOP, which is also described as “individual change in IOP” [ΔIOP]), and MYOC.mt1 and APOE(−491T) as independent (“between-groups”) factors. IOPs at diagnosis and at inclusion in the study were not correlated, and ΔIOP was normally distributed. In the whole group of patients, as expected, the between-subjects variation of IOP was highly significant (P=1×10-12), reflecting overall efficient IOP lowering in treated patients. The significance level of the effect of APOE(−491T) was increased (P=.0004). As we reported elsewhere, the effect of MYOC.mt1 was also significant (P=.001) (Colomb et al. 2001). Moreover, there was an interaction between both genetic markers (P=.012).
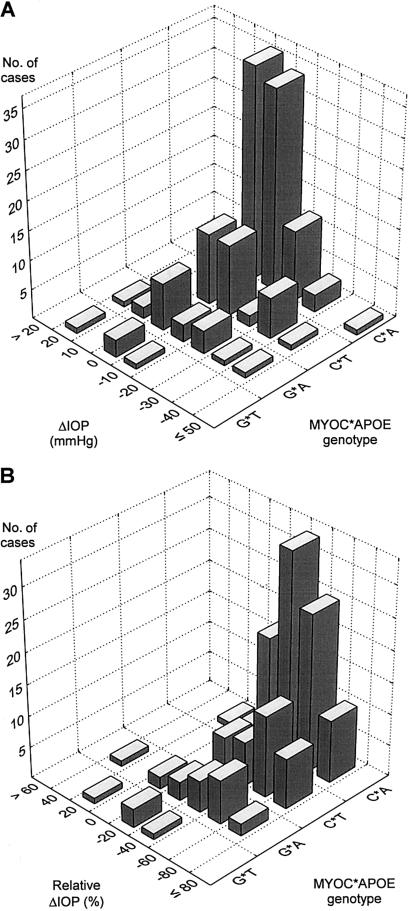
Figure 1 shows the frequency histograms of the distributions of ΔIOP and relative ΔIOP (i.e., ΔIOP divided by IOP at diagnosis), grouped according to the four SNP combinations. Table 3 shows the means and standard deviations in the same genetic groups for these two variables and also for the IOPs at diagnosis and at inclusion in the study. The individual changes in IOP were significant in all patient groups, except in those that were positive for both MYOC.mt1 and APOE(−491T), as shown by post hoc tests and also by the nonparametric Wilcoxon matched-pairs test (table 3). In other words, the treatment protocols used between diagnosis and the time of the study failed to decrease IOP significantly in patients who carried both MYOC.mt1 and APOE(−491T). By several criteria, the patients who carried both MYOC.mt1 and APOE(−491T) did not differ from the other patients included in the study. In particular, their age at diagnosis and the time between the diagnosis and the inclusion in the study were not different. The patients had received an appropriate treatment, including a topical β-blocker and a cholinomimetic agent, an inhibitor of carbonic anhydrase (two patients), and surgery (two patients). Therefore, it does not seem that the patients had been undertreated. Their IOP levels, however, remained well above 20 mmHg. In contrast, and as reported elsewhere (Colomb et al. 2001), the decrease of IOP in patients who did not carry the MYOC.mt1 polymorphism was very significant, and the decline in IOP was >30%, resulting in mean levels <20 mmHg. Subjects who carried MYOC.mt1 but not APOE(−491T) had an intermediate status. Their final IOP level was, on average, >20 mmHg, and their relative decrease in IOP was less pronounced than that of patients who were negative for MYOC.mt1 (P=.04). Because the magnitude of ΔIOP is correlated with the IOP levels, the effect of genetic markers on ΔIOP and on relative ΔIOP was reanalyzed, introducing the IOP at diagnosis, or alternatively, the mean of IOPs at diagnosis and at inclusion in the study, as covariates. As shown in table 4, this analysis of covariance considerably increased the power to detect the effects of MYOC.mt1 and APOE(−491T). The interaction term also became highly significant.
Figure 1.
Absolute and relative IOP changes in patients with POAG, depending on MYOC(−1000C/G) and APOE(−491A/T) SNPs. A, Bivariate distribution histograms of ΔIOP. B, Bivariate distribution histograms of relative ΔIOP in the four genotypic groups defined by the presence or absence of MYOC(−1000G) and APOE(−491T) alleles.
Table 3.
Evolution of IOP Depending on MYOC.mt1 and on APOE(−491T)
| MYOC.mt1/APOE(−491T) | N | Mean IOP atDiagnosis ± SD(mmHg) | Mean IOP atInclusion ± SD(mmHg) | ΔIOPa ± SD(mmHg) | Relative ΔIOPb ± SD(%) | Pc | Pd |
| −/− | 89 | 31.4 ± 8.9 | 19.4 ± 4.6 | −12 ± 9.8 | −34.3 ± 21 | 3 × 10−5 | <1 × 10−12 |
| −/+ | 34 | 35.1 ± 10.2 | 18.7 ± 4.6 | −16.4 ± 11.5 | −42.5 ± 20.2 | 3 × 10−5 | 1 × 10−6 |
| +/− | 20 | 32 ± 10.1 | 21 ± 7.2 | −10.9 ± 13.5 | −27.9 ± 31.4 | 3 × 10−4 | 3 × 10−3 |
| +/+ | 5 | 35.4 ± 7.1 | 35.2 ± 7.9 | −.2 ± 8.2 | −.7 ± 21.7 | NS | NS |
ΔIOP between inclusion in the study and the diagnosis.
ΔIOP divided by IOP at diagnosis.
Post hoc comparison between IOP at diagnosis and IOP at inclusion in the study, with Tukey honest significant difference test, after three-factor ANOVA.
Wilcoxon matched-pairs test.
Table 4.
Significance Levels from Analysis of Covariance and Interaction of MYOC.mt1 and APOE(−491T)[Note]
| ΔIOP |
Relative ΔIOP |
|||||
| Genetic Factor | None | IOP at Diagnosis | Mean IOP | None | IOP at Diagnosis | Mean IOP |
| MYOC.mt1 | .003 | 1×10−9 | 2×10−7 | 7 ×10−5 | 7 ×10−8 | 2×10−6 |
| APOE(−491T) | NS | 4×10−6 | 9×10−4 | NS | 5 ×10−4 | 1×10−2 |
| Interaction term | .01 | 4×10−7 | 1×10−5 | .003 | 9 ×10−5 | 3×10−4 |
Note.— A two-way analysis of variance of individual IOP changes (ΔIOP or IOP at inclusion in the study minus IOP at diagnosis) or of relative IOP changes (ΔIOP variation divided by either IOP at diagnosis or initial IOP) was performed either directly or by introduction, as covariate, of either IOP at diagnosis or mean IOP.
These findings extend and refine our previous observation of an association of MYOC.mt1 with resistance to IOP control, and they strengthen the idea that this resistance probably has a genetic basis. At present, it cannot be determined whether poor IOP control reflects an intrinsic evolution of the pathogenic process or a failure of the treatments, which primarily included topical β-blockers, with or without cholinomimetic agents. Knowledge that a patient is likely to show resistance to IOP-lowering treatment should nevertheless improve management of his or her disease.
As mentioned above, IOP elevation does not fully explain glaucomatous optic neuropathy. In this regard, our initial observation of the APOE-promoter variant deserves final emphasis. The individual effect of APOE(−219) SNP on optic nerve damage parameters potentially provides a first genetic basis for the lack of good correlation between IOP level and optic nerve damage in patients with POAG and in subjects with ocular hypertension (Quigley et al. 1994; Drance 1997; Martinez-Bello et al. 2000). At the molecular level, both APOE(−219) and (−491) SNP, but not APOE(−427), were shown to alter transcriptional activity of the APOE gene, using promoter activity and electrophoretic mobility–shift assays (Artiga et al. 1998). A quantitative analysis of APOE gene expression in the eye tissue of patients with POAG would be helpful, in much the same way as studies of APOE expression in the brains of patients with Alzheimer disease (Lambert et al. 1998). Our present observation lends support to the hypothesis that transcriptional regulation of APOE expression plays an important role in pathogenesis, independently of allelic forms of APOE protein (Theuns and van Broeckhoven 2000). What leads to a different effect of the two SNPs at the clinical level—and that effect could depend on the cell type or the tissue or, perhaps, on an inducing stimulus—is also open to investigation.
Since the discovery of MYOC and the recognition that the gene has implications for the phenotype and predisposition of adult and juvenile forms of POAG, several loci have been identified that may be related to this prevalent form of glaucoma, but none of the candidates have been defined at the molecular level. Our data indicate that APOE could be an additional important locus, in which specific genetic alterations at the molecular level are observed to modify the POAG phenotype. Alterations in APOE might account also for the excess of haplotype sharing among pairs of siblings with POAG, which was previously detected on chromosome 19q after a genome scan (Wiggs et al. 2000), and for the increased frequency of glaucoma that was recently reported in patients with Alzheimer disease (Bayer et al. 2002). A modification of POAG phenotype by APOE is also consistent with the ideas expressed elsewhere concerning early-stage role(s) for cellular injury and/or inflammation-signaling pathway in increases of IOP in POAG (Wang et al. 2001). In addition, APOE's interaction with the MYOC(−1000G)-promoter SNP takes on a special significance in light of the role of MYOC in regulation of IOP at the level of the trabecular meshwork and of the regulation of MYOC expression by oxidative stress (Polansky et al. 1997, 2000; Tamm et al. 1999).
Thus, our findings point toward separate roles of APOE in pathological processes in both the anterior and posterior part of the eye of patients with glaucoma. The identification of APOE as a new candidate gene that influences important clinical parameters in POAG also supports the importance of glaucoma research that focuses on the role of oxidative and other forms of stress in the pathogenesis of the disease. More generally, our study demonstrates the usefulness of a candidate-gene approach based on SNPs and, whenever possible, on a combinatorial, multivariate analysis of their modifying effects on a complex trait. In the case of POAG, we are left with important questions of gene interactions involving the MYOC gene, as well as other genes that may soon be identified in glaucoma.
Acknowledgments
This study was funded by INSERM (Institut National de la Santé et de la Recherche Médicale), by Insite Vision, and by the Assistance Publique des Hôpitaux de Paris grant DRRC-AOM96110. We are most grateful to Dr. Jon R. Polansky (Department of Ophthalmology, University of California at San Francisco) for fruitful discussion and critical reading of the manuscript.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM): http://www.ncbi.nlm.nih.gov/Omim/ for POAG [MIM 137760], MYOC [MIM 601652], and APOE [MIM 107741]
References
- Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ (1997) Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet 6:2091–2097 [DOI] [PubMed] [Google Scholar]
- Anderson DR (1989) Glaucoma: the damage caused by pressure: XLVI Edward Jackson memorial lecture. Am J Ophthalmol 108:485–495 [DOI] [PubMed] [Google Scholar]
- Artiga MJ, Bullido MJ, Sastre I, Recuero M, Garcia MA, Aldudo J, Vazquez J, Valdivieso F (1998) Allelic polymorphisms in the transcriptional regulatory region of apolipoprotein E gene. FEBS Lett 421:105–108 [DOI] [PubMed] [Google Scholar]
- Baskin F, Smith GM, Fosmire JA, Rosenberg RN (1997) Altered apolipoprotein E secretion in cytokine treated human astrocyte cultures. J Neurol Sci 148:15–18 [DOI] [PubMed] [Google Scholar]
- Bayer AU, Keller ON, Ferrari F, Maag KP (2002) Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer's disease and Parkinson's disease. Am J Ophthalmol 133:135–137 [DOI] [PubMed] [Google Scholar]
- Brezin AP, Bechetoille A, Hamard P, Valtot F, Berkani M, Belmouden A, Adam MF, Dupont de Dinechin S, Bach JF, Garchon HJ (1997) Genetic heterogeneity of primary open angle glaucoma and ocular hypertension: linkage to GLC1A associated with an increased risk of severe glaucomatous optic neuropathy. J Med Genet 34:546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullido MJ, Artiga MJ, Recuero M, Sastre I, Garcia MA, Aldudo J, Lendon C, Han SW, Morris JC, Frank A, Vazquez J, Goate A, Valdivieso F (1998) A polymorphism in the regulatory region of APOE associated with risk for Alzheimer's dementia. Nat Genet 18:69–71 [DOI] [PubMed] [Google Scholar]
- Colomb E, Nguyen TD, Béchetoille A, Dascotte J-C, Valtot F, Brézin AP, Berkani M, Copin B, Gomez L, Polansky JR, Garchon H-J (2001) Association of a single nucleotide polymorphism in the TIGR/MYOCILIN gene promoter with the severity of primary open-angle glaucoma. Clin Genet 60:220–225 [DOI] [PubMed] [Google Scholar]
- Drance SM (1997) Glaucoma: a look beyond intraocular pressure. Am J Ophthalmol 123:817–819 [DOI] [PubMed] [Google Scholar]
- Fautsch MP, Bahler CK, Jewison DJ, Johnson DH (2000) Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci 41:4163–4168 [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, Stengard JH, Salomaa V, Vartiainen E, Perola M, Boerwinkle E, Sing CF (2000) Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am J Hum Genet 67:881–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver CC, Kliffen M, Van Duijn CM, Hofman A, Cruts M, Grobbee DE, Van Broeckhoven C, de Jong PT (1998) Genetic association of apolipoprotein E with age-related macular degeneration (erratum 63:1252 [1998]). Am J Hum Genet 63:200–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E, Riley J, Purvis I, Roses A (1998) A 4-Mb high-density single nucleotide polymorphism-based map around human APOE. Genomics 54:31–38 [DOI] [PubMed] [Google Scholar]
- Lambert JC, Berr C, Pasquier F, Delacourte A, Frigard B, Cottel D, Perez-Tur J, Mouroux V, Mohr M, Cecyre D, Galasko D, Lendon C, Poirier J, Hardy J, Mann D, Amouyel P, Chartier-Harlin MC (1998) Pronounced impact of Th1/E47cs mutation compared with −491 AT mutation on neural APOE gene expression and risk of developing Alzheimer's disease. Hum Mol Genet 7:1511–1516 [DOI] [PubMed] [Google Scholar]
- Lambert JC, Brousseau T, Defosse V, Evans A, Arveiler D, Ruidavets JB, Haas B, Cambou JP, Luc G, Ducimetiere P, Cambien F, Chartier-Harlin MC, Amouyel P (2000) Independent association of an APOE gene promoter polymorphism with increased risk of myocardial infarction and decreased APOE plasma concentrations: the ECTIM study. Hum Mol Genet 9:57–61 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y (1999) Apolipoprotein E: from atherosclerosis to Alzheimer's disease and beyond. Curr Opin Lipidol 10:207–217 [DOI] [PubMed] [Google Scholar]
- Martin ER, Lai EH, Gilbert JR, Rogala AR, Afshari AJ, Riley J, Finch KL, Stevens JF, Livak KJ, Slotterbeck BD, Slifer SH, Warren LL, Conneally PM, Schmechel DE, Purvis I, Pericak-Vance MA, Roses AD, Vance JM (2000) SNPing away at complex diseases: analysis of single-nucleotide polymorphisms around APOE in Alzheimer disease. Am J Hum Genet 67:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Bello C, Chauhan BC, Nicolela MT, McCormick TA, Leblanc RP (2000) Intraocular pressure and progression of glaucomatous visual field loss. Am J Ophthalmol 129:302–308 [DOI] [PubMed] [Google Scholar]
- Miyata M, Smith JD (1996) Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat Genet 14:55–61 [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR (1998) Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem 273:6341–6350 [DOI] [PubMed] [Google Scholar]
- Nickerson DA, Taylor SL, Fullerton SM, Weiss KM, Clark AG, Stengard JH, Salomaa V, Boerwinkle E, Sing CF (2000) Sequence diversity and large-scale typing of SNPs in the human apolipoprotein E gene. Genome Res 10:1532–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J (1994) Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci 17:525–530 [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, Lutjen-Drecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD (1997) Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211:126–139 [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Zimmerman CC (2000) Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye 14:503–514 [DOI] [PubMed] [Google Scholar]
- Quigley HA (1993) Open-angle glaucoma. N Engl J Med 328:1097–1106 [DOI] [PubMed] [Google Scholar]
- Quigley HA, Enger C, Katz J, Sommer A, Scott R, Gilbert D (1994) Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol 112:644–649 [DOI] [PubMed] [Google Scholar]
- Roses AD (1996) Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med 47:387–400 [DOI] [PubMed] [Google Scholar]
- Shanmugaratnam J, Berg E, Kimerer L, Johnson RJ, Amaratunga A, Schreiber BM, Fine RE (1997) Retinal Muller glia secrete apolipoproteins E and J which are efficiently assembled into lipoprotein particles. Brain Res Mol Brain Res 50:113–120 [DOI] [PubMed] [Google Scholar]
- Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G (1998) The ε4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol 125:353–359 [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, MacKey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC (1997) Identification of a gene that causes primary open angle glaucoma. Science 275:668–670 [DOI] [PubMed] [Google Scholar]
- Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J (1999) Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci 40:2577–2582 [PubMed] [Google Scholar]
- Theuns J, Van Broeckhoven C (2000) Transcriptional regulation of Alzheimer's disease genes: implications for susceptibility. Hum Mol Genet 9:2383–2394 [DOI] [PubMed] [Google Scholar]
- Wang N, Chintala SK, Fini ME, Schuman JS (2001) Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med 7:304–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs JL, Allingham RR, Hossain A, Kern J, Auguste J, Delbono EA, Broomer B, Graham FL, Hauser M, Pericak-Vance M, Haines JL (2000) Genome-wide scan for adult onset primary open angle glaucoma. Hum Mol Genet 9:1109–1117 [DOI] [PubMed] [Google Scholar]