Abstract
In this report, we reinvestigate the only patient ever reported with a deficiency of peroxisomal 3-ketoacyl-CoA thiolase (THIO). At the time when they were described, the abnormalities in this patient, which included accumulation of very-long-chain fatty acids and the bile-acid intermediate trihydroxycholestanoic acid, were believed to be the logical consequence of a deficiency of the peroxisomal β-oxidation enzyme THIO. In light of the current knowledge of the peroxisomal β-oxidation system, however, the reported biochemical aberrations can no longer be explained by a deficiency of this thiolase. In this study, we show that the true defect in this patient is at the level of d-bifunctional protein (DBP). Immunoblot analysis revealed the absence of DBP in postmortem brain of the patient, whereas THIO was normally present. In addition, we found that the patient had a homozygous deletion of part of exon 3 and intron 3 of the DBP gene, resulting in skipping of exon 3 at the cDNA level. Our findings imply that the group of single–peroxisomal β-oxidation–enzyme deficiencies is limited to straight-chain acyl-CoA oxidase, DBP, and α-methylacyl-CoA racemase deficiency and that there is no longer evidence for the existence of THIO deficiency as a distinct clinical entity.
In 1986, Goldfischer et al. described a patient with clinical features similar to those of patients with Zellweger syndrome (MIM 214100) (Goldfischer et al. 1986). In contrast to patients with Zellweger syndrome who lack functional peroxisomes, however, this patient had apparently normal peroxisomes in liver and kidney. There was an accumulation of very-long-chain fatty acids (VLCFAs) in plasma and of 3α,7α,12α-trihydroxycholestanoic acid (THCA) in duodenal aspirate of the patient (Goldfischer et al. 1986). Later studies, by Clayton et al. (1990), showed that 3α,7α,12α,24-tetrahydroxycholestanoic acid (varanic acid), an intermediate in the formation of cholic acid from THCA, was present in body fluids of the patient. Immunoblot experiments by Schram et al. (1987) revealed the absence of 3-ketoacyl-CoA thiolase (THIO) in postmortem liver of the patient, whereas normal levels were found for other peroxisomal matrix enzymes (i.e., acyl-CoA oxidase, bifunctional protein, and catalase). These results led to the conclusion that the strongly reduced rate of peroxisomal β-oxidation measured in liver of the patient and the accumulation of VLCFAs and THCA in body fluids were caused by a deficiency of THIO (MIM 261510). After the identification, in 1991, of the gene encoding human THIO, molecular studies in this patient were performed, but no large DNA rearrangements involving the thiolase gene were observed in Southern blot experiments (Bout et al. 1991).
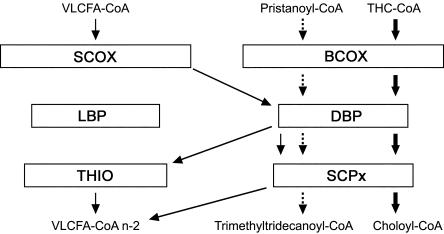
At the time when the patient was described, it was believed that the peroxisomal β-oxidation system consisted of only a single set of enzymes: an acyl-CoA oxidase catalyzing the first step, a bifunctional protein catalyzing the second and third step, and a thiolase responsible for the last step of the β-oxidation process. During the past few years, however, a number of studies have shed new light on the enzymology of the peroxisomal β-oxidation system (for recent reviews, see van Veldhoven et al. 2001; Wanders et al. 2001a, 2001b). These studies have shown that peroxisomes contain two sets of β-oxidation enzymes, which differ in substrate specificity (fig. 1). In addition to the original acyl-CoA oxidase, which is now called “straight-chain acyl-CoA oxidase” (SCOX), a second oxidase, called “branched-chain acyl-CoA oxidase” (BCOX), has been identified. SCOX is responsible for the oxidation of VLCFAs such as C26:0 and C24:0, whereas BCOX is involved in the β-oxidation of pristanic acid and of the bile-acid intermediates THCA and dihydroxycholestanoic acid (DHCA) (fig. 1). The second bifunctional protein that has been identified has been named “d-bifunctional protein” (DBP), because it forms and dehydrogenates d-3-hydroxyacyl-CoAs, in contrast to the original protein, l-bifunctional protein (LBP), which produces l-hydroxy intermediates. Both in vitro studies performed with the purified bifunctional proteins and the identification of patients with a deficiency of DBP (MIM 261515 and 601860) (Suzuki et al. 1997; van Grunsven et al. 1998, 1999a, 1999b) have provided unequivocal evidence that DBP is involved in the degradation of VLCFAs, as well as of the branched-chain fatty acids, pristanic acid, and DHCA/THCA. At present, the physiological function of LBP remains elusive. Both peroxisomal thiolases are believed to be involved in the degradation of VLCFAs. In addition, sterol carrier protein X (SCPx), the second peroxisomal thiolase that has been identified, which contains both a thiolase domain and a sterol carrier–protein domain, is the key enzyme in the β-oxidation of pristanic acid and DHCA/THCA.
Figure 1.
Schematic representation of fatty-acid β-oxidation machinery in human peroxisomes catalyzing the oxidation of very-long-chain fatty acyl-CoAs (VLCFA-CoA) and branched-chain fatty acyl-CoAs (i.e., pristanoyl-CoA and THC-CoA). Oxidation of VLCFA-CoAs C24:0 and C26:0 involves SCOX, DBP, and both THIO and SCPx, whereas oxidation of branched-chain fatty acyl-CoAs involves BCOX, DBP, and SCPx (for a review, see Wanders et al. 2001a).
Since the new insights into the peroxisomal β-oxidation system and into the physiological function of the different β-oxidation enzymes no longer provide an explanation for the biochemical findings in the reported patient, we reinvestigated this unique case. In the original report, it was concluded that there was a deficiency of peroxisomal THIO in the index patient, because immunoblot experiments revealed the normal presence of SCOX and LBP, but no THIO, in postmortem liver material (Schram et al. 1987). To determine whether this thiolase deficiency is caused by mutations in the gene encoding THIO, we sequenced the cDNA, amplified by RT-PCR, from RNA isolated from postmortem brain and kidney material from the patient and from a control subject, in two overlapping fragments, by use of primers THIOF-58 (5′-TGT TAA CTC CGC GGT CAG TTC CCG GAC TGG-3′), THIOR 484 (5′-CCA GGG TTC CCT CTG TCA GCC AGG GAC ATG-3′), THIOF 403 (5′-GTG GCA TCA GAA ATG GGT CTT ATG ACA TTG-3′), and THIOR 1326 (5′-GCT GCT AGA GCA GCA GGA CTG TCT GCG TAG-3′). Unfortunately, no liver material from the patient could be used for this study, because the liver samples used for the studies described by Schram et al. (1987) were no longer available. No mutations were identified by sequence analysis of the cDNA encoding the thiolase from both brain and kidney in the patient.
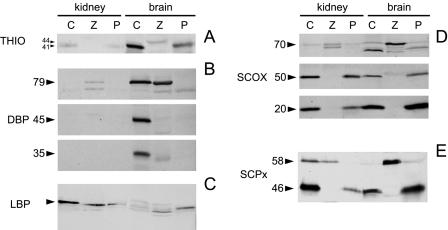
The absence of mutations in the cDNA encoding the thiolase, in conjunction with the current view that peroxisomes contain two sets of β-oxidation enzymes, prompted us to reinvestigate the patient, at the biochemical level. To this end, we performed immunoblot experiments using antibodies against the different enzymes, except BCOX, since no antibody against this enzyme was available. The results are shown in figure 2. In contrast to the previous data for liver, the mature 41-kD form of THIO was normally present in both brain and kidney from the index patient (fig. 2A). Also, the other peroxisomal thiolase, SCPx, as well as the 70-, 50-, and 20-kD components of SCOX and LBP were normally present. DBP, however, was deficient in brain from the index patient, whereas it was normally present in brain of the control subject. The full-length protein, 79 kD, was not detectable; nor were the two proteolytically processed polypeptides: the 45-kD band corresponding to the enoyl-CoA hydratase component of DBP and the 35-kD band corresponding to the 3-hydroxyacyl-CoA dehydrogenase component of DBP. In kidney, no DBP could be detected in either the control subject or the index patient (fig. 2B).
Figure 2.
Immunoblot analysis in postmortem kidney and brain of a control subject (lanes C), a patient suffering from Zellweger syndrome (lanes Z), and the index patient (lanes P). Antibodies were used against (A) peroxisomal THIO (Tager et al. 1985), (B) DBP (Jiang et al. 1996), (C) LBP (Tager et al. 1985), (D) SCOX (Tager et al. 1985), and (E) SCPx (Ossendorp et al. 1996). In A, the arrowheads indicate the 44-kD precursor form and the 41-kD mature form of THIO; in B, the arrowheads indicate the 79-kD full-length protein, the 45 kD enoyl-CoA hydratase component of DBP, and the 35-kD 3-hydroxyacyl-CoA dehydrogenase component of DBP; in C, the arrowhead indicates the 79-kD full-length LBP; in D, the arrowheads indicate the 70-, 50-, and 20-kD components of SCOX; and, in E, the arrowheads indicate the 58-kD full-length protein and the 46-kD thiolase component of SCPx.
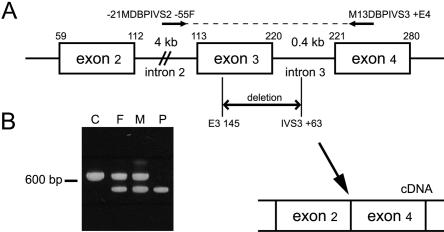
Since no skin fibroblasts from the index patient were available, we measured DBP activity (as described in the report by van Grunsven et al. [1998]) in fibroblasts of the patient’s parents and found partially reduced activity (table 1), which is in agreement with heterozygosity for DBP deficiency. To confirm the apparent DBP deficiency in the index patient at the molecular level, we amplified the cDNA encoding DBP, from brain and kidney, by PCR, in three overlapping fragments, as described by van Grunsven et al. (1998), and subsequently sequenced the PCR products. We found a homozygous deletion of bp 113–220, corresponding to exon 3 of the DBP gene (Leenders et al. 1998). We also analyzed the cDNA encoding DBP in fibroblasts of the patient’s mother and found a heterozygous deletion of exon 3. To determine the cause of skipping of exon 3 in the patient, the 3′ end of intron 2, exon 3, and intron 3 of the DBP gene were amplified from brain and kidney DNA, by use of primers –21MDBPIVS2−55F (5′-CAC ATT TTG AAA GTC TAG AA-3′) and M13DBPIVS3+E4 (5′-CAC CTA TTC TTC CAA AAG CAT CC-3′), and the PCR products were sequenced. This revealed a deletion of 138 bp, encompassing bp 145–220 of exon 3 and the first 63 bp of intron 3 (fig. 3). In fibroblasts from the parents of the patient, the same deletion was identified, in heterozygous form.
Table 1.
Activity Measurements of the Enoyl-CoA Hydratase and 3-hydroxyacyl-CoA Dehydrogenase Component of d-Bifunctional Protein, in Fibroblasts of the Patient’s Parents and in Control Subjects
|
Measurement in(pmol/min/mg) |
|||
| Activity | Patient'sMother | Patient'sFather | Controls (n=24)a |
| Hydratase (formation of 24-OH-THC-CoA) | 86 | 127 | 240 ± 65 |
| Dehydrogenase (formation of 24-keto-THC-CoA) | 10 | 26 | 73 ± 33 |
Values are mean ± SD.
Figure 3.
A, Schematic representation of exons 2–4 of d-bifunctional protein (DBP) and the intervening intron sequences. The deletion in the DBP gene in the index patient is indicated (from bp 145 in exon 3 through the first 63 bp of intron 3). This was determined by amplification of part of the DBP gene by primers –21MDBPIVS2−55F and M13DBPIVS3+E4, which are depicted, and subsequent sequencing of the PCR products. On the genomic level, the deletion results in skipping of exon 3, at the cDNA level. B, Products of amplification of the DBP gene by primers –21MDBPIVS2−55F and M13DBPIVS3+E4, in brain of a control subject (lane C), the patient’s father (lane F), the patient’s mother (lane M), and the index patient (lane P).
The data presented in this study show that the true defect in the only patient documented with a deficiency of THIO is at the level of DBP. No DBP protein could be detected by immunoblot analysis in brain of the patient, whereas THIO was normally present. These results were confirmed by cDNA analysis in brain and kidney. The cDNA encoding THIO was completely normal, whereas the patient had a homozygous deletion of exon 3 in DBP cDNA. Studies at the genomic level revealed that skipping of exon 3 in this patient is caused by a deletion of part of exon 3 and the 5′ end of intron 3. The parents of the patient, who were consanguineous, are heterozygous for this deletion, which results in partially reduced DBP activity, as measured in their fibroblasts. Exon 3 consists of 108 bp, and skipping of this exon leads to an in-frame deletion of 36 amino acids. Since neither the full-length, 79-kD band nor the 45- and 35-kD bands, corresponding to the enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase components of DBP, respectively, were present in brain from the patient, the mutated protein, if produced at all, is probably unstable and rapidly degraded.
Our immunoblot experiments showed the normal presence of THIO in kidney and brain from the index patient, a finding that contrasts with the earlier data, reported by Schram et al. (1987), showing the absence of thiolase in liver. The most likely explanation for these discrepant results is that the quality of the liver material used by Schram et al. (1987), which was obtained postmortem, was very poor. Unfortunately, this possibility cannot be investigated, since this liver material is no longer available.
The first patient with a deficiency of DBP was described in 1997, 10 years after the reported thiolase deficiency in the index patient (Suzuki et al. 1997). Since then, several other cases of DBP deficiency have been reported in the literature (reviewed by Wanders et al. [2001a]), and, to date, DBP deficiency constitutes one of the most frequently occurring single-peroxisomal-enzyme–deficiency disorders. The clinical as well as the biochemical abnormalities in the index patient were similar to those reported in patients with an established DBP deficiency. The mutation identified in the patient has not been reported before. Our findings have significant implications, since they imply that the group of single–peroxisomal β-oxidation–enzyme deficiencies is limited to SCOX (Poll-The et al. 1988), DBP (Suzuki et al. 1997; van Grunsven et al. 1998, 1999a, 1999b), and α-methylacyl-CoA racemase deficiency (Ferdinandusse et al. 2000) and that THIO deficiency is no longer a distinct disease entity. In conclusion, this study stresses the importance, now that the knowledge of the peroxisomal β-oxidation system and of the enzymes involved has improved greatly during recent years, of reinvestigation of patients who, in the literature, have been described as having an unknown defect of peroxisomal β-oxidation. The elucidation of the true defect in these patients will further increase our understanding of the peroxisomal β-oxidation system and its substrates and will be important for prenatal diagnosis in this group of patients.
Acknowledgments
We are grateful to Prof. Hashimoto (Shinshu University, Matsumoto, Japan) for kindly providing the antibodies raised against SCOX, DBP, LBP, and THIO, and to Prof. Wirtz (Utrecht University, Utrecht) for kindly providing the antibodies raised against SCPx. This work was supported by the Princess Beatrix Fund (The Hague).
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Zellweger syndrome [MIM 214100], peroxisomal 3-ketoacyl-CoA thiolase deficiency [MIM 261510], and DBP deficiency [MIM 261515 and MIM 601860])
References
- Bout A, Franse MM, Collins J, Blonden L, Tager JM, Benne R (1991) Characterization of the gene encoding human peroxisomal 3-oxoacyl-CoA thiolase (ACAA): no large DNA rearrangement in a thiolase-deficient patient. Biochim Biophys Acta 1090:43–51 [DOI] [PubMed] [Google Scholar]
- Clayton PT, Patel E, Lawson AM, Carruthers RA, Collins J (1990) Bile acid profiles in peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. J Clin Invest 85:1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandusse S, Denis S, Clayton PT, Graham A, Rees JE, Allen JT, McLean BN, Brown AY, Vreken P, Waterham HR, Wanders RJ (2000) Mutations in the gene encoding peroxisomal α-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat Genet 24:188–191 [DOI] [PubMed] [Google Scholar]
- Goldfischer S, Collins J, Rapin I, Neumann P, Neglia W, Spiro AJ, Ishii T, Roels F, Vamecq J, Van Hoof F (1986) Pseudo-Zellweger syndrome: deficiencies in several peroxisomal oxidative activities. J Pediatr 108:25–32 [DOI] [PubMed] [Google Scholar]
- Jiang LL, Miyazawa S, Hashimoto T (1996) Purification and properties of rat d-3-hydroxyacyl-CoA dehydratase: d-3-hydroxyacyl-CoA dehydratase/d-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J Biochem (Tokyo) 120:633–641 [DOI] [PubMed] [Google Scholar]
- Leenders F, Dolez V, Begue A, Moller G, Gloeckner JC, de Launoit Y, Adamski J (1998) Structure of the gene for the human 17β-hydroxysteroid dehydrogenase type IV. Mamm Genome 9:1036–1041 [DOI] [PubMed] [Google Scholar]
- Ossendorp BC, Voorhout WF, van Amerongen A, Brunink F, Batenburg JJ, Wirtz KW (1996) Tissue-specific distribution of a peroxisomal 46-kDa protein related to the 58-kDa protein (sterol carrier protein x: sterol carrier protein 2/3-oxoacyl-CoA thiolase). Arch Biochem Biophys 334:251–260 [DOI] [PubMed] [Google Scholar]
- Poll-The BT, Roels F, Ogier H, Scotto J, Vamecq J, Schutgens RB, Wanders RJ, van Roermund CW, van Wijland MJ, Schram AW, Tager JM, Saudubray J-M (1988) A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy). Am J Hum Genet 42:422–434 [PMC free article] [PubMed] [Google Scholar]
- Schram AW, Goldfischer S, van Roermund CW, Brouwer-Kelder EM, Collins J, Hashimoto T, Heymans HS, van den Bosch H, Schutgens RB, Tager JM, Wanders RJ (1987) Human peroxisomal 3-oxoacyl-coenzyme A thiolase deficiency. Proc Natl Acad Sci USA 84:2494–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Jiang LL, Souri M, Miyazawa S, Fukuda S, Zhang Z, Une M, Shimozawa N, Kondo N, Orii T, Hashimoto T (1997) d-3-Hydroxyacyl-CoA dehydratase/d-3-hydroxyacyl-CoA dehydrogenase bifunctional protein deficiency: a newly identified peroxisomal disorder. Am J Hum Genet 61:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager JM, Van der Beek WA, Wanders RJ, Hashimoto T, Heymans HS, Van den Bosch H, Schutgens RB, Schram AW (1985) Peroxisomal β-oxidation enzyme proteins in the Zellweger syndrome. Biochem Biophys Res Commun 126:1269–1275 [DOI] [PubMed] [Google Scholar]
- van Grunsven EG, Mooijer PA, Aubourg P, Wanders RJ (1999a) Enoyl-CoA hydratase deficiency: identification of a new type of d-bifunctional protein deficiency. Hum Mol Genet 8:1509–1516 [DOI] [PubMed] [Google Scholar]
- van Grunsven EG, van Berkel E, IJlst L, Vreken P, de Klerk JB, Adamski J, Lemonde H, Clayton PT, Cuebas DA, Wanders RJ (1998) Peroxisomal d-hydroxyacyl-CoA dehydrogenase deficiency: resolution of the enzyme defect and its molecular basis in bifunctional protein deficiency. Proc Natl Acad Sci USA 95:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grunsven EG, van Berkel E, Mooijer PA, Watkins PA, Moser HW, Suzuki Y, Jiang LL, Hashimoto T, Hoefler G, Adamski J, Wanders RJ (1999b) Peroxisomal bifunctional protein deficiency revisited: resolution of its true enzymatic and molecular basis. Am J Hum Genet 64:99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veldhoven PP, Casteels M, Mannaerts GP, Baes M (2001) Further insights into peroxisomal lipid breakdown via α- and β-oxidation. Biochem Soc Trans 29:292–298 [DOI] [PubMed] [Google Scholar]
- Wanders RJA, Barth PG, Heymans HSA (2001a) Single peroxisomal enzyme deficiencies. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The molecular and metabolic bases of disease. McGraw-Hill, New York, pp 3219–3256 [Google Scholar]
- Wanders RJ, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, Van Roermund CW, Van Grunsven EG (2001b) Peroxisomal fatty acid α- and β-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans 29:250–267 [DOI] [PubMed] [Google Scholar]