Abstract
Objective
To assess the humoral immune response to low-dose AS03-adjuvanted and standard-dose nonadjuvanted 2009 pandemic H1N1 influenza A vaccine in HIV-infected aviremic individuals receiving antiretroviral therapy and in uninfected individuals.
Design
A three-arm study.
Setting
Two clinics: one at the National Institutes of Health in Bethesda, Maryland, USA; and the other at the Maple Leaf Medical Clinic in Toronto, Ontario, Canada.
Participants
HIV-infected and HIV-uninfected adults.
Intervention
Single intramuscular 15µg dose of the monovalent inactivated 2009 pandemic H1N1 influenza A vaccine without adjuvant or 3.75µg dose of the same strain with adjuvant AS03.
Main outcomes
Immunogenicity, as measured by hemagglutination inhibition (HAI) antibody titers and vaccine-specific memory B-cell responses.
Results
A total of 74 participants were enrolled. Twenty-one HIV-infected individuals received the low-dose adjuvanted 2009 pandemic H1N1 influenza A vaccine. Twenty-nine HIV-infected and 24 HIV-uninfected individuals received the standard-dose nonadjuvanted vaccine. There were no significant differences in antibody responses at 9 weeks postvaccination among the three groups studied. However, the IgG memory B-cell response against the vaccine was significantly higher in the HIV-infected group that received the low-dose adjuvanted vaccine when compared to the HIV-infected and uninfected groups that received the standard-dose nonadjuvanted vaccine. Conclusions remained unchanged after regression adjustment for age, gender, CD4+ T-cell count, and baseline HAI titer.
Conclusion
These data suggest that adjuvants could be used to expand coverage through dose sparing and improve humoral immune responses in immunocompromised individuals.
Keywords: adjuvants, antibody response, HIV infection, memory B-cell response, pandemic influenza, vaccination
Introduction
The rapid emergence of the 2009 pandemic H1N1 influenza A virus led to unprecedented global efforts to vaccinate large numbers of people within a short period of time. Several high-risk groups were identified for priority vaccination, including immunocompromised individuals [1]. Among this priority list are HIV-infected individuals [1]. Longstanding recommendations to vaccinate HIV-infected individuals against seasonal influenza stem from several reports that HIV disease is associated with higher influenza-related morbidity and mortality rates compared to the general population [2–5].
Vaccination remains the most efficacious and cost-effective strategy to prevent the spread of influenza and to reduce influenza-related morbidities and mortalities [6]. However, as seasonal influenza viruses vary from year to year (antigenic drift) and novel strains emerge (antigenic shift), such as the 2009 pandemic H1N1 influenza A virus, current vaccination production and implementation approaches may not be adequate with regard to the amount of vaccine available (surge capacity), the strength of the immune response that is elicited, or the breadth of coverage against divergent strains. The use of adjuvants provides the opportunity to spare dosage as well as to increase the strength and breadth of elicited responses [7, 8]. Several studies performed on healthy individuals have demonstrated that inclusion of adjuvant in influenza vaccines is safe [7–11]. Two of these studies reported an increase in immunogenicity and cross-reactivity for an adjuvanted H5N1 vaccine [7, 9], whereas results were mixed in the three other studies reporting on the immunogenicity of the 2009 pandemic H1N1 vaccine. Modest enhancing effects were shown in one interim report using the oil-in-water adjuvant MF59 [10] and in one preliminary report using another oil-in-water adjuvant, AS03A [8]. However, decreased immunogenicity was shown in the third study in which alum was used as adjuvant [11]. Furthermore, adjuvants have been shown to enhance the strength of immune responses to vaccines in immunocompromised individuals [12], including against seasonal influenza in HIV-infected individuals [13]. The enhancing effects of adjuvants may be particularly important for influenza vaccines, given the numerous reports showing an impaired humoral immune response to nonadjuvanted seasonal influenza vaccination in HIV-infected individuals [14–17]. Reduced responsiveness to seasonal influenza vaccination has also been associated with HIV disease progression [14, 16, 18, 19], which in turn is associated with increasing incidences of influenza-related morbidity and mortality [2, 4, 5]. However, in a recent report on the 2009 pandemic H1N1 strain, antibody responses to a nonadjuvanted vaccine were poor even among well controlled HIV-infected individuals [20]. Thus, it would be important to attempt to increase the strength of the immune response to influenza vaccination in HIV-infected individuals, especially against new emerging strains of influenza such as the 2009 pandemic H1N1 wherein preexisting immunity is expected to be low. The induction and maintenance of a humoral immune response following vaccination with an inactivated influenza virus formulation is necessary to confer protection against infection [21]. Serologic antibody titers, the most widely reported component of the humoral immune response following vaccination, are maintained by long-lived plasma cells residing primarily in the bone marrow and the spleen [22, 23].However, humoral immunity is alsomaintained by antigen-specific memory B cells [22, 23], a less frequently reported component of the humoral response that may be as essential as plasma cells in maintaining memory for rapid antibody responses [24]. In this regard, whereas pathogen-specific antibodies induced by vaccination are the first line of defense following re-exposure, pathogen-specific memory B cells are rapidly induced to undergo affinity maturation, a process that increases the effectiveness of the antibody response and helps prevent the emergence of clinical manifestations associated with the pathogen [23].
In this study, we report on the humoral immune response against the inactivated 2009 pandemic H1N1 influenza A virus whole-virion vaccine, administered either at full dose without adjuvant or at one-quarter dose in the presence of an oil-in-water emulsion-based adjuvant AS03. The effect of adjuvant was studied in two groups of HIV-infected individuals both of which had been rendered aviremic by antiretroviral therapy (ART) and one group of HIV-uninfected individuals. The responses measured included hemagglutination inhibition (HAI) antibody titers in serum as well as vaccine-specific memory B-cell frequencies in the peripheral blood. Our findings indicate that adjuvants may increase the immunogenicity of influenza vaccines in immunocompromised individuals, especially against new emerging pandemic strains to which individuals may not have been previously exposed and against which there may be little or no preexisting, cross-reacting immunity.
Methods
Participants
We recruited 74 participants over the age of 18 years between October 2009 and January 2010 to receive a single dose of vaccine against the 2009 pandemic H1N1 influenza A virus. Participants recruited at the National Institutes of Health (NIH) in the United States included 24 HIV-uninfected (NIH-HIV-negative) and 29 HIV-infected (NIH-HIV-positive) individuals. The NIH-HIV-negative group consisted of healthcare workers who were identified for priority vaccination against the 2009 pandemic H1N1 influenza A virus. In addition, 21 HIV-infected participants were recruited at the Maple Leaf Medical Clinic in Toronto, Ontario, Canada (TO-HIV-positive). All participants in the two HIV-infected groups were receiving effective ART and maintained a level of HIV plasma viremia below the limit of detection of 50 copies of HIV RNA per milliliter at the time of study (branched DNA assay; Bayer Diagnostics, New York, New York, USA, and Chiron Corp, Emeryville, California, USA). Lymphocyte counts were performed at core facilities of the two sites. All participants provided informed consent for procedures approved by the Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (NIAID), at the NIH, USA, the University of Toronto, Toronto, Canada, and by the Office of Human Subjects Research at the NIH, USA.
Vaccines
Participants recruited at the NIH site received one dose of inactivated split-virion vaccine derived from A/California/ 7/2009 H1N1-like X-179A strain containing 15µg hemagglutinin (Novartis Vaccines, Cambridge, Massachusetts, USA), in accordance with recommendations from the US Centers for Disease Control and Prevention. Participants recruited at the Toronto site received one dose of an adjuvanted inactivated split-virion vaccine derived from the above strain and containing 3.75µg hemagglutinin (GlaxoSmithKline, Research Triangle Park, North Carolina, USA), in accordance with recommendations from the Public Health Agency of Canada. The adjuvanted vaccine was administered following emulsification with the adjuvant AS03 (containing 11.86 mg DL-α-tocopherol, 10.69 mg squalene, and 4.86 mg polysorbate 80 per dose), as per the manufacturer’s recommendations.
Procedures
Serum was obtained prior to vaccination and at day 63+/− 7 days postvaccination. In two groups, NIH-HIV-negative and TO-HIV-positive, additional serum samples were available between day 7 and day 28 postvaccination. Antibody titers against A/California/7/2009 H1N1 were measured in duplicate on stored serum samples by the HAI assay in a standard microtiter format as previously described [25]. Whole blood was obtained at day 63 postvaccination and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation. To evaluate the memory B-cell response, PBMCs were stimulated for 4 days with Staphylococcus aureus Cowan and CpG oligonucleotide and then used to measure frequencies of antibody-secreting cells (ASCs) by ELI-SPOT as previously described [14], with the following modifications. Acrowell polyvinylidene fluoride filter plates (Pall, Port Washington, New York, USA) were coated with the nonadjuvanted H1N1 vaccine preparation. Bound antibodies were detected using alkaline phosphatase conjugated antihuman IgG and peroxidase conjugated antihuman IgA (KPL, Gaithersburg, Maryland, USA) with subsequent development using alkaline phosphatase and peroxidase substrate kits (Vector Laboratories, Burlingame, California, USA). ASC frequencies were normalized to the number of B cells in each sample by evaluating percentage CD19+ B cells in cultured PBMCs using cytofluorometric analysis.
Statistical analysis
Geometric mean titers (GMTs) of HAI responses were calculated for each group and reported with 95% confidence intervals. Differences in HAI titers and ASC frequencies between the three groups were analyzed using the Kruskal–Wallis test followed by pairwise analyses using the Wilcoxon rank-sum test. Regression analyses were performed to statistically address potential confounding between groups and CD4+ T-cell count, age, gender, and baseline HAI titer. The variables age and CD4+ T-cell count were compared pairwise using theWilcoxon ranksum test, whereas gender was compared pairwise using Fisher’s exact test. Key analyses were repeated after omission of five patients with suspected exposure to natural influenza. All reported P values are two-sided with no adjustment for multiple testing. Analyses were performed using SAS (Version 9.2; SAS Institute, Cary, North Carolina, USA) and Prism software (Version 5.0; GraphPad Software, La Jolla, California, USA).
Results
Table 1 shows group demographics for the 74 participants. There were no significant differences in age among the three groups. As expected, there was a significant difference in CD4+ T-cell counts between the NIH-HIV-negative group and the two HIV-positive groups (P=0.0008 for NIH-HIV-positive and P=0.0004 for TO-HIV-positive). However, there was no significant difference between the two HIV-positive groups. There were significant differences between groups regarding gender that were beyond the control of the study design, given that no exclusion other than HIV plasma viremia was considered for the HIV-positive groups and that the majority of healthcare workers who volunteered for the study at NIH were women. The P values for gender differences between each group were as follows: 0.0061 for NIH-HIV-negative vs. NIH-HIV-positive; <0.0001 for NIH-HIV-negative vs. TO-HIV-positive; and 0.0147 for NIH-HIV-positive vs. TO-HIV-positive. However, based on previous findings [26], the gender bias was likely to have a negligible effect on immune response outcomes.
Table 1.
Description of study groups.
| NIH-HIV-negative | NIH-HIV-positive | TO-HIV-positive | |
|---|---|---|---|
| Number of participants | 24 | 29 | 21 |
| Age (years)a | 43 (23–61) | 45 (24–73) | 47 (26–62) |
| Men | 9 (38%) | 21 (72%) | 21 (100%) |
| CD4+ T-cell count (cells/µl)a | 805 (440–1401) | 590 (194–962) | 520 (190–1230) |
| HIV RNA (copies/ml plasma) | N/A | <50 | <50 |
| H1N1 vaccine | 15 µg without AS03 | 15 µg without AS03 | 3.75 µg with AS03 |
| Evidence of H1N1 prereactivityb | 3 (12%) | 4 (14%) | 6 (29%) |
| Baseline HAI titer ≥1 : 40 | N/A | 3 (15%) | 3 (14%) |
N/A, not applicable.
Median (range).
Detectable hemagglutination inhibition (HAI) titer (≥1 : 10) prior to vaccination.
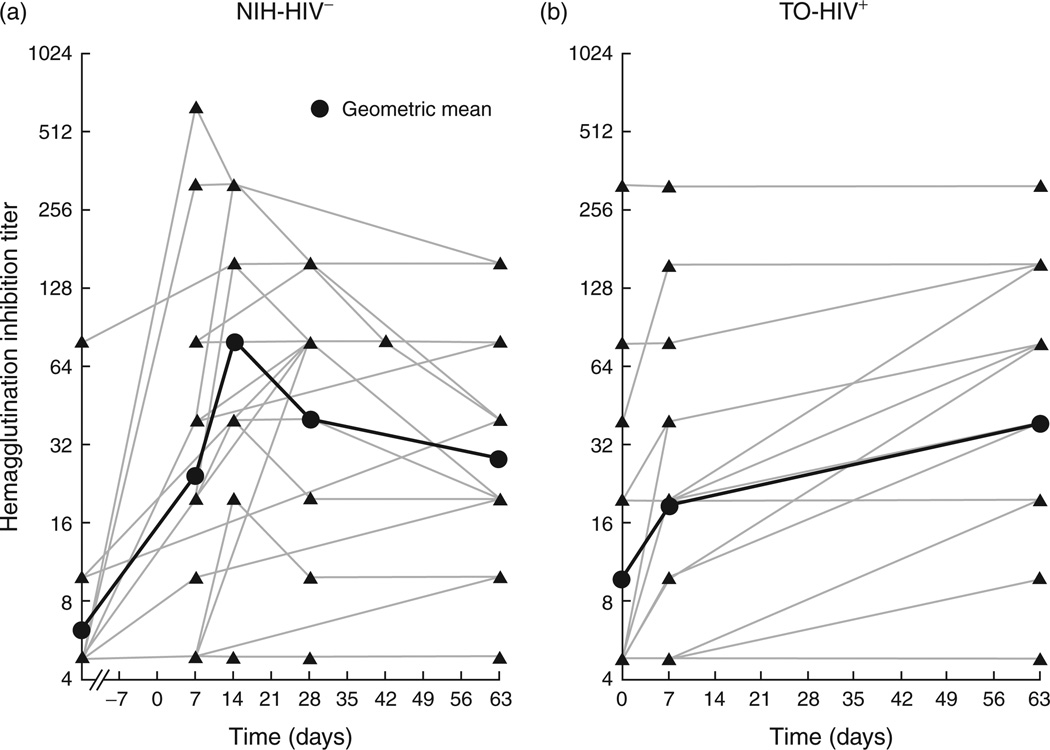
Evidence of preexposure to or the existence of cross-reactive immunity to 2009 pandemic H1N1 influenza was evaluated using various parameters, depending on the availability and timing of pre and postvaccination serum samples and clinical observations. In the NIH-HIV-negative group, sequential serum samples were available on several participants, and baseline serum samples were obtained several weeks prior to vaccination. As shown in Fig. 1(a), peak antibody responses occurred at 14 days postvaccination, consistent with preliminary findings for the 2009 pandemic H1N1 influenza vaccine [1, 10]. Based on expected HAI kinetics and clinical reporting, evidence of recent exposure to H1N1 was identified in three of the 24 NIH-HIV-negative participants. A similar level of preexposure was observed in the NIH-HIV-positive participants (Table 1). In the TO-HIV-positive group, serum samples were available at day 0, as well as day 7 and day 63 postvaccination (Fig. 1b). The kinetics of HAI and clinical reporting suggest that two individuals may have been recently exposed (Fig. 1a), and another four had prevaccination reactivity to a virus or vaccine similar to the 2009 pandemic H1N1 influenza A virus (Table 1).
Fig. 1. Kinetics of hemagglutination inhibition antibody response to 2009 pandemic H1N1 influenza A vaccine.
Graphs are shown for (a) NIH-HIV-negative and (b) TO-HIV-positive groups. The bold line represents the geometric mean titer (GMT) for each group. The broken line on the X-axis in (a) indicates that baseline serologies were measured several weeks prior to vaccination at day 0.
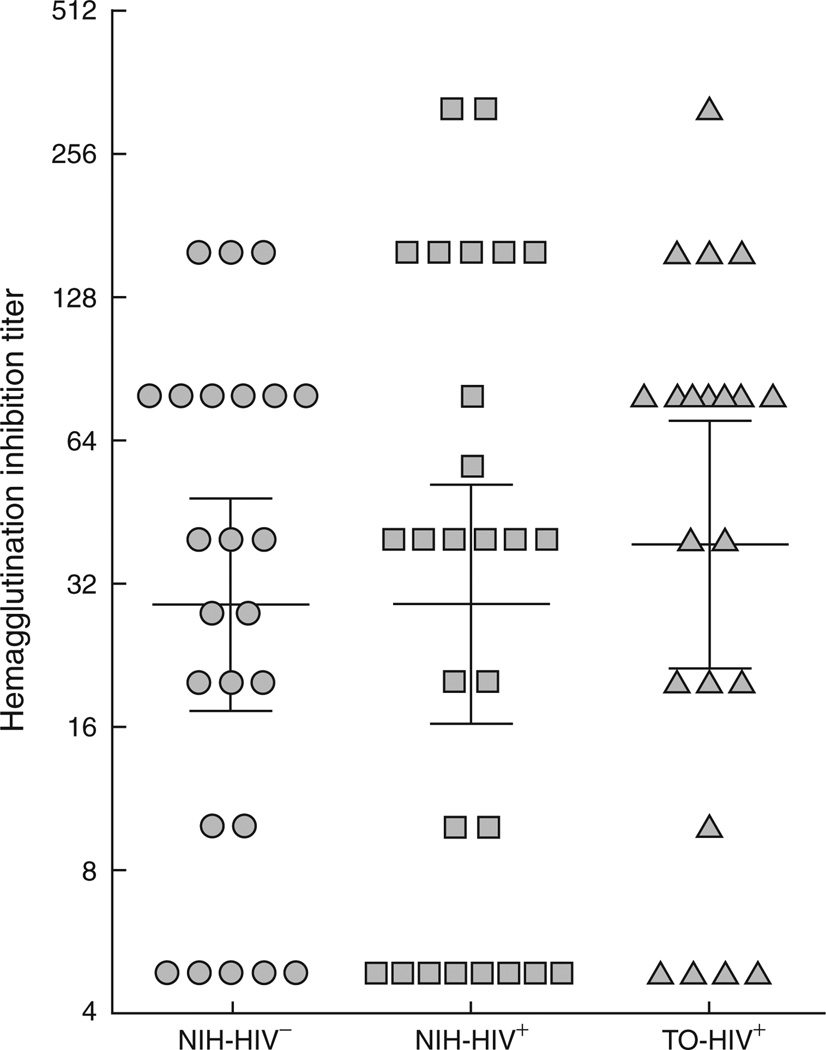
HAI titers for each group are shown at day 63 postvaccination in Fig. 2, with statistical analyses shown in Table 2. There was no significant difference in HAI titers between the three groups studied and the differences in log HAI titers among the three groups remained nonsignificant after regression adjustments for baseline HAI titer.
Fig. 2. Hemagglutination inhibition titers against the 2009 pandemic H1N1 influenza A virus at 9 weeks postvaccination.
Horizontal bars represent the geometric mean titer (GMT) and whiskers indicate the 95% confidence interval for each group.
Table 2.
Antibody titers and memory B-cell responses postvaccination.
| Outcome measurements |
NIH-HIV- negative A |
NIH-HIV- positive B |
TO-HIV- positive C |
Pairwise Wilcoxon test P value |
Overall Kruskal–Wallis test P value |
|---|---|---|---|---|---|
| HAI H1N1 | 34 | 40 | 80 | A vs. B: 1.0000 A vs. C: 0.4412 B vs. C: 0.4765 |
0.69 |
| Mem IgG Total | 15728 | 42282 | 49123 | A vs. B: 0.0017 A vs. C: 0.0003 B vs. C: 0.6088 |
0.0001 |
| Mem IgA Total | 5621 | 22420 | 19444 | A vs. B: 0.0014 A vs. C: 0.0035 B vs. C: 0.9279 |
0.0008 |
| Mem IgG H1N1 | 95 | 62 | 170 | A vs. B: 0.2680 A vs. C: 0.0105 B vs. C: 0.0011 |
0.0012 |
| Mem IgA H1N1 | 3 | 0 | 7 | A vs. B: 0.5103 A vs. C: 0.5467 B vs. C: 0.4044 |
0.6089 |
Data are median or P values. HAI, hemagglutinin inhibition antibody titer; Mem, memory B-cell antibody-secreting cell (ASC) frequency per 106 B cells.
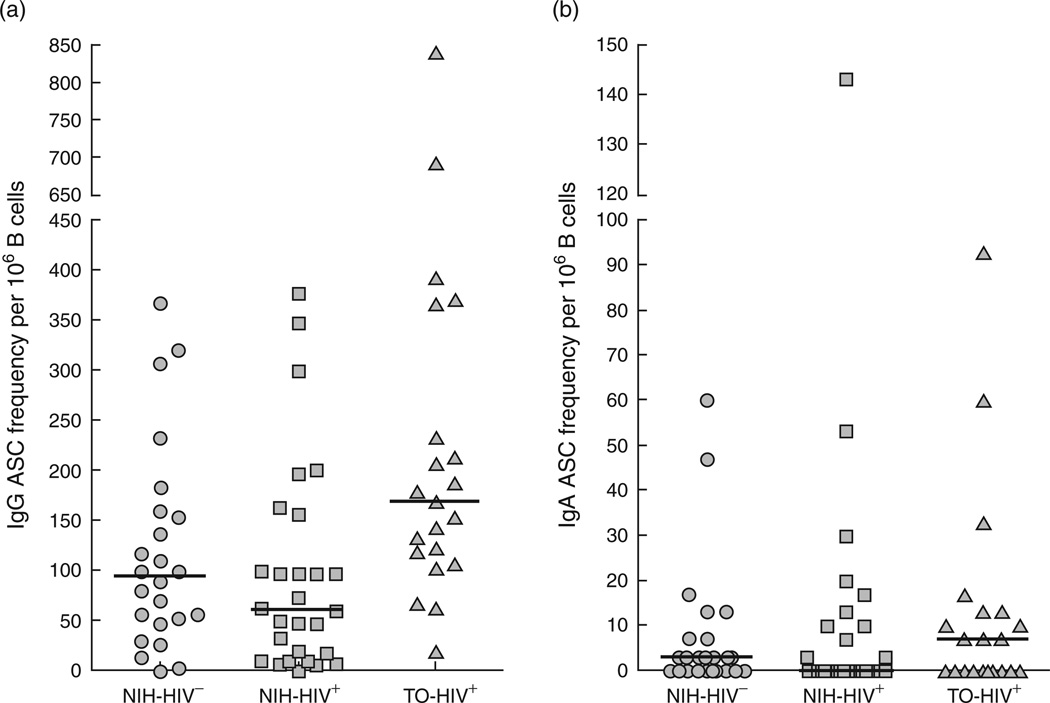
Frequencies of memory B-cell response against the 2009 pandemic H1N1 influenza vaccine at day 63 postvaccination were measured by ELISPOT after in-vitro stimulation (Fig. 3). Several subsets of memory B cells exist in the peripheral blood of HIV-infected individuals, especially those with ongoing viremia [27]. However, we have shown that the majority of influenza-specific memory B cells are CD27+ B cells [28], and more specifically CD21hi/CD27+ B cells in HIV-aviremic individuals [29]. Although the amount of blood obtained in the current study precluded precise determination of the phenotype of H1N1-specific memory B cells, preculture analysis confirmed that the majority of memory B cells in both HIV-aviremic groups and HIV-negative individuals were of the CD21hi/CD27+ phenotype (data not shown). Furthermore, plasma cells present at the start of the culture period were unlikely to contribute significantly to these frequencies given that they represented less than 2% of peripheral blood B cells in the cohorts studied here (data not shown) and that they preferentially die during the in-vitro stimulation period [28]. Controls for nonspecific binding (Keyhole limpet hemocyanin) and total immunoglobulin-secreting capacity (IgG and IgA) were also included for each sample. The latter control also served to verify that delays in transportation of blood from Toronto did not alter overall B-cell properties. There was no significant difference in total immunoglobulin-secreting capacity (IgG and IgA) between the two HIV-positive groups, although they were both significantly higher compared to the NIH-HIV-negative group (Table 2), consistent with residual B-cell hyperactivity in HIV-infected individuals receiving ART [27]. Finally, H1N1 vaccine-specific memory B-cell IgG responses were significantly higher in the TO-HIV-positive group having received the adjuvanted quarter-dose vaccine formulation compared to both the NIH-HIV-negative and NIH-HIV-positive groups having received the nonadjuvanted standard-dose vaccine formulation (Fig. 3 and Table 2). H1N1 IgA-specific responseswere not significantly different between the three groups (Table 2). However, IgA responses constituted a minor component of the H1N1-specific response when compared to total IgA and H1N1-specific IgG responses (see Table 1 and Fig. 3). Regression analyses using frequency and log frequency of B-cell responses as outcomes while adjusting for CD4+ T-cell count, age, gender, and baseline HAI titer were all consistent with the between group comparisons of Table 2. Results were unchanged when five participants with evidence of exposure to natural influenza were excluded from analyses.
Fig. 3. Memory B-cell responses against the 2009 pandemic H1N1 influenza A vaccine at 9 weeks postvaccination.
Frequencies of vaccine-specific antibody-secreting cells (ASCs) were measured from peripheral blood mononuclear cells (PBMCs) after 4 days of stimulation and reported per million B cells for (a) IgG and (b) IgA. Horizontal bars represent the median for each group.
Discussion
In the present study, we showed that vaccination of HIV-infected individuals with a low-dose AS03-adjuvanted 2009 pandemic H1N1 influenza A formulation induced a higher memory B-cell response and a similar antibody response when compared to vaccination with a standard full-dose nonadjuvanted vaccine in both HIV-infected and HIV-uninfected individuals. The enhanced response observed with an adjuvanted vaccine given at one-quarter the dose of the nonadjuvanted formulation raises two important concepts: that adjuvants such as AS03 may help extend coverage among a greater number of individuals in times of limited vaccine supply through dose-sparing; and that adjuvants may help improve immune responses, especially against neoantigens such as the 2009 pandemic H1N1 strain, in immunocompromised individuals, including HIV-infected individuals. Previous studies on seasonal influenza have shown that HIV-infected individuals with low CD4+ T-cell counts and ongoing HIV viremia have lower antibody and B-cell responses compared to healthy individuals [14–17]. A more recent study on the immunogenicity of a nonadjuvanted 2009 pandemic H1N1 influenza vaccine found low seroconversion rates even among HIV-infected individuals with favorable immunologic and virologic profiles [20]. Given the evidence that adjuvants can safely induce and boost humoral immune responses to vaccines in immunocompromised individuals [12, 13, 30, 31], our present findings suggest that adjuvants may provide new opportunities for improving protection against influenza in high-risk individuals.
A recent study reported a low rate of seroconversion in HIV-infected individuals who received an AS03-adjuvanted vaccine against pandemic 2009 H1N1 influenza [32]; however, there was no healthy control group and results were discussed in relation to recent reporting on response to this vaccine formulation. Caution should be exerted when comparing studies in which differences in HAI assays are inevitable [33], especially in light of the fact that for a majority of individuals pandemic 2009 H1N1 influenza represents a neoantigen. Our data showed the induction of low HAI titers for all groups studied. However, we measured the antibody response at day 63, several weeks past the peak of day 21 reported in other studies [8, 10, 11]. Furthermore, there are indications that protection against influenza, and in particular against pandemic 2009 H1N1 influenza, can be conferred in the absence of measurable HAI titers [26]. In this regard, although there is evidence that antibody responses are correlated with memory B-cell responses for certain antigens [24], this may not be the case for influenza in which memory B cells can be detected in the absence of antibodies [34]. However, regardless of the nature of the antigen and correlations between responses, antibodies secreted from long-lived plasma cells and affinity-matured antibodies arising from memory B cells, are both thought to be essential for maintaining humoral immunity [22, 23].
Our study contained a number of limitations, including a relatively small sample size and time constraints that precluded randomization and complete serologic baseline data. The low sample size limited our ability to detect potential differences in HAI titers between the groups studied. Nonetheless, our data indicated that the adjuvanted low-dose was similar to the nonadjuvanted standard-dose formulation in inducing antibodies against the vaccine. With regard to baseline serologies, the absence of serum samples in the NIH-HIV-negative groups was compensated by numerous samples prior to and following vaccination, in addition to extensive clinical documentation and evidence from regression analyses, which indicated that imbalances between the groups did not affect outcomes. Our results provide a rational basis for designing a larger randomized study.
Thus, on the basis of our findings, we recommend further investigation into the use of adjuvants in influenza vaccine formulations, particularly when used in immunocompromised individuals against new emerging pandemic strains against which there may be little or no preexisting, cross-reacting immunity. Previous studies have shown AS03-based formulations to be safe [8, 9], and our study showed evidence of enhanced immunogenicity with a low-dose formulation. Future studies on immunogenicity and protection should include the assessment of antibody as well as memory B-cell responses.
Acknowledgements
We thank the volunteers for their participation in this study. We thank Catherine A. Rehm, Erika Benko, Marie A. O’Shea, and Laura Heytens for their assistance with participant recruitment and sample processing. We thank J. Shawn Justement, Danielle Murray, and Clarisa M. Buckner for technical assistance. This work was funded by the Intramural Research Program of NIAID at the NIH, and in part with federal funds from the National Cancer Institute, NIH, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
J.H., S.M., T.-W.C., and A.S.F. designed the study; C.K. and M.S. provided research participants; J.H., W.W., J.G.P., R.D., T.-W.C., and M.T.R. implemented the study; J.H., S.M., R.D., W.G., D.F., T.W.C., and A.S.F. interpreted the data; J.H., S.M., and A.S.F. wrote the article.
References
- 1.National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC) Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–8. [PubMed] [Google Scholar]
- 2.Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med. 2001;161:441–446. doi: 10.1001/archinte.161.3.441. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Reed GW, Mitchel EF, Jr, Griffin MR. Influenza-associated morbidity and mortality in young and middle-aged women. JAMA. 1999;281:901–907. doi: 10.1001/jama.281.10.901. [DOI] [PubMed] [Google Scholar]
- 4.Radwan HM, Cheeseman SH, Lai KK, Ellison IR. Influenza in human immunodeficiency virus-infected patients during the 1997–1998 influenza season. Clin Infect Dis. 2000;31:604–606. doi: 10.1086/313985. [DOI] [PubMed] [Google Scholar]
- 5.Safrin S, Rush JD, Mills J. Influenza in patients with human immunodeficiency virus infection. Chest. 1990;98:33–37. doi: 10.1378/chest.98.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine. 2008;26(Suppl 4):D17–D22. doi: 10.1016/j.vaccine.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 7.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 8.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1 v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Levie K, Leroux-Roels I, Hoppenbrouwers K, Kervyn AD, Vandermeulen C, Forgus S, et al. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis. 2008;198:642–649. doi: 10.1086/590913. [DOI] [PubMed] [Google Scholar]
- 10.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 11.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 12.Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 2008;46:1310–1314. doi: 10.1086/533467. [DOI] [PubMed] [Google Scholar]
- 13.Iorio AM, Francisci D, Camilloni B, Stagni G, De Martino M, Toneatto D, et al. Antibody responses and HIV-1 viral load in HIV-1-seropositive subjects immunised with either the MF59- adjuvanted influenza vaccine or a conventional nonadjuvanted subunit vaccine during highly active antiretroviral therapy. Vaccine. 2003;21:3629–3637. doi: 10.1016/s0264-410x(03)00408-0. [DOI] [PubMed] [Google Scholar]
- 14.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 15.Nelson KE, Clements ML, Miotti P, Cohn S, Polk BF. The influence of human immunodeficiency virus (HIV) infection on antibody responses to influenza vaccines. Ann Intern Med. 1988;109:383–388. doi: 10.7326/0003-4819-109-5-383. [DOI] [PubMed] [Google Scholar]
- 16.Amendola A, Boschini A, Colzani D, Anselmi G, Oltolina A, Zucconi R, et al. Influenza vaccination of HIV-1-positive and HIV-1-negative former intravenous drug users. J Med Virol. 2001;65:644–648. doi: 10.1002/jmv.2085. [DOI] [PubMed] [Google Scholar]
- 17.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine. 2000;18:3040–3049. doi: 10.1016/s0264-410x(00)00079-7. [DOI] [PubMed] [Google Scholar]
- 18.Kroon FP, Rimmelzwaan GF, Roos MT, Osterhaus AD, Hamann D, Miedema F, et al. Restored humoral immune response to influenza vaccination in HIV-infected adults treated with highly active antiretroviral therapy. AIDS. 1998;12:F217–F223. doi: 10.1097/00002030-199817000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Evison J, Farese S, Seitz M, Uehlinger DE, Furrer H, Muhlemann K. Randomized, double-blind comparative trial of subunit and virosomal influenza vaccines for immunocompromised patients. Clin Infect Dis. 2009;48:1402–1412. doi: 10.1086/598193. [DOI] [PubMed] [Google Scholar]
- 20.Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;14:2184–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 21.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 25.Dowdle W, Kendal A, Noble G, editors. Influenza viruses. 5th ed. Washington, DC: American Public Health Association; 1979. [Google Scholar]
- 26.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–1108. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 27.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010 doi: 10.1182/blood-2010-05-285528. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol. 2008;15:253–259. doi: 10.1128/CVI.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. CurrOpin Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, Stephan C, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV- 1-infected patients. AIDS. 2010;24:F31–F35. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 33.Allwinn R, Geiler J, Berger A, Cinatl J, Doerr HW. Determination of serum antibodies against swine-origin influenza A virus H1N1/09 by immunofluorescence, haemagglutination inhibition, and by neutralization tests: how is the prevalence rate of protecting antibodies in humans? Med Microbiol Immunol. 2010;199:117–121. doi: 10.1007/s00430-010-0143-4. [DOI] [PubMed] [Google Scholar]
- 34.Baer J, Santiago F, Yang H, Wu H, Holden-Wiltse J, Treanor J, et al. B cell responses to H5 influenza HA in human subjects vaccinated with a drifted variant. Vaccine. 2010;28:907–915. doi: 10.1016/j.vaccine.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]