Abstract
Bemisia tabaci, the whitefly vector of Tomato yellow leaf curl virus (TYLCV), seriously reduces tomato production and quality. Here, we report the first evidence that infection by TYLCV alters the host preferences of invasive B. tabaci B (Middle East-Minor Asia 1) and Q (Mediterranean genetic group), in which TYLCV-free B. tabaci Q preferred to settle on TYLCV-infected tomato plants over healthy ones. TYLCV-free B. tabaci B, however, preferred healthy tomato plants to TYLCV-infected plants. In contrast, TYLCV-infected B. tabaci, either B or Q, did not exhibit a preference between TYLCV-infected and TYLCV-free tomato plants. Based on gas chromatography-mass spectrometry (GCMS)analysis of plant terpene volatiles, significantly more β-myrcene, thymene, β-phellandrene, caryophyllene, (+)-4-carene, and α-humulene were released from the TYLCV-free tomato plants than from the TYLCV-infected ones. The results indicate TYLCV can alter the host preferences of its vector Bemisia tabaci B and Q.
Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) originated in the tropics and subtropics1 and has rapidly spread as a consequence of the international trade in flowers and other nursery stock. Because of its wide host range, rapid propagation, and superior ability to transmit virus, B. tabaci has become one of the most important pests in field crops worldwide2. B. tabaci is a complex of numerous genetically distinct populations, previously referred to as biotypes and now recognized as cryptic species2,3,4. There are about 24 cryptic species of B. tabaci, including the two most widely distributed and invasive biotypes, B and Q, hereafter referred to as B and Q whiteflies5. B. tabaci is the only known vector of Tomato yellow leaf curl virus (TYLCV), which seriously reduces tomato production and quality. TYLCV is a single-stranded DNA (ssDNA) plant virus in the genus begomovirus, family Geminiviridae, that originated in the Middle East6,7. Begomoviruses are transmitted by B. tabaci in a circulative manner and persist in the whitefly vector8,9,10,11.
Plant–pathogen–vector systems are characterized by complex direct and indirect interactions12,13. Virus-induced plant reactions can influence the behavior, physiology, and dynamics of insect vectors in plant populations, sometimes causing behavioral changes in the vectors that favor virus transmission14. For example, a recent paper by Stafford et al. (2011)15 demonstrated that plant-infecting viruses can directly alter vector feeding behavior. The authors found that Tomato spotted wilt virus infected male thrips spent more time feeding than that of non-infected thrips. However, modification of virus “behavior” within the host plant in response to attack by herbivorous insect vectors has been addressed only very recently16.
B. tabaci B was introduced into China in the mid-1990's17, but the first incidence of TYLCV was not recorded until 2006 in Shanghai18, following the introduction and spread of the Q whitefly in 200319. The virus has since spread throughout most of China20, and the pattern of its spread has followed that of B. tabaci Q21. Researchers have hypothesized that the spread of TYLCV is closely related with the establishment and spread of B. tabaci Q20,22,23. Our recent study showed that TYLCV is benefit B. tabaci Q, but harm B. tabaci B22. In addition, TYLCV infected weed (Datura stramonium) also affects the host preference and performance of B. tabaci Q23. The results indicated that B. tabaci Q preferentially settled and oviposited on TYLCV-infected plants rather than on healthy plants. In addition, B. tabaci Q performed better on TYLCV-infected plants than on healthy plants23.
Prior studies18,19,20,21 suggest a closer mutualistic relationship that TYLCV spreads following B. tabaci Q, rather than B. In the present study, we compared the host preference of B and Q whiteflies for TYLCV-infected and healthy (i.e., virus-free) tomato plants. We also compared the volatile compounds released by healthy and TYLCV-infected tomato plants to explain the alteration in the host-selection behavior of B. tabaci. This information increases our understanding of TYLCV spread and outbreaks.
Results
Symptoms and viral load in TYLCV-infected and healthy tomato plants
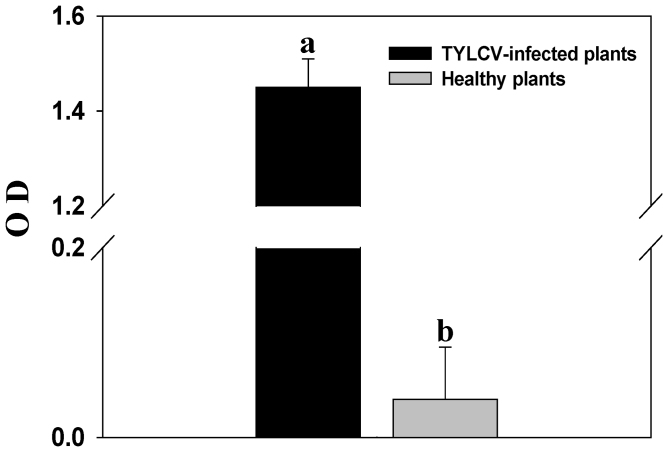
Compared to the leaves of healthy tomato plants (Fig. 1A), the leaves of TYLCV-infected plants curl upward and are yellow and stunted (Fig. 1B). The viral load was significantly higher in the TYLCV-infected plants than in the healthy plants (F1, 22 = 45367.531, p < 0.0001, Fig. 2).
Figure 1. Symptoms of (A) healthy and (B) TYLCV-infected tomato plants.
Photographs by Yong Fang.
Figure 2. Viral load in healthy and TYLCV-infected tomato plants.
Host selection
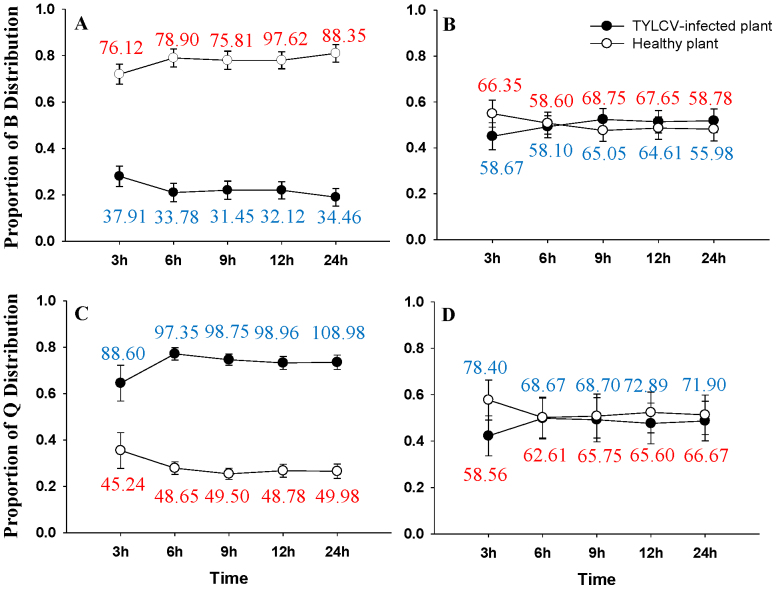
TYLCV-free B whiteflies (reared on virus-free plants) preferred to settle on TYLCV-free tomato plants over TYLCV-infected tomato plants (Fig. 3A) (F1, 56 = 50.060, p < 0.0001), whereas TYLCV-free Q whiteflies displayed the opposite behavior, settling in significantly greater proportions on TYLCV-infected plants than on TYLCV-free plants (Fig. 3C) (F1, 56 = 40.856, p < 0.0001). In contrast, the TYLCV-infected whiteflies of both B. tabaci B and Q showed no preference between the TYLCV-free and the TYLCV-infected tomato plants (Fig. 3B, D) (F1, 56 = 0.0001, p = 1.000 and F1, 56 = 0.0001, p = 1.000, respectively).
Figure 3. Proportion of B. tabaci B and Q individuals that settled on healthy vs. TYLCV-infected tomato plants in a choice test.
B. tabaci B and Q settling on TYLCV-free (open circles) versus TYLCV-infected tomato plants (closed circles) in two choice laboratory bioassays: (A) noninfected B. tabaci B; (B) TYLCV-infected B. tabaci B; (C) noninfected B. tabaci Q; and (D) TYLCV-infected B. tabaci Q. The numbers of adult whiteflies are also shown in the figure: the red number indicates the number of whiteflies on the healthy plant, and the blue number indicates the number of whiteflies on the TYLCV-infected plant.
Volatiles released by TYLCV-infected and non-infected tomato plants
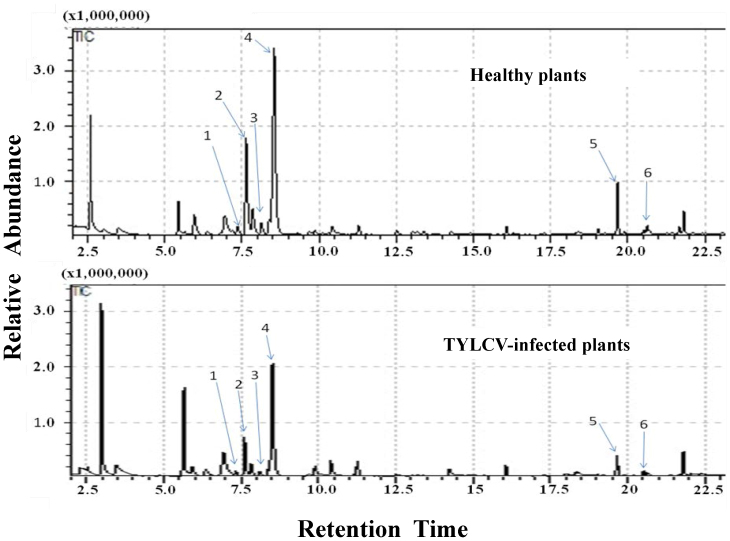
GC–MS chromatograms of volatiles from the TYLCV-free and the TYLCV-infected tomato plants exhibited significant qualitative and/or quantitative differences in chemical composition (Fig. 4, Table 1). TYLCV-free tomato plants emitted significantly more β-Myrcene, Thymene, β-Phellandrene, Caryophyllene, and α-Humulene than did TYLCV-infected tomato plants. Furthermore, (+)-4-carene was detected only from TYLCV-free tomato plants (Fig. 4, Table 1).
Figure 4. Total ion chromatograms of volatile compounds released by the healthy tomato plants and the TYLCV-infected tomato plants.
The identified compounds which have significant difference between the healthy tomato plants and the TYLCV-infected tomato plants:1 = β-Myrcene, 2 = (+)-4-Carene, 3 = Thymene, 4 = β-Phellandrene, 5 = β-Caryophyllene, 6 = α-Humulene.
Table 1. The peak areas (×1000) of volatile constituents released from TYLCV-infected and TYLCV-free tomato plants (mean ± SE).
| Compound | Retention time | TYLCV-infected plants | TYLCV-free plants | P-value |
|---|---|---|---|---|
| β-Myrcene | 7.33 | 127.02 ± 20.20 a | 229.38 ± 24.67 b | 0.022 |
| (+)-4-Carene | 7.62 | 0 a | 2411.89 ± 482.71 b | 0.008 |
| Thymene | 8.37 | 768.81 ± 22.34 a | 1240.71 ± 296.90 b | 0.036 |
| β-Phellandrene | 8.52 | 5607.10 ± 217.80 a | 9078.92 ± 2135.28 b | 0.038 |
| β-Caryophyllene | 19.67 | 227.70 ± 14.90 a | 375.22 ± 80.17 b | 0.024 |
| α-Humulene | 20.63 | 91.724 ± 4.18 a | 148.30 ± 35.15 b | 0.035 |
| Butylated Hydroxytoluene | 21.82 | 208.16 ± 42.35 a | 351.00 ± 65.31 b | 0.374 |
| α-Phellandrene | 7.83 | 1210.39 ± 37.56 a | 1742.48 ± 678.30 a | 0.097 |
| α-Terpinene | 8.12 | 254.93 ± 5.89 a | 376.00 ± 133.86 a | 0.076 |
| γ-Butyrolactone | 5.85 | 29.52 ± 15.18 a | 53.81 ± 5.23 a | 0.141 |
| α-Pinene | 5.93 | 623.57 ± 5.65 a | 979.58 ± 267.56 a | 0.052 |
Within each row, different letters indicate significant differences between virus-infected and virus-free plants (P < 0.05).
Discussion
We have demonstrated that the host-preference of B. tabaci is shaped by: TYLCV infected and non-infected plants; TYLCV infected and non-infected B. tabaci B and Q insects. TYLCV-free B. tabaci B were attracted to TYLCV-free tomato plants, whereas TYLCV-free B. tabaci Q were attracted to TYLCV-infected tomato plants. In addition, TYLCV-infected B. tabaci B and Q showed no preference between TYLCV-free and TYLCV-infected tomato plants. The host preferences we observed together with our recent studies20,22,23,24 to some extent explain why the spread of TYLCV in China appears to have been closely associated with the spread of B. tabaci Q rather than B.
The relationship of plant, pathogen, and vector insect includes direct and indirect interactions that can be beneficial or harmful, depending on the species25,26,27. Plant viruses infect their vectors and likely affect them in at least some instances. For example, the infection with TYLCV is harmful to B. tabaci B but beneficial to Q in performance, preference of feeding behaviors and virus transmission22,25,26,27. In addition, relative to their TYLCV-free B feeding on cotton (a non-host for TYLCV), TYLCV-infected B exhibited significant reductions in survival from egg to adult; fecundity; female and male body size, whereas TYLCV-infected Q showed only marginal reductions22. While Q performed better on TYLCV-infected tomato plants than on uninfected ones, whereas B performed better on uninfected tomato plants than on TYLCV-infected ones22. The transmission of plant viruses by insect vectors has been explored for over a century28. Several studies have shown that virus-induced plant reactions shape the behavior, physiology, and dynamics of the insect vectors, sometimes inducing changes in the insect vectors that favor virus transmission25,27.
Pathogen-induced plant responses may result from the changes of plant volatiles. Plant defensive compounds, specifically terpenoids, play a key role in mediating vector–pathogen mutualistic relationships. Our results show that TYLCV-free plants released significantly more β-myrcene, thymene, β-phellandrene, β-caryophyllene, and α-humulene than TYLCV-infected plants (Table 1, Fig. 4). This result is consistent with that of Luan et al. (2013)29, who reported that elevation in terpenoid levels (via exogenous stem applications) reduced whitefly fitness and that suppression of terpenoid synthesis via gene silencing increased whitefly fitness. Previous study has shown that the monoterpene (+)-3-carene is associated with resistance of Sitka spruce (Picea sitchensis) to white pine weevil (Pissodes strobi) and that resistant trees contained significantly more (+)-3-carene than susceptible trees30. In the current study, (+)-4-carene was detected in the TYLCV-free tomato plants but not in the infected ones. In addition, virus infection often alters plant morphology, nutrition, and color, and these changes could affect the host preference of herbivorous vectors. With respect to nutrition, virus infection can change the amino acid composition in the phloem or in other ways change the nutritional composition of the plant tissue13,31 and thereby change the host selection by herbivorous vectors31,32. Further experiments are needed to investigate how virus-induced changes in plant volatiles, morphology, nutrition, and color affect host selection by B. tabaci B and Q.
The status of the vector (virus-infected or virus-free) can also influence its behavior in a way that benefits the virus. Recently, we used the electrical penetration graph (EPG) technique to study the effect of TYLCV infection of tomato plants on vector (B. tabaci B and Q) feeding behavior24. Both B. tabaci B and Q appeared to find TYLCV-infected plants more attractive than healthy plants, probing them more quickly and exhibiting a greater number of feeding bouts. Interestingly, virus-infected whiteflies fed more often than virus-free insects, and they spend more time in feeding. Because vector salivation is essential for viral transmission, this virus-mediated alteration of behavior should directly benefit TYLCV fitness24.
To our knowledge, we provide the first evidence for a direct effect of a plant virus (TYLCV) on its vector, and the resulting behavioral change in B. tabaci Q may have greatly contributed to the spread of TYLCV. However, the cause of the shift in host preference between TYLCV-infected and TYLCV-free B. tabaci is unknown and should be investigated.
Methods
Plant and insect rearing
B. tabaci B was originally collected from infested cabbage, Brassica oleracea L. cv. Jing feng 1, in Beijing, China in 200433. B. tabaci Q was collected from poinsettia, Euphorbia pulcherrima Wild. ex Klotz., in Beijing, China in 200920. B and Q whiteflies were reared on healthy tomato plants, Solanum lycopersicum Mill. cv. Zhongza 9, in separate, whitefly-proof screen cages in a greenhouse under natural lighting and controlled temperature (26 ± 2°C) for six generations. The purity of each B. tabaci was monitored by sampling 15 adults per generation using a molecular diagnostic technique, CAPS (cleavage amplified polymorphic sequence), and a molecular marker, mitochondrial cytochrome oxidase I genes (mtCOI)34. Tomato plants were grown in insect-proof cages under natural lighting and ambient temperatures. Inoculation was mediated by Agrobacterium tumefaciens using a cloned TYLCV genome (GenBank accession ID: AM282874), which was originally isolated from tomato plants in Shanghai, China18. Similar plants were not inoculated with the virus. TYLCV-infected and uninfected tomato plants with the same height were selected for experiments. Healthy and TYLCV-inoculated tomato plants at the seven true-leaf stage were used to test for TYLCV with a triple antibody sandwich enzyme-linked immunosorbent assay (TAS-ELISA). A 0.1-g sample of leaf tissue (the third leaf from the top) was ground in 1 ml of extraction buffer. Each of the two treatments was represented by 12 replicates23. A kit supplied by Adgen Phytodiagnotics (Neogen Europe (Ayr), Ltd) was used, and the manufacturer's protocol was followed. Absorbance was read with a fluorescence microplate reader at 405 nm (SpectraMax M2e, Molecular Devices). The samples were considered positive for TYLCV when the mean optical density (OD) values at 405 nm were greater than three times those of the healthy controls.
Detection of TYLCV in insect and plant samples
Genomic DNA was extracted from individual whiteflies according to De Barro and Driver (1997)35 and Frohlich et al. (1999)3. The nucleic acids from plants were extracted using the Plant Genomic DNA Extraction Kit (BioTeke Biotechnology, Beijing Co, Ltd). A ~410-bp TYLCV DNA fragment was amplified using the primer pairs C473 and V6136. The resultant PCR products were electrophoresed on a 2.0% agarose gel in a 0.5 × TBE buffer and visualized by Gelview staining.
Acquisition of TYLCV by Bemisia tabaci B and Q
Plants selected to be infected with virus were inoculated at the three true-leaf stage and were assumed to be infected with TYLCV when they developed characteristic leaf-curl symptoms; TYLCV was confirmed by molecular analysis as described in the previous paragraph. About 1000 newly emerged (0–8 h post-emergence) B or Q whiteflies were placed in small cages containing the TYLCV-infected tomato plants or healthy tomato plants for 72 h.
Two-choice bioassays to assess Bemisia tabaci B and Q preferences
We determined the proportion of TYLCV-infected and TYLCV-free whiteflies that settled on TYLCV-infected and virus-free plants after 24 h. Two tomato plants of similar size and with same number of true leaves (one infected with TYLCV and the other virus free) were placed in a cage (60 cm long, 55 cm wide, 70 cm high), and about 150 B. tabaci adults of one biotype (B or Q) and one infection status (virus-infected or not infected) were released into the center of the cage (Fig. 5). The position of the two plants in the cage was randomized, and cages were kept under laboratory conditions (25 ± 1°C, natural lighting). There were eight replicate cages for each of the four kinds of whiteflies: 1) infected B whiteflies (n = 8 replicates); 2) uninfected B whiteflies (n = 8); 3) infected Q whiteflies (n = 8); and 4) uninfected Q whiteflies (n = 8). The number of B. tabaci settling on each plant was recorded 3, 6, 9, 12, and 24 h after release.
Figure 5. Selection of healthy vs. TYLCV-infected tomato plants by Bemisia tabaci.
Photographs by Yong Fang.
Volatile collection and analysis
Leaf samples were collected from five TYLCV-free and five TYLCV-infected tomato plants. There were thus two treatments: volatile compounds released by the healthy tomato plants (n = 5 replicates); volatile compounds released by the TYLCV-infected tomato plants (n = 5). A 0.3-g quantity of leaf from each plant was subjected to gas chromatography–mass spectrometry (GCMS-2010, Shimadzu) using a VF-5MS column (0.25 mm × 30 mm, J&W Scientific, Folsom, CA). The temperature program was as follows: an initial temperature of 50°C was held for 1 min, increased at 5°C/min to 240°C, held for 2 min, and then increased at 30°C/min to 300°C and held for 5 min. The injection temperature was 270°C. Relative quantification was based on the peak area of each component of the volatiles. The mass spectrometer was operated in EI ionization mode at 70 eV. The temperature of the source was kept at 200°C, and the interface temperatures were 280°C.
Data analysis
One-way ANOVAs were used to compare the viral load in the healthy and TYLCV-infected tomato plants. The host-settling preference between B. tabaci B and Q was tested by repeated-measures ANOVA. The concentration of individual volatile compounds emitted by TYLCV-free (healthy) versus TYLCV-infected tomato plants was compared with a Student's t-test. Statistical analyses were performed with SPSS (version 13.0; SPSS Inc., Chicago, IL, USA).
Author Contributions
Y.J.Z., Y.F., H.P.P. designed the experiment. Y.F., X.B.S., G.C. performed the experiment. W.X., S.L.W., Q.J.W., Q.S., X.Y. contributed reagents/materials. Y.F., X.G.J., H.P.P., Y.J.Z. wrote the paper.
Acknowledgments
The authors thank Jonathan Sweeney (Canadian Forest Service-Atlantic Forestry Centre) for his comments and constructive criticisms. This work was funded by the National Science Fund for Distinguished Young Scholars (31025020), the Beijing Natural Science Foundation (6131002), the 973 Program (2013CB127602), and the Beijing Key Laboratory for Pest Control and Sustainable Cultivation of Vegetables. The granting agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Mound L. A. In Plant Virus Epidemiology: The Spread and Control of Insect-borne Viruses. (eds. Plumb R. T., & Thresh J. M.) 305–311 (Blackwell Ltd., 1983). [Google Scholar]
- Brown J. K., Frohlich D. R. & Rosell R. C. The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu. Rev. Entomol. 40, 511–534 (1995). [Google Scholar]
- Frohlich D. R., Torres-Jerez I., Bedford I. D., Markham P. G. & Brown J. K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 8, 1683–1691 (1999). [DOI] [PubMed] [Google Scholar]
- De Barro P. J., Driver F., Trueman J. W. & Curran J. Phylogenetic relationships of world populations of Bemisia tabaci (Gennadius) using ribosomal ITS1. Mol. Phylogenet. Evol. 16, 29–36 (2000). [DOI] [PubMed] [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M. & Dinsdale A. B. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011). [DOI] [PubMed] [Google Scholar]
- Varma A. & Malathi V. G. Emerging geminivirus problems: a serious threat to crop production. Ann. Appl. Biol. 142, 145–164 (2003). [Google Scholar]
- Cohen S. & Harpaz I. Periodic, rather than continual acquisition of a new tomato virus by its vector, the tobacco whitefly (Bemisia tabaci Gennadius). Entomol. Exp. Appl. 7, 155–166 (1964). [Google Scholar]
- Brown J. K. Molecular markers for the identification and global tracking of whitefly vector-begomovirus complexes. Virus Res. 71, 233–260 (2000). [DOI] [PubMed] [Google Scholar]
- Brown J. K. & Czosnek H. Whitefly transmission of plant viruses. Adv. Bot. Res. 36, 65–76 (2002). [Google Scholar]
- Brown J. K. In Bemisia: Bionomics and Management of a Global Pest (eds. Stansly P. A., & Naranjo S. E.) 31–67 (Springer Ltd., 2010). [Google Scholar]
- Gill R. J. & Brown J. K. In Bemisia: Bionomics and Management of a Global Pest (eds. Stansly P. A., & Naranjo S. E.) 5–30 (Springer Ltd., 2010). [Google Scholar]
- Belliure B., Janssen A., Maris P. C., Peters D. & Sabelis M. W. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79 (2005). [Google Scholar]
- Stout M. J., Thaler J. S. & Thomma B. P. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 51, 663–689 (2006). [DOI] [PubMed] [Google Scholar]
- Bosque-Pérez N. A. & Eigenbrode S. D. The influence of virus-induced changes in plants on aphid vectors: insights from luteovirus pathosystems. Virus Res. 159, 201–205 (2011). [DOI] [PubMed] [Google Scholar]
- Stafford C. A., Walker G. P. & Ullman D. E. Infection with a plant virus modifies vector feeding behavior. P. Natl. Acad. Sci. USA. 108, 9350–9355 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc S., Uzest M. & Drucker M. New research horizons in vector-transmission of plant viruses. Curr. Opin. Microbiol. 14, 483–491 (2011). [DOI] [PubMed] [Google Scholar]
- Luo C. et al. The use of mitochondrial cytochrome oxidase I (mtCOI) gene sequences for the identification of biotype of Bemisia tabaci (Gennadius) in China. Acta Entomol. Sin. 45, 759–763 (2002). [Google Scholar]
- Wu J. B., Dai F. M. & Zhou X. P. First report of Tomato yellow leaf curl virus in China. Ann. Appl. Biol. 155, 439–448 (2006). [DOI] [PubMed] [Google Scholar]
- Chu D. et al. The introduction of the exotic Q biotype of Bemisia tabaci from the Mediterranean region into China on ornamental crops. Fla. Entomol. 89, 168–174 (2006). [Google Scholar]
- Pan H. P. et al. Rapid spread of Tomato yellow leaf curl virus in China is aided differentially by two invasive whiteflies. PLoS One 7, e34817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H. P. et al. Further spread of and domination by Bemisia tabaci biotype Q on field crops in China. J. Econ. Entomol. 104, 978–985 (2011). [DOI] [PubMed] [Google Scholar]
- Pan H. P. et al. Differential effects of an exotic plant virus on its two closely related vectors. Sci. Rep. 3, 2230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. et al. Virus infection of a weed increases vector attraction to and vector fitness on the weed. Sci. Rep. 3, 2253 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. M. et al. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and Tomato yellow leaf curl virus. J. Virol. 87, 4929–4937 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Delafuente A., Garzo E., Moreno A. & Fereres A. A. Plant virus manipulates the behavior of its whitefly vector to enhance its transmission efficiency and spread. PLoS One 8, e61543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluth S., Kruess A. & Tscharntke T. Insects as vectors of plant pathogens: mutualistic and antagonistic interactions. Oecologia 133, 193–199 (2002). [DOI] [PubMed] [Google Scholar]
- Gutiérrez S., Michalakis Y., Munster M. & Blanc S. Plant feeding by insect vectors can affect life cycle, population genetics and evolution of plant viruses. Funct. Ecol. 27, 610–622 (2013). [Google Scholar]
- Fernández-Calvino L., LóPez-Abella D. & LóPez-Moya J. J. In General Concepts in Integrated Pest and Disease Management (eds Ciancio A., & Mukerji K. G.) 269–293 (Springer Ltd, 2007). [Google Scholar]
- Luan J. B. et al. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Eco. Lett. 16, 390–398 (2013). [DOI] [PubMed] [Google Scholar]
- Hall D. E. et al. An integrated genomic, proteomic and biochemical analysis of (+)-3-carene biosynthesis in Sitka spruce (Picea sitchensis) genotypes that are resistant or susceptible to white pine weevil. Plant J. 65, 936–948 (2011). [DOI] [PubMed] [Google Scholar]
- Mauck K. E., De Moraes C. M. & Mescher M. C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. P. Natl. Acad. Sci. U. S. A. 107, 3600–3605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awmack C. S. & Leather S. R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844 (2002). [DOI] [PubMed] [Google Scholar]
- Xie W. et al. Induction effects of host plants on insecticide susceptibility and detoxification enzymes of Bemisia tabaci (Hemiptera: Aleyrodidae). Pest. Manag. Sci. 67, 87–93 (2011). [DOI] [PubMed] [Google Scholar]
- Chu D., Wan F. H., Zhang Y. J. & Brown J. K. Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ. Entomol. 39, 1028–1036 (2010). [DOI] [PubMed] [Google Scholar]
- De Barro P. J. & Driver F. Use of RAPD PCR to distinguish the B biotype from other biotypes of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Aust. J. Entomol. 2, 149–152 (1997). [Google Scholar]
- Ghanim M., Sobol I., Ghanim M. & Czosnek H. Horizontal transmission of begomoviruses between Bemisia tabaci biotypes. Arthropod-Plant Inte. 1, 195–204 (2007). [Google Scholar]