Abstract
Cholesteryl ester rich apolipoprotein B100 (apoB100) lipoproteins accumulate in Bruch’s membrane before the development of age-related macular degeneration. It is not known if these lipoproteins come from the circulation or local ocular tissue. Emerging, but incomplete evidence suggests that the retinal pigmented epithelium (RPE) can secrete lipoproteins. The purpose of this investigation was to determine 1) whether human RPE cells synthesize and secrete apoB100, and 2) whether this secretion is driven by cellular cholesterol, and if so, 3) whether statins inhibit this response. The established, human derived ARPE-19 cells challenged with 0–0.8mM oleic acid accumulated cellular cholesterol, but not triglycerides. Oleic acid increased the amount of apoB100 protein recovered from the medium by both Western blot analysis and 35S-radiolabeled immunoprecipitation while negative stain electron microscopy showed lipoprotein-like particles. Of nine statins evaluated, lipophilic statins induced HMG-CoA reductase mRNA expression the most. The lipophilic Cerivastatin (5µM) reduced cellular cholesterol by 39% and abrogated apoB100 secretion by 3-fold. In contrast, the hydrophilic statin Pravastatin had minimal effect on apoB100 secretion. These data suggest that ARPE-19 cells synthesize and secrete apoB100 lipoproteins, that this secretion is driven by cellular cholesterol, and that statins can inhibit apoB100 secretion by reducing cellular cholesterol.
Keywords: age-related macular degeneration, apolipoprotein B100, basal deposits, Bruch’s membrane, drusen, retinal pigmented epithelium
Introduction
With aging, there is a striking accumulation of neutral lipids in Bruch’s membrane (BrM) that continues through adulthood. A significant proportion of this neutral lipid is esterified cholesterol contained within 60–80 nm diameter lipoprotein particles that has an apolipoprotein B100 (apoB100) backbone. Due to the presence of apoB100, these lipoproteins are most similar to very low density lipoproteins (VLDL) and low density lipoproteins (LDL), and not chylomicrons, which contain apoB48, the product of Apolipoprotein B mRNA editing, as the backbone of their lipoprotein particles. These particles also accumulate in basal laminar deposits, basal linear deposits, and drusen, the pathognomonic histopathologic BrM lesions of age-related macular degeneration (AMD), the most common cause of blindness among the elderly in the United States (Green et al. 1985; Curcio et al. 2001; Malek et al. 2003; Ruberti et al. 2003; Curcio et al. 2005b; Curcio et al. 2005a; Li et al. 2005a). The identical topographical location of lipoprotein deposition within Bruch’s membrane prior to the development of these hallmark lesions suggests that lipoproteins play a critical role in the pathophysiology of AMD.
The lipoprotein particles recovered from Bruch’s membrane are distinct from plasma lipoproteins because of their distinct morphology, lipid composition (i.e. cholesteryl ester rich), and density profile (Li et al. 2005b; Li et al. 2006; Wang et al. 2009). This difference could result from their local ocular production rather than systemic delivery of plasma lipoproteins. In fact, there is emerging, but incomplete evidence that the RPE can synthesize and secrete apoB100 lipoprotein particles. Malek et al identified apoB100 transcripts and protein in the RPE of human globes, although it is not clear whether the cellular apoB was synthesized or internalized as a lipoprotein particle (Malek et al. 2003). In monkey eyes, Tserentsoodol et al reported apoB immunolabeling in the RPE (Tserentsoodol et al. 2006b). It was not specified however, whether the antibody used was specific for apoB100 or whether it also recognized apoB48.
The established human ARPE-19 cell line has been found to express mRNA for apoB100 and microsomal triglyceride transfer protein (MTP), which is required for apoB lipoprotein assembly (Wetterau et al. 1997; Li et al. 2005a). Sterol O-acyltransferase 1, the enzyme that converts cholesterol to cholesteryl esters to maintain cellular cholesterol availability (Rudel et al. 2001), was also expressed by ARPE-19 cells (Li et al. 2005a). Neutral lipid and lipid particles were identified in the cell culture supernatant (Li et al. 2005a). However, this study did not identify synthesis and secretion of apoB100, which would help to define the specific lipoprotein particle. Furthermore, sterol O-acyltransferase 2, the isoform that converts cholesterol to cholesteryl esters specifically for lipoprotein assembly (Rudel et al. 2001), was not expressed by ARPE-19 cells (Li et al. 2005a). With these subtle inconsistencies, it remains unclear whether apoB100 lipoproteins are produced by human RPE cells.
In rats, neutral lipids and apoB100 protein have been shown to be secreted by RPE cells (Wang et al. 2006; Wang et al. 2009). However, there are major differences in lipoprotein biology between rodents and humans. For example, rodents predominantly express the splice variant apoB48 rather than apoB100, and their plasma cholesterol is typically found in high density lipoproteins (Hofker et al. 1998). As a result, mice are resistant to atherosclerosis. These differences suggest that rodent cells are suboptimal for studying apoB100 lipoprotein secretion by RPE cells, and highlight the importance of fully characterizing whether human RPE cells are capable of synthesizing and secreting apoB100.
Lipoprotein apoB100 secretion is driven by available cellular lipids, such as triglycerides and cholesterol. The high cholesteryl ester content of lipoproteins recovered from Bruch’s membrane points toward cholesterol as an influencing factor in lipoprotein secretion by the RPE (Wang et al. 2009). The statins are well recognized HMG-CoA reductase (HMG-CoA R) inhibitors that reduce endogenous cholesterol production that results in the up-regulation of the low-density lipoprotein receptor (LDLR) and the lowering of plasma LDL cholesterol (Davidson and Toth 2004; Ito et al. 2006). A diverse group of studies all suggest that inhibiting cholesterol synthesis by statins decreases the hepatic assembly and secretion of apolipoprotein B-containing lipoproteins, mainly VLDLs, as reviewed in (Huff and Burnett 1997). A large number of statins are available on the market to treat hypercholesterolemia. Historically, they have been divided into fungally derived and synthetic statins. However, a functionally useful classification has defined the statins by their solubility in octanol (lipophilicity) or water (hydrophilicity) since the solubility characteristics will influence the drug’s ability to enter a cell. The impact of statins on RPE cells at this time, is unknown.
Given the differences in lipoprotein biology between species, the subtle inconsistencies found in previous studies on human RPE cells, and the potential role that local secretion of apoB100 lipoproteins could have on the accumulation of Bruch’s membrane lipoproteins, demonstrating definitive apoB100 secretion by human RPE cells would be a significant contribution that would enable further studies to delineate how lipoproteins accumulate in Bruch’s membrane from the RPE. In this investigation, we therefore sought to perform a systematic evaluation to establish definitive evidence of apoB100 secretion by human RPE cells. Because of the high cholesteryl ester content of lipoproteins recovered from Bruch’s membrane, we additionally sought to determine whether the lipoprotein secretion is driven by cellular cholesterol, and if so, to determine if statins can reduce cellular cholesterol and reduce lipoprotein secretion by human RPE cells.
Materials and Methods
Cell Culture
The routine maintenance of the established, immortalized human RPE cell line, ARPE-19, was previously described (Handa et al. 1998). ARPE-19 cells were grown in 6-well culture plates or T75 cm2 culture flasks, and maintained in Dulbecco's modified Eagle's medium /F12 (DMEM/F12; Invitrogen Corporation, Carlsbad, CA) containing 10% fetal bovine serum (FBS, Invitrogen), and 2 mM L-glutamine solution (Invitrogen).
Viability
Cell viability was assessed with the CellTiter 96 Aqueous One Solution cell proliferation assay (Promega Inc., Madison, WI) according to the manufacturer’s directions. ARPE19 cells were treated with statins (0–10 µM) for 1–3 days. The statins that were evaluated included: Atorvastatin, Lovastatin, Mevastatin, Simvastatin (Molcan Corp., Toronto, Canada), Cerivastatin (HalloChem Pharma Co, Ltd, Chongging, China), Fluvastatin (Hisoar Pharm. Co., Ltd, Zhejiang, China), Itavastatin, Pravastatin, and Rosuvastatin (Sequoia Research Products, Oxford, United Kingdom). Cells were treated with MTS assay solution for 2 h, and the absorbance was measured at 490 nm.
RNA Extraction
Total RNA was extracted from ARPE-19 cells using the RNeasy Mini Kit (Qiagen, Inc. Valencia, CA) using the manufacturer’s instructions. The purity of RNA was estimated by the 260/280 absorbance ratio. Total RNA was treated with DNase I (Qiagen, Inc.) during RNA purification.
Real-Time RT- qPCR
HMG-CoA reductase expression was determined by RT-qPCR (LightCycler, Roche Diagnostics, Nutley, NJ) as previously described (Ishibashi et al. 2004). The following primer pair for HMG-CoA reductase was used: 5’-3' (forward): CCCAGTTGTGCGTCTTCCA and 5’-3’ (Reverse): CACTGCGAACCCTTCAGATGT that were designed with Primer 3 (http://fokker.wi.mit.edu/primer3). PCR products were quantified using the second derivate maximum values calculated with the Light-Cycler analysis software. Negative controls without template were produced for each run. Expression levels were normalized to expression levels of GAPDH (5’-CGACCACTTTGTCAAGCTCA-3’ (sense) 5’-AGGGGTCTACATGGCAACTG-3’ (anti-sense). All PCR products were checked by melting point analysis, and reaction specificity was verified by melting curve analysis. Results are reported as the mean ± SD. P < 0.05 was considered significant using the Student’s unpaired two-tailed t-test.
Nile Red Histochemistry
A Nile red assay was performed to visualize cellular neutral lipid accumulation in ARPE- 19 cells treated with 0–0.8mM oleic acid (Complexed to fatty acid free BSA, Sigma Aldrich, Inc. St. Louis, MO), as previously described (McMillian et al. 2001). Cells were seeded at 3 × 103 cells/cm2 in 96 well, clear-bottomed plates using DMEM/F12+10%FBS for 24 h and switched to DMEM/F12 + 1% BSA for 48 h. Cells were incubated in 100 µl of 1 µM Nile Red (Sigma Aldrich, Inc) with 1% Pluronic F-127 (Sigma Aldrich, Inc) and 0.1% DMSO. Incubation was carried for 4 h at room temperature in the dark, and cells were washed and then incubated with Hanks' balanced salt solution (HBSS; Invitrogen, Inc.) for 16 h. Fluorescence was read with an epifluorescence microscope (Ellipse, TE 2000-U; Nikon, Tokyo, Japan) integrated with a digital camera and accompanying software (Spot RT and software ver. 3.5; Diagnostic Imaging, Sterling Heights, MI). Background fluorescence was read at emission wavelength 590 nm with the excitation wavelength at 544 nm.
Filipin Histochemistry
Filipin is a polyene macrolide antibiotic that is fluorescent and specifically binds to free non-esterified 3beta-hydroxy sterols, such as cholesterol, in a one-to-one molar stoichiometry, and that filipin fluorescence intensity is proportional to the amount of sterol bound (Severs and Simons 1986). ARPE-19 cells were seeded at 3 × 103 cells/cm2 in 96 well, clear-bottomed plates in DMEM+10%FBS for 24 h and switched to DMEM/F12 + 1% BSA for 48 h. Cells were fixed with 4% paraformaldehyde. Cells were preincubated with 1.5 mg/ml glycine in PBS for 30 minutes, then incubated with filipin (125 µg/ml; Sigma Aldrich, Inc.) in PBS for 1 hour at room temperature, as previously published (Sugii et al. 2003). Staining was imaged with a Zeiss Axiovert 200M microscope (Zeiss Microimaging, Inc., Thornwood, NY. Fluorescence was read at (excitation 355 nm, emission 420 nm).
Cellular Cholesterol and Triglyceride
Cells were seeded at 1 × 105/cm2 into T-75 cm2 flasks. After statin treatment, cells were cultured in DMEM containing 1% BSA for 48 h. The cells were washed with PBS and lysed. Total and free cellular cholesterol were determined using a colorimetric assay kit (Biovision Inc., Mountain View, CA) according to the manufacturer’s recommendations. Cellular triglyceride levels were quantified using a colorimetric assay kit (Zen-Bio, Inc., Research Triangle Park, NC), according to the manufacturer’s protocol. Measurements were made with a Synergy HT microplate reader (BioTek, Inc, Winooski, VT). The quantity of lipid was normalized to cellular protein by the Bradford method.
Measurement of Apolipoprotein B100 (apoB100) Secretion
ARPE-19 cells were seeded at 1 ×105 cells/cm2 in 6-well-plates and grown to near confluence in DMEM+10% FBS, DMEM/F12+1%BSA (fatty acid free) for 24 h, and then incubated with DMEM/F12 plus 0–0.8mM oleic acid-BSA (fatty acid free; Sigma Aldrich, Inc.) for 24h . ApoB100 protein secreted into the culture medium was measured using a previously published protocol (Boren et al. 1994). Briefly, cells were pre-incubated for 1 h with incubation medium (methionine- and cysteine-free DMEM/F12 with 2.0mM GlutaMAX1 (Invitrogen, Inc.), and 2.0mM Na Pyruvate, then for 6 h in 1.0ml of incubation medium with protease inhibitors (Complete Mini; Roche) containing 230µCi 35S-methionine/cysteine (GE Healthcare Biosciences, Inc. Piscataway, NJ ). The supernatant was collected and cells were lysed in RIPA buffer (Upstate Biotechnology Inc, Lake Placid, NY). Each sample was incubated with 20µl of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) for 30 min and centrifuged for 2 min at 13,000xg. A polyclonal human apoB antibody (1:1000; Abcam Inc, Cambridge, MA) was added to the supernatant for 16 h at 4°C, precipitated with Protein A/G Plus-Agarose (Sigma) for 2 h, and centrifuged for 2 min at 13,000×g. The 35S-labeled immunoprecipitated proteins were separated by SDS-PAGE with Novex 4–20% tris-glycine gels (Invitrogen), fixed, dried, and exposed to a phosphorimager plate to visualize labeled apoB100.
Density Gradient Ultracentrifugation
Lipoprotein particles were prepared from the culture medium according to a previously published method (Li et al. 2005a). ARPE-19 cells in T-75 cm2 flasks were treated with 0.4 mM oleic acid. After cells were washed twice with PBS, 10 ml of serum-free DMEM/F12 containing 1% BSA was added and incubated for 48h. The medium was harvested and cellular debris was pelleted by centrifugation at 2, 000 rpm for 20 min at 4°C. Density was adjusted to 1.24 g/ml by adding solid KBr (Sigma Aldrich Inc). Ten ml of sample was added to a centrifuge tube, overlaid with 2 ml of 1.21 g/ml KBr, and centrifuged at 40,000 rpm for 36 h at 10°C in a Beckman L90 ultracentrifuge using a SW41Ti rotor. Supernatant (0.2 ml) was drawn from the tube top.
Negative Stain Electron Microscopy
Lipoprotein-like particles in the d≤1.21 g/ml fraction were visualized as described (Forte and Nordhausen 1986) with modification. Supernatant (0.2 ml) was drawn from the tube top and dialyzed overnight in 0.125 M ammonium acetate, 2.6 mM ammonium carbonate, and 0.26 mM tetrasodium EDTA (pH 7.4, 4°C) and then mixed with an equal volume of 2% potassium phosphotungstate. A 5 µl droplet was placed onto the center of freshly discharged 400 mesh copper grids (Ted Pella, Inc. Redding, CA) with carbon coated parlodion support film. After 5 min absorption, samples were air dried. Specimens were viewed with a Hitachi H-7600 TEM operating at 80 Kv and images were digitally captured with an AMT 2K camera. The dialysis buffer was examined as a negative control.
H2DCFA assay
To quantify the degree of cellular oxidative stress, we used a fluorometric microplate assay to measure reactive oxygen intermediates (ROI). ARPE-19 cells were seeded in 96-well plates and maintained in culture for 7 days to confluence. The medium was removed, and cells were washed with HBSS plus calcium and magnesium, and loaded with 10µM 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen Inc.) diluted in HBSS plus calcium and magnesium. After cells were incubated for 30 minutes at 37°C, the dye was removed and cells were washed with HBSS. Various doses of tert-butyl hydroperoxide (tBOOH) and statins were added to the cells for 30 minutes. The intracellular ROI production was measured and quantified using a plate reader (SpectraMax plate reader, MDS Analytical Technologies, Sunnyvale, CA; excitation wavelength=485 nm; emission wavelength=535 nm.)
Results
ARPE-19 cells accumulate lipids after fatty acid loading
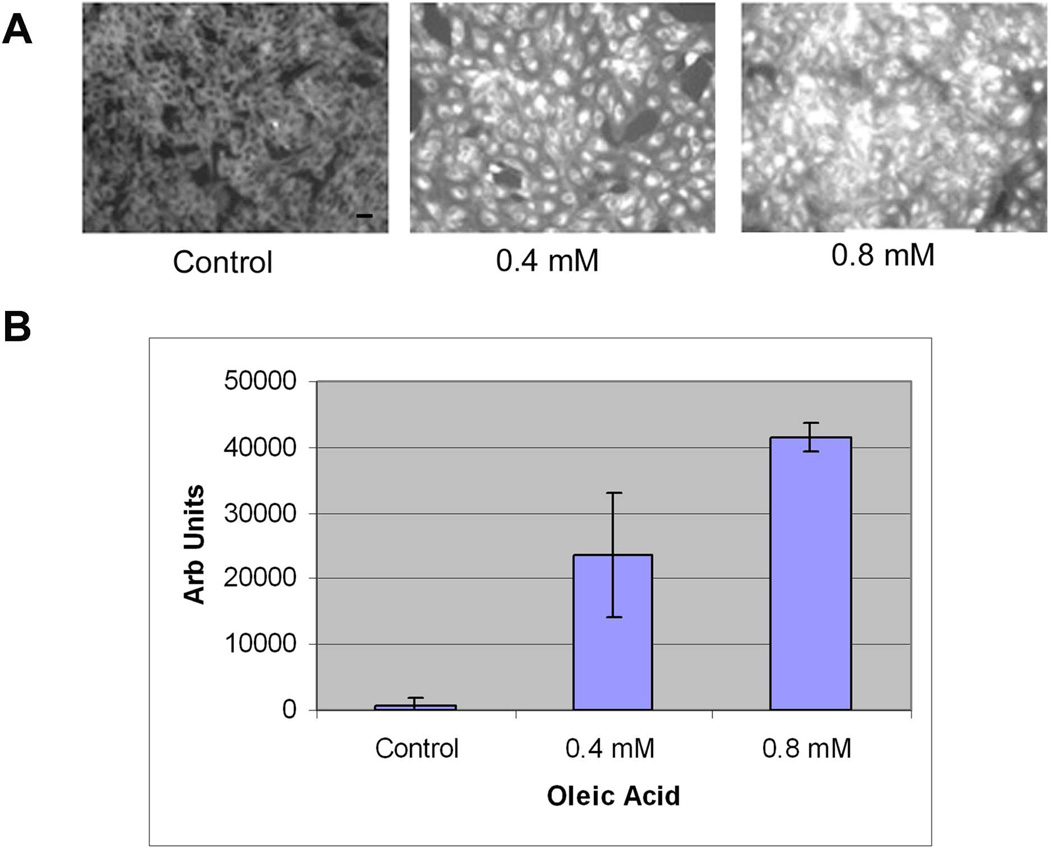
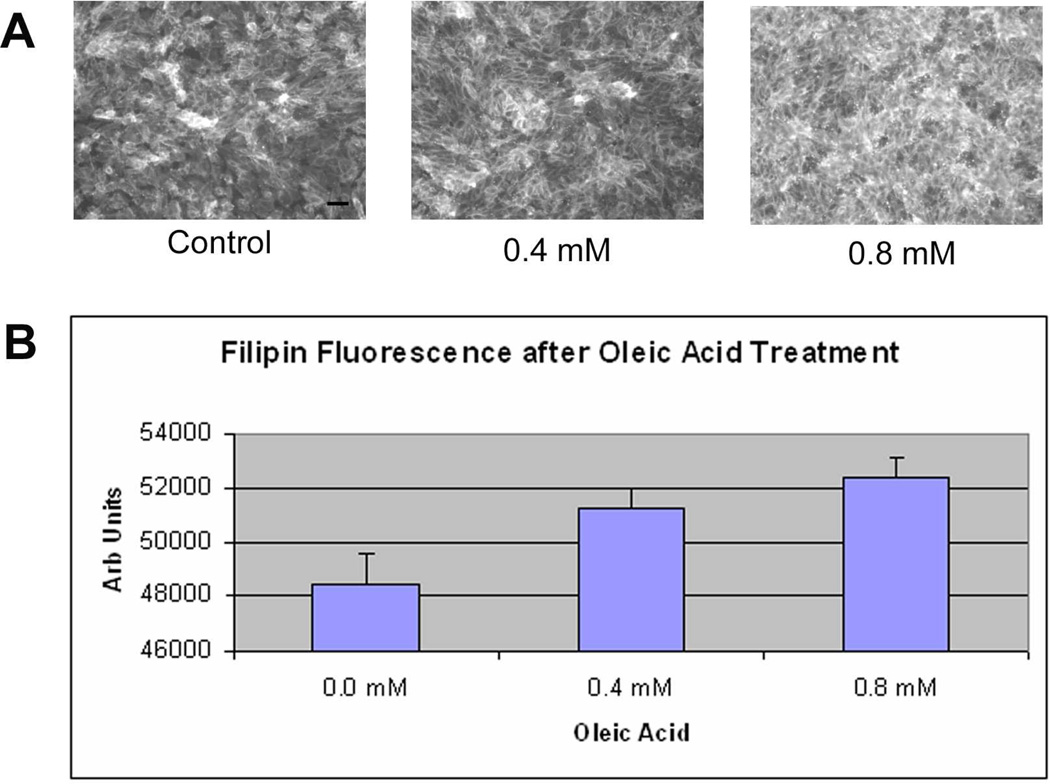
Oleic acid increases cellular cholesterol, stimulates apoB100 lipoprotein secretion in some cell types (Goh and Heimberg 1973; Fungwe et al. 1994; Zhang et al. 2004), and is a relevant fatty acid for study of RPE cells because it is a significant component of photoreceptor outer segment tips (Futterman and Andrews 1964; Martinez et al. 1988; van Kuijk and Buck 1992). ARPE-19 cells were grown to near confluence in DMEM/F12 +10% FBS, medium with 1%BSA (fatty acid free) for 24h, and loaded with oleic acid-BSA (fatty acid free) containing medium devoid of lipoprotein for 24h. These doses of oIeic acid did not affect cell viability (data not shown). Figure 1 shows a dose dependent accumulation of neutral lipids by Nile Red fluorescence microscopy 24 hours after stimulating cells with oleic acid (ANOVA, p<0.001). Figure 2 shows by Filipin histochemistry, that cellular cholesterol accumulates within ARPE-19 cells after oleic acid loading (ANOVA, p<0.001 for each dose compared to control; p<0.01 for 0.4 vs. 0.8mM). As expected, much of the labeling localized to the cell membrane, but at higher doses, the labeling appeared within cells. Using a biochemical assay, 0.4–0.8mM oleic acid treatment did not raise cellular triglycerides (n=3; p=0.3). The same oleic acid treatment increased total cholesterol (31% increase at 0.4mM and 51% increase at 0.8mM oleic acid; n=3; p<0.05) and cholesterol ester (33% increase at 0.4mM and 83% increase at 0.8mM oleic acid; n=3; p<0.05) in a dose dependent fashion.
Figure 1.
Nile Red fluorescence microscopy after 0–0.8mM oleic acid treatment. A) ARPE-19 cells show progressive increased fluorescence with increasing doses of oleic acid. Bar = 15µm. B) Graph of the net fluorescence of cells treated with oleic acid (0–0.8mM) after background absorbance (control cells) was subtracted (n=4 exp). P<0.001 for each dose compared to control, and between 0.4mM and 0.8mM oleic acid.
Figure 2.
Filipin fluorescence microscopy after 0–0.8 mM oleic acid treatment. A) ARPE-19 cells demonstrate increasing fluorescence with increasing doses of oleic acid. Note how cell membranes are predominantly labeled with 0.4mM oleic acid treatment, but fluorescence becomes intracellular at 0.8mM oleic acid. Bar = 15µm. B) Graph of the net fluorescence of cells treated with oleic acid after background absorbance was subtracted (n=4 exp). p<0.001 for each dose compared to control; p<0.05 0.4mM vs. 0.8mM oleic acid.
ARPE-19 cells secrete ApoB100 protein
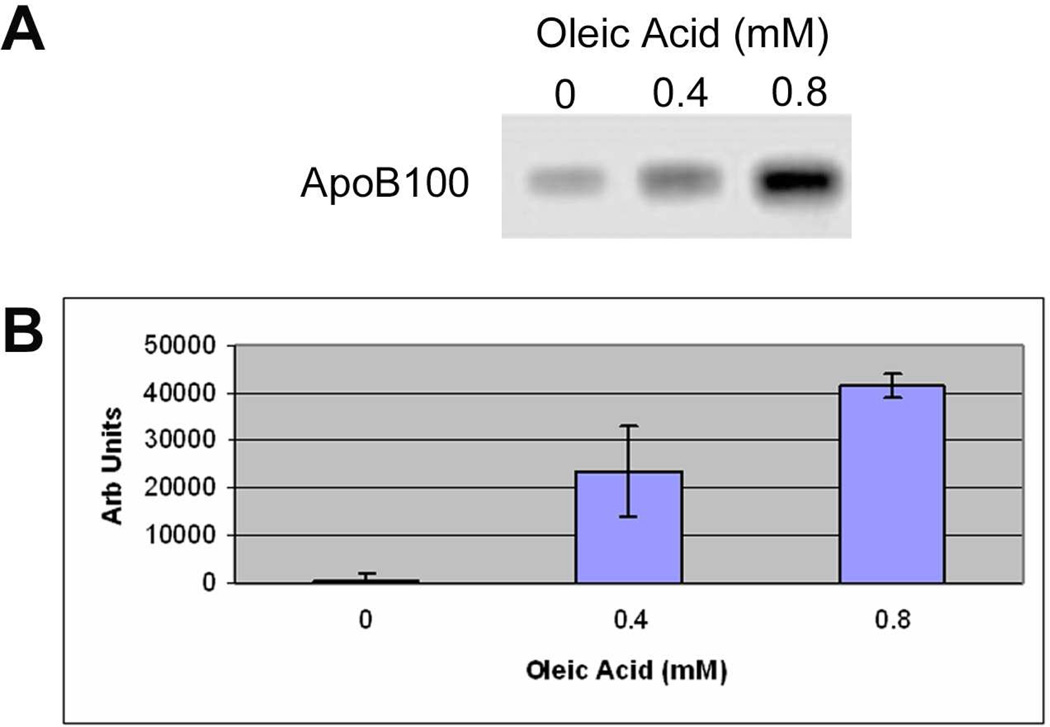
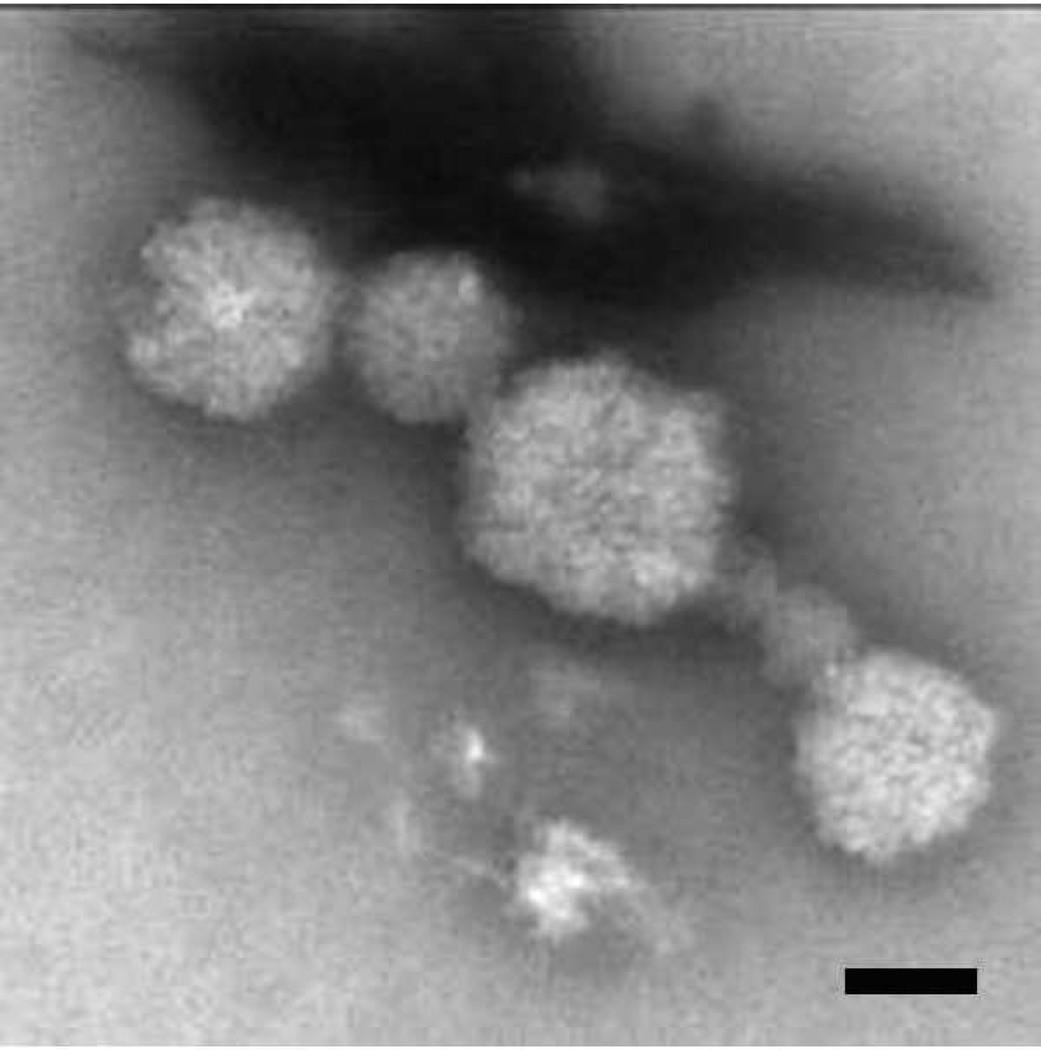
We next performed Western blot analysis to determine if apoB100 protein was produced by ARPE-19 cells and secreted into the culture medium. Unstimulated cells secreted low levels of apoB100 protein. With oleic acid, a dose dependent increase in apoB100 protein was observed in the culture medium (p<0.001 vs. control, n=3 exp), as shown in Figure 3. ApoB100 is found in bovine serum, and therefore, could be a contaminant in Western blot analysis (Sakamoto et al. 2007). To demonstrate that apoB100 is not from lipoproteins contained in bovine serum, ARPE- 19 cells were treated with 0–0.8 mM oleic acid-BSA and 35S-methionine/cysteine for 24h. 35S-apoB100 from the medium was immunoprecipitated with an anti-apoB100 antibody, separated on a 5% SDS-PAGE gel, and quantified by phosphorimager analysis. Figure 4 shows a typical autoradiogram of a dose dependent increase in radiolabeled apoB100 protein that was synthesized by ARPE-19 cells, and secreted into the culture medium after oleic acid challenge. The assay was repeated twice more, and the increase in apoB100 protein was increased by an average of 3-fold at 0.4mM and 6.7-fold at 0.8mM oleic acid stimulation, above controls (p<0.001). These results indicate that apoB100 is synthesized and secreted by ARPE-19 cells. Finally, we used negative stain electron microscopy to determine whether intact lipoprotein-like particles were secreted by ARPE-19 cells. Figure 5 shows the secreted lipoprotein-like particles after ARPE-19 cells were treated with 0.4mM oleic acid for 24h. Particles in the d≤1.21 g/ml fraction of conditioned medium had an average diameter of 79nm, with a range in diameter from 44–130nm.
Figure 3.
Western blot analysis of ApoB100 protein in the culture medium. A) Western blot showing increased labeling for ApoB100 protein at 512KDa. B) Graph of increasing apoB100 protein with increasing dose of oleic acid. p<0.001 vs. control, and for 0.4mM vs. 0.8mM oleic acid; n=3 exp.
Figure 4.
Secretion of radiolabeled ApoB100 protein into the culture medium. A) Typical autoradiogram of a dose dependent increase in 35S-radiolabeled apoB100 protein that was synthesized by ARPE-19 cells and secreted into the culture medium after oleic acid challenge. B) Graph showing increasing radiolabeled apoB100 protein that was measured in the culture medium. P<0.001 vs. control, and for 0.4mM vs. 0.8mM oleic acid. n=3 exp.
Figure 5.
Negative stain electron microscopy of presumed lipoprotein particles. Particles in the d ≤ 1.21 g/ml fraction of conditioned medium had an average diameter of 79nm, with a range in diameter from 44–130 nm. Bar = 50nm.
Selection of a statin for treatment on ARPE-19 cells
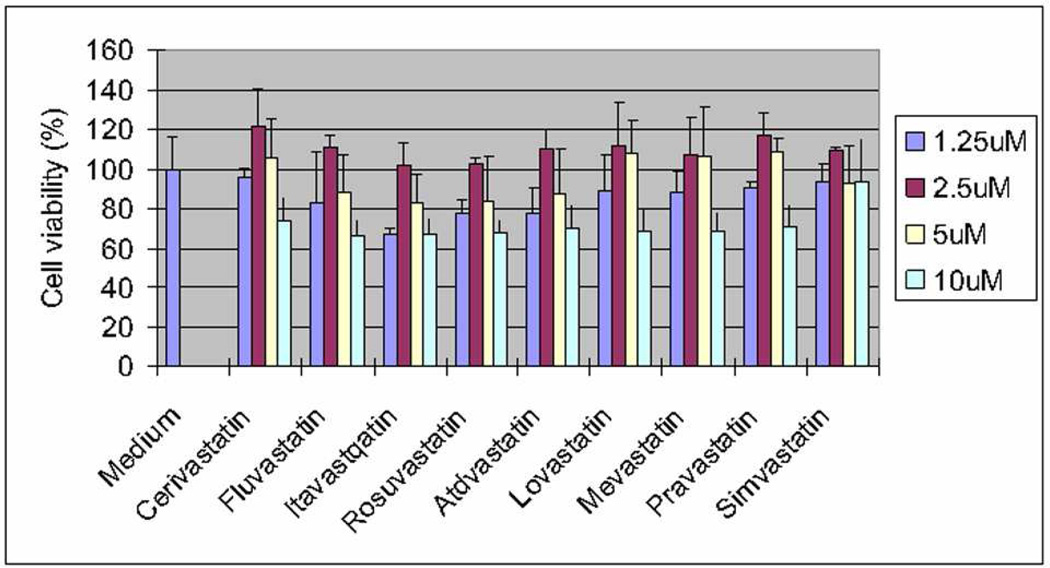
The statins are well known HMG-CoA R inhibitors, which reduce cellular cholesterol (Ito et al. 2006). The response of each statin is cell type specific (Soma et al. 1992). First, ARPE-19 cells were treated with 9 different statins, and assessed for cell viability by MTT assay after 24 hours of exposure. Figure 6 shows that most of the statins showed no toxicity up to and including 5µM, but all were cytotoxic at 10µM (p<0.05 at 10µM for all statins except Simvastatin (p=0.07), versus control).
Figure 6.
Viability of ARPE-19 cells after treatment with 9 statins. ARPE-19 cells were treated with statins (0–10µM) for 24h. Toxicity was observed at 10µM doses for all nine statins. p<0.05 at 10µM for all statins except Simvastatin (p=0.07), versus control.
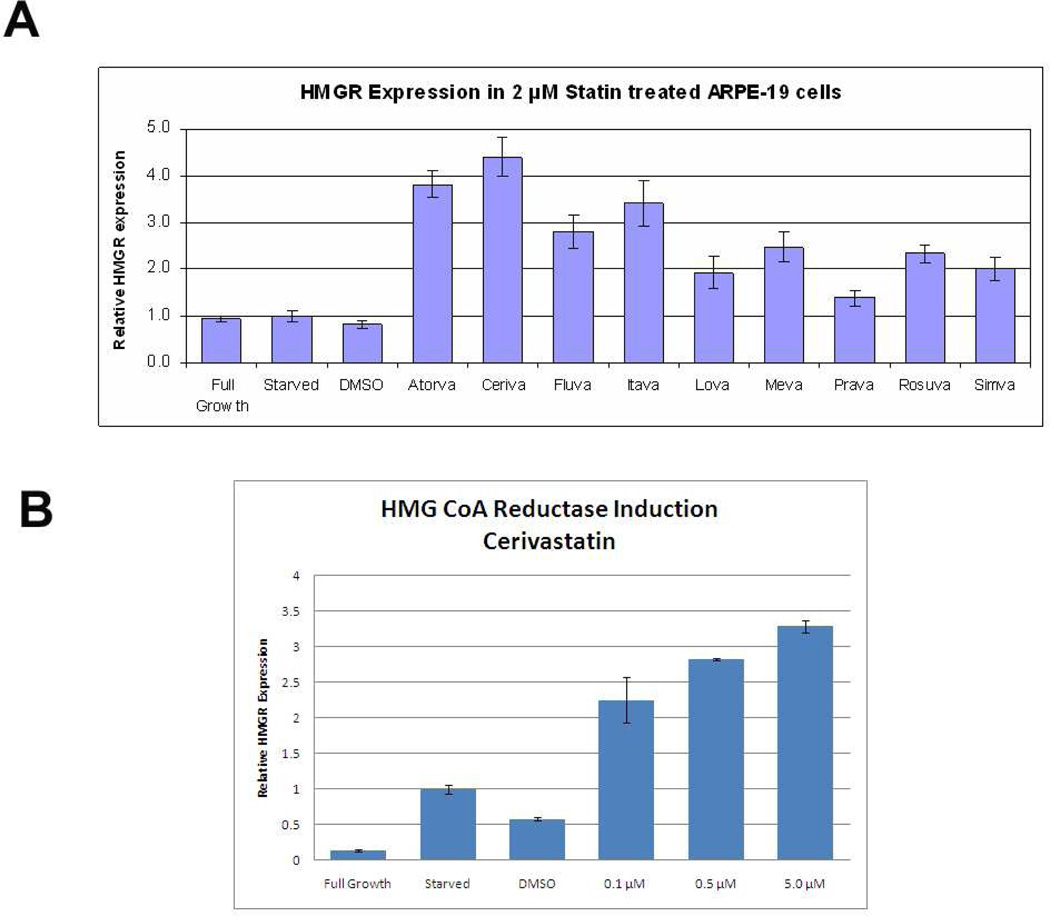
Statins that reach the endoplasmic reticulum cause an early mRNA induction of HMGCoA reductase as a compensatory response (Thelen et al. 2006). We used this response as a biomarker of whether statins penetrated ARPE-19 cells and exerted a functional response. Cells were treated with 2µM statins for 24h in serum-free media, and HMG-CoA R mRNA was determined by RT-qPCR. Figure 7 shows the variability in induction of HMG-CoA R expression by the different statins. Since the lipophilic Cerivastatin showed the highest induction of HMGCoA R expression, a follow-up set of dose response experiments were conducted using Cerivastatin.
Figure 7.
Induction of HMG-CoA reductase mRNA after Statin Treatment. A) ARPE-19 cells were treated with 9 statins (2µM) for 24h, and induction of HMG-CoA reductase mRNA was measured by RT-qPCR analysis and normalized to GAPDH expression. Variable expression is seen among the statins. Full growth control indicates cells grown in DMEM+10%FBS while starved indicates cells grown in DMEM+1% BSA. P<0.01 for all statins vs. each control. (n=3 exp). B) Induction of HMG-CoA reductase by Cerivastatin 0.1–5µM after 24h. P<0.01 for all doses vs. each control. (n=3 exp).
Cerivastatin reduces cellular cholesterol
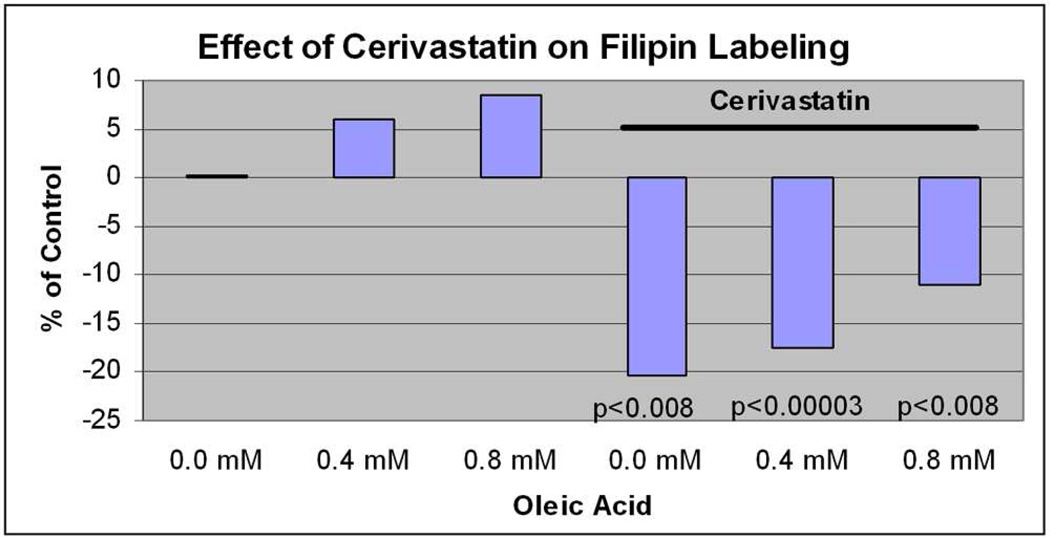
Since it was the most potent inducer of HMG-CoA reductase expression in our system, the remaining experiments focused on Cerivastatin. We next evaluated changes in cellular lipid and apoB100 secretion as two indicators of a pharmacologic effect by Cerivastatin. When challenged with oleic acid, ARPE-19 cells showed a dose dependent increase in filipin staining. While 5µM Cerivastatin had no effect on cellular cholesterol after a 24h incubation, filipin staining was reduced up to 20% (p<0.001) after 3 days compared to controls (Figure 8). Using a biochemical assay, after 0.4mM oleic acid treatment, 3 days of Cerivastatin 5 µM reduced total cholesterol levels by 39% (p<0.05). Cerivastatin did not have an effect on cellular triglyceride content after ARPE-19 cells were treated with oleic acid (data not shown).
Figure 8.
The effect of Cerivastatin on Filipin fluorescence labeling. ARPE-19 cells were treated with oleic acid 0–0.8mM for show a dose dependent increase in Filipin labeling. Treatment with Cerivastatin 5µM caused a marked reduction in Filipin labeling at each dose.
Cerivastatin inhibits apoB100 protein secretion
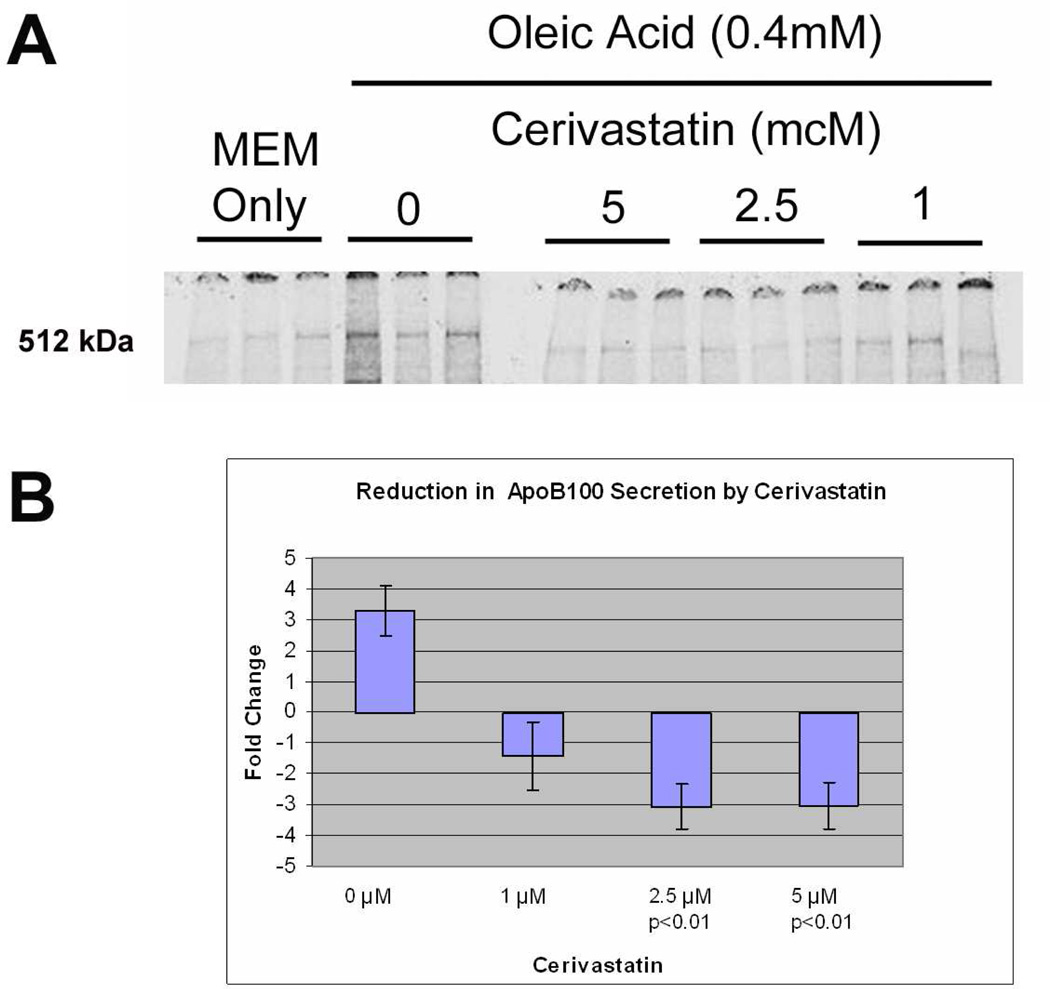
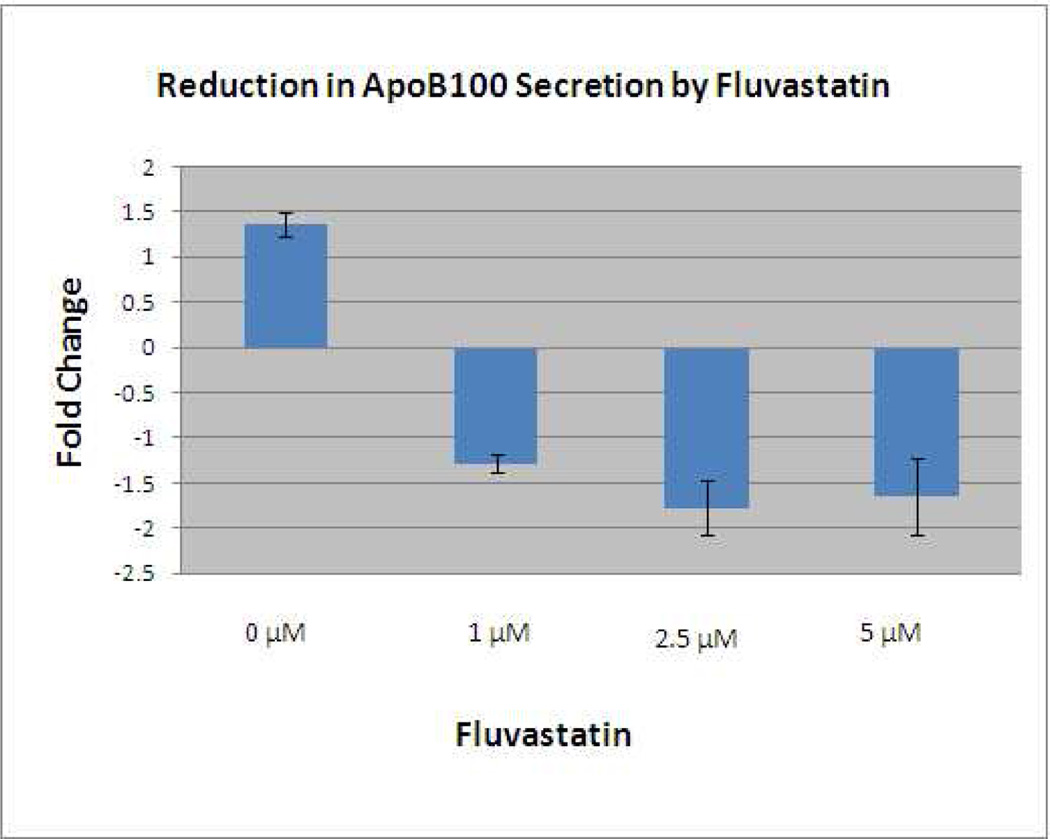
Cerivastatin inhibited the synthesis and secretion of apoB100 protein into the culture medium. Figure 9A shows that oleic acid caused a 3.3-fold increase in radiolabeled apoB100 protein secreted into the medium while incubation with Cerivastatin (2.5–5µM) significantly reduced apoB100 secretion (p<0.01). The experiment was repeated twice with similar results, as summarized in Figure 9B. Most statins enter cells through passive diffusion, which is dependent upon their lipophilicity (Thelen et al. 2006). To demonstrate that the suppressive effect on ApoB100 secretion is not unique to Cerivastatin, we next evaluated the effect of Fluvastatin, another lipophilic statin. A dose response experiment with Fluvastatin 1–5µM showed up to a 1.8-fold reduction in secretion of radiolabeled apoB100 protein at a dose of 2.5µM (p<0.05; Figure 10). The experiment was repeated with a similar reduction of 1.6-fold with 2.5µM (p<0.05). On the other hand, Pravastatin, a highly hydrophilic statin, showed no effect on ApoB100 secretion (data not shown).
Figure 9.
Reduction in 35S-radiolabeled ApoB100 protein secreted into the culture medium by Cerivastatin after oleic acid stimulation. A) Autoradiogram of 35S-radiolabeled ApoB100 protein secreted in the culture medium by ARPE-19 cells. Treatment with Cerivastatin 1–5µM reduced the 512KDa band. B) Graph showing reduced 35S-radiolabeled apoB100 protein recovered from the culture medium after Cerivastatin treatment.
Figure 10.
Reduction in 35S-radiolabeled ApoB100 protein secreted into the culture medium after treatment with Fluvastatin after oleic acid stimulation. A similar decrease in 35S-radiolabeled apoB100 protein recovered from the culture medium after Fluvastatin 1–5µM.
Antioxidant effect of Cerivastatin
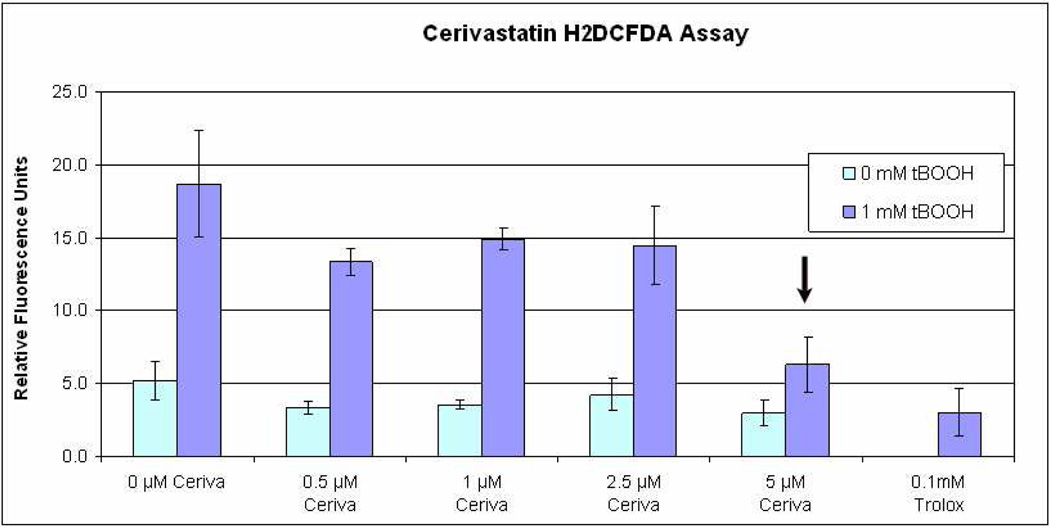
Oxidative stress is an important factor in AMD development. The statins have been reported to display a number of effects other than cholesterol lowering, including anti-oxidant effects (Ramasubbu et al. 2008). To explore this possible benefit of statins, ARPE-19 cells were treated with 1mM tBOOH and assessed for ROI by the H2DCF assay. A trend toward an antioxidant effect was observed with increasing dose, with similar protection at 5µM Cerivastatin compared to the antioxidant Trolox (Figure 11).
Figure 11.
Cerivastatin reduces Reactive oxygen intermediates after Tert-butyl hydroperoxide (tBOOH) using H2DCFDA labeling. A significant decrease (p<0.01 vs. other doses of Cerivastatin) was seen at Cerivastatin (5µM), and was similar to the antioxidant Trolox (p=0.17, Cerivastatin 5µM vs. Trolox).
Discussion
Herein, we provide strong evidence that human RPE cells synthesize and secrete apoB100 lipoproteins, and that this secretion is driven by cellular cholesterol content. Unstimulated human ARPE-19 cells neither accumulated lipid nor synthesized and secreted apoB100 protein. On the other hand, after oleic acid stimulation, we first demonstrated that ARPE-19 cells contained increased cellular cholesterol, as assessed by Filipin histochemical staining and an enzymatic assay, while triglycerides, a principal cellular neutral lipid, were unchanged by oleic acid treatment. Several different assays were then used to show that apoB100 lipoprotein particle secretion, including increased apoB100 protein in the culture medium by Western blot analysis, and immunoprecipitation of 35S-radiolabeled apoB100 protein in the medium. This result indicates definitively, that ARPE-19 cells synthesize and secrete apoB100 protein, and removes doubt that apoB100 could be a contaminant from serum in the culture medium (Sakamoto et al. 2007). Using negative stain electron microscopy, we visualized lipoprotein-like particles in the culture medium of ARPE-19 cells. These data show that oleic acid loading of ARPE-19 cells induces increased cellular cholesterol which stimulates the secretion of apoB100 lipoprotein particles. In contrast, reducing cellular cholesterol by statin treatment reduced ApoB100 synthesis and secretion. Taken together, these data support the emerging hypothesis that human RPE cells secrete apoB100 lipoproteins. Due to significant differences in lipoprotein biology across species such as rodents and humans, our findings will help to facilitate future studies addressing why human RPE cells secrete lipoproteins, and if their secretion contributes to AMD.
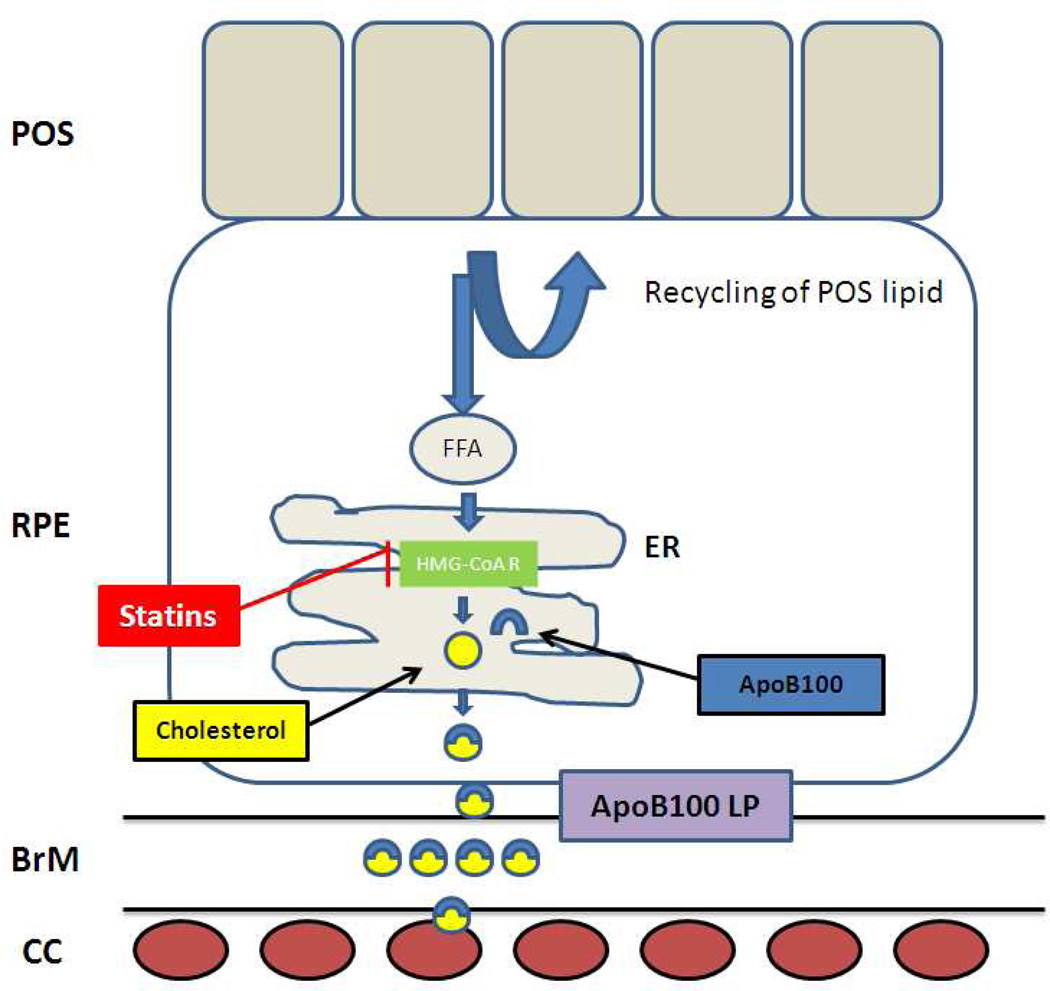
Why would the RPE secrete lipoproteins? The RPE has tremendous lipid processing responsibilities due to the constant recycling of 30,000 lipid rich photoreceptor outer segments per day (Gulcan et al. 1993; Ershov and Bazan 2000). The ingestion of systemically derived LDLs via LDL receptors by the RPE (Tserentsoodol et al. 2006b), is an additional potential lipid burden, especially if hyperlipidemia resulting from western diets would increase LDL ingestion. With aging, there is a reduction in energy metabolism, and a commensurate reduced utilization of lipids (Lee et al. 2002; Tian et al. 2005). Collectively, these factors would promote cellular lipid accumulation. Since lipid overload is extremely toxic to the cell and capable of inducing lipoapoptosis (Listenberger and Schaffer 2002), cells must compensate by excreting unwanted lipid. The RPE can transport cholesterol out of the cell by reverse cholesterol transport to high density lipoproteins (HDLs) (Tserentsoodol et al. 2006a). However, HDLs are an inefficient method for eliminating lipid (Yokoyama et al. 2004). In addition, the accumulation of the lipofuscin fluorofluore A2E that is seen in AMD, can inhibit the reverse cholesterol pathway, and result in the accumulation of cholesteryl esters in RPE cells (Lakkaraju et al. 2007). The most efficient mechanism to remove unwanted cellular lipid is with apoB100 lipoproteins (Yokoyama et al. 2004). The increase in cholesteryl esters resulting from A2E, would drive apoB100 lipoprotein secretion by the RPE, as suggested by our studies. This scenario would be analogous to the secretion of apoB100 lipoproteins by lipid overloaded cardiac myocytes as a protective response to prevent lipotoxic cardiomyopathy (Yokoyama et al. 2004). A schematic of our current working hypothesis is outlined in Figure 12. While apoB100 lipoprotein secretion may be designed to protect against lipotoxicity to the RPE, chronic lipoprotein secretion could cause lipoproteins to accumulate in Bruch’s membrane, especially if their clearance to the choriocapillaris is impaired. With the high oxidative stress environment of the fundus, retained lipoproteins would be at risk for becoming oxidized, as we have previously shown in early AMD specimens (Yamada et al. 2008). Oxidized lipoproteins are potent activators of complement mediated inflammation (Bhakdi et al. 1995; Bhakdi et al. 1999; Kislinger et al. 1999; Kalousova et al. 2003; Miller et al. 2003; Uesugi et al. 2004). Given the role of complement in AMD, future studies aimed at further understanding the factors regulating RPE lipoprotein secretion would be worthwhile.
Figure 12.
Diagram of ApoB100 secretion by the RPE and its potential consequences consequences. Lipids accumulation in part, from incompletely digested photoreceptor outer segments (POS) that are transported to the endoplasmic reticulum (ER) where free fatty acids (FFA) are synthesized into cholesterol. ApoB100 is constitutively produced, and cholesterol reduces ApoB100 protein degradation, and thereby promotes the formation of apoB100 lipoprotein particles, which are secreted and pass through Bruch’s membrane (BrM) to the choriocapillaris (CC). The statins inhibit HMG-CoA R, reduce cholesterol synthesis, and reduce apoB100 lipoprotein (LP) formation.
The statins are well known inhibitors of HMG-CoA reductase, the rate limiting step in cholesterol synthesis. We investigated the statins as a tool to study apoB100 secretion because of the known high content of cholesteryl ester in lipoproteins recovered from Bruch’s membrane. A significant factor in the efficacy of any drug at the cellular level is its ability to penetrate into the cell. Since statins enter cells passively, a lipophilic statin is expected to enter a cell easier than a hydrophilic one (Pasha et al. 2006). In our survey of statins, we found that lipophilic statins, such as Cerivastatin, Atorvastatin, Itavastatin, and Fluvastatin, induced HMG-CoA reductase, as an acute marker of cellular internalization, better than hydrophilic statins such as Pravastatin and Rosuvastatin. With focused studies using Cerivastatin, cellular cholesterol and apoB100 secretion were reduced after cells were stimulated with oleic acid. We presume that the reduced cellular cholesterol was the lipid substrate that influenced reduced apoB100 secretion since triglyceride content was unchanged after oleic acid stimulation. We hypothesize that reduced apoB100 lipoprotein secretion would reduce lipoprotein accumulation in Bruch’s membrane, and thereby reduce drusen formation. At present, it is unclear how the overall lipid composition of the RPE is influenced by statins. It is possible that inhibiting apoB100 lipoprotein secretion might increase the overall cellular lipid content, and drive the cell into lipoapoptosis. The cytotoxic effects would depend upon how the cellular lipid composition is altered with the most toxic effects resulting from excessive free fatty acids. While somewhat cell type specific, HMG-CoA R inhibition has been shown to alter cellular triglycerides, fatty acids, and phospholipids (Hrboticky et al. 1997; Rise et al. 2005; Leszczynska et al. 2009). Within the concentration of statins that we tested, there was no cytoxicity, which suggests that any changes in lipid composition were not toxic. Further delineation of the specific cellular lipid composition through mass spectroscopic analysis induced by statins would be helpful in future studies, so we could determine in more detail, whether the changes in lipid composition are potentially cytotoxic.
We recognize that there are considerable data on apoB100 secretion by statins, and the results appear to be variable and cell type dependent (Huff and Burnett 1997; Mohammadi et al. 1998; Wilcox et al. 1999; Twisk et al. 2000). The other principal mechanism of statins is upregulation of LDL receptors, which causes both increased uptake at the cell membrane as well as potential presecretory degradation (Twisk et al. 2000). Using flow cytometry, in preliminary data, we found that LDLR density was increased in a dose dependent manner with both LDL and oxLDL treatment. However, LDLR density did not change after a 24 hour incubation with Cerivastatin. This suggests that LDLR density change is not a significant factor in reducing apoB100 secretion in RPE cells. We recognize that intracellular LDLR, which would be missed by flow cytometry, could affect apoB100 secretion. Further work will be necessary to delineate the exact mechanism of action by statins in human RPE cells. We have outlined in Figure 12, our current understanding of how statins influence apoB100 lipoprotein secretion.
How do the results of our study influence interpretation of the studies that have shown no treatment benefit for AMD after systemic administration of statins (McGwin et al. 2003; McGwin et al. 2005; Smeeth et al. 2005; McGwin et al. 2006; Klein et al. 2007; Tan et al. 2007)? Efficacy from systemically administered statins assumes that Bruch’s membrane lipoproteins are either derived from the systemic circulation via their production in the liver, or that the statins exert a local effect on lipoprotein production after penetrating the blood retinal barrier. Clearly, the statins reduce plasma LDL-cholesterol by inhibiting cholesterol synthesis in the liver. If the systemic circulation is the predominant source of Bruch’s membrane lipoproteins, then systemic administration of statins would likely have shown some benefit.
The ineffectiveness by the statins does not eliminate them as a potential treatment if lipoproteins are locally secreted by the RPE. An ocular effect by the statins would depend largely upon their ability to penetrate the blood retinal barrier, and in particular, the outer blood retinal barrier, which is comprised mainly of the RPE. At present, the only available pharmacokinetic clues must be extrapolated from studies evaluating penetration of the blood brain barrier, an analogous structure to the blood retinal barrier. Penetration of the blood brain barrier is dependent upon the statin’s lipophilicity. For example, when given systemically, the lipophilic Simvastatin, penetrates the blood brain barrier well, and inhibits brain cholesterol synthesis (Thelen et al. 2006; Vuletic et al. 2006). On the other hand, high doses of the hydrophilic Pravastatin are required to achieve a similar biologic effect (Thelen et al. 2006). All of the studies evaluating the statins on AMD published to date, have lumped all statins together so an assessment of a local ocular effect is not possible.
While commonly used to simulate RPE cell function, we recognize that the results obtained in ARPE-19 cells may not represent the behavior of RPE cells in vivo. Nevertheless, the data from our study support the secretion of apoB100 protein and lipoprotein-like particles by human RPE cells, which will enable us to study why RPE cells secrete lipoproteins. Treatment with the statins inhibited apoB100 secretion. Locally administered statins, particularly with the emergence of several different sustained released intraocular platforms, could be an intriguing avenue to pursue in an attempt to reduce lipoprotein accumulation since this appears to be an important, early event in the development of AMD.
Acknowledgements
Grant Support: EY14005 (JTH), a grant from QLT, Inc. (JTH), Robert Bond Welch Professorship (JTH), an unrestricted grant from Research to Prevent Blindness (Wilmer Eye Institute), and generous gifts from Ric and Sandy Forsythe, the Kwok family, the Merlau family, and Aleda Wright.
Abbreviations
- AMD
age-related macular degeneration
- apoB100
apolipoprotein B100
- RPE
retinal pigmented epithelium
- ROI
reactive oxygen intermediates
Footnotes
Conflict of interest: none.
References
- Bhakdi S, Torzewski M, Klouche M, Hemmes M. Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Dorweiler B, Kirchmann R, Torzewski J, Weise E, Tranum-Jensen J, Walev I, Wieland E. On the pathogenesis of atherosclerosis: enzymatic transformation of human low density lipoprotein to an atherogenic moiety. J Exp Med. 1995;182:1959–1971. doi: 10.1084/jem.182.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren J, Rustaeus S, Olofsson SO. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J Biol Chem. 1994;269:25879–25888. [PubMed] [Google Scholar]
- Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:265–274. [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Millican CL, Medeiros NE. Basal deposits and drusen in eyes with age-related maculopathy: evidence for solid lipid particles. Exp Eye Res. 2005a;80:761–775. doi: 10.1016/j.exer.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, Kruth HS. Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res. 2005b;81:731–741. doi: 10.1016/j.exer.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Davidson MH, Toth PP. Comparative effects of lipid-lowering therapies. Prog Cardiovasc Dis. 2004;47:73–104. doi: 10.1016/j.pcad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ershov AV, Bazan NG. Photoreceptor phagocytosis selectively activates PPARgamma expression in retinal pigment epithelial cells. J Neurosci Res. 2000;60:328–337. doi: 10.1002/(SICI)1097-4547(20000501)60:3<328::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Forte TM, Nordhausen RW. Electron microscopy of negatively stained lipoproteins. Methods Enzymol. 1986;128:442–457. doi: 10.1016/0076-6879(86)28086-6. [DOI] [PubMed] [Google Scholar]
- Fungwe TV, Fox JE, Cagen LM, Wilcox HG, Heimberg M. Stimulation of fatty acid biosynthesis by dietary cholesterol and of cholesterol synthesis by dietary fatty acid. J Lipid Res. 1994;35:311–318. [PubMed] [Google Scholar]
- Futterman S, Andrews JS. The fatty acid composition of human retinal vitamin A ester and the lipids of human retinal tissue. Invest Ophthalmol. 1964;3:441–444. [PubMed] [Google Scholar]
- Goh EH, Heimberg M. Stimulation of hepatic cholesterol biosynthesis by oleic acid. Biochem Biophys Res Commun. 1973;55:382–388. doi: 10.1016/0006-291x(73)91098-x. [DOI] [PubMed] [Google Scholar]
- Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92:615–627. [PubMed] [Google Scholar]
- Gulcan HG, Alvarez RA, Maude MB, Anderson RE. Lipids of human retina, retinal pigment epithelium, and Bruch's membrane/choroid: comparison of macular and peripheral regions. Invest Ophthalmol Vis Sci. 1993;34:3187–3193. [PubMed] [Google Scholar]
- Handa JT, Reiser KM, Matsunaga H, Hjelmeland LM. The advanced glycation endproduct pentosidine induces the expression of PDGF-B in human retinal pigment epithelial cells. Exp Eye Res. 1998;66:411–419. doi: 10.1006/exer.1997.0442. [DOI] [PubMed] [Google Scholar]
- Hofker MH, van Vlijmen BJ, Havekes LM. Transgenic mouse models to study the role of APOE in hyperlipidemia and atherosclerosis. Atherosclerosis. 1998;137:1–11. doi: 10.1016/s0021-9150(97)00266-9. [DOI] [PubMed] [Google Scholar]
- Hrboticky N, Becker A, Kruse HJ, Weber PC. Increased cellular triglyceride levels in human monocytic and rat smooth muscle cells after lovastatin. Biochim Biophys Acta. 1997;1349:211–221. doi: 10.1016/s0005-2760(97)00136-7. [DOI] [PubMed] [Google Scholar]
- Huff MW, Burnett JR. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and hepatic apolipoprotein B secretion. Curr Opin Lipidol. 1997;8:138–145. doi: 10.1097/00041433-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Tian J, Handa JT. Similarity of mRNA phenotypes of morphologically normal macular and peripheral retinal pigment epithelial cells in older human eyes. Invest Ophthalmol Vis Sci. 2004;45:3291–3301. doi: 10.1167/iovs.04-0168. [DOI] [PubMed] [Google Scholar]
- Ito MK, Talbert RL, Tsimikas S. Statin-associated pleiotropy: possible beneficial effects beyond cholesterol reduction. Pharmacotherapy. 2006;26:85S–97S. doi: 10.1592/phco.26.7part2.85S. discussion 98S–101S; quiz 106S–108S. [DOI] [PubMed] [Google Scholar]
- Kalousova M, Zima T, Tesar V, Sulkova S, Fialova L. Relationship between advanced glycoxidation end products, inflammatory markers/acute-phase reactants, and some autoantibodies in chronic hemodialysis patients. Kidney Int Suppl. 2003:S62–S64. doi: 10.1046/j.1523-1755.63.s84.19.x. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Klein R, Knudtson MD, Klein BE. Statin use and the five-year incidence and progression of age-related macular degeneration. Am J Ophthalmol. 2007;144:1–6. doi: 10.1016/j.ajo.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proc Natl Acad Sci U S A. 2002;99:14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynska A, Burzynska B, Plochocka D, Kaminska J, Zimnicka M, Kania M, Kiliszek M, Wysocka-Kapcinska M, Danikiewicz W, Szkopinska A. Investigating the effects of statins on cellular lipid metabolism using a yeast expression system. PLoS One. 2009;4:e8499. doi: 10.1371/journal.pone.0008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Clark ME, Chimento MF, Curcio CA. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Invest Ophthalmol Vis Sci. 2006;47:3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- Li CM, Presley JB, Zhang X, Dashti N, Chung BH, Medeiros NE, Guidry C, Curcio CA. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J Lipid Res. 2005a;46:628–640. doi: 10.1194/jlr.M400428-JLR200. [DOI] [PubMed] [Google Scholar]
- Li CM, Chung BH, Presley JB, Malek G, Zhang X, Dashti N, Li L, Chen J, Bradley K, Kruth HS, Curcio CA. Lipoprotein-like particles and cholesteryl esters in human Bruch's membrane: initial characterization. Invest Ophthalmol Vis Sci. 2005b;46:2576–2586. doi: 10.1167/iovs.05-0034. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Schaffer JE. Mechanisms of lipoapoptosis: implications for human heart disease. Trends Cardiovasc Med. 2002;12:134–138. doi: 10.1016/s1050-1738(02)00152-4. [DOI] [PubMed] [Google Scholar]
- Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA. Apolipoprotein B in cholesterol-containing drusen and basal deposits of human eyes with age-related maculopathy. Am J Pathol. 2003;162:413–425. doi: 10.1016/S0002-9440(10)63836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Ballabriga A, Gil-Gibernau JJ. Lipids of the developing human retina: I. Total fatty acids, plasmalogens, and fatty acid composition of ethanolamine and choline phosphoglycerides. J Neurosci Res. 1988;20:484–490. doi: 10.1002/jnr.490200412. [DOI] [PubMed] [Google Scholar]
- McGwin G, Jr, Xie A, Owsley C. The use of cholesterol-lowering medications and age-related macular degeneration. Ophthalmology. 2005;112:488–494. doi: 10.1016/j.ophtha.2004.10.027. [DOI] [PubMed] [Google Scholar]
- McGwin G, Jr, Owsley C, Curcio CA, Crain RJ. The association between statin use and age related maculopathy. Br J Ophthalmol. 2003;87:1121–1125. doi: 10.1136/bjo.87.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGwin G, Jr, Modjarrad K, Hall TA, Xie A, Owsley C. 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors and the presence of age-related macular degeneration in the Cardiovascular Health Study. Arch Ophthalmol. 2006;124:33–37. doi: 10.1001/archopht.124.1.33. [DOI] [PubMed] [Google Scholar]
- McMillian MK, Grant ER, Zhong Z, Parker JB, Li L, Zivin RA, Burczynski ME, Johnson MD. Nile Red binding to HepG2 cells: an improved assay for in vitro studies of hepatosteatosis. In Vitr Mol Toxicol. 2001;14:177–190. doi: 10.1089/109793301753407948. [DOI] [PubMed] [Google Scholar]
- Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Curr Opin Lipidol. 2003;14:437–445. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Mohammadi A, Macri J, Newton R, Romain T, Dulay D, Adeli K. Effects of atorvastatin on the intracellular stability and secretion of apolipoprotein B in HepG2 cells. Arterioscler Thromb Vasc Biol. 1998;18:783–793. doi: 10.1161/01.atv.18.5.783. [DOI] [PubMed] [Google Scholar]
- Pasha MK, Muzeeb S, Basha SJ, Shashikumar D, Mullangi R, Srinivas NR. Analysis of five HMG-CoA reductase inhibitors-- atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin: pharmacological, pharmacokinetic and analytical overview and development of a new method for use in pharmaceutical formulations analysis and in vitro metabolism studies. Biomed Chromatogr. 2006;20:282–293. doi: 10.1002/bmc.561. [DOI] [PubMed] [Google Scholar]
- Ramasubbu K, Estep J, White DL, Deswal A, Mann DL. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:415–426. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Rise P, Ghezzi S, Priori I, Galli C. Differential modulation by simvastatin of the metabolic pathways in the n-9, n-6 and n-3 fatty acid series, in human monocytic and hepatocytic cell lines. Biochem Pharmacol. 2005;69:1095–1100. doi: 10.1016/j.bcp.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Ruberti JW, Curcio CA, Millican CL, Menco BP, Huang JD, Johnson M. Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch's membrane. Invest Ophthalmol Vis Sci. 2003;44:1753–1759. doi: 10.1167/iovs.02-0496. [DOI] [PubMed] [Google Scholar]
- Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol. 2001;12:121–127. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Tsuji K, Muul LM, Lawler AM, Petricoin EF, Candotti F, Metcalf JA, Tavel JA, Lane HC, Urba WJ, Fox BA, Varki A, Lunney JK, Rosenberg AS. Bovine apolipoprotein B-100 is a dominant immunogen in therapeutic cell populations cultured in fetal calf serum in mice and humans. Blood. 2007;110:501–508. doi: 10.1182/blood-2007-01-066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs NJ, Simons HL. Caveolar bands and the effects of sterol-binding agents in vascular smooth muscle plasma membrane. Single and double labeling with filipin and tomatin in the aorta, pulmonary artery, and vena cava. Lab Invest. 1986;55:295–307. [PubMed] [Google Scholar]
- Smeeth L, Cook C, Chakravarthy U, Hubbard R, Fletcher AE. A case control study of age related macular degeneration and use of statins. Br J Ophthalmol. 2005;89:1171–1175. doi: 10.1136/bjo.2004.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma MR, Corsini A, Paoletti R. Cholesterol and mevalonic acid modulation in cell metabolism and multiplication. Toxicol Lett. 1992;64–65(Spec No):1–15. doi: 10.1016/0378-4274(92)90167-i. [DOI] [PubMed] [Google Scholar]
- Sugii S, Reid PC, Ohgami N, Shimada Y, Maue RA, Ninomiya H, Ohno-Iwashita Y, Chang TY. Biotinylated theta-toxin derivative as a probe to examine intracellular cholesterol-rich domains in normal and Niemann-Pick type C1 cells. J Lipid Res. 2003;44:1033–1041. doi: 10.1194/jlr.D200036-JLR200. [DOI] [PubMed] [Google Scholar]
- Tan JS, Mitchell P, Rochtchina E, Wang JJ. Statins and the long-term risk of incident age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:685–687. doi: 10.1016/j.ajo.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Thelen KM, Rentsch KM, Gutteck U, Heverin M, Olin M, Andersson U, von Eckardstein A, Bjorkhem I, Lutjohann D. Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J Pharmacol Exp Ther. 2006;316:1146–1152. doi: 10.1124/jpet.105.094136. [DOI] [PubMed] [Google Scholar]
- Tian J, Ishibashi K, Ishibashi K, Reiser K, Grebe R, Biswal S, Gehlbach P, Handa JT. Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: A comprehensive transcriptional response. Proc Natl Acad Sci U S A. 2005;102:11846–11851. doi: 10.1073/pnas.0504759102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006a;12:1319–1333. [PubMed] [Google Scholar]
- Tserentsoodol N, Sztein J, Campos M, Gordiyenko NV, Fariss RN, Lee JW, Fliesler SJ, Rodriguez IR. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006b;12:1306–1318. [PubMed] [Google Scholar]
- Twisk J, Gillian-Daniel DL, Tebon A, Wang L, Barrett PH, Attie AD. The role of the LDL receptor in apolipoprotein B secretion. J Clin Invest. 2000;105:521–532. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi N, Sakata N, Nangaku M, Abe M, Horiuchi S, Hisano S, Iwasaki H. Possible mechanism for medial smooth muscle cell injury in diabetic nephropathy: glycoxidation-mediated local complement activation. Am J Kidney Dis. 2004;44:224–238. doi: 10.1053/j.ajkd.2004.04.027. [DOI] [PubMed] [Google Scholar]
- van Kuijk FJ, Buck P. Fatty acid composition of the human macula and peripheral retina. Invest Ophthalmol Vis Sci. 1992;33:3493–3496. [PubMed] [Google Scholar]
- Vuletic S, Riekse RG, Marcovina SM, Peskind ER, Hazzard WR, Albers JJ. Statins of different brain penetrability differentially affect CSF PLTP activity. Dement Geriatr Cogn Disord. 2006;22:392–398. doi: 10.1159/000095679. [DOI] [PubMed] [Google Scholar]
- Wang L, Li CM, Rudolf M, Curcio CA, Peng D, Liu X, Zeng S. Gene expression of apolipoprotein and lipids synthesis and secretion in RPE-J cells. Yan Ke Xue Bao. 2006;22:244–251. [PubMed] [Google Scholar]
- Wang L, Li CM, Rudolf M, Belyaeva OV, Chung BH, Messinger JD, Kedishvili NY, Curcio CA. Lipoprotein particles of intraocular origin in human Bruch membrane: an unusual lipid profile. Invest Ophthalmol Vis Sci. 2009;50:870–877. doi: 10.1167/iovs.08-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- Wilcox LJ, Barrett PH, Huff MW. Differential regulation of apolipoprotein B secretion from HepG2 cells by two HMG-CoA reductase inhibitors, atorvastatin and simvastatin. J Lipid Res. 1999;40:1078–1089. [PubMed] [Google Scholar]
- Yamada Y, Tian J, Yang Y, Cutler RG, Wu T, Telljohann RS, Mattson MP, Handa JT. Oxidized Low Density Lipoproteins Induce a Pathologic Response by Retinal Pigmented Epithelial Cells. J Neurochem. 2008;105:1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Yagyu H, Hu Y, Seo T, Hirata K, Homma S, Goldberg IJ. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem. 2004;279:4204–4211. doi: 10.1074/jbc.M311995200. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Hernandez-Ono A, Ko C, Yasunaga K, Huang LS, Ginsberg HN. Regulation of hepatic apolipoprotein B-lipoprotein assembly and secretion by the availability of fatty acids I. Differential response to the delivery of fatty acids via albumin or remnant-like emulsion particles. J Biol Chem. 2004;279:19362–19374. doi: 10.1074/jbc.M400220200. [DOI] [PubMed] [Google Scholar]