Abstract
Increased susceptibility to tuberculosis following HIV-1 seroconversion contributes significantly to the tuberculosis epidemic in sub-Saharan Africa. Lung-specific mechanisms underlying the interaction between HIV-1 and Mycobacterium tuberculosis infection are incompletely understood. Here we address these questions by examining the effect of HIV-1 and latent M. tuberculosis co-infection on the expression of viral-entry receptors and ligands in bronchoalveolar lavage (BAL) of HIV-1-infected and -uninfected patients with and without latent M. tuberculosis infection. Irrespective of HIV-1 status, T cells from BAL expressed higher levels of the beta-chemokine receptor (CCR)5 than peripheral blood T cells, in particular the CD8+ T cells of HIV-1-infected persons showed elevated CCR5 expression. The concentrations of the CCR5 ligands RANTES and MIP-1β were elevated in the BAL of HIV-1-infected persons compared with that in HIV-1-uninfected controls. CCR5 expression and RANTES concentration correlated strongly with HIV-1 viral load in the BAL. In contrast, these alterations were not associated with M. tuberculosis sensitisation in vivo, nor did M. tuberculosis infection of BAL cells ex vivo change RANTES expression. These data suggest ongoing HIV-1 replication predominantly drives local pulmonary CCR5+ T-cell activation in HIV/latent M. tuberculosis co-infection.
Keywords: BAL, CCR5, RANTES, TB, Viral load
Introduction
Tuberculosis and AIDS are amongst the leading causes of death worldwide. In the majority of cases in immunocompetent persons, Mycobacterium tuberculosis is successfully controlled by local immune responses leading to isolation of infected alveolar macrophages and the formation of granulomas. In HIV-1 infection, the rate of active tuberculosis following primary infection and the rate of reactivation of tuberculosis in individuals with latent M. tuberculosis infection is greatly increased although the underlying mechanisms are not well understood 1.
T-cell mediated immunity is critical for immune control of tuberculosis. HIV-1 replicates in activated CD4+ T cells, monocytes or dendritic cells and leads to immunodeficiency characterised by progressive CD4+ T-cell depletion. Moreover, despite relatively normal numbers of circulating CD4+ T cells HIV-1-infected persons are already at increased risk of tuberculosis in the first year following seroconversion 2. HIV-1 infection impairs not only the quantity but also the quality of M. tuberculosis-specific immune responses 3. In addition mortality remains higher after successful anti-tuberculosis treatment of HIV-1-infected persons 4, even though CD4+ T-cell numbers partially reconstitute after initiation of anti-retroviral therapy (ART) 5.
T-cell activation is an important mechanism of HIV-1 pathogenesis 6 and persistent cell expression of activation markers predicts the progression into AIDS 7. So far only a few studies have investigated the activation markers CD38, CD69 and Ki67 on BAL cells at the site of active tuberculosis disease 6, 8, 9. Chronic antigen exposure in latent M. tuberculosis infection may lead to a persistent localised immune activation that facilitates HIV-1 entry into CD4+ T cells in lungs. The alpha (CXCR)4 and beta chemokine receptors (CCR)5 are the most important coreceptors for HIV-1 entry and infection of CD4+ T cells 10, 11. CCR5 expression on CD8+ T cells mediates the migration of antigen-specific effector and differentiated memory CD8+ T cells to the site of inflammation and it has been suggested that these CD8+ T cells are important in eradication of virus-infected CD4+ T cells. The ß-chemokines MIP1α, MIP1β and RANTES attract CCR5+ T cells to the region 12.
To evaluate the hypothesis that HIV-1 infection or M. tuberculosis exposure influence CCR5 and CXCR4 receptor and agonist expression, receptor expression and chemokine profiles from the peripheral blood and BAL mononuclear cells were compared between in HIV-1-infected and -uninfected persons with and without evidence of M. tuberculosis sensitisation.
Results
Participants
Blood and BAL cells from age- and sex-matched groups of 15 HIV-1-infected and 21 HIV-1-uninfected persons from an area of high tuberculosis incidence and high HIV-1 prevalence were investigated 3 (Table 1). Ten HIV-1-infected and 11 HIV-1-uninfected participants were diagnosed with latent M. tuberculosis infection by a positive ESAT-6 and/or CFP-10 specific IFN-γ immune response in an ELISPOT assay performed with PBMCs in the absence of active tuberculosis disease. HIV-1-infected persons had a median CD4+ count of 226 cells/μL. Frequencies of CD4+ CD3+ T cells were significantly lower in blood (median 10.0 versus 42.4%, p < 0.001) and BAL lymphocytes (median 7.7 versus 37.7%, p < 0.001) when compared with those in HIV-1-uninfected persons (Table 1).
Table 1.
Characteristics of persons enrolled to the study
| HIV-1 infected | HIV-1 uninfected | p-value | |
|---|---|---|---|
| N | 15 | 21 | |
| Sex: female/male, n | 11/4 | 13/8 | 0.564 |
| Age, mean years (range) | 34.3 (24–51) | 32.7 (21–55) | 0.531 |
| CD4, median CD4 cells/μL (range) | 226 (61–595) | 786 (461–1225) | <0.001a) |
| Blood CD4+, median CD4+ as % of CD3+ T cells (range) | 10.0 (3.45–28.9) | 42.4 (9.46–67.8) | <0.001a) |
| BAL CD4+, median CD4+ as % of CD3+ T cells (range) | 7.7 (1.33–31.30) | 37.7 (12.5–78.7) | <0.001a) |
| Blood viral load, median RNA copies/mL, (range) | 22 000 (590–660 000) | ||
| BAL viral load, median RNA copies/mL, (range) | 1964 (855–709 088) | ||
| Response to ESAT-6/CFP-10, blood: positive/negative | 10/5 | 11/10 | 0.190b) |
Differences between the HIV-1-infected and -uninfected groups were compared using the Mann–Whitney U-test.
Difference of ESAT-6/CFP-10-induced IFNγ-response in PBMCs between HIV-1-infected and -uninfected groups was compared using Fisher’s exact test of probability.
HIV-1 viral load
Stratification of BAL urea level by HIV-1 status revealed no difference between HIV-1-uninfected and -infected people (p = 0.64, data not shown). To compare the tissue load of HIV-1 in the lungs with the levels in blood, the viral load was determined in serum and BAL (Fig. 1A). In 5 out of 15 HIV-1-infected persons the viral load in BAL was below the detection limit (individuals marked in the graphs). The median viral load in serum was 22 000 RNA copies/mL (range 590–660 000 copies/mL). A significantly lower median of 1964 RNA copies/mL (range 855–709 088 copies/mL) was found in BAL (p = 0.030). Nevertheless, in three cases the viral load in the BAL was higher than the viral load in serum. There was a positive correlation between the viral load in serum and in lungs (ρ = 0.787, p < 0.001, Fig. 1B). Figure 1C demonstrates an inverse correlation (ρ = −0.586, p = 0.022) between viral load and the proportion of CD4+ in BAL.
Figure 1.
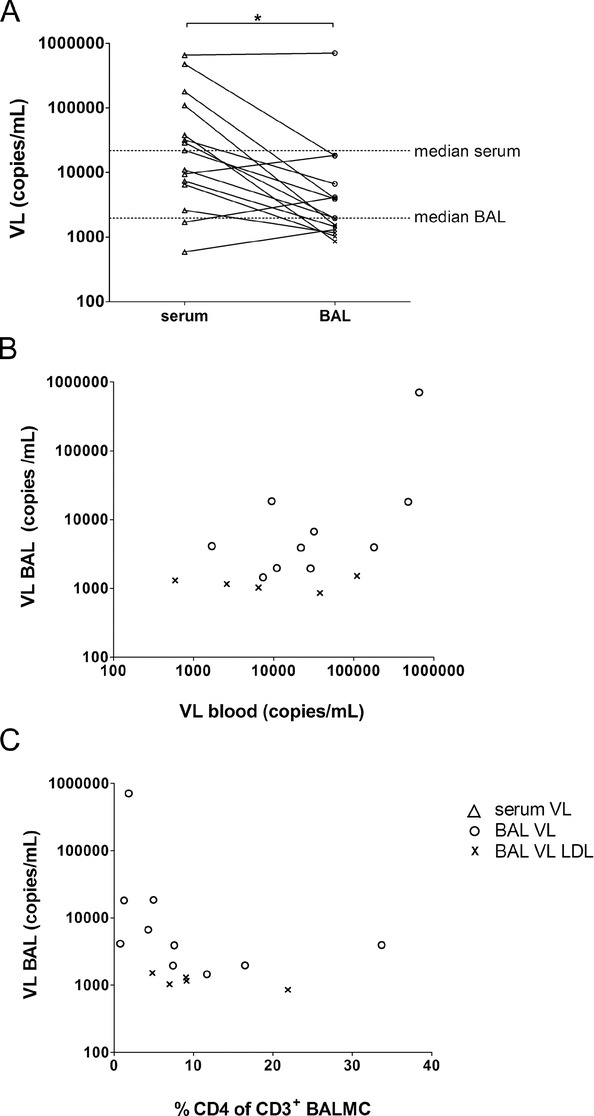
Viral load (VL) in bronchoalveolar lavage (BAL). (A) Paired VL in serum (n = 15, open triangles) and BAL fluid (n = 15) was measured by nucleic acid amplification tests of gag for the quantitation of human HIV-1 RNA. The detection limit was 20 copies/800 μL of pure BAL fluid. BAL VLs above the detection limit are depicted with open circles (n = 10). BAL VL values lower the detection limit (LDL) were set to a value of 19 copies/mL and normalised according to the urea method (n = 5, symbol ×). Each symbol represents an individual sample, horizontal lines represent median VL of serum and BAL. *p = 0.030 between serum and BAL VL, Wilcoxon signed rank test. (B) Correlation between VL in serum and BAL was assessed by Pearson (ρ = 0.787, p < 0.001) and (C) correlation between VL BAL and the relative frequency of CD4+ CD3+ BAL T cells was assessed by Spearman (ρ = −0.586, p = 0.022). Data shown are pooled data from 15 experiments performed.
HIV-1 receptor expression on bronchoalveolar T cells
Several mechanisms of increased HIV-1 replication have been described at the site of M. tuberculosis infection 13. In the previous study 3 which was performed with the same participants, no differences were found in the T-cell memory phenotype between HIV-1-infected and -uninfected persons, but in both groups there was a shift to the predominance of CD4+ effector T cells in BAL when compared with blood. As these activated effector T cells in the lungs may facilitate HIV-1 entry, BAL mononuclear cells (BALMCs) were characterised for their expression of the HIV-1 entry receptors CCR5 and CXCR4. Comparing the compartments, CD4+ (Fig. 2A) and CD8+ BALMCs (Fig. 2B) invariably had significantly higher expression of the CCR5 receptor than Peripheral blood mononuclear cells (PBMCs) (p < 0.001), measured by median fluorescence intensity (MFI). No differences in CCR5 expression by CD4+ blood T cells were found between HIV-1-infected and -uninfected persons, whereas CCR5 expression by CD4+ T cells in BAL was slightly higher in HIV-1-infected persons than in HIV-1-uninfected participants (178 versus 153) although the difference was not significant (p = 0.37, Fig. 2A). Similarly, CCR5 expression by peripheral CD8+ T cells did not differ between HIV-1-infected and -uninfected persons. In contrast, CCR5+ expression by CD8+ T cells in BAL was moderately higher in HIV-1-infected persons (222.5 versus 116.5, p = 0.026, Fig. 2B). Levels of CCR5 on CD4+ and CD8+ BAL T cells were directly correlated with viral load in BAL (ρ = 0.706, p = 0.005, Fig. 2C and ρ = 0.793, p < 0.001, Fig. 2D). In contrast, stratifying CCR5 expression on CD4+ and CD8+ BALMCs by M. tuberculosis infection status did not reveal any difference (data not shown).
Figure 2.
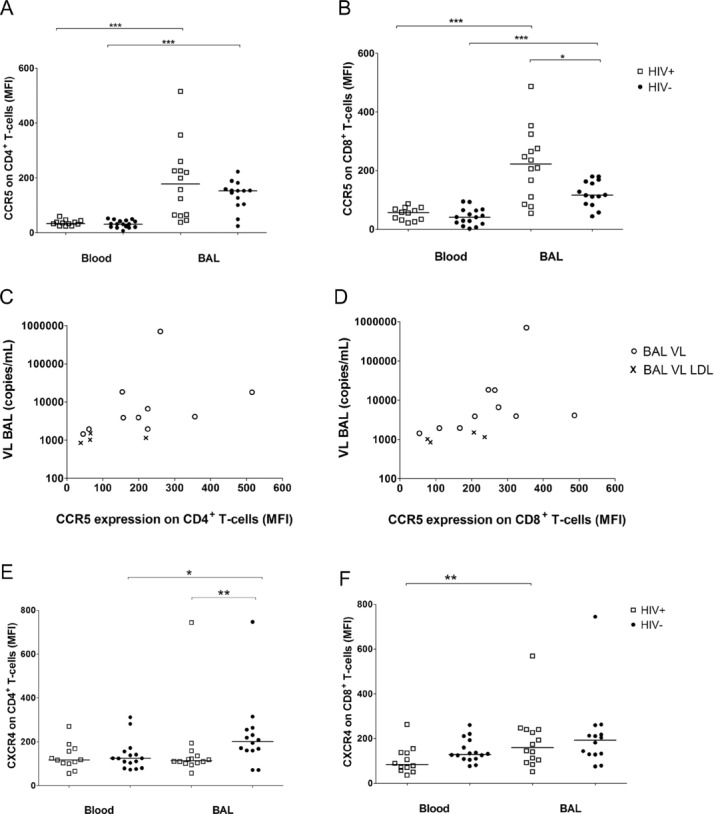
CCR5 and CXCR4 expression on CD4+ or CD8+ T cells in blood and BAL. (A, B, E, F) The median fluorescence intensity (MFI) of (A, B) CCR5 and (E, F) CXCR4 receptor expression was measured by flow cytometry on (A, E) CD4+ and (B, F) CD8+ cells from the blood and BAL of HIV-1-infected (n = 12 in blood, n = 14 in BAL, open squares) and HIV-1-uninfected persons (n = 16 in blood, n = 14 in BAL, solid circles), each sign represents one individual, bars represent medians. Differences between CCR5 expression on CD4+ and CD8+ T cells from paired blood and BAL samples were calculated by Wilcoxon signed rank test (***p < 0.001). The CCR5 expression on CD8+ T cells in the BAL was significantly higher in HIV-1-infected compared to HIV-1-uninfected persons (*p = 0.026, by Mann–Whitney U-test). (C, D) The correlation between viral load (VL) in BAL and MFI of (C) CCR5+ on CD4+ (ρ = 0.706, p = 0.005) or (D) CD8+ (ρ = 0.793, p < 0.001) BAL T cells of HIV-1-infected participants was assessed by Spearman. Open circles, BAL VL; symbol ×, BAL VL LDL were set to a value of 19 copies/mL and normalised according to the urea method. (E) The difference of CXCR4 expression on CD4+ paired blood and BAL T cells was measured by Wilcoxon signed rank test (*p = 0.049). MFI of CXCR4+ CD4+ BALMCs was significantly higher in the HIV-1-uninfected control group when compared with that of HIV-1-infected persons (**p = 0.009, Mann–Whitney U-test). (F) BAL CD8+ T cells expressed higher levels of CXCR4 than PBMCs in HIV-1-infected persons (**p = 0.003), differences between the HIV-1 status were assessed by Mann–Whitney U-test. Data shown are pooled data from experiments on 12 blood and 14 BAL samples of HIV-1-infected and 16 blood and 14 BAL samples of HIV-1-uninfected persons.
The pattern of CXCR4 expression on T cells differed from CCR5 expression. Levels of CXCR4 expression on CD4+ T cells trended to be higher on BALMCs than on PBMCs in HIV-1-uninfected subjects (201.5 versus 124.5, p = 0.049), whereas no difference in CXCR4 expression was observed between BAL or blood CD4+ T cells of HIV-1-infected persons. CXCR4 expression on CD4+ BALMCs was significantly higher in the HIV-1-uninfected control group when compared with that of HIV-1-infected persons (201.5 versus 114.5, p = 0.009, Fig. 2E). No correlation was found between CXCR4 expression by CD4+ BALMCs and viral load in BAL in HIV-1-infected persons (data not shown).
CXCR4+ expression on CD8+ PBMCs did not differ by HIV-1 status. BALMCs CD8+ T cells expressed higher levels of CXCR4+ than PBMCs in HIV-1-infected persons (159.5 versus 83.9, respectively, p = 0.003, Fig. 2F), whereas the slightly higher CXCR4 expression on CD8+ BAL T cells compared with that of PBMCs in the HIV-1-uninfected group was not significant (193 versus 129.5, p = 0.059).
Level of CCR5 ligands
Blockade of the CCR5 receptor by drugs, such as Maraviroc, can prevent the entry of CCR5-tropic HIV-1 into target cells 14, 15. As differences were observed in the expression of CCR5 in BAL compared with that in blood, CCR5 ligand levels were ascertained in the two compartments by measuring the transcript levels of the three CCR5 agonists RANTES, MIP-1β and MIP-1α in BALMCs. A lower threshold cycle (ΔCT) represents a higher abundance (Fig. 3). RANTES abundance was significantly higher in HIV-1-infected than in uninfected participants (median ΔCT 2.68 versus 7.27, p = 0.003). The mean abundance of MIP-1β was slightly but not significantly higher in HIV-1-infected (median ΔCT 5.70), when compared with that of HIV-1-uninfected persons (median ΔCT 7.02, p = 0.225), whereas MIP-1α transcript abundance in BAL was comparable in HIV-1-infected and uninfected persons.
Figure 3.

Constitutive transcript abundance of CCR5 ligands in BAL cells. Constitutive transcript abundance was assessed in freshly isolated BALMCs of HIV-1-infected persons (n = 12, open squares) and HIV-1-uninfected persons (n = 10, solid circles). ΔCT = (CT gene of interest) − (CT β-actin). A lower ΔCT indicates higher transcript abundance. Each symbol represents one sample, bars represent medians. Differences between constitutive RANTES transcript abundance of HIV-1-infected and -uninfected persons was determined by the Mann–Whitney U-test (**p = 0.003). Data shown are pooled data from experiments on 12 HIV-1-infected and 10 HIV-1-uninfected persons.
Having observed differences in transcript abundance, the protein levels of RANTES, MIP-1β and MIP-1α were investigated by multiplex bead array in blood and BAL. RANTES was found at high concentrations in serum, but at significantly lower concentrations in the BAL (p < 0.001). The level of this chemokine in serum was independent of HIV-1 status, whereas significantly higher RANTES levels were recorded in the BAL of HIV-1-infected versus HIV-1-uninfected persons (2725 pg/mL versus 594.6 pg/mL, p < 0.001, Fig. 4A). The RANTES levels in BAL also correlated with viral load in the same compartment (ρ = 0.635, p = 0.011, Fig. 4D), whereas elevated RANTES levels were not associated with M. tuberculosis sensitisation status (data not shown).
Figure 4.
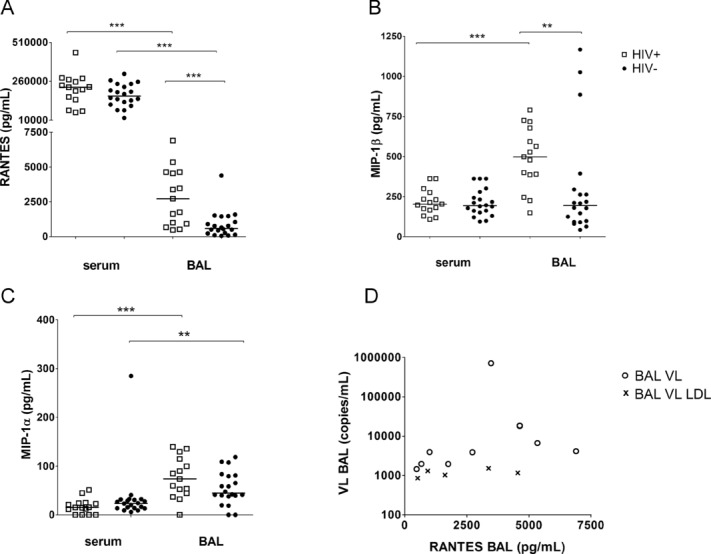
Chemokine concentration of RANTES, MIP-1β and MIP-1α in serum and BAL of HIV-1-infected (n = 15, open squares) or HIV-1-uninfected (n = 20, solid circles) persons. Chemokine concentration was measured by multiplex bead array; each symbol represents one sample, bars represent medians. (A) Differences of RANTES concentration between paired serum and BAL samples of HIV-1-infected (***p < 0.001) and HIV-1-uninfected persons were assessed by Wilcoxon signed rank test. The RANTES level in BAL in HIV-1-infected in comparison with HIV-1-uninfected (***p < 0.001) was calculated by Mann–Whitney U-test. (B) Difference of MIP-1β concentration between paired serum and BAL samples of HIV-1-infected participants (***p < 0.001) was assesses by Wilcoxon signed rank test. MIP-1β level in BAL was higher in HIV-1-infected versus HIV-1-uninfected persons (**p = 0.004, Mann–Whitney U-test). (C) Wilcoxon signed rank test was used to compare MIP-1α level in paired blood and BAL samples (***p < 0.001 in HIV-1-infected, **p = 0.01 in HIV-1-uninfected persons). (D) Correlation between RANTES concentration in BAL and viral load (VL) in BAL were assessed by Spearman (ρ = 0.635, p = 0.011). Open circles, BAL VL; symbol ×, BAL VL LDL were set to a value of 19 copies/mL and normalised according to the urea method. Data shown are pooled data from experiments on 15 HIV-1-infected and 20 HIV-1-uninfected persons.
MIP-1β levels in blood were comparable in HIV-1-uninfected and -infected persons. In contrast, MIP-1β was elevated in the BAL of HIV-1-infected compared with that of HIV-1-uninfected persons (497.2 pg/mL versus 194.3 pg/mL, p = 0.004). Furthermore, MIP-1β levels in the BAL of HIV-1-infected participants were significantly higher when compared with that of the blood compartment (497.2 pg/mL versus 202.8 pg/mL, p < 0.001, Fig. 4B).
Only low concentrations of MIP-1α were detected in both blood and serum. The serum levels of MIP-1α in HIV-1-infected participants did not differ from uninfected persons. The MIP-1α level in the BAL tended to be slightly higher in HIV-1-infected versus uninfected persons (73.7 pg/mL versus 44.7 pg/mL, p = 0.113). Comparing both, blood and BAL compartments, significantly higher levels of MIP-1α were found in the BAL compared with that in the serum of HIV-1-infected (73.7 pg/mL versus 15.2 pg/mL, respectively, p < 0.001) and HIV-1-uninfected participants (44.7 pg/mL versus 23.5 pg/mL, p = 0.01, Fig. 4C).
No difference in the concentration of CXCR4 ligand stromal cell-derived factor 1 (SDF-1α) in serum was observed between HIV-1-infected and -uninfected persons. Levels of SDF-1α in BAL samples were all below the detection limit of 18.9 pg/mL (data not shown).
Effect of M. tuberculosis infection on CCR5 ligand expression
To investigate if differences in CCR5 ligand expression were due to HIV-1 infection or M. tuberculosis, BALMCs were cultured in the presence or absence of M. tuberculosis H37Rv for 24 h and transcript abundance measured by RT-PCR. Incubation with H37Rv decreased RANTES expression in BALMCs from HIV-1-infected persons (median 0.84-fold induction), BALMCs of HIV-1-uninfected persons showed a slight increase in RANTES expression (median 1.20-fold, p = 0.034, the difference is within experimental error). H37Rv induced little increase in MIP-1β (medians 1.28-fold in HIV-1-infected in comparison to 1.89-fold in HIV-1-uninfected). Similarly, median fold increases of 1.31 and 1.69 were observed in MIP-1α transcripts in HIV-1-infected and -uninfected persons, respectively. Therefore the influence of M. tuberculosis on the gene expression level of CCR5 agonists was not great, with RANTES gene expression even diminished in HIV-1-infected persons (Fig. 5).
Figure 5.
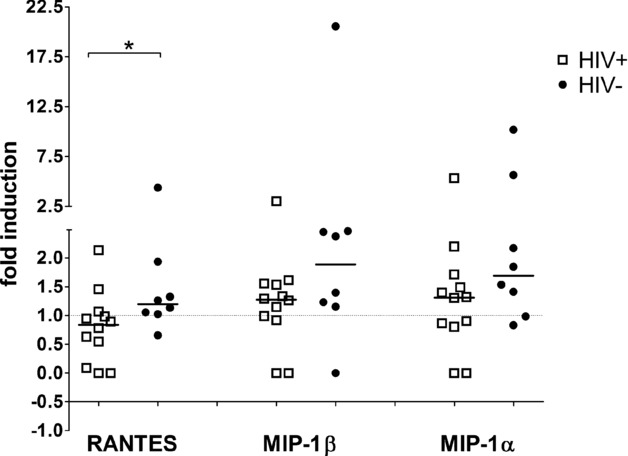
Fold induction of CCR5 ligands in bronchoalveolar lavage cells after 24 h M. tuberculosis stimulation. 5 × 105 BALMCs of HIV-1-infected (n = 12, open squares) and HIV-1-uninfected participants (n = 8, solid circles) were cultured for 24 h in the presence or absence of M. tuberculosis H37Rv at MOI 1:1. Fold induction was calculated as (ΔCT in the presence of H37Rv) − (ΔCT in the absence of H37Rv), values normalised by power 2× transformation. Each symbol is representative of one sample, bars represent medians. Differences between RANTES constitutive transcript abundance of HIV-1-infected and -uninfected persons (*p = 0.034) were assessed by Mann–Whitney test. Data shown are pooled data from experiments on 12 HIV-1-infected and 8 HIV-1-uninfected persons.
Discussion
The immunological mechanisms underlying the increased risk of tuberculosis in persons with HIV-1 co-infection, and those that exazerbate in the course of both diseases, are unclear. The aim of this study was to investigate the influence of HIV-1 infection on immune activation of resident bronchopulmonary lymphocytes and a possible permissive environment for HIV-1 entry and replication. Therefore, chemokine receptors and chemokine profiles of the two compartments lavage and blood of HIV-1-infected and -uninfected persons from an area of high tuberculosis exposure were compared. The key findings of this study are: (i) viral load correlates inversely with the number of CD4+ T cells in BAL, (ii) CD8+ BAL T cells of HIV-1-infected persons express higher levels of CCR5 compared with HIV-1-uninfected persons, and (iii) the observed elevation of MIP-1β and RANTES levels in BAL of HIV-1-infected persons was not further upregulated by ex vivo M. tuberculosis infection.
In this study HIV-1 viral load in BAL correlated directly with viral load in serum and inversely with CD4+ cell count in BAL. These data support previous observations of HIV-1 replication in lungs 16, 17. Previous studies have demonstrated that HIV-1 replicates in alveolar macrophages 18, 19, others showed that CCR5+ CD4+ BAL T cells support replication 5, 20. These results show in some people an elevation of HIV-1 RNA concentration in BAL fluid compared to serum, which supports compartmentalised local viral replication in human lungs. Furthermore, lung segments affected by tuberculosis disease have significantly higher HIV-1 viral load than uninvolved paired lung segment 21. This may be partially explained by the finding that activated T cells display greater permissiveness to infection and viral replication, compared with quiescent cells 22. To this end, HIV-1 replication was recently associated with M. tuberculosis-specific CD4+ T cells, compared with CMV-specific CD4+ T cells 23.
This study compared expression of the HIV-1 entry receptors on BAL and blood T cells of HIV-1-infected and -uninfected persons living in an area of high tuberculosis incidence. There was a wide range of CXCR4 expression levels on T cells, with significantly decreased levels of CXCR4+ on CD4+ BAL T cells of HIV-1-infected compared with those of HIV-1-uninfected persons. However, CXCR4 expression by CD4+ BALMCs was not associated with HIV-1 viral load in the same compartment, confirming previous findings 24.
Higher levels of CCR5 expression were observed on CD4+ BAL T cells by comparison with peripheral blood cells. This may be due to the fact that CCR5 is mainly expressed on effector memory (CD45RA− and CD27−) T cell 25, the predominant T-cell subset in BAL 3. As CCR5 expression increases permissiveness to HIV-1 infection 11, the elevated expression of CCR5 on CD4+ BAL T cells might increase BALMC susceptibility to HIV-1 infection. Our finding that HIV-1 viral load correlated with CCR5 expression on CD4+ BAL T cells supports this hypothesis. Therefore these data add to previous descriptions of BALMCs expression of CCR5 5, 9, 20, 26 at the site of M. tuberculosis infection.
Findings from this study suggest that HIV-1 infection induces the elevation of CCR5+ expression on CD8+ BAL T cells. Although increased expression of CCR5 on CD8+ T cells is not directly related to HIV-1 susceptibility, CD8+ T cells play a critical role in the control of viral infection. During HIV-1 infection cytotoxic T-lympocytes eliminate HIV-1-infected cells and secrete β-chemokines 27, which recruit monocytes and T cells to inflammatory sites. Because our results showed an increased presence of CD8+ T cells at the site of infection, we expected to find an elevation of the β-chemokines RANTES, MIP-1β and MIP-1α in BAL. This hypothesis was confirmed as higher levels of RANTES and MIP-1β were found in the BAL of HIV-1-infected in comparison to HIV-1-uninfected persons. It should be noted that soluble chemokines can be used to determine relative changes of these peptides during an inflammatory process but may more represent evidence of inflammation than a biological relevant form of these mediators. Since minimal concentrations of RANTES required for activation of CCR5 on T cells are between 1 and 10 nM 28, about threefold lower levels found in BAL derived from HIV-infected patient (2700 pg/mL; ∼0.35 nM) are unlikely able to modulate the receptor status, but indicate to a higher status of cell activation in the lungs of HIV-1-infected persons.
RANTES levels directly correlated with viral load in BAL. On restimulation of BAL cells with M. tuberculosis no upregulation of the RANTES gene expression was observed. This suggests that CCR5 agonist levels in BALMCs are influenced rather by HIV-1 status than by M. tuberculosis infection. Regulation of β-chemokine expression in BAL cells may differ from peripheral blood cells, as the latter showed higher β-chemokine levels in active tuberculosis, compared with healthy controls 29 and peripheral levels remained elevated in HIV-1 and M. tuberculosis coinfected persons despite anti-tuberculosis treatment 30.
HIV-1 viral replication in the lungs may drive the production of MIP-1β and RANTES. We speculate that this, at least in part, reflects compartmentalised cytolytic activity of HIV-1-specific CD8+ T cells 31, 32. Circulating T cells that have been activated by β-chemokines and migrate to the site of infection, may thus be preferentially infected by HIV-1 33. By contrast, locally primed antigen-specific T cells are less permissive to HIV-1 infection, as CCR5 agonists compete with HIV-1 for binding sites of the CCR5 receptor resulting in an inhibitory effect for HIV-1 entry 14, 30, 33.
Our study had several limitations. Due to restricted numbers of BAL cells we were unable to better characterise the CCR5+ T-cell populations in respect of memory or effector phenotyping and to investigate the HIV-1-specific immune response. Such investigations would have allowed us to address whether, as shown for peripheral blood cells, antigen-specific CD4+ BAL T cells that express high levels of CCR5 are preferentially eliminated 34 or if ongoing viral replication would be a similar predictor of HIV-1-specific CD8+ T-cell loss in BAL 35. Secondly, it might be possible that HIV-1 infection preferentially depletes M. tuberculosis-specific CCR5+ CD4+ T cells 9, 23, 36. Although we have not addressed this question specifically, data from the same participants show that M. tuberculosis-specific T-cell responses in lungs of HIV-1-infected persons are markedly impaired 3. It will be important to also study these effects of HIV infection in patients with active pulmonary tuberculosis.
Conclusion
Elevated levels of CCR5, the coreceptor for HIV-1, on CD4+ BAL T cells in comparison to peripheral blood cells suggest a local permissive environment for HIV-1 infection in human lungs. HIV-1-infected persons also exhibited higher expression of CCR5 by CD8+ BAL T cells, suggesting that CCR5 may play an important role in the recruitment of HIV-1-specific CD8+ effector T cells into inflamed tissue where these CD8+ T cells may mediate killing of HIV-infected cells. These results provide further evidence that ongoing HIV-1 replication is an important factor in the elevation of MIP-1β and RANTES levels in BAL from persons with HIV/latent M. tuberculosis co-infection.
Materials and methods
Participants
The study was approved by the Research Ethics Committees of the Universities of Cape Town, South Africa (REC 381/2006), and Lübeck, Germany (05-096) and all participants provided written informed consent. These investigations were performed as part of a larger study. Cells from the same patient samples were used for different experiments, these results focussing on the influence of HIV-1 on M. tuberculosis-specific T-cell immune responses have been previously published 3.
Briefly, participants were recruited at the Khayelitsha Site B Clinic in Cape Town, South Africa. In compliance with South African national guidelines HIV-1 care including ART was offered to all HIV-1-infected persons. A symptom screen and physical examination were performed, persons with active or a past history of tuberculosis, or isoniazid preventive therapy were excluded. Smoking, pregnancy, chronic cardiovascular or metabolic illnesses, immunosuppressive medication, and age less than 21 years also constituted exclusions. All participants had negative cultures for M. tuberculosis in BAL and had no radiological evidence of lung disease.
BAL and blood collection and processing
Bronchoscopy and blood collection were performed before patients were initiated on ART. Standard flexible diagnostic bronchoscopy including a BAL of the middle lobe with 300 mL sterile saline and isolation of the bronchoalveolar mononuclear cells (BALMCs) were conducted as described previously 3, 37. BAL fluid was harvested and aliquots were frozen immediately at −80°C. PBMCs were prepared as described previously 3, 37. Serum samples were centrifugated at 3000 rpm for 15 min at 20°C, aliquoted and frozen at −80°C.
Latent M. tuberculosis infection
Sensitisation by M. tuberculosis was defined by the immune responses to the M. tuberculosis-specific antigens early secreted antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10 measured in PBMCs by IFN-γ ELISPOT assay, MABTECH (Nacka, Sweden) as reported previously 3.
HIV-1 viral load
Plasma HIV-1 viral load was detected by Nuclisens (BioMerieux, Randburg, South Africa). Viral load in BAL fluid was measured by Cobas TaqMan HIV-1 test (Roche Diagnostics GmbH, Grenzach-Wyhlen, Germany). Both assays are accredited in vitro nucleic acid amplification tests of gag for the quantitation of human HIV-1 RNA and both determine the results in copies/mL. The limit of agreement between the two assays is reported to be 0.126 copies/ mL 38. The detection limit was 20 copies/800 μL pure BAL fluid. For analysis, values below the detection limit were assigned 19 copies/800μL BAL fluid. These values are specifically marked in the figures. To allow direct comparison between viral load in serum and BAL fluid, the dilution factor of the BAL procedure was assesed by the urea method 39. Urea was measured by BUN Flex reagent cartridge (DF21, Siemens Healthcare Diagnostics GmbH, Eschborn, Germany). All data refer to copies/mL alveolar lining fluid, for easier reading the term copies/mL BAL will be used throughout the text.
Flow cytometry
For phenotypic analysis freshly isolated PBMCs and BALMCs were stained with the surface marker antibodies (BD Biosciences, Johannesburg, South Africa) anti-CD3 Pacific Blue (UCTH1), anti-CD4 Alexa Fluor 700 (RPA-T4), anti-CD8 PerCP-Cy5.5 (SK1), anti-CD184 allophycocyanin (12G5/CXCR4) and anti-CD195 PE (2D7/CCR5). Staining and acquisition was performed as previously described 3. The flow cytometric gating strategy is illustrated in the Supporting Information Fig. 1. Photomultiplier voltage settings were not changed in between group comparison. Data analysis was performed with FlowJo software version 9.2 (TreeStar, Ashland, TX, USA).
mRNA analysis
To estimate constitutive transcript abundance RNA samples were extracted immediately after BALMCs isolation. To compare the differences in transcript abundance threshold cycle (CT) values for β-actin were subtracted from the CT values of the gene of interest. To analyse M. tuberculosis induced changes in transcript levels, 5 × 105 BALMC were cultured for 24 h in a 24-well plate with 1 mL RPMI and 10% heat-inactivated fetal calf serum (Gibco, Mowbray, South Africa) in the presence or absence of M. tuberculosis H37Rv at MOI 1:1. RNA isolation and quantitative RT-PCR were performed as previously described 40. The fold induction of genes was calculated by the ΔΔCT method (ΔCT in the presence of H37Rv minus ΔCT in the absence of H37Rv in culture) and values normalised by power 2x transformation. RANTES, MIP-1α and MIP-1β primers and probes were obtained from Applied Biosystems (Foster City, CA, USA).
Multiplex bead array
MIP-1α, MIP-1β, RANTES and SDF-1α levels in serum and BAL fluid were assayed in batches by multiplex bead array (Bio-Rad Laboratories, Munich, Germany). Serum testing was performed according to the manufacturer’s instructions (User Bulletin #10014905 Rev C, download from http://bio-rad.com/bioplex). 500 μL BAL fluid was incubated with multiplex beads, 1% bovine serum albumin (Sigma-Aldrich, Steinheim, Germany) and protease inhibitor (Roche, Mannheim, Germany) on a roller device at 4°C overnight. BAL fluid volume was subsequently reduced on the 96-well Biorad plate with vacuum manifold. Samples were read on the Biorad Luminex reader using Bioplex manager 4.1 software. Chemokine levels in BAL fluid were normalised by the urea method as pg/mL alveolar lining fluid, which for easier reading is reported as pg/mL BAL throughout the manuscipt.
Data analysis
Due to relatively small cell numbers in individual samples, not all analyses could be performed on all subjects. Statistical tests between groups were performed by the Mann–Whitney U-test, for paired data with the Wilcoxon Signed Rank test and for 2 × 2 tables Fisher’s exact test of probability. Non-parametric correlation was assessed by Spearman coefficient, association between normally distributed data was tested by Pearson’s correlation test. Comparison in figures are indicated as *p < 0.05; **p < 0.005 and ***p < 0.0005.
Acknowledgments
We are grateful to the study participants and thank the staff at Ubuntu Clinic for assistance in recruitment. We thank Prof. Mark Nicol for providing the M. tuberculosis strain, H37Rv.
BK was funded by the German Research Foundation (DFG SCHE1556) and German National Respiratory Society (DGP). TJS, WH and RJW are funded by the Wellcome Trust (088316, 080929, 084323). TJS and WH have additional support from the Aeras Global TB Vaccine Foundation, Gates Foundation and the NIH (RO1-AI-087915 and NO1-AI-70022). KS and CL were supported by the HW & J Hector Foundation, Weinheim, Germany. RJW also has additional support from the MRC (U.1175.02.002.00014.01) and European Union (Sante/2006/105-061).
Glossary
- ART
anti-retroviral therapy
- BAL
bronchoalveolar lavage
- BALMC
BAL mononuclear cell
- CCR5
beta chemokine receptor 5
- CFP-10
culture filtrate protein 10
- CT
threshold cycle
- CXCR4
alpha chemokine receptor 4
- ESAT-6
early secreted antigenic target 6
- LDL
lower detection limit
- SDF-1
stromal cell-derived factor 1
Conflict of interest
The authors declare no financial or commercial conflict of interest.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Flow cytometric gating strategy of CCR5 and CXCR4 receptor expression on CD4+/CD8+ BALMCs. (A) The gating strategy used to identify CD4+ and CD8+ T cells from BAL is shown in representative density plots from a single person. From left to right, lymphocytes were selected using forward scatter/side scatter-area, subsequently followed by gating on CD3+ T cells and thereafter selecting CD4+ and CD8+ T cells. (B–E) Representative histograms show (B, D) CCR5 and (C, E) CXCR4 expression on (B, C) CD4+ and (D, E) CD8+ BALMCs. The grey histograms give one representative example of an HIV-1-uninfected person, the black histograms show one representative example of an HIV-1-infected person. The same strategy was used for phenotyping of PBMCs.
References
- 1.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin. Infect. Dis. 2010;50(Suppl 3):201–207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 3.Kalsdorf B, Scriba TJ, Wood K, Day CL, Dheda K, Dawson R, Hanekom WA, et al. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 2009;180:1262–1270. doi: 10.1164/rccm.200907-1011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manas E, Pulido F, Pena JM, Rubio R, Gonzalez-Garcia J, Costa R, Perez-Rodriguez E, et al. Impact of tuberculosis on the course of HIV-infected patients with a high initial CD4 lymphocyte count. Int. J. Tuberc. Lung Dis. 2004;8:451–457. [PubMed] [Google Scholar]
- 5.Knox KS, Vinton C, Hage CA, Kohli LM, Twigg HL, III, Klatt NR, Zwickl B, et al. Reconstitution of CD4 T cells in bronchoalveolar lavage fluid after initiation of highly active antiretroviral therapy. J Virol. 2010;84:9010–9018. doi: 10.1128/JVI.01138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry SM, Johnson MA, Janossy G. Increased proportions of activated and proliferating memory CD8 +T lymphocytes in both blood and lung are associated with blood HIV viral load. J. Acquir. Immune Defic. Syndr. 2003;34:351–357. doi: 10.1097/00126334-200312010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 8.Raju B, Tung CF, Cheng D, Yousefzadeh N, Condos R, Rom WN, Tse DB. In situ activation of helper T cells in the lung. Infect. Immun. 2001;69:4790–4798. doi: 10.1128/IAI.69.8.4790-4798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santucci MB, Bocchino M, Garg SK, Marruchella A, Colizzi V, Saltini C, Fraziano M. Expansion of CCR5+ CD4+ T-lymphocytes in the course of active pulmonary tuberculosis. Eur. Respir. J. 2004;24:638–643. doi: 10.1183/09031936.04.000105403. [DOI] [PubMed] [Google Scholar]
- 10.Morris L, Cilliers T, Bredell H, Phoswa M, Martin DJ. CCR5 is the major coreceptor used by HIV-1 subtype C isolates from patients with active tuberculosis. AIDS Res. Hum. Retroviruses. 2001;17:697–701. doi: 10.1089/088922201750236979. [DOI] [PubMed] [Google Scholar]
- 11.Gorry PR, Ancuta P. Coreceptors and HIV-1 pathogenesis. Curr. HI/AIDS Rep. 2011;8:45–53. doi: 10.1007/s11904-010-0069-x. [DOI] [PubMed] [Google Scholar]
- 12.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J. Immunol. 2002;168:2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 13.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J. Infect. Dis. 2003;188:1146–1155. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 14.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 15.Vandekerckhove L, Verhofstede C, Vogelaers D. Maraviroc: integration of a new antiretroviral drug class into clinical practice. J. Antimicrob. Chemother. 2008;61:1187–1190. doi: 10.1093/jac/dkn130. [DOI] [PubMed] [Google Scholar]
- 16.Koziel H, Kim S, Reardon C, Li X, Garland R, Pinkston P, Kornfeld H. Enhanced in vivo human immunodeficiency virus-1 replication in the lungs of human immunodeficiency virus-infected persons with Pneumocystis carinii pneumonia. Am. J. Respir. Crit. Care Med. 1999;160:2048–2055. doi: 10.1164/ajrccm.160.6.9902099. [DOI] [PubMed] [Google Scholar]
- 17.Wood KL, Chaiyarit P, Day RB, Wang Y, Schnizlein-Bick CT, Gregory RL, Twigg HL., III Measurements of HIV viral loads from different levels of the respiratory tract. Chest. 2003;124:536–542. doi: 10.1378/chest.124.2.536. [DOI] [PubMed] [Google Scholar]
- 18.Toossi Z, Nicolacakis K, Xia L, Ferrari NA, Rich EA. Activation of latent HIV-1 by Mycobacterium tuberculosis and its purified protein derivative in alveolar macrophages from HIV-infected individuals in vitro. J. Acquir. Immune Defic. Syndr Hum Retrovirol. 1997;15:325–331. doi: 10.1097/00042560-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Kaner RJ, Santiago F, Rahaghi F, Michaels E, Moore JP, Crystal RG. Adenovirus vectors block human immunodeficiency virus-1 replication in human alveolar macrophages by inhibition of the long terminal repeat. Am. J. Respir. Cell Mol. Biol. 2010;43:234–242. doi: 10.1165/rcmb.2008-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenchley JM, Knox KS, Asher AI, Price DA, Kohli LM, Gostick E, Hill BJ, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakata K, Rom WN, Honda Y, Condos R, Kanegasaki S, Cao Y, Weiden M. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am. J. Respir. Crit. Care Med. 1997;155:996–1003. doi: 10.1164/ajrccm.155.3.9117038. [DOI] [PubMed] [Google Scholar]
- 22.Owen RE, Heitman JW, Hirschkorn DF, Lanteri MC, Biswas HH, Martin JN, Krone MR, et al. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS. 2010;24:1095–1105. doi: 10.1097/QAD.0b013e3283377a1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J. Exp. Med. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshino Y, Tse DB, Rochford G, Prabhakar S, Hoshino S, Chitkara N, Kuwabara K, et al. Mycobacterium tuberculosis-induced CXCR4 and chemokine expression leads to preferential X4 HIV-1 replication in human macrophages. J. Immunol. 2004;172:6251–6258. doi: 10.4049/jimmunol.172.10.6251. [DOI] [PubMed] [Google Scholar]
- 25.Groot F, van Capel TM, Schuitemaker J, Berkhout B, de Jong EC. Differential susceptibility of naive, central memory and effector memory T cells to dendritic cell-mediated HIV-1 transmission. Retrovirology. 2006;3:52–61. doi: 10.1186/1742-4690-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraziano M, Cappelli G, Santucci M, Mariani F, Amicosante M, Casarini M, Giosue S, et al. Expression of CCR5 is increased in human monocyte-derived macrophages and alveolar macrophages in the course of in vivo and in vitro Mycobacterium tuberculosis infection. AIDS Res. Hum. Retroviruses. 1999;15:869–874. doi: 10.1089/088922299310575. [DOI] [PubMed] [Google Scholar]
- 27.Gulzar N, Copeland KF. CD8 +T-cells: function and response to HIV infection. Curr. HIV Res. 2004;2:23–37. doi: 10.2174/1570162043485077. [DOI] [PubMed] [Google Scholar]
- 28.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation: beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 29.Mayanja-Kizza H, Wajja A, Wu M, Peters P, Nalugwa G, Mubiru F, Aung H, et al. Activation of beta-chemokines and CCR5 in persons infected with human immunodeficiency virus type 1 and tuberculosis. J. Infect. Dis. 2001;183:1801–1804. doi: 10.1086/320724. [DOI] [PubMed] [Google Scholar]
- 30.Wolday D, Tegbaru B, Kassu A, Messele T, Coutinho R, van Baarle D, Miedema F. Expression of chemokine receptors CCR5 and CXCR4 on CD4+ T cells and plasma chemokine levels during treatment of active tuberculosis in HIV-1-coinfected patients. J. Acquir. Immune Defic. Syndr. 2005;39:265–271. doi: 10.1097/01.qai.0000163027.47147.2e. [DOI] [PubMed] [Google Scholar]
- 31.Wagner L, Yang OO, Garcia-Zepeda EA, Ge Y, Kalams SA, Walker BD, Pasternack MS, et al. Beta-chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature. 1998;391:908–11. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 32.Stegelmann F, Bastian M, Swoboda K, Bhat R, Kiessler V, Krensky AM, Roellinghoff M, et al. Coordinate expression of CC chemokine ligand 5, granulysin, and perforin in CD8 + T cells provides a host defense mechanism against. M. tuberculosis. J. Immunol. 2005;175:7474–83. doi: 10.4049/jimmunol.175.11.7474. [DOI] [PubMed] [Google Scholar]
- 33.Kelly MD, Naif HM, Adams SL, Cunningham AL, Lloyd AR. Dichotomous effects of beta-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J. Immunol. 1998;160:3091–3095. [PubMed] [Google Scholar]
- 34.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J. Infect. Dis. 2003;187:769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 35.Geldmacher C, Gray C, Nason M, Currier JR, Haule A, Njovu L, Geis S, et al. A high viral burden predicts the loss of CD8 T-cell responses specific for subdominant gag epitopes during chronic human immunodeficiency virus infection. J. Virol. 2007;81:13809–13815. doi: 10.1128/JVI.01566-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jambo KC, Sepako E, Fullerton DG, Mzinza D, Glennie S, Wright AK, Heyderman RS, et al. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax. 2011;66(5):375–382. doi: 10.1136/thx.2010.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jafari C, Ernst M, Strassburg A, Greinert U, Kalsdorf B, Kirsten D, Lange C. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur. Respir. J. 2008;31:261–265. doi: 10.1183/09031936.00096707. [DOI] [PubMed] [Google Scholar]
- 38.Scott LE, Noble LD, Moloi J, Erasmus L, Venter WDF, Stevens W. Evaluation of the Abbott m2000 real time human immunodeficiency virus type 1 (HIV-1) assay for HIV load monitoring in South Africa compared to the Roche Cobas AmpliPrep-Cobas Amplicor, Roche Cobas AmpliPrep-Cobas TaqMan HIV-1, and BioMerieux NucliSENS EasyQ HIV-1 assays. J. Clin. Microbiol. 2009;47:2209–17. doi: 10.1128/JCM.01761-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 40.Tadokera R, Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Seldon R, Chegou NN, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur. Respir. J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometric gating strategy of CCR5 and CXCR4 receptor expression on CD4+/CD8+ BALMCs. (A) The gating strategy used to identify CD4+ and CD8+ T cells from BAL is shown in representative density plots from a single person. From left to right, lymphocytes were selected using forward scatter/side scatter-area, subsequently followed by gating on CD3+ T cells and thereafter selecting CD4+ and CD8+ T cells. (B–E) Representative histograms show (B, D) CCR5 and (C, E) CXCR4 expression on (B, C) CD4+ and (D, E) CD8+ BALMCs. The grey histograms give one representative example of an HIV-1-uninfected person, the black histograms show one representative example of an HIV-1-infected person. The same strategy was used for phenotyping of PBMCs.


