Abstract
Objective
To determine how intraoperative microelectrode recordings (MER) and intraoperative lead placement acutely influence tremor, rigidity, and bradykinesia. Secondarily, to evaluate whether the longevity of the MER and lead placement effects were influenced by target location (subthalamic nucleus (STN) or globus pallidus interna (GPi)).
Background
Currently most groups who perform deep brain stimulation (DBS) for Parkinson disease (PD) use MER, as well as macrostimulation (test stimulation), to refine DBS lead position. Following MER and/or test stimulation, however, there may be a resultant “collision/implantation” or “microlesion” effect, thought to result from disruption of cells and/or fibres within the penetrated region. These effects have not been carefully quantified.
Methods
47 consecutive patients with PD undergoing unilateral DBS for PD (STN or GPi DBS) were evaluated. Motor function was measured at six time points with a modified motor Unified Parkinson Disease Rating Scale (UPDRS): (1) preoperatively, (2) immediately after MER, (3) immediately after lead implantation/collision, (4) 4 months following surgery—off medications, on DBS (12 h medication washout), (5) 6 months postoperatively—off medication and off DBS (12 h washout) and (6) 6 months—on medication and off DBS (12 h washout).
Results
Significant improvements in motor scores (p<0.05) (tremor, rigidity, bradykinesia) were observed as a result of MER and lead placement. The improvements were similar in magnitude to what was observed at 4 and 6 months post-DBS following programming and medication optimisation. When washed out (medications and DBS) for 12 h, UPDRS motor scores were still improved compared with preoperative testing. There was a larger improvement in STN compared with GPi following MER (p<0.05) and a trend for significance following lead placement (p<0.08) but long term outcome was similar.
Conclusion
This study demonstrated significant acute intraoperative penetration effects resulting from MER and lead placement/collision in PD. Clinicians rating patients in the operating suite should be aware of these effects, and should consider pre- and post-lead placement rating scales prior to activating DBS. The collision/implantation effects were greater intraoperatively with STN compared with GPi, and with greater disease duration there was a larger effect.
Currently, most groups who perform deep brain stimulation (DBS) for Parkinson disease (PD) use microelectrode recording (MER), as well as macro-stimulation (test stimulation) to refine DBS lead positioning.1–6 Following MER and/or test stimulation there may, however, be a resultant “collision/implantation or microlesion” effect,7,8 thought to result from disruption of cells and/or fibres within the penetrated region. These effects have not been carefully quantified but could prove important to the clinician, especially when testing lead effectiveness in the operating room to confirm and to refine final DBS lead placement. We sought to compare intraoperative changes and clinical outcome of STN or GPi DBS.
METHODS
Study design
A consecutive series of patients undergoing unilateral DBS for PD (either STN or GPi DBS) were evaluated. All patients signed informed consents to have their data stored in a database in accordance with the Declaration of Helsinki. Motor function was measured at six time points to evaluate the acute and long term effects of surgical collision/implantation. These time points were: (1) pre-operatively (on the day of surgery), (2) immediately after MER, (3) immediately after lead implantation, (4) 4 months following surgery while off medications on DBS (12 h medication washout), (5) 6 months after surgery while off medication and off DBS (12 h washout) and (6) 6 months on medication and off DBS (12 h washout).
Measures
A modified motor Unified Parkinson Disease Rating Scale (UPDRS) was performed in the operative setting following an overnight withdrawal of dopaminergic medication (≥12 h). The modified scale was necessary as the head and neck were immobilised by a stereotactic ring used for the surgical procedure. Resting tremor (upper and lower limb), rigidity (upper and lower limb) and bradykinesia (upper and lower limb, finger taps, opening and closing of the hand, over and back movements of the hand, and foot tapping) were quantified in the contralateral extremity (with respect to the hemisphere being operated). The items on the modified UPDRS were graded identically to what would be obtained in a clinic setting, with the exception that all patients were in a supine position. The items were scored by a movement disorders neurologist (MSO). The rater was not blinded to the state (ie, post-MER). Immediately following all MER penetrations a second modified UPDRS was obtained. Finally, immediately after the DBS lead was placed and prior to activation or testing, a final modified UPDRS scale was recorded. Chronic changes documented at clinic visits at approximately 4 months (off medication, on DBS) and approximately 6 months (on medication, off DBS and off medication, off DBS) post-DBS were analysed by extracting comparable items on the UPDRS. Analyses included comparisons between preoperative values and the immediate post-MER, immediate post-DBS lead placement and follow-up UPDRS scales visits.
We created four composite motor score outcome variables based on the modified UPDRS motor scores: total, bradykinesia, tremor and rigidity. Bradykinesia was defined as the sum of leg agility, finger tapping, hand movements and rapid alternating arm movements. Tremor was defined as the sum of the lower and upper extremity tremor measures. Rigidity was defined as the sum of upper and lower extremity rigidity items. Total score was defined as the sum of all of the modified UPDRS items.
Statistical methods
Initially, we calculated statistics and graphed box and whisker plots to identify missing values and outliers and/or impossible or implausible values, to summarise the data, and to check for distributional forms. We used a two sided alpha = 0.05 as the level of significance. Statistical analyses were performed using SAS V.9.1 (SAS Institute, Cary, North Carolina, USA).
To test for significant change between presurgery, intra-operative and postoperative time points, differences in motor scores were computed (presurgery versus the other conditions). The Wilcoxon signed rank test was then used to test the null hypothesis of no change in motor score. A generalised linear mixed model (GLMM) was used to investigate the effects of various patient and surgical variables on the slope of change in motor function over time. In our model, the response was the vector of repeated motor scores at preoperative, postoperative MER, postoperative lead collision/implantation and postoperative values (4 months). Explanatory variables considered in the model were duration of illness, severity of illness (measured by Hoehn and Yahr), number of macrostimulation passes, number of microelectrode (MER) passes and the target site of DBS. We assumed that the slope of motor scores was linear and we modelled within subject correlations with an autoregressive covariance structure. To compare DBS target sites on magnitude of motor score change, we used the Wilcoxon rank sum test.
RESULTS
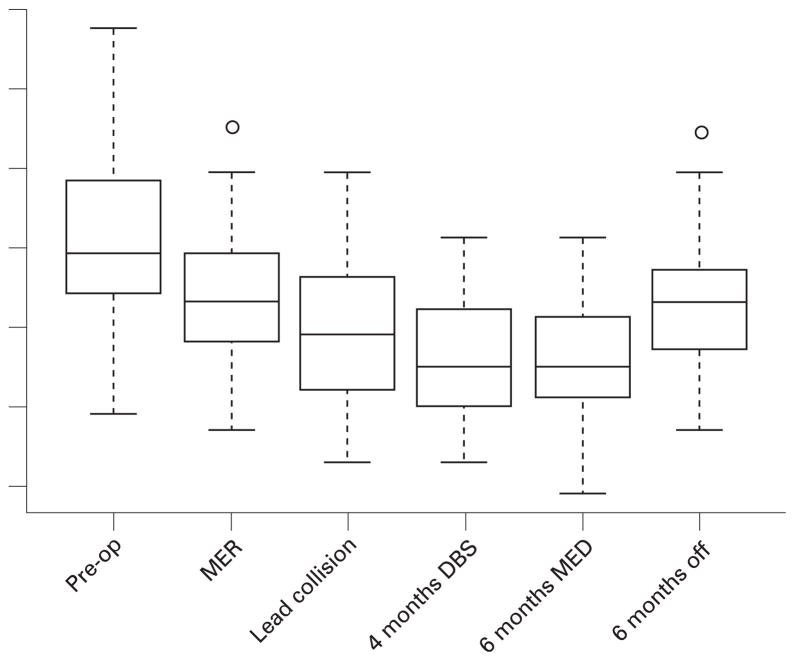
Forty-seven subjects with an average age of 59 years (SD 7.3 years) were included in the study; 74% (n = 35) were male. Table 1 summarises the cohort characteristics of PD duration, severity and characteristics of the DBS surgery. Figure 1 is a graphical representation of the UPDRS data using box plots to illustrate the modified UPDRS total motor score over time, while table 2 describes the summary statistics (mean, median, SD) for the modified UPDRS total motor score and the composite bradykinesia, tremor and rigidity scores for each time point.
Table 1.
Parkinson’s disease and surgical characteristics of the cohort
| Characteristic | |
|---|---|
| Duration of illness (years) | |
| Mean | 11.9 |
| Median | 11.0 |
| SD | 4.3 |
| Handedness (%) | |
| Right | 91 (n = 43) |
| Left | 9 (n = 4) |
| Hoehn and Yahr stage (%) | |
| Stage 1 | 0 (n = 0) |
| Stage 1.5 | 2 (n = 1) |
| Stage 2 | 10 (n = 5) |
| Stage 2.5 | 15 (n = 7) |
| Stage 3 | 59 (n = 27) |
| Stage 4 | 13 (n = 6) |
| Stage 5 | 2 (n = 1) |
| Levodopa equivalent dose | |
| Mean | 1042.0 |
| Median | 933.4 |
| SD | 494.6 |
| Mini-Mental State Examination | |
| Mean | 28.4 |
| Median | 29.0 |
| SD | 1.7 |
| DBS side (%) | |
| Right | 38 (n = 18) |
| Left | 62 (n = 29) |
| DBS target (%) | |
| Globus pallidus interna | 34 (n = 16) |
| Subthalamic nucleus | 66 (n = 31) |
| No of macrostimulation passes | |
| Mean | 1.3 |
| Median | 1.0 |
| SD | 0.5 |
| No of microelectrode passes | |
| Mean | 4.2 |
| Median | 4.0 |
| SD | 1.1 |
| Days between time points DBS surgery to 4 month clinic | |
| Mean | 142.4 |
| Median | 131 |
| SD | 33.5 |
| 4 month clinic to 6 month clinic | |
| Mean | 65.1 |
| Median | 60 |
| SD | 40.4 |
DBS, deep brain stimulation.
Figure 1.
Box plots of the modified Unified Parkinson’s Disease Rating Scale (UPDRS) total scores. DBS, deep brain stimulation; MER, microelectrode recording.
Table 2.
Summary statistics for the modified UPDRS total score, bradykinesia, tremor and rigidity scores over surgical course
| Preop off medication | Post MER penetration off medication | Post lead penetration off medication | 4 months off medication on DBS | 6 months on medication off DBS | 6 months off medication off DBS | |
|---|---|---|---|---|---|---|
| Total motor score | ||||||
| Mean | 15.5 | 12.5 | 10.1 | 8.5 | 8.3 | 11.8 |
| Median | 15.0 | 12.0 | 10.0 | 8.0 | 8.0 | 12.0 |
| SD | 4.9 | 4.6 | 4.4 | 3.8 | 3.3 | 4.3 |
| Bradykinesia | ||||||
| Mean | 2.2 | 1.8 | 1.6 | 1.4 | 1.4 | 1.8 |
| Median | 2.0 | 2.0 | 1.5 | 1.3 | 1.5 | 1.8 |
| SD | 0.6 | 0.7 | 0.8 | 0.7 | 0.6 | 0.7 |
| Tremor | ||||||
| Mean | 1.2 | 0.7 | 0.5 | 0.3 | 0.3 | 0.7 |
| Median | 1.0 | 0.5 | 0.5 | 0.0 | 0.0 | 0.5 |
| SD | 1.0 | 0.8 | 0.6 | 0.7 | 0.6 | 0.8 |
| Rigidity | ||||||
| Mean | 2.2 | 1.8 | 1.4 | 1.3 | 1.0 | 1.6 |
| Median | 2.5 | 2.0 | 1.5 | 1.5 | 1.0 | 1.5 |
| SD | 0.9 | 0.9 | 0.8 | 0.8 | 0.7 | 0.7 |
DBS, deep brain stimulation; MER, microelectrode recording; UPDRS, Unified Parkinson’s Disease Rating Scale.
Improvements in motor function observed due to penetration were maintained over the 6 month follow-up period. Bradykinesia, tremor and rigidity scores revealed similar improvements in all domains over time. Hoehn and Yahr stage, the number of macrostimulation passes and the number of MER passes were not significantly related to the slope of motor score over time. However, disease duration and DBS target were significantly related to the improvement in total motor score slope over time (p<0.03 and p<0.02, respectively). Longer duration of illness reduced the slope (GLMM beta coefficient of 0.11 (SE 0.05)) of the change in motor score over time. The STN target had more MER penetration effects (p<0.05) and trended toward more lead penetration effects (p<0.08) than the GPi target. All other time points in this analysis were not significant.
DISCUSSION
This study sought to examine the effects of MER and lead collision/implantation in PD patients with STN or GPi DBS. The results suggest that significant improvements in motor scores (tremor, rigidity, bradykinesia) were observed as a result of acute collision/implantation with MER and from lead placement. It is interesting that these improvements were similar in magnitude to what was observed at 4 and 6 months post-DBS following programming and medication optimisation. When washed out (medications and DBS) for 12 h, these patients remained improved compared with preoperative testing. These observations suggest that collision/implantation/penetration alone provides therapeutic benefit. MER penetration into the STN produced greater motor improvements then when GPi was targeted. There was a trend for greater improvement from lead placement into the STN as well. The results from this study suggest that the surgical team must consider the possibility that the procedure itself (MER plus lead placement) may result in the therapeutic benefit seen intra-operatively. This finding can influence the macrostimulation testing and the decision to adjust or not to adjust the lead into a different brain location. However, the long term collision/implantation/penetration effects were similar between targets.
Most surgeons performing DBS and lesion therapy are clinically aware of strong microlesion or collision/penetration effects in at least a subset of patients.7,8 A recent study of STN DBS observed a microlesion effect that was present at least several days following the surgery.7,8 However, there are few large scale studies that have carefully examined the influence of collision/implantation/penetration, and no investigation that has specifically examined the intraoperative effects.
The difference in collision/implantation/penetration effects in the two DBS targets identified in this study is noteworthy. One potential explanation is that the smaller STN (158 mm3 compared with 458 mm3 for GPi)9 may render this structure more susceptible to lesional effects. This was recently shown in a study from our centre where STN DBS resulted in a verbal fluency decline even off medications and off DBS.10
Similar to what was seen in the ELLDOPA cohort,11,12 washout of medications and DBS did not result in a return of motor function to preoperative values. Explanations for this finding may include collision/implantation/penetration effects, inadequate washout or disease modifying benefits. Most experts, however, would suggest that penetration alone would not be enough to modify disease progression.11,12 It would have been useful to have had UPDRS scores from early programming sessions as this may have also shed some light on this issue.
While novel and informative, this study had many limitations. The evaluations were not blinded and therefore bias may have been introduced into the measurement of all postoperative data points. The UPDRS with its 0–4 ordinal scale design may have also been inadequate in detecting subtle differences that more objective observations or tools may have revealed. Additionally, more patients in this cohort had STN DBS compared with GPi and this may have potentially impacted the validity of between group comparisons. The size of the leads, clustering of MER passes and lead locations were not considered in this study, and although the intraoperative examinations were done by one rater, the postoperative examinations were gathered from multiple raters. Despite these limitations, this study demonstrated significant MER and lead placement intraoperative collision/implantation/penetration effects in PD. Clinicians rating patients in the operating suite should be aware of these effects, and should consider pre- and post-lead placement rating scales prior to activating DBS and testing its effects. There seemed to be more collision/penetration effects intraoperatively with STN compared with GPi, and overall there was a greater collision/penetration effect with longer disease durations. Future studies may improve on these results by increasing sample size, blinding raters and utilising more objective measures.
Acknowledgments
Funding: We would like to acknowledge the support of the National Parkinson Foundation Center of Excellence, the McKnight Brain Institute, UF and Shands, and NIH/NINDS K23 NS044997.
Footnotes
Competing interests: None.
Ethics approval: Ethics approval was obtained.
References
- 1.Hariz MI, Fodstad H. Do microelectrode techniques increase accuracy or decrease risks in pallidotomy and deep brain stimulation? A critical review of the literature. Stereotact Funct Neurosurg. 1999;72:157–69. doi: 10.1159/000029720. [DOI] [PubMed] [Google Scholar]
- 2.Hutchison WD, Allan RJ, Opitz H, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson’s disease. Ann Neurol. 1998;44:622–8. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 3.Ondo WG, Bronte-Stewart H. The North American survey of placement and adjustment strategies for deep brain stimulation. Stereotact Funct Neurosurg. 2005;83:142–7. doi: 10.1159/000088654. [DOI] [PubMed] [Google Scholar]
- 4.Romanelli P, Heit G, Hill BC, et al. Microelectrode recording revealing a somatotopic body map in the subthalamic nucleus in humans with Parkinson disease. J Neurosurg. 2004;100:611–18. doi: 10.3171/jns.2004.100.4.0611. [DOI] [PubMed] [Google Scholar]
- 5.Starr PA, Turner RS, Rau G, et al. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. Neurosurg Focus. 2004;17:E4. doi: 10.3171/foc.2004.17.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Vitek JL, Bakay RA, Hashimoto T, et al. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027–43. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Lee JY. Long-lasting microthalamotomy effect after temporary placement of a thalamic stimulating electrode. Stereotact Funct Neurosurg. 2004;82:127–30. doi: 10.1159/000079844. [DOI] [PubMed] [Google Scholar]
- 8.Maltete D, Derrey S, Chastan N, et al. Microsubthalamotomy: an immediate predictor of long-term subthalamic stimulation efficacy in Parkinson disease. Mov Disord. 2008;23:1047–50. doi: 10.1002/mds.22054. [DOI] [PubMed] [Google Scholar]
- 9.Sudhyadhom A, Bova FJ, Foote KD, et al. Limbic, associative, and motor territories within the targets for deep brain stimulation: potential clinical implications. Curr Neurol Neurosci Rep. 2007;7:278–89. doi: 10.1007/s11910-007-0043-1. [DOI] [PubMed] [Google Scholar]
- 10.Okun MS, Fernandez HH, Wu S, et al. Late breaking abstract: Cognition and mood in Parkinson disease after unilateral STN versus GPi DBS: The COMPARE Trial. 12th International Congress of Parkinson Disease and Movement Disorders; June 22–26, 2008; Chicago Hilton, Chicago, USA. [Google Scholar]
- 11.Fahn S. Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J Neurol. 2005;252(Suppl 4):IV37–42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- 12.Fahn S. A new look at levodopa based on the ELLDOPA study. J Neural Transm Suppl. 2006;70:419–26. doi: 10.1007/978-3-211-45295-0_63. [DOI] [PubMed] [Google Scholar]