Abstract
This Letter describes the synthesis and structure–activity-relationships (SAR) of isoform-selective PLD inhibitors. By virtue of the installation of alternative halogenated piperidinyl benzimidazolone privileged structures, in combination with a key (S)-methyl group, novel PLD inhibitors with low nM potency and unprecedented levels of PLD1 isoform selectivity (~1700-fold) over PLD2 were developed.
Keywords: Phospholipase D, Cancer, Isoform, PLD1, PLD2
Phospholipase D (PLD) isozymes mediate the parallel reactions of phospholipid hydrolysis and transphosphatidylation. There are two mammalian isoforms of PLD, coined PLD1 and PLD2, and despite conserved regulatory and catalytic domains, studies indicate distinct modes of activation and functional roles for PLD1 and PLD2.1 From a therapeutic perspective, PLD has been implicated in a human cancer cell progression (breast, renal, gastric and colorectal) as well as actin cytoskeleton reorganization and cell motility. 2–8 Thus, small molecules that selectively inhibit PLD1 or PLD2 could represent a novel approach for the treatment of cancer. The lack of isoform selective and direct-acting inhibitors has hindered the PLD field for decades. Instead, the study of PLD has been facilitated for decades by the use of n-butanol or indirect, non-selective inhibitors such as trans-diethylstilbestrol, resveratrol, honokiol and SCH420789, or non-selective, direct-acting inhibitors such as raloxifene and tamoxifen.9–15
Recently, Monovich and co-workers reported that halopemide 1 and some related congeners, identified in a PLD high throughput screen (HTS) inhibited PLD2.16 As we have recently reported, this work did not discuss activity for these compounds on PLD1, and in fact, we found that these compounds are a combination of dual PLD1/2 inhibitors and modestly PLD1-preferring inhibitors–none of the analogs disclosed showed any PLD2-preferring inhibition.17 In the course of our initial investigation of this report, we developed a series of small molecule, isoform-selective PLD inhibitors which included a dual PLD1/2 inhibitor 2, two PLD1 selective (>100-fold) inhibitors 3 and 4 (>100-fold), and the only known PLD2 preferring (>9-fold) inhibitor 5 (Figure 1). Inhibition of PLD with these direct-acting inhibitors leads to decreased invasive migration in breast cancer cell lines (i.e., MDA-231, 4T1 and PMT), and siRNA confirmed the role of PLD in this response.17 Thus, PLD inhibitors represent a new class of antimetastatic agents. However, to further probe PLD and the function and role of the individual PLD isoforms, more potent inhibitors with a greater degree of PLD isoform specificity are required.
Figure 1.
Halopemide 1, and our recently reported isoform-selective PLD inhibitors: dual PLD1/2 inhibitor 2, PLD1-selective (>100-fold) inhibitors 3 and 4, and PLD2 preferring (>9-fold) inhibitor 5.
Our initial library was based on a diversity-oriented approach utilizing commercial (1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one, and the analogous 5-chloro congener, as key scaffolds which afforded inhibitors 2–4, but was limited in scope. This screen also identified the (S)-methyl group on the ethyl diamine linker as a PLD1-inhibition enhancing moiety.17 In order to refine these inhibitors, we employed our iterative parallel synthesis approach, 18 and synthesized libraries to address the potential SAR depicted in Figure 2.
Figure 2.

Library strategy to refine PLD inhibitors to improve potency and PLD isoform selectivity.
The alternative halogenated (4-F, 5-F, 6-F, 5-Br) (1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-ones 6 were not commercially available and were synthesized as previously described.19 The remaining monomers were readily available and the libraries were prepared according to the general route depicted in Scheme 1. In the event, a halogenated (4-F, 5-F, 6-F, 5-Br) or unsubstituted (1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one 6 underwent a reductive amination with either N-Boc glycinal, a functionalized alinal 7 or ahomologated/cyclic constrainedN-Boc amino aldehyde 8 to yield 9. Subsequent removal of the Boc group with 4 N HCl and standard acylation chemistry provides analogs 10. All compounds were then purified to >98% purity by mass-directed preparative HPLC.20
Scheme 1.
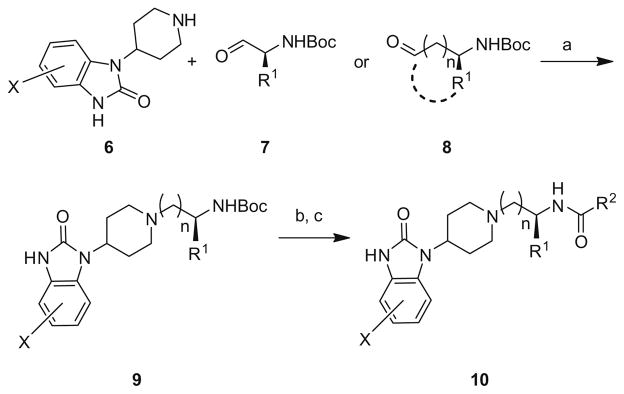
Reagents and conditions: (a) MP-B(OAc)3, DCE, rt, 16 h (77–97%); (b) 4 N HCl/dioxane, MeOH (98%); (c) R2COCl, DCM, DIEA, rt (65–95%) or (i) R2COH, PS-DCC, HOBt, DCM, DIEA; (ii) MP-CO32−(58–90%).
Robust, tractable SAR was observed in the 10 libraries (~250 compounds) synthesized in an iterative fashion over several months–refining library design with new biochemical data.18 All of the halogenated (4-F, 5-F, 6-F, 5-Br) (1-(piperidin-4-yl)-1Hbenzo[ d]imidazol-2(3H)-one provided PLD inhibitors, and a diverse array of alternative amides were also tolerated. In contrast, the ethyl diamino linker was essential—homologation to the corresponding 3- and 4-carbon tethers were inactive, as were cyclic constraints. Only H or (S)-methyl substitution was tolerated on the ethyl diamino linker. All library members were evaluated for their ability to inhibit PLD1 and PLD2 in a cellular assay (Calu-1 and HEK293-gfpPLD2, respectively) as well as a biochemical assay with recombinant PLD1 and PLD2 enzymes.17 The cellular assays were the ‘workhorse’ assays that drove the SAR, with routine confirmation in the in vitro biochemical assay. Table 1 highlights unsubstituted (X = H) 6 congeners 11 without the (S)-methyl group and examines only alternative amides.
Table 1.
Structures and activities of analogs 11

| ||||
|---|---|---|---|---|
| Cmpd | R1 | PLD1 IC50a(nM) | PLD2 IC50b (nM) | Fold PLD1-selective |
| 2 |

|
21 | 380 | 18 |
| 4 |

|
35 | 3900 | 111 |
| 11a |

|
8 | 42 | 5.1 |
| 11b |

|
40 | 730 | 18 |
| 11c |
|
70 | 740 | 10.5 |
| 11d |
|
110 | 860 | 7.8 |
| 11e |

|
120 | 850 | 7 |
Cellular PLD1 assay with Calu-1 Cells.
Cellular assay with HEK293-gfp PLD2 cells. Each IC50 was determined in triplicate. For details see Ref. 17.
While a number of amide caps afforded good PLD1 inhibition, the 2-naphthyl analog 11a provided single digit nanomolar levels of PLD1 inhibition, but poor selectivity versus PLD2 (~5.1-fold). The trans-phenyl cyclopropane congener 4 provided a 35 nM PLD1 inhibitor with excellent selectivity against PLD2 (111-fold).17
Next, we evaluated halogenated (5-F, 6-F, 5-Cl and 5-Br) (1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one analogs 12 without the (S)-methyl group and explored alternative amides (Table 2). Robust, tractable SAR was observed, with this exploration identifying a combination of dual PLD1/2 inhibitors (12e and 12i) with ~3-fold PLD1 selectivity to an inhibitor 12b (X = 5-Cl, trans-phenyl cyclopropane amide) with improved PLD1 selectivity (250-fold) compared to 4. In general, halogen substitution increased PLD1 inhibition to the low nanomolar range irrespective of the halogen (F, Cl or Br) or the position (5- or 6-substitution). However, the degree of PLD2 inhibition also increased relative to analogs 11. Thus, efforts now centered on installation of the key PLD1 enhancing (S)-methyl group in an attempt to further improve PLD1 inhibition while diminishing activity towards PLD2 inhibition.
Table 2.
Structures and activities of halogenated analogs 12

| |||||
|---|---|---|---|---|---|
| Cmpd | X | R1 | PLD1 IC50a(nM) | PLD2 IC50b (nM) | Fold PLD1-selective |
| 1 | 5-Cl |

|
21 | 300 | 14.2 |
| 12a | 5-Cl |

|
10 | 240 | 24 |
| 12b | 5-Cl |
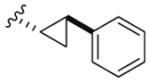
|
2 | 520 | 250 |
| 12c | 5-F |
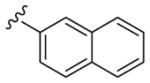
|
4 | 140 | 35 |
| 12d | 5-F |
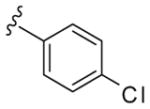
|
18 | 61 | 3.3 |
| 12e | 5-F |

|
12 | 375 | 31 |
| 12f | 5-Br |

|
3 | 97 | 32 |
| 12g | 5-Br |
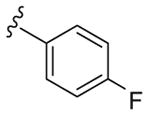
|
4 | 76 | 19 |
| 12h | 6-F |

|
7 | 42 | 6 |
| 12i | 6-F |
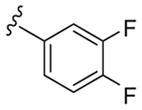
|
4 | 14 | 3.2 |
Cellular PLD1 assay with Calu-1 Cells.
Cellular assay with HEK293-gfp PLD2 cells. Each IC50 was determined in triplicate. For details see Ref. 17.
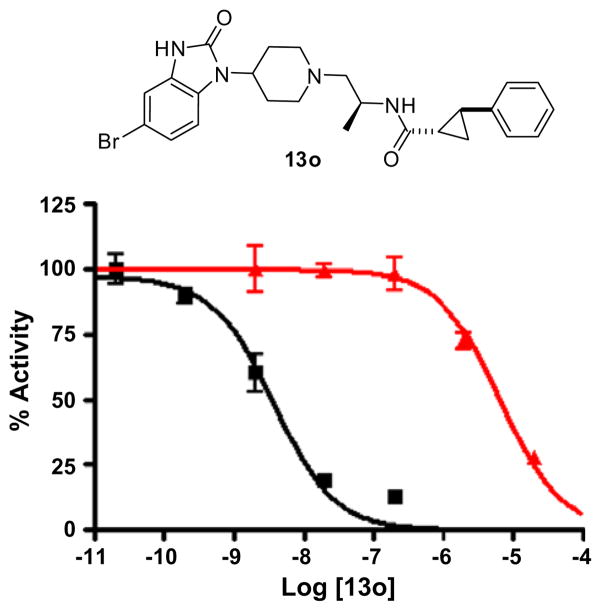
The combination of halogenated (4-F, 5-F, 6-F, 5-Cl and 5-Br) (1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-ones 6 with the (S)-methyl group and optimized amides led to some phenomenal, synergistic results (Table 3). With only two exceptions (13b and 13l) PLD1 selectivity for analogs 13 exceeded 140-fold. Importantly, the combination of halogen substitution on the benzimidazolone ring and the (S)-methyl group on the diamino ethyl linker performed as we had hypothesized, and increased PLD1 activity into the single digit nanomolar range, while dramatically decreasing PLD2 inhibition into the micromolar range. Without question, the trans-phenyl cyclopropane amide was the optimal amide moiety, uniformly providing 140- to 1700-fold PLD1 versus PLD2 selectivity. In terms of potency and selectivity, the 5-Br (13l–m) and 6-F (13p and q) substitutions offered the best results, providing PLD1 IC50s ranging from 2 nM to 12 nM and with high PLD1 selectivity. Of all of these analogs, 13o was the standout molecule and possesses unprecedented selectivity for PLD1 inhibition. Compound 13o, with the 5-Br-(1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one, the (S)-methyl group linker and the racemic trans-phenyl cyclopropane amide is the most potent (IC50 = 3.7 nM) and selective versus PLD2 (IC50 = 6.4 μM, ~1700-fold selective) PLD1 inhibitor described to date. The concentration-response-curves (CRC) for the cellular PLD1 (Calu-1) and PLD2 (HEK293-gfpPLD2) assays17 are shown in Figure 3.
Table 3.
Structures and activities of halogenated analogs 13

| |||||
|---|---|---|---|---|---|
| Cmpd | X | R1 | PLD1 IC50a(nM) | PLD2 IC50b (nM) | Fold PLD1-selective |
| 3 | 5-CI |

|
46 | 933 | 163 |
| 13a | 4-F |
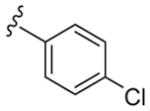
|
43 | 12,000 | 280 |
| 13b | 4-F |

|
130 | 10,000 | 77 |
| 13c | 4-F |

|
38 | 14,000 | 370 |
| 13d | 4-F |

|
66 | 13,000 | 200 |
| 13e | 4-F |

|
100 | >20,000 | >200 |
| 13f | 5-F |

|
11 | 3100 | 180 |
| 13g | 5-F |

|
7.4 | 1040 | 140 |
| 13h | 5-CI |

|
10 | 1400 | 140 |
| 13i | 5-CI |

|
6.4 | 1200 | 190 |
| 13j | 5-CI |

|
8 | 1150 | 140 |
| 13k | 5-CI |

|
3 | 730 | 240 |
| 131 | 5-Br |
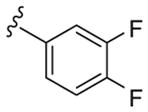
|
3.5 | 187 | 53 |
| 13m | 5-Br |

|
5.5 | 3900 | 700 |
| 13n | 5-Br |
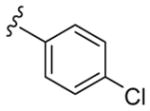
|
4 | 890 | 222 |
| 13o | 5-F, |

|
3.7 | 6400 | 1700 |
| 13p | 6-F |

|
2 | 360 | 180 |
| 13q | 6-F |

|
12 | 3800 | 320 |
Cellular PLD1 assay with Calu-1 Cells.
Cellular assay with HEK293-gfp PLD2 cells. Each IC50 was determined in triplicate. For details see Ref. 17.
Figure 3.
CRCs for cellular PLD1 ■ (Calu-1) assay and PLD2 ▲ (HEK293-gfpPLD2) assay, highlighting the unprecedented PLD1 versus PLD2 selectivity (1700-fold).
How can we account for this unprecedented level of PLD1 isoform selectivity? Preliminary evidence suggests that these inhibitors do not interact with the catalytic site of PLD, but may bind, and inhibit PLD via an allosteric site.21 Further work to confirm this allosteric mode of isoform-selective PLD inhibition is in progress.
In summary, the discovery of these highly potent and selective PLD1 inhibitors highlights the power of an iterative analog library synthesis approach for lead optimization, coupled with biochemical assays and mass spectrometric lipid profiling of cellular responses. PLD inhibitor 13o (PLD1 IC50 = 3.7 nM, PLD2 IC50 = 6400 nM, 1700-fold selective) represents the most potent and selective PLD1 small molecule inhibitor reported to date, and will serve as an invaluable tool to study and dissect the role of PLD1 in blocking invasiveness in metastatic breast cancer models and other signaling pathways in which PLD is thought to play key regulatory roles. In order to better understand the role of PLD, a more potent PLD2 selective inhibitor is still required; however, PLD2 preferring inhibitors with the same order of selectivity of those generated for PLD1 remain elusive thus far in this chemical series. Work in this field, as well as additional studies to confirm an allosteric mode of inhibition are ongoing.
Acknowledgments
The authors thank the Vanderbilt Department of Pharmacology, the Vanderbilt Institute of Chemical Biology and the A.B. Hancock Family Foundation for Cancer Research and the Sartain-Lanier Family Foundation (J.R.B./C.W.L.) for support of this research. J.A.L. and R.L. are supported by an NIH ITTD training grant (T90DA022873). P.E.S. and S.L.S. are supported by a Pharmacology Training grant (NIH 5T326M007628-30).
References and notes
- 1.Ponting CP, Kerr ID. Protein Sci. 1996;5:914. doi: 10.1002/pro.5560050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noh DY, Ahn SJ, Lee RA, Park IA, Kim JH, Suh PG, Lee KH, Han JS. Cancer Lett. 2000;161:207. doi: 10.1016/s0304-3835(00)00612-1. [DOI] [PubMed] [Google Scholar]
- 3.Uchida N, Okamura S, Nagamachi Y, Yamshita S. J Cancer Res Clin Oncol. 1997;123:280. doi: 10.1007/BF01208639. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Ehara H, Akao Y, Shamoto M, Nakagawa Y, Banno Y, Deguchi T, Ohishi N, Yagi K, Nozawa Y. Biochem Biophys Res Commun. 2000;278:140. doi: 10.1006/bbrc.2000.3719. [DOI] [PubMed] [Google Scholar]
- 5.Uchida N, Okamura S, Kuwano H. Anticancer Res. 1999;19:671. [PubMed] [Google Scholar]
- 6.Yamada Y, Hamajima N, Kato T, Iwata H, Yamamura Y, Shinoda M, Suyama M, Mitsudomi T, Tajima K, Kusakabe S, Yoshida H, Banno Y, Akao Y, Tanaka M, Nozawa Y. J Mol Med. 2003;81:126. doi: 10.1007/s00109-002-0411-x. [DOI] [PubMed] [Google Scholar]
- 7.Min DS, Kwon TK, Park WS, Chang JS, Park SK, Ahn BH, Ryoo ZY, Lee YH, Lee YS, Rhie DJ, Yoon SH, Hahn SJ, Kim MS, Jo YH. Carcinogenesis. 2001;22:1641. doi: 10.1093/carcin/22.10.1641. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan FG, McReynolds M, Couvillon A, Kam Y, Holla VR, DuBois RN, Exton JA. Proc Natl Acad Sci USA. 2005;102:1638. doi: 10.1073/pnas.0406698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tou JS, Urbizo C. Steroids. 2008;73:216. doi: 10.1016/j.steroids.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Tou JS, Urbizo C. Cell Signalling. 2001;13:191. doi: 10.1016/s0898-6568(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 11.Garcia A, Zheng Y, Zhao C, Toschi A, Fan J, Shraibman N, Brown HA, Bar-Sagi D, Foster DA, Arbiser JL. Clin Cancer Res. 2008;14:4267. doi: 10.1158/1078-0432.CCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puar MS, Barrabee E, Hallade M, Patel M. J Antibiot. 2000;53:837. doi: 10.7164/antibiotics.53.837. [DOI] [PubMed] [Google Scholar]
- 13.McDonald LA, Barbieri LR, Bernan VS, Janso J, Lassota P, Carter GT. J Nat Prod. 2004;67:1565. doi: 10.1021/np049924g. [DOI] [PubMed] [Google Scholar]
- 14.Levy BD, Hickey L, Morris AJ, Larvie M, Keledjian R, Petasis NA, Bannenberg G, Serhan CN. Br J Pharmacol. 2005;146:344. doi: 10.1038/sj.bjp.0706338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisen SF, Brown HA. Mol Pharmacol. 2002;62:911. doi: 10.1124/mol.62.4.911. [DOI] [PubMed] [Google Scholar]
- 16.Monovich L, Mugrage B, Quadros E, Toscano K, Tommasi R, LaVoie S, Liu E, Du Z, LaSala D, Boyar W, Steed P. Bioorg Med Chem Lett. 2007;17:2310. doi: 10.1016/j.bmcl.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 17.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Nat Chem Biol. 2009;5:108. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy JP, Williams L, Bridges TM, Daniels RN, Weaver D, Lindsley CW. J Comb Chem. 2008;10:345. doi: 10.1021/cc700187t. [DOI] [PubMed] [Google Scholar]
- 19.Miller NR, Daniels RN, Bridges TM, Brady AE, Conn PJ, Lindsley CW. Bioorg Med Chem Lett. 2008;18:5443. doi: 10.1016/j.bmcl.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leister WH, Strauss KA, Wisnoski DD, Zhao Z, Lindsley CW. J Comb Chem. 2003;5:322. doi: 10.1021/cc0201041. [DOI] [PubMed] [Google Scholar]
- 21.Lewis JA, Lebois EP, Lindsley CW. Curr Opin Chem Biol. 2008;12:269. doi: 10.1016/j.cbpa.2008.02.014. [DOI] [PubMed] [Google Scholar]