Abstract
Object
Gamma Knife surgery (GKS) has been reported as an effective modality for treating brain metastases from renal cell carcinoma (RCC). The authors aimed to determine if targeted agents such as tyrosine kinase inhibitors, mammalian target of rapamycin inhibitors, and bevacizumab affect the patterns of failure of RCC after GKS.
Methods
Between 1999 and 2010, 61 patients with brain metastases from RCC were treated with GKS. A median dose of 20 Gy (range 13–24 Gy) was prescribed to the margin of each metastasis. Kaplan-Meier analysis was used to determine local control, distant failure, and overall survival rates. Cox proportional hazard regression was performed to determine the association between disease-related factors and survival.
Results
Overall survival at 1, 2, and 3 years was 38%, 17%, and 9%, respectively. Freedom from local failure at 1, 2, and 3 years was 74%, 61%, and 40%, respectively. The distant failure rate at 1, 2, and 3 years was 51%, 79%, and 89%, respectively. Twenty-seven percent of patients died of neurological disease. The median survival for patients receiving targeted agents (n = 24) was 16.6 months compared with 7.2 months (n = 37) for those not receiving targeted therapy (p = 0.04). Freedom from local failure at 1 year was 93% versus 60% for patients receiving and those not receiving targeted agents, respectively (p = 0.01). Multivariate analysis showed that the use of targeted agents (hazard ratio 3.02, p = 0.003) was the only factor that predicted for improved survival. Two patients experienced post-GKS hemorrhage within the treated volume.
Conclusions
Targeted agents appear to improve local control and overall survival in patients treated with GKS for metastastic RCC.
Keywords: renal cell carcinoma, brain metastasis, stereotactic radiosurgery, targeted agent
Approximately 10% of patients with RCC develop brain metastases.20 Previous series have found that as many as 76% of patients with brain metastases from RCC who are treated with WBRT will ultimately die of these metastases.20 Moreover, GKS has become a standard option in the treatment of brain metastases from RCC; several single-institution series have suggested a survival benefit in patients undergoing radiosurgical management.12 Local control rates from multiple single-institution series have been high, and predictors for longer-term survival after radiosurgery include fewer brain lesions, RPA classification, and interval from diagnosis of RCC to time to development of brain metastasis.11,16,17
Recent series of patients with brain metastases of all histological types treated with GKS have suggested a trend toward improving overall survival in patients who were treated in the post-2005 era.9 One of the hypotheses for this recent improvement in survival is the advent of novel systemic therapies, or so-called “targeted therapies,” in that time period. In particular, RCC has undergone a dramatic improvement in systemic disease management as agents such as sunitinib (Sutent, Pfizer), sorafenib (Nexavar, Bayer), temsirolimus (Torisel, Wyeth), and bevacizumab (Avastin, Genentech) have all been shown to improve outcomes in major randomized trials.3,8,10,21 As a result, it was decided to compare the clinical outcomes of patients treated with targeted agents with the cohort managed with previously considered standard options including immunotherapy, metastasectomy, cytotoxic chemotherapy, and those who were followed expectantly.
We present a retrospective series of patients treated with GKS at a single institution between 1999 and 2010. In our analysis, we concentrate particularly on how the evolving systemic management of RCC has affected the outcomes of metastatic brain disease after GKS.
Methods
Data Acquisition
This study was approved by the Wake Forest University Institutional Review Board. The Wake Forest University Medical Center Department of Radiation Oncology Gamma Knife Tumor Registry was searched for all patients who underwent GKS and had a diagnosis of RCC. Sixty-one patients with RCC were identified who were treated with GKS between November 1999 and June 2010 at Wake Forest University Baptist Medical Center in Winston-Salem, North Carolina. Patient outcomes were determined using the patients’ electronic medical records.
Patient Characteristics
Patient characteristics are summarized in Table 1. Patient factors such as age, RPA class, status of extracranial metastatic disease, MSKCC risk group, and previous systemic therapeutic regimens and numbers of such cycles were determined from patients’ electronic medical records. The RPA class was defined as per the Radiation Therapy Oncology Group analysis reported by Gaspar et al.5 The status of extracranial metastatic disease was recorded as none, oligometastatic, or widespread. Oligometastatic disease was defined as 5 or fewer nonbrain metastases in areas such as bone, the chest, or the pelvis. Widespread metastatic disease was diagnosed in patients with more than 5 metastases or diffuse organ involvement. A modified MSKCC risk group was also assigned to each patient similar to that reported by Eggener et al.2 The risk grouping was modified because of an insufficient number of patients with lactate dehydrogenase values (Table 2). Of 61 patients in the series, 24 received targeted agents, classified as 1 or more of tyrosine kinase inhibitors, mTOR inhibitors, and/or bevacizumab. Significant time intervals that were recorded included time to local failure, distant failure, and death. Neurological death was also determined as reported previously by Patchell et al.13 The number of brain metastases and the prescription dose delivered to the margin of each metastasis were all recorded. Post-GKS steroid use, hemorrhage, and any reoperations for radiation necrosis were also recorded.
TABLE 1.
Modified MSKCC risk grouping*
| Characteristic | Points Assigned |
|---|---|
| recurrence <1 yr after nephrectomy | 1 |
| calcium >10 mg/dl | 1 |
| hemoglobin (g/dl) | |
| <13.0 (male) | 1 |
| <11.5 (female) | 1 |
| KPS score <80 | 1 |
KPS = Karnofsky Performance Scale.
TABLE 2.
Patient characteristics*
| Characteristic | Value |
|---|---|
| no. of patients | 61 |
| age (yrs) | |
| median | 62 |
| range | 43–89 |
| sex | |
| female | 11 |
| male | 50 |
| extent of extracranial disease | |
| none | 5 |
| oligometastatic | 28 |
| widespread | 27 |
| KPS score | |
| median | 80 |
| range | 60–90 |
| RPA class | |
| I | 4 |
| II | 49 |
| III | 7 |
| modified MSKCC risk group | |
| 0 | 6 |
| 1 | 27 |
| 2 | 21 |
| 3 | 5 |
| 4 | 1 |
| prior WBRT | 9 |
| no. of lesions | |
| median | 2 |
| range | 1–12 |
| minimal marginal dose (Gy) | |
| median | 20 |
| range | 13–24 |
| time to GKS (mos) | |
| median | 13.5 |
| range | 7–42 |
Values indicate the number of patients unless specified otherwise.
Radiosurgical Technique
After evaluation by a radiation oncologist and neurosurgeon, informed consent for GKS was obtained. Patients were treated using a Leksell Model B, C, or Perfexion unit (Elekta AB). Prior to radiosurgery, the patients underwent a high-resolution contrast-enhanced stereotactic MRI study of the brain. Treatment planning was performed using the Leksell GammaPlan Treatment Planning System (Elekta AB). A median dose of 20 Gy (range 13–24 Gy) was prescribed to the margin of each metastasis. The dose prescription was determined based on the size and volume of the metastasis following guidelines published by Shaw et al.15 for single-fraction radiosurgical treatment of brain metastases.
Patient Follow-Up and Salvage
Patients underwent additional MRI of the brain 4–8 weeks after the initial GKS procedure and then every 3 months thereafter. Distant brain failures were generally treated using additional GKS, if feasible. Local failures were treated with resection or WBRT, or they were observed until they became symptomatic.
Statistical Analysis
Kaplan-Meier analysis was performed to determine actuarial local control, distant brain control, and the overall survival of our patient population. The log-rank test was used to determine statistical differences between survival times. The chi-square test was used to determine differences in likelihood of neurological death between cohorts. Multivariate analysis using Cox proportional hazard regression was performed to determine factors that predicted for improved survival, local control, and distant brain control.
Results
Survival
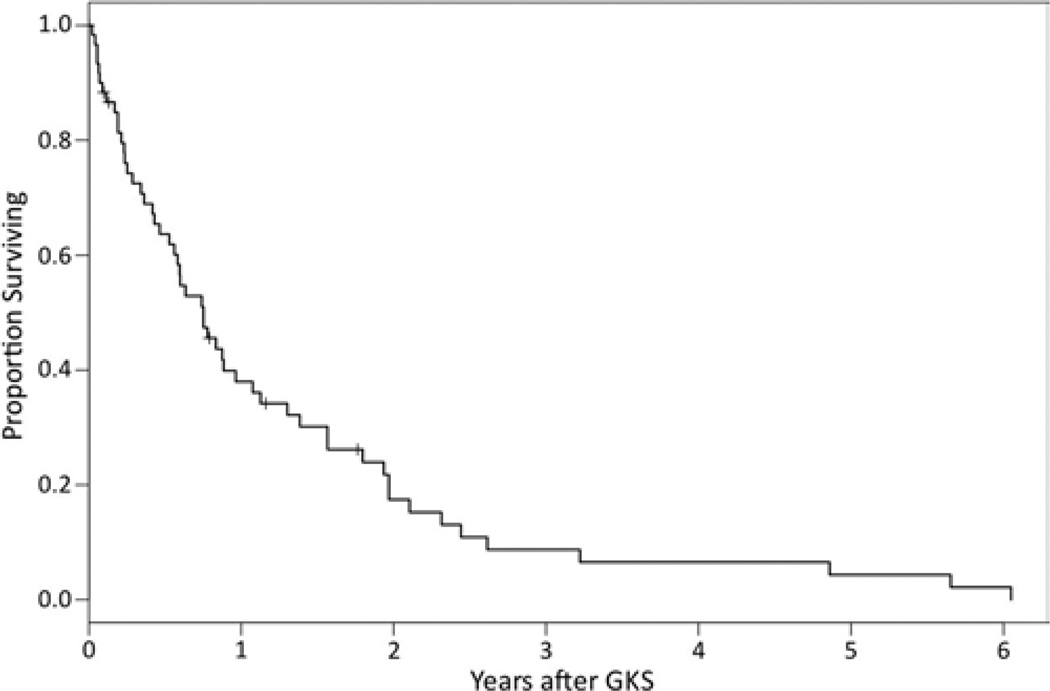
At the time of last follow-up, 53 of 61 patients in the series had died. The Kaplan-Meier method was used to calculate overall survival. The Kaplan-Meier survival curve is depicted in Fig. 1. The median survival time of the entire population was 9.0 months. Actuarial 1-, 2-, and 3-year survival probability was 38%, 17.4%, and 8.7%, respectively. Of the 52 deaths with known cause, 14 (26.9%) were determined to be neurological deaths.
Fig. 1.
Kaplan-Meier plot of overall survival.
Patterns of Failure
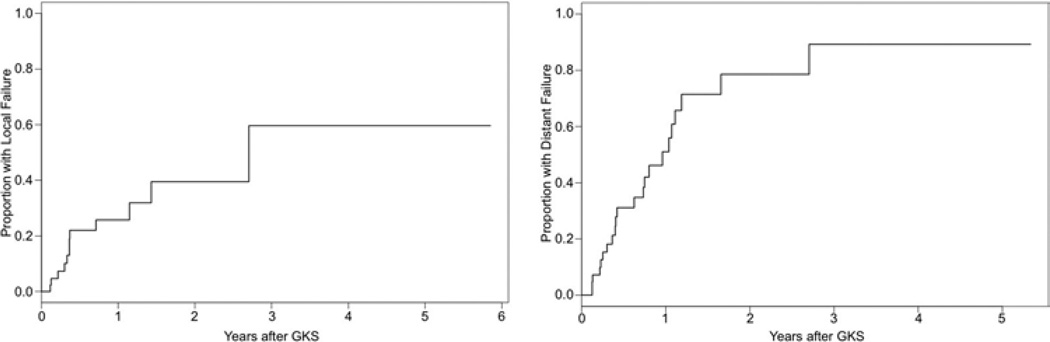
The Kaplan-Meier method was used to calculate local and distant failure rates. Results are depicted in Fig. 2. The median time to local failure was 32.5 months. Actuarial 1-, 2-, and 3-year freedom from local failure rates were 74.3%, 60.5%, and 40.3%, respectively. Actuarial 1-, 2-, and 3-year local control of individually treated lesions was 93%, 85%, and 35%, respectively. The median time to distant failure was 11.5 months. Actuarial 1-, 2-, and 3-year distant failure rates were 51%, 78.6%, and 89.3%, respectively.
Fig. 2.
Left: Kaplan-Meier plot of time to local failure. Right: Kaplan-Meier plot of time to distant failure.
Effects of Targeted Agents
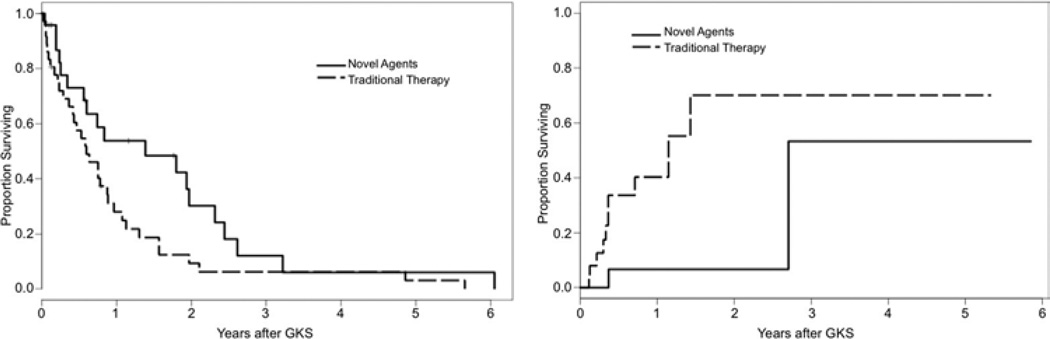
The Kaplan-Meier method was used to determine survival, local control, and distant failure rates between the cohort of patients (n = 24) that received tyrosine kinase inhibitors, mTOR inhibitors, or bevacizumab, and the cohort (n = 37) that did not receive these agents. The log-rank test was used to determine statistical differences between the cohorts. The Kaplan-Meier curves are depicted in Fig. 3. The median survival was 16.6 months for the patients receiving targeted agents versus 7.2 months for patients undergoing traditional chemo- or immunotherapy (p = 0.04). Patients receiving targeted agents had improved local control (HR 5.9, p = 0.01). Local control at 1 year was 93.3% and 60% for patients treated with and without targeted agents, respectively. There were no significant differences in distant brain failure rates between patients receiving and those not receiving targeted agents (HR 1.0, p = 0.98). There were also no differences in the likelihood of neurological death between patients receiving targeted agents and those not receiving targeted agents (21.1% vs 30.3%, p = 0.47). Characteristics of the cohorts receiving targeted agents and those receiving conventional therapies are summarized in Table 3.
Fig. 3.
Left: Kaplan-Meier plot of overall survival comparing cohorts treated with novel agents and those not treated with novel agents. Right: Kaplan-Meier plot of time to local failure comparing cohorts treated with novel agents and those not treated with novel agents.
TABLE 3.
Comparisons of populations receiving targeted versus conventional therapy
| Characteristic | Targeted Agents | Conventional Therapy |
|---|---|---|
| no. of patients | 24 | 37 |
| age (yrs) | ||
| median | 60 | 63 |
| range | 43–89 | 33–82 |
| sex | ||
| female | 4 | 7 |
| male | 20 | 30 |
| extent of extracranial disease | ||
| none | 2 | 3 |
| oligometastatic | 9 | 19 |
| widespread | 13 | 14 |
| no. of brain metastases | ||
| 1 | 8 | 20 |
| 2–4 | 16 | 14 |
| >4 | 0 | 2 |
| modified MSKCC risk score | ||
| 0–1 | 14 | 18 |
| 2–4 | 10 | 18 |
| RPA class | ||
| I | 2 | 2 |
| II | 19 | 30 |
| III | 3 | 4 |
| KPS score | ||
| <70 | 3 | 4 |
| 70–80 | 13 | 16 |
| 90 | 8 | 16 |
Predictive Factors
Multivariate analysis was performed to determine factors that predicted improved outcomes. The use of targeted agents (HR 3.02, p = 0.003) was the only factor that predicted improved survival. The results of the multivariate analysis for survival are summarized in Table 4. The use of targeted agents was the only factor that predicted local control, although this did not reach statistical significance (HR 5.28, p = 0.06). There were no factors that were predictive of distant failure, although there was a trend in those who received previous WBRT (HR 2.08, p = 0.17). An increased MSKCC risk score was suggestive of a decreased likelihood of neurological death (HR 0.06, p = 0.06).
TABLE 4.
Multivariate analysis of factors affecting overall survival
| Factor | HR (95% CI) | p Value |
|---|---|---|
| age | 0.99 (0.96–1.03) | 0.56 |
| sex | ||
| female | 1.00 (reference) | 0.15 |
| male | 2.41 (0.74–7.90) | |
| systemic therapy | ||
| targeted agents | 1.00 (reference) | 0.003 |
| other | 3.02 (1.46–6.25) | |
| modified MSKCC risk score | ||
| 0–1 | 1.00 (reference) | 0.58 |
| 2–4 | 0.77 (0.30–1.95) | |
| minimal dose | 0.97 (0.84–1.12) | 0.71 |
| no. of brain metastases | 1.10 (0.89–1.36) | 0.40 |
Toxicity
Six patients experienced radiation-induced edema or necrosis after GKS, which required a course of steroids to resolve. Of patients experiencing radiation necrosis, 3 had previously been treated with targeted agents and 3 had not. One patient required long-term steroid therapy for radiation necrosis. Two patients experienced hemorrhage within the GKS treatment volume. Both patients experiencing hemorrhage were hospitalized, but neither required surgery.
Discussion
The use of GKS in the treatment of brain metastases from RCC stems from historical series suggesting poor outcomes in patients treated with WBRT alone. A retro-spective series published from the M. D. Anderson Cancer Center described 200 patients with metastastic brain disease from RCC treated with WBRT and showing a median survival of 4.4 months and a likelihood of neurological death in 76%.20 Other series have compared patients receiving WBRT alone with patients receiving some sort of surgical or radiosurgical management, revealing a survival advantage in patients receiving surgery or radiosurgery.12 The likelihood of neurological death in radiosurgical series for RCC has ranged from 4% to 29%.17 In the current series, the median survival was 9.0 months with neurological death occurring in 27% of patients. It also appeared that patients with longer life expectancies, specifically those with lower MSKCC risk scores, had an increased likelihood of experiencing neurological death.
The improvement in outcomes in patients with RCC in radiosurgical series is likely due to an improvement in local control. It has been hypothesized that the improved local control from hypofractionation is due to a decrease in the ability of radioresistant clonogens to undergo DNA repair,1 and the ability of hypofractionated radiotherapy to also damage the tumor vasculature.18 Local control in prior series in the literature has ranged from 83% to 100%.7,11,16,17 The freedom from local failure seen in the current series was 74% at 1 year; absolute local control at 1 year was 93%. The difference between these 2 numbers is due to some patients receiving radiosurgery to multiple lesions. Another rationale for the use of radiosurgery is the fact that RCC tends to have a greater latency between distant brain failures than other histological types. The median time to distant failure in our series was 11.5 months. Whole-brain radiation therapy is a modality that is generally used once. As such, it is often best reserved for multiple distant brain failures or unresectable local failures.
Renal cell carcinoma is traditionally considered to be resistant to cytotoxic chemotherapy. In fact, surgical and ablative therapies to both the primary tumor as well as to isolated metastases have played significant roles in the management of patients with metastatic disease.4,19 Furthermore, immunotherapies such as interleukin-2 and interferon-α had been considered the mainstays of systemic therapy. Since 2006, however, several systemic agents have emerged in the treatment of RCC. Bevacizumab is a monoclonal antibody targeting vascular endothelial growth factor. Sunitinib and sorafenib are molecular inhibitors of multiple tyrosine kinases, while temsirolimus is an mTOR inhibitor that affects downstream regulation of angiogenic factors and cyclins. All 4 of these targeted agents have been found in randomized controlled trials to improve progression-free survival.3,8,10,21 Sunitinib and temsirolimus have demonstrated improvements over interferon-α and were previously considered standard treatment options. Currently, sunitinib, bevacizumab, interferon, and the multitargeted tyrosine kinase inhibitor, pazopanib (Votrient, GlaxoSmithKline), are considered to be equivalent first-line options for metastastic RCC. Temsirolimus is a first-line therapy for patients with poor-risk RCC. Everolimus (Zortress, Novartis), an mTOR inhibitor, is used in the setting of failures of tyrosine kinase inhibitors, while sorafenib is commonly used for failures of cytokine therapy. There are no clear lines of therapy, and the aforementioned agents are used for salvage as well.
In the current series, the use of targeted agents improved both local control and overall survival over patients who did not receive these agents. There was no effect, however, on distant brain failure. While a significant portion of the improvement in outcomes in the current series is probably a result of the targeted agents on extracranial metastatic disease, there is a suggestion that these agents also alter the natural history of disease within the brain as well. A recently published report from the Royal Marsden Hospital assessed for the safety profile of sunitinib in the setting of patients with RCC with brain metastases and found a 12% objective response in 213 patients with brain metastases and a 5.6-month progression-free survival.6 In our own study, freedom from local failure was improved from 60% to 93% at 1 year with the use of the targeted agents, suggesting a synergistic effect within the CNS between GKS and the targeted agents. It is possible that patients with an increased risk of local failure such as those with larger metastases may benefit from the use of targeted agents in conjunction with their radiosurgery.
The question of why targeted agents had no effect on the development of new brain metastases is a difficult one to answer. A recent study suggested that the major risk factors for distant brain failure in patients who receive radiosurgery without WBRT for brain metastases include the presence of systemic disease and a greater number of brain metastases.14 It is possible that the failure of systemic therapies precipitates the development of new brain metastases. Insufficient data were available in the present study to make this determination. The only factor that predicted for distant brain failure in this study was an increasing number of lesions (HR 1.27 per lesion treated), but this did not reach statistical significance (p = 0.14).
There are several limitations of the current study. First of all, the retrospective nature of the study limits the findings to hypothesis generation. While the cohorts appeared to be balanced in terms of RPA class and MSKCC risk groups, there is the possibility of unknown bias. Furthermore, the study was not powered to assess for differences between the various systemic agents because of the limited numbers and the fact that several patients received multiple targeted agents. Prospective trials are necessary to elucidate the effect of novel targeted agents on metastatic brain disease from RCC.
Conclusions
Targeted agents appear to improve overall survival and local control in patients with brain metastases from RCC treated with GKS. Use of modern management strategies including GKS has decreased the likelihood of neurological death as compared with historical series using WBRT.
Abbreviations used in this paper
- GKS
Gamma Knife surgery
- HR
hazard ratio
- MSKCC
Memorial Sloan-Kettering Cancer Center
- mTOR
mammalian target of rapamycin
- RCC
renal cell carcinoma
- RPA
recursive partitioning analysis
- WBRT
whole-brain radiation therapy
Footnotes
Disclosure
The authors’ institution is a Leksell Center of Radiosurgery by Elekta, the manufacturer of the GKS system used in this work.
Author contributions to the study and manuscript preparation include the following. Conception and design: Chan, Aklilu, Lovato, Bourland, Urbanic, McMullen, Shaw, Tatter, Ellis. Acquisition of data: Chan, Cochran, Alphonse, Bourland, Ellis. Analysis and interpretation of data: all authors. Drafting the article: Chan, Cochran, Bourland, Ellis. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Chan. Statistical analysis: Chan, Cochran, Lovato, McMullen, Ellis. Administrative/technical/material support: Chan, Cochran, Aklilu, Alphonse, Bourland, Urbanic, McMullen, Shaw, Ellis. Study supervision: Chan, Cochran, Ellis.
References
- 1.DiBiase SJ, Valicenti RK, Schultz D, Xie Y, Gomella LG, Corn BW. Palliative irradiation for focally symptomatic metastatic renal cell carcinoma: support for dose escalation based on a biological model. J Urol. 1997;158:746–749. doi: 10.1097/00005392-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101–3106. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 6.Gore ME, Hariharan S, Porta C, Bracarda S, Hawkins R, Bjarnason GA, et al. Sunitinib in metastatic renal cell carcinoma patients with brain metastases. Cancer. 2011;117:501–509. doi: 10.1002/cncr.25452. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez L, Zamorano L, Sloan A, Fontanesi J, Lo S, Levin K, et al. Gamma knife radiosurgery for renal cell carcinoma brain metastases. J Neurosurg. 2002;97(5 Suppl):489–493. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 9.Jensen CA, Chan MD, McCoy TP, Bourland JD, deGuzman AF, Ellis TL, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. Clinical article. J Neurosurg. 2011;114:1585–1591. doi: 10.3171/2010.11.JNS10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Muacevic A, Kreth FW, Mack A, Tonn JC, Wowra B. Stereotactic radiosurgery without radiation therapy providing high local tumor control of multiple brain metastases from renal cell carcinoma. Minim Invasive Neurosurg. 2004;47:203–208. doi: 10.1055/s-2004-818511. [DOI] [PubMed] [Google Scholar]
- 12.Nieder C, Spanne O, Nordøy T, Dalhaug A. Treatment of brain metastases from renal cell cancer. Urol Oncol. 2011;29:405–410. doi: 10.1016/j.urolonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 14.Sawrie SM, Guthrie BL, Spencer SA, Nordal RA, Meredith RF, Markert JM, et al. Predictors of distant brain recurrence for patients with newly diagnosed brain metastases treated with stereotactic radiosurgery alone. Int J Radiat Oncol Biol Phys. 2008;70:181–186. doi: 10.1016/j.ijrobp.2007.05.084. [DOI] [PubMed] [Google Scholar]
- 15.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003;98:342–349. doi: 10.3171/jns.2003.98.2.0342. [DOI] [PubMed] [Google Scholar]
- 17.Shuto T, Inomori S, Fujino H, Nagano H. Gamma knife surgery for metastatic brain tumors from renal cell carcinoma. J Neurosurg. 2006;105:555–560. doi: 10.3171/jns.2006.105.4.555. [DOI] [PubMed] [Google Scholar]
- 18.Truman JP, García-Barros M, Kaag M, Hambardzumyan D, Stancevic B, Chan M, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5:e12310. doi: 10.1371/journal.pone.0012310. [Erratum in PLoS One 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogl UM, Zehetgruber H, Dominkus M, Hejna M, Zielinski CC, Haitel A, et al. Prognostic factors in metastatic renal cell carcinoma: metastasectomy as independent prognostic variable. Br J Cancer. 2006;95:691–698. doi: 10.1038/sj.bjc.6603327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrónski M, Maor MH, Davis BJ, Sawaya R, Levin VA. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997;37:753–759. doi: 10.1016/s0360-3016(97)00006-0. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]