Abstract
We show that highly efficient depletion of sphingolipids in two different cell lines does not abrogate the ability to isolate Lubrol-based DRMs (detergent-resistant membranes) or detergent-free lipid rafts from these cells. Compared with control, DRM/detergent-free lipid raft fractions contain equal amounts of protein, cholesterol and phospholipid, whereas the classical DRM/lipid raft markers Src, caveolin-1 and flotillin display the same gradient distribution. DRMs/detergent-free lipid rafts themselves are severely depleted of sphingolipids. The fatty acid profile of the remaining sphingolipids as well as that of the glycerophospholipids shows several differences compared with control, most prominently an increase in highly saturated C16 species. The glycerophospholipid headgroup composition is unchanged in sphingolipid-depleted cells and cell-derived detergent-free lipid rafts. Sphingolipid depletion does not alter the localization of MRP1 (multidrug-resistance-related protein 1) in DRMs/detergent-free lipid rafts or MRP1-mediated efflux of carboxyfluorescein. We conclude that extensive sphingolipid depletion does not affect lipid raft integrity in two cell lines and does not affect the function of the lipid-raft-associated protein MRP1.
Keywords: caveolin, detergent-free lipid raft, flotillin, multidrug-resistance-related protein 1 (MRP1), Neuro-2a cell, Src
INTRODUCTION
Lipid rafts have been established as an important feature in cell membranes with regard to signal transduction, cell adhesion and protein sorting [1]. The physical properties of these membrane microdomains are distinct from those of the surrounding membrane. This is generally considered to be the result of a different lipid composition in these domains compared with the surrounding membrane. Membrane microdomains are enriched in cholesterol and sphingolipids with a high degree of saturation in their fatty acyl chains. The presence of these lipids is thought to stabilize membrane domains, because of the ability of saturated sphingolipids and cholesterol to pack tightly in the plane of the membrane [2,3]. In model membranes, cholesterol induces tight packing of sphingolipids into a liquid-ordered state. It is not known how membrane microdomains arise in cells, but it is likely that this process is initiated by small-scale protein–lipid interactions followed by an increase in domain size because of protein–protein interactions [4]. Lipid rafts in cells are operationally defined as complexes of molecules that are insoluble at low temperature in detergents, such as Lubrol and Triton X-100 [5,6]. We refer to these isolated lipid rafts as DRMs (detergent-resistant membranes). Most studies on the occurrence, properties and functions of lipid rafts in cells rely on the use of detergents for their isolation, but detergent-free isolation methods have also been developed [7]. In the present paper, we use the term detergent-free lipid rafts exclusively for membrane domains isolated using the detergent-free isolation procedure. Finally, the term lipid raft is only used in the present paper to refer to the theoretical concept of membrane microdomains, according to the hypothesis based on a body of literature [8].
ABC (ATP-binding cassette) transporter proteins, such as Pgp (P-glycoprotein) (or ABCB1) and MRP1 (multidrug-resistance-related protein 1) (ABCC1) have been associated with DRMs. Originally Pgp was localized in caveolae [9,10], but later studies showed localization of both Pgp and MRP1 in non-caveolar DRMs [11,12]. Both ABC transporters were more strongly enriched in Lubrol-based DRMs compared with Triton X-100-based DRMs [11]. Given their localization in DRMs, the function of ABC transporters may well be dependent on or modulated by sphingolipids and/or cholesterol. Indeed, some evidence exists for modulation of Pgp function by cholesterol and involvement of DRMs in this process [13,14]. Concerning a role for sphingolipids in modulation of ABC transporter function, several hypotheses exist, but a coherent picture has not yet emerged. In MDR (multidrug-resistant) cells overexpressing Pgp or MRP1, several changes in sphingolipid metabolism have been observed, including accumulation of GlcCer (glucosylceramide). The latter has been related to increased GCS (glucosylceramide synthase) (also known as UDP-glucose:ceramide glucosyltransferase) activity [15]. Because GCS is responsible for metabolic removal of Cer (ceramide) from the sphingolipid pool, an increased activity of this enzyme is beneficial to tumour cells that are under stress of cytostatics or other stress factors that induce Cer formation and subsequent apoptosis. On the other hand, enhanced GlcCer formation may be part of a more comprehensive response involving up-regulation of the total glycolipid pool, including gangliosides. The latter may be involved in MDR, either related to or independent of ABC transporters [16]. One study described that gangliosides activate Pgp through modulation of its phosphorylation state [17]. Another study reached the conclusion that gangliosides are not involved in modulation of ABC transporter localization and function [18].
Most evidence for a role of lipid rafts in cell function is based on studies showing DRM association of proteins and modulation of function of such proteins by procedures aimed at removing or modifying membrane cholesterol, such as methyl-β-cyclodextrin treatment [19]. Only a few studies (for example [20]) have employed depletion of sphingolipids as a means to perturb lipid raft association of proteins, even though sphingolipids are widely recognized as important building blocks of lipid raft membranes. In the present study, we investigated the role of sphingolipids in the integrity of DRMs in two different cell lines and showed that highly efficient depletion of these lipids from cells and DRMs does not abrogate the ability to isolate DRMs employing Lubrol. DRM integrity was confirmed by protein content and gradient distribution of the classical DRM markers Src, Cav-1 (caveolin-1) and flotillin. In addition, detergent-free lipid rafts could be isolated from sphingolipid-depleted cells. Again, no effects on lipid raft integrity were observed upon sphingolipid depletion, as indicated by similar protein, cholesterol and phospholipid content and lipid raft marker distributions compared with lipid rafts from control cells. The fatty acid profile of the remaining sphingolipids as well as that of the glycerophospholipids showed several changes compared with control in whole cells and detergent-free lipid rafts, most prominently towards highly saturated short-chain species.
Sphingolipid depletion did not affect MRP1 localization in DRMs/detergent-free lipid rafts or MRP1 efflux activity. We conclude that very low levels of sphingolipids are sufficient to sustain DRM/detergent-free lipid raft integrity as well as MRP1 localization in lipid rafts and its efflux activity.
MATERIALS AND METHODS
Materials
MK571 was a gift from Professor A.W. Ford-Hutchinson (Merck-Frosst, Kirkland, Canada). All cell culture plastic was from Corning. Cell culture media, HBSS (Hanks balanced salt solution), antibiotics, L-glutamine, sodium pyruvate and trypsin were from Gibco. FBS (fetal bovine serum) was from Bodinco. L-[U-14C]Serine was purchased from GE Healthcare. HPTLC (high-performance TLC) plates were from Merck. C12-fatty acid homologues of Cer, SM (sphingomyelin), GlcCer and LacCer (lactosylceramide) were from Avanti Polar Lipids. Gangliosides were purchased from Alexis Biochemicals. CFDA (5-carboxyfluorescein diacetate) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] were from Sigma–Aldrich. Lubrol was obtained from Serva. ISP-1 was from Biomol Research Laboratories. Lipofectamine™ 2000 reagent, Silencer Select Pre-designed siRNA (small interfering RNA) for SPTLC (serine palmitoyltransferase long-chain base subunit) 1 and SPTLC2 were purchased from Invitrogen. OptiPrep was from Axis-Shield PoC AS. The rat monoclonal anti-MRP1 antibody was obtained from Signet Laboratories. The polyclonal anti-Cav-1 antibody and the monoclonal anti-flotillin antibodies were from Transduction Laboratories. The polyclonal anti-c-Src antibody was from Santa Cruz Biotechnology. Purified mouse anti-LCB (long-chain base subunit) 1 was purchased from BD Transduction Laboratories. Anti-(serine palmitoyltransferase) antibody (detecting LCB2) was from Abcam.
Cell culture
The murine neuroblastoma cell line Neuro-2a was purchased from the A.T.C.C. (Manassas, VA, USA). These cells were grown as adherent monolayer cultures in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10 % FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine and 1 mM sodium pyruvate, under standard incubator conditions (humidified atmosphere, 5 % CO2, 37 °C). The BHK (baby hamster kidney) cell line stably expressing the human MRP1/ABCC1 gene, BHK/MRP1(ABCC1), was a gift from Dr Riordan (Mayo Clinic Arizona, S.C. Johnson Medical Research Center, Scottsdale, AZ, U.S.A. [21]). These cells were grown as adherent monolayer cultures in DMEM/nutrient mixture F-12 (1:1) supplemented with 10 %FBS, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine, under standard incubator conditions. The BHK/MRP1 cells were kept under selective pressure by growing them in the presence of 100 μM methotrexate.
Isolation of DRMs
DRM fractions were isolated from cells as described in [22]. For each isolation, confluent cells from two 75 cm2 flasks were washed once with TNE buffer (20 mM Tris/HCl, pH 7.4, 150 mM NaCl and 1 mM EDTA) and harvested by scraping in 2 ml of ice-cold TNE buffer containing 0.5 %Lubrol and protease inhibitors. After 30 min of incubation on ice, cells were homogenized by passing the lysate 20 times through a 25-gauge needle. Then 2 ml of the lysate was transferred to a centrifuge tube and mixed with 2 ml of 80 % (w/v) sucrose in TNE. On top of this, 4 ml of 35 % (w/v) and 3 ml of 5 %(w/v) sucrose in TNE were successively loaded, resulting in a discontinuous gradient. Gradients were centrifuged in a Beckman SW41 swing-out rotor at 40 000 rev./min for 18–20 h at 4 °C. A total of 11 fractions of 1 ml each were collected (from top to bottom), vortex-mixed and stored at −80 °C. The protein content [23] of all fractions was measured using BSA as a standard.
Isolation of detergent-free lipid rafts
Detergent-free lipid rafts were isolated as described in [7]. The whole procedure was performed on ice. In short, confluent cells of two 75 cm2 flasks were washed with base buffer (20 mM Tris/HCl, pH 7.8, and 250 mM sucrose) supplemented with 1 mM CaCl2 and 1 mM MgCl2. The cells were collected by scraping in this solution and centrifuging at 250 g for 2 min. The resulting pellet was suspended in 1 ml of base buffer supplemented with 1 mM CaCl2, 1 mM MgCl2 and protease inhibitors. After homogenization by passage through a 25-gauge needle 20 times, another centrifugation step at 1000 g for 10 min followed. The resulting PNS (post-nuclear supernatant) was collected and transferred to a separate tube. The pellet was homogenized again in 1 ml of base buffer supplemented with 1 mM CaCl2, 1 mM MgCl2 and protease inhibitors, sheared through the needle 20 times and centrifuged at 1000 g for 10 min. The second PNS was combined with the first. Protein content of the combined PNS was determined [23] and samples were processed for gradient analysis based on equal amounts of protein, adjusted to 2 ml volumes. Subsequently, 2 ml of base buffer containing 50 % OptiPrep was added to this 2 ml of PNS. By using a gradient mixer, an 8 ml gradient of 0–20 % OptiPrep in base buffer was poured on top of this 4 ml in a centrifugation tube. After centrifugation at 22 000 rev./min for 90 min at 4 °C in a Beckman SW41 rotor, fractions of 1.34 ml were collected (from top to bottom) and stored at −80 °C. The protein content [24] of all the fractions was measured using BSA as a standard.
Analysis of cholesterol and phospholipid content
Lipids were extracted from detergent-free lipid raft fractions [25]. In the extract, the cholesterol concentration was determined spectrophotometrically using a cholesterol oxidase/peroxidase assay [26]. The phosphorus content, as a measure for the phospholipid content in the fractions, was determined using a phosphate assay [27].
Equilibrium radiolabelling and analysis of cellular sphingolipids
Sphingolipid pools were metabolically radiolabelled by growing the cells for 72 h in the presence of L-[U-14C]serine (0.5 μCi/ml), a precursor molecule for sphingolipid biosynthesis. Cells were harvested by scraping and were centrifuged at 900 g for 5 min, followed by lipid extraction from the cell pellet [25]. Aliquots of the lipid extracts were taken for determination of the total amount of lipid-incorporated radioactivity. Glycerolipids were hydrolysed during a 1 h incubation at 37 °C in chloroform/methanol (1:1, v/v) containing KOH (0.1 M). The remaining lipids were re-extracted and applied on to HPTLC plates. Plates were developed in chloroform/methanol/water (14:6:1, by vol.) in the first dimension. Plates were then sprayed with 2.5 % (w/v) boric acid in methanol and developed in chloroform/methanol/25 % (w/v) NH4OH (13:7:1, by vol.) in the second dimension. After autoradiography, GlcCer-, LacCer- and SM-containing spots were identified with the aid of standards and scraped from the plates. Plates were then developed in the second dimension, but now in the reverse direction, in chloroform/acetic acid (9:1, v/v). Plates were dried and, after staining in I2 vapour, Cer- containing spots were scraped. Radioactivity was measured by scintillation counting (Packard Topcount microplate scintillation counter). Levels of a specific lipid class were expressed as d.p.s. incorporated in that specific lipid class per 103 d.p.s. of total lipid-incorporated radioactivity.
LC (liquid chromatography)–ESI (electrospray ionization)–MS/MS (tandem MS)
Details can be found in the Supplementary Online Data at http://www.BiochemJ.org/bj/430/bj4300519add.htm.
Ganglioside analysis
Gangliosides were isolated from 4 × 107 cells. Pelleted cells were extracted in chloroform/methanol (1:1, v/v) and chloroform/methanol (2:1, v/v). The supernatants were pooled and dried (in N2), and lipids were redissolved and sonicated in chloroform/methanol (1:1, v/v). After centrifugation at 2000 g for 15 min and overnight storage at −20 °C, the supernatants were collected and dried, and their phospholipid content was determined [27]. Aliquots containing equal amounts of phospholipid were redissolved in di-isopropyl ether/butan-1-ol (3:2, v/v), and 17 mM NaCl was added. The aqueous phase was re-extracted with di-isopropyl ether/butan-1-ol and subsequently freeze-dried. Samples were dissolved in methanol/water (1:1, v/v) and loaded on to pre-washed Sep-Pak C18 cartridges. After rinsing in water, gangliosides were eluted with methanol and chloroform/methanol (1:1, v/v). The eluate was concentrated and loaded on to HPTLC plates, which were developed in chloroform/methanol/0.2 % CaCl2 (11:9:2, by vol.) and stained with Ehrlich reagent. Ganglioside band intensities were quantified using Scion Image Beta 4.0.2 (Scion Corporation) software and compared with a standard curve of GM3, ranging from 10 pmol to 5 nmol. In some experiments, gangliosides were metabolically radiolabelled by growing the cells for 72 h in the presence of L-[U-14C]serine (1.0 μCi/ml). After autoradiography, ganglioside-containing spots were identified with the aid of standards and scraped from the plates. Radioactivity was measured by scintillation counting (Packard Topcount microplate scintillation counter).
Immunoblot analysis
In the case of equal protein loading, an equal amount of protein from each gradient fraction was precipitated with trichloroacetic acid and resuspended in sample buffer (5 % SDS, 5 % 2-mercaptoethanol, 0.125 M Tris/HCl, pH 6.8, and 40 %glycerol). In the case of equal volume loading, protein from equal volume samples of each gradient fraction was precipitated with trichloroacetic acid and resuspended in sample buffer. The samples were resolved by SDS/PAGE (10 % minigels) and subsequently electrotransferred on to a nitrocellulose membrane (Trans-Blot Transfer Medium membrane, Bio-Rad Laboratories). The membranes were rinsed with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 and 1.76 mM KH2PO4, pH 7.4) and incubated for 1 h at room temperature (22 °C) with Odyssey blocking buffer/PBS (1:1, v/v). Membranes were incubated at 4 °C overnight with a primary antibody against MRP1 (1:1000), Src (1:1000), Cav-1 (1:1000) or flotillin (1:1000) in Odyssey blocking buffer/PBS/0.1 % Tween 20. Membranes were rinsed in washing buffer (PBS containing 0.1 % Tween 20) and subsequently incubated for 1 h with the appropriate IR-dye-conjugated secondary antibody (1:5000) (LI-COR) in Odyssey blocking buffer/PBS/0.1 %Tween 20. After rinsing with washing buffer followed by PBS, the immunoblots were scanned with the Odyssey instrument (LI-COR) to visualize the immunoreactive complexes, according to the manufacturer’s instructions. Relative quantification of the complexes was performed using the Odyssey software. In some cases, membranes were rinsed with PBS and incubated at room temperature for 1–2 h with 5 % (w/v) non-fat dried skimmed milk in PBS. Membranes were rinsed in washing buffer and incubated for at least 2 h at room temperature with a primary antibody against MRP1 (1:500), Src (1:1000), Cav-1 (1:1000) or flotillin (1:1000) in washing buffer containing 0.5 % non-fat dried skimmed milk. Membranes were rinsed in washing buffer and subsequently incubated for 2 h at room temperature with the appropriate horseradish-peroxidase-conjugated secondary antibody (1:2000) (GE Healthcare) in washing buffer containing 0.5 % non-fat dry milk. Membranes were incubated in ECL (enhanced chemiluminescence) substrate solution (GE Healthcare), according to the manufacturer’s instructions, and immunoreactive complexes were visualized by exposure to a Konica Minolta medical film and quantified using Scion Image Beta 4.0.2 software.
Detection of MRP1-mediated efflux by flow cytometric analysis
Cells were harvested by trypsinization, washed with HBSS and incubated with the MRP1 substrate CFDA (0.5 μM in HBSS) at 10 °C for 60 min. Cells were transferred on ice and washed with ice-cold HBSS. Subsequently, the cells were incubated at 37 °C in the presence or absence of the MRP1 inhibitor MK571 (20 μM) for various times. All subsequent steps were performed on ice. The efflux of the fluorescent substrate was stopped by cold centrifugation at 1000 g for 1 min and the cells were resuspended in ice-cold HBSS containing MK571. Retention of fluorescence was determined by flow cytometric analysis using an Elite™ flow cytometer (Beckman Coulter). For each sample, 5000 events were collected and analysed using Win-list 5.0 software (Verity Software House).
RNAi (RNA interference) of serine palmitoyltransferase
Neuro-2a cells were plated at a density of 40 000 cells/ml. After 24 h, cells were transfected with 6 nM siRNA using 2 μg/ml Lipofectamine™ 2000 reagent according to the manufacturer’s protocol. After 24 h, the medium was refreshed and the cells were allowed to grow for another 24 h. RNAi treatment was combined with ISP-1 treatment (0.5 μM) during the entire incubation period.
Measurement of cell viability (MTT assay)
Either 1000 (Neuro-2a) or 5000 (BHK/MRP1) cells/well were plated in microtitre plates. For sphingolipid depletion, cells were incubated with ISP-1 (0.5 μM) for 3 days, followed by determination of viable cells. In the case of 7 days of treatment, cells were pre-incubated with ISP-1 (0.5 μM) for 4 days, subsequently trypsinized and plated in the presence of ISP-1. At 3 days after plating, viable cells were determined. Alternatively, during the last day of the 7-day treatment, incubation with ISP-1 was performed in serum-free medium. The MTT assay was performed as described previously [28]. Briefly, 100 μg of MTT was added to each well, and cells were incubated for 2 h at 37 °C. Plates were then centrifuged at 900 g for 15 min and the supernatants were removed. Pellets were dissolved in DMSO and absorbance was measured in a microtitre plate reader (μQuant, Bio-Tek Instruments) at 570 nm. The background absorbance was subtracted from all values and data are expressed as percentages compared with untreated control cells (=100 %). Alternatively, the MTT signal was expressed relative to protein content, as determined in a parallel set of cells.
RESULTS
Efficient depletion of sphingolipid content
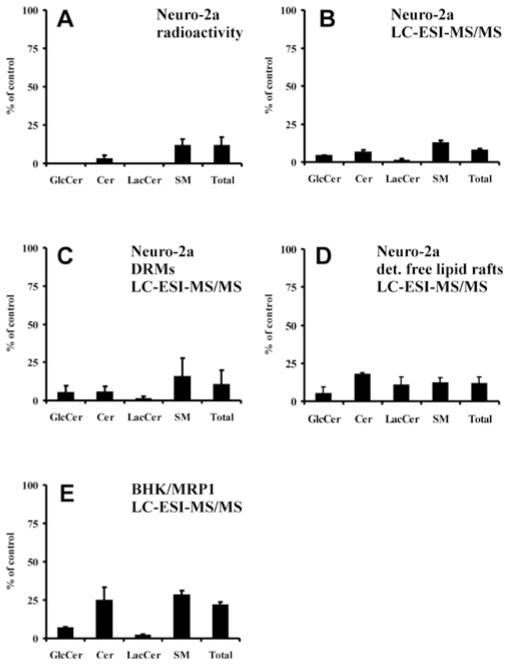
The serine palmitoyltransferase inhibitor ISP-1 (0.5 μM) efficiently depleted sphingolipid content in Neuro-2a cells upon a 3-day incubation. The pool of sphingolipids was reduced by 88 ± 5 % (n = 3), as determined by equilibrium radiolabelling (Figure 1A). Depletion was highly efficient for Cer and glycosphingolipids and slightly less for SM (Figure 1A). Measurement of endogenous sphingolipid mass using LC–ESI–MS/MS gave similar results: levels of Cer and glycosphingolipids were depleted by at least 90 % in whole cells (Figure 1B). SM depletion again was slightly less efficient (87 %). Importantly, when sphingolipids were analysed in DRM fractions, a highly efficient depletion by ISP-1 was also observed, indicating that depletion was not confined to non-lipid raft membrane regions (Figure 1C). Highly efficient depletion of sphingolipids by ISP-1 was also confirmed in detergent-free lipid raft fractions (Figure 1D). In BHK/MRP1 cells, similar results were obtained with ISP-1, although depletion was somewhat less efficient (Figure 1E). In addition, we measured the level of gangliosides in Neuro-2a cells (Figure 2). HPTLC analysis showed that GM3, the only ganglioside present in Neuro-2a cells, was undetectable in ISP-1-treated cells, indicating that ganglioside mass was very efficiently reduced by ISP-1 treatment. In BHK/MRP1 cells, gangliosides were undetectable in control cells, both by Ehrlich staining and by autoradiography after radiolabelling using L-[U-14C]serine. Using a standard curve of GM3, we estimated the amount of gangliosides in these cells to be less than 10 pmol/106 cells, which is less than 0.7 % of the total sphingolipid pool. In Neuro-2a cells, the amount of GM3 is 350 pmol/106 cells, which is 5.6 %of the total sphingolipid pool.
Figure 1. Sphingolipids are efficiently depleted by ISP-1 treatment in whole cells, DRMs and detergent-free lipid rafts.
(A) The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on sphingolipid levels in Neuro-2a cells was established by equilibrium radiolabelling. (B) The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on sphingolipid levels in Neuro-2a cells was established by LC–ESI–MS/MS. (C) Sphingolipids of Neuro-2a-derived DRMs with equal protein content were extracted and analysed by LC–ESI–MS/MS. (D) Sphingolipids of Neuro-2a-derived detergent-free lipid raft fractions with equal protein content were extracted and analysed by LC–ESI–MS/MS. (E) The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on sphingolipid levels in BHK/MRP1 cells was established by LC–ESI–MS/MS. Results for each lipid as well as the sum of all lipids are expressed as a percentage of those from untreated cells (100 %) and are means + S.D. for three independent experiments.
Figure 2. Ganglioside depletion by ISP-1 treatment.
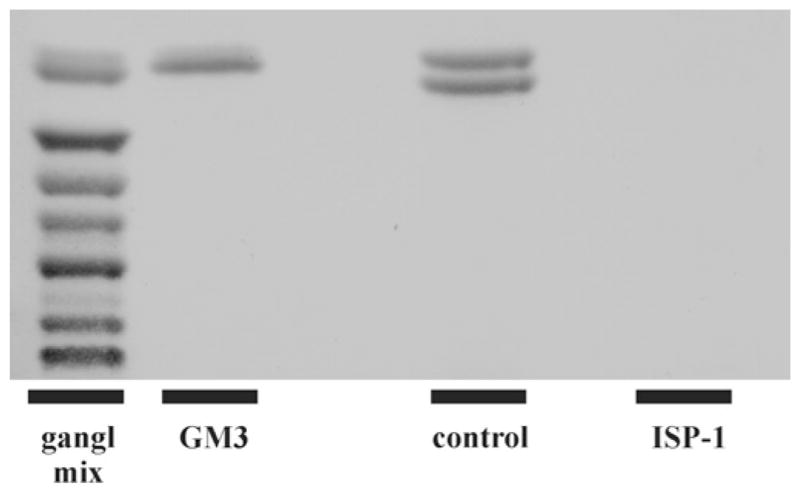
Mass analysis of GM3, the only ganglioside present in Neuro-2a cells, shows that this ganglioside is undetectable and thus strongly reduced in ISP-1-treated cells (lane 4) compared with control cells (lane 3), which present as a characteristic double band. A ganglioside mixture (gangl mix; lane 1) and GM3 (lane 2) were applied as references.
The depletion of sphingolipids using ISP-1 did not significantly (unpaired Student’s t test; P > 0.05) affect Neuro-2a cell viability, as determined using a MTT assay. Cell viability was 88.4 ± 16.8 % (n = 6) for ISP-1-treated cells compared with control cells (=100 %).
Depletion of sphingolipids does not abrogate Lubrol-based DRM isolation
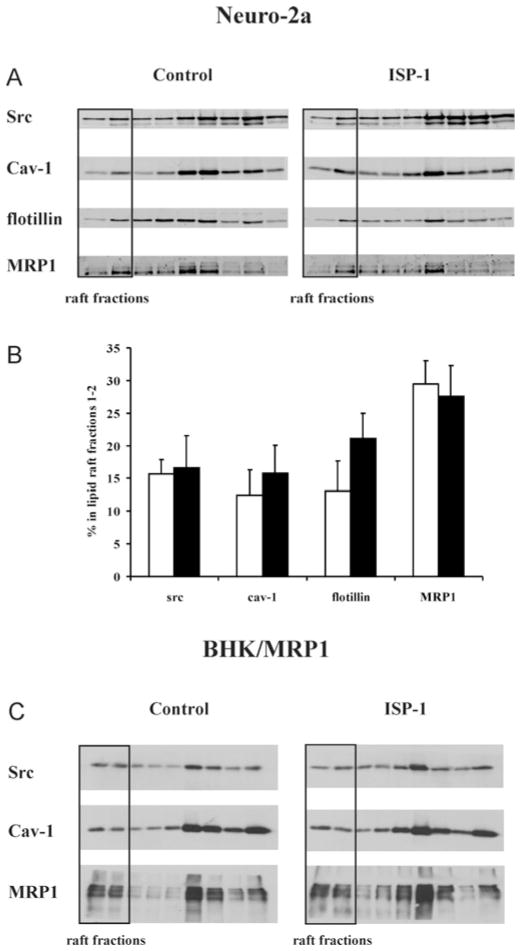
Surprisingly, when Neuro-2a cells were efficiently depleted of sphingolipids, we were still able to isolate Lubrol-based DRMs from these cells (see Supplementary Figure S1 at http://www.BiochemJ.org/bj/430/bj4300519add.htm). First of all, the protein content of these DRMs, i.e. the protein profile of the sucrose gradient fractions, was identical with that of control cells (see Supplementary Figure S1A). Also the gradient distributions of established DRM protein markers Src, Cav-1 and flotillin were indistinguishable between ISP-1-treated and control cells (Figure 3A). Indeed, quantification of these typical blots using the Odyssey protocol showed that the percentage of these lipid raft markers found to be associated with lipid rafts did not differ between control and ISP-1-treated cells (Figure 3B). This was also the case in two other independent experiments, when quantification was performed using Scion Image software on protein bands stained with the ECL procedure (results not shown). Given these results, it was important to establish that sphingolipid depletion actually diminished the DRM-associated lipid pools. Alternatively, DRM-associated sphingolipids could have been spared upon treatment with ISP-1. However, as indicated above, results with isolated Lubrol-based DRMs (Figure 1C) showed that Cer and glycosphingolipids were depleted by at least 90 % and for SM again somewhat less efficiently (84 %). We conclude that efficient DRM depletion of sphingolipids did not hamper the potential to isolate DRMs from Neuro-2a cells.
Figure 3. ISP-1 treatment does not affect DRM marker distribution in Lubrol-based gradients of Neuro-2a cells.

(A) After ISP-1 treatment, Lubrol-based DRMs were isolated from Neuro-2a cells and analysed for Src, Cav-1, flotillin and MRP1 distribution along the gradient. Gradient samples were applied on SDS/PAGE based on equal volume. DRM fractions are indicated. Gels are typical of three independent experiments. (B) Quantification of the relative amount of a specific protein found in the lipid raft fractions of this typical experiment. White bars, control; black bars, ISP-1.
Similar results were obtained when equal amounts of protein from all gradient fractions were processed for immunoblotting instead of equal volumes of gradient fractions. The difference is that with equal volume loading, the final result reflects the distribution of the absolute amount of a given protein on a gradient, whereas with equal protein loading, the result reflects the abundance of a given protein relative to total protein in a gradient fraction. The latter thus indicates the enrichment of a given protein in DRM fractions. Also with this procedure, raft association of the markers Src and Cav-1 was similar in control and ISP-1-treated cells (see Supplementary Figure S2A at http://www.BiochemJ.org/bj/430/bj4300519add.htm).
Similar observations were made in BHK/MRP1 cells. Also in these cells, ISP-1 treatment did not abrogate the potential to isolate DRMs, based on gradient protein profile (see Supplementary Figure S1B) and gradient distribution of Src and Cav-1 (see Supplementary Figure S2B). Thus these properties are not restricted to a single cell type.
Depletion of sphingolipids does not abrogate detergent-free lipid raft isolation
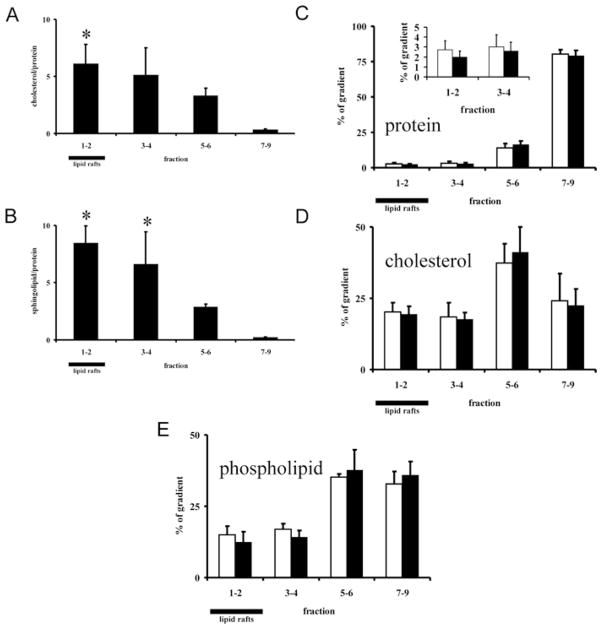
It is conceivable that the properties of DRMs as isolated by detergents, such as Lubrol, are strongly influenced by the use of detergent. Therefore we additionally used a detergent-free method for the isolation of lipid rafts and tested the effect of sphingolipid depletion on the integrity of these domains. We first characterized the gradient fractions in terms of cholesterol and sphingolipid enrichment. For this purpose, fractions 1 and 2 were pooled, as well as fractions 3 and 4, 5 and 6, and 7–9. Fractions 1 and 2 were most strongly enriched in both cholesterol (Figure 4A) and sphingolipids (Figure 4B), and to a lesser extent also fractions 3 and 4. This indicates that fractions 1 and 2, with the lowest buoyant density, optimally fulfil the criteria for lipid rafts. Also with this detergent-free approach, lipid rafts could be isolated from sphingolipid-depleted Neuro-2a cells with protein profiles similar to those of control cells (Figure 4C). In addition, the cholesterol profile (Figure 4D) was unchanged in ISP-1-treated cells, as well as the phospholipid profile (Figure 4E). The gradient distributions of the classical lipid raft markers Src, Cav-1 and flotillin were not changed upon treatment with ISP-1 (Figure 5A). This was confirmed by quantification of the blots using the Odyssey protocol showing that the percentage of these lipid raft markers found to be associated with lipid rafts did not differ between control and ISP-1-treated cells (Figure 5B). It is important to note that sphingolipid depletion by ISP-1 was equally effective in detergent-free lipid raft fractions as in whole cells (Figure 1D). Also in BHK/MRP1 cells, the gradient distributions of Src and Cav-1 were not changed by ISP-1 (Figure 5C), again indicating that the effect is not cell-type-specific.
Figure 4. ISP-1 treatment does not affect protein, cholesterol and phopholipid profiles in detergent-free lipid raft gradients of Neuro-2a cells.
(A and B) Detergent-free lipid raft gradients were analysed for cholesterol (A) and sphingolipid (B) profiles. These lipid classes were measured in pooled gradient fractions and are expressed relative to protein content of the pooled gradient fractions. To calculate the cholesterol/protein and sphingolipid/proteins ratios, the percentage values in the gradient of each of cholesterol, sphingolipids and protein were used. This ratio is therefore unitless and represents the relative enrichment in gradient fractions of cholesterol and sphingolipids respectively relative to protein enrichment in the respective fractions. Results are means + S.D. for three independent experiments. *P < 0.05 compared with fractions 5–6 (non-lipid raft membrane fractions) as determined by Student’s t test. (C–E) After ISP-1 treatment, detergent-free lipid rafts were isolated from Neuro-2a cells and analysed for protein (C), cholesterol (D) and phospholipid (E) profiles. Lipid raft fractions are indicated. White bars, control; black bars, ISP-1. Results are means + S.D. for three independent experiments. No significant differences between ISP-1-treated and control cells were found using Student’s t test.
Figure 5. ISP-1 treatment does not affect lipid raft marker distribution in detergent-free lipid raft gradients of Neuro-2a and BHK/MRP1 cells.
(A) After ISP-1 treatment, detergent-free lipid rafts were isolated from Neuro-2a cells and analysed for Src, Cav-1, flotillin and MRP1 distribution along the gradient. Gradient samples were applied on SDS/PAGE based on equal volume. Lipid raft fractions are indicated. Gels are typical of three independent experiments. (B) Quantification of the relative amount of a specific protein found in the lipid raft fractions. Results are means + S.D. for three independent experiments. No significant differences between ISP-1-treated (black bars) and control (white bars) cells were found using Student’s t test. (C) After ISP-1 treatment, detergent-free lipid rafts were isolated from BHK/MRP1 cells and analysed for Src, Cav-1 and MRP1 distribution along the gradient. Gradient samples were applied on SDS/PAGE based on equal volume. Lipid raft fractions are indicated.
Depletion of sphingolipids does not result in major changes in glycerophospholipid composition and cholesterol content in cells or detergent-free lipid rafts
It is conceivable that the glycerophospholipid composition had changed in cells and/or lipid rafts with low sphingolipid levels after ISP-1 treatment as a compensation mechanism for the loss of sphingolipids. If this were the case, we would expect to find increased levels of long-chain saturated fatty acids in glycerophospholipids, since these are considered to be enriched in lipid rafts. In addition, changes in headgroup composition of glycerophospholipids could have occurred. We employed LC–MS to analyse glycerophospholipids in terms of headgroup and fatty acid composition as well as total amount of glycerophospholipids. In whole Neuro-2a cells after 3 days of ISP-1 treatment, there were no significant changes in the total glycerophospholipid content (Figure 6A) or in the content of PA (phosphatidic acid), PC (phosphatidylcholine), PE (phosphatidylethanolamine), PG (phosphatidylglycerol), PI (phosphatidylinositol) or PS (phosphatidylserine) (Figure 6F). There were also no significant changes in the content of the lyso-forms of PE, PG and PI, or plasmalogens (results not shown). Concerning fatty acid composition of glycerophospholipids, minor changes were observed: several relatively short-chain PAs, PCs, PEs and PIs were significantly reduced upon ISP-1 treatment (see Supplementary Table S1 at http://www.BiochemJ.org/bj/430/bj4300519add.htm).
Figure 6. ISP-1 treatment does not affect the amount or headgroup composition of glycerophospholipids or the amount of cholesterol.
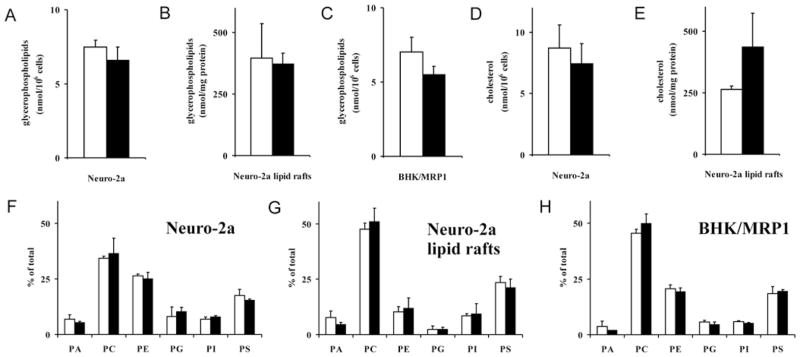
(A–C) The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on glycerophospholipid levels in Neuro-2a cells (A), Neuro-2a-derived detergent-free lipid rafts (B) and BHK/MRP1 cells (C) was established by LC–MS. (D and E) The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on cholesterol content of Neuro-2a cells (D) and Neuro-2a-derived detergent-free lipid rafts (E) was analysed with a cholesterol assay. (F–H) The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on glycerophospholipid headgroup composition in Neuro-2a cells (F), Neuro-2a-derived detergent-free lipid rafts (G) and BHK/MRP1 cells (H) was established by LC–MS. White bars, control; black bars, ISP-1. Results are means + S.D. for three independent experiments. No significant differences between ISP-1-treated and control cells were found using Student’s t test.
Next we analysed the glycerophospholipid composition of detergent-free lipid rafts isolated from Neuro-2a cells. First, it became apparent that headgroup class composition of detergent-free lipid rafts was quite different from that of whole cells, detergent-free lipid rafts being enriched in PC and PS and relatively poor in PE and PG, whereas PA and PI were similar (compare Figure 6G with Figure 6F). Relatively short-chain and saturated fatty acid species contributed most to the enrichment of PC and PS in detergent-free lipid rafts (results not shown). Secondly, the effect of ISP-1 on glycerophospholipid composition of detergent-free lipid rafts was confined to a significant decrease in several PA species, whereas the decrease in the total pool of PA was not significant, and a highly significant 38 % increase in PC (C32:0) (see Supplementary Table S1). Similar to the situation in whole Neuro-2a cells, there were no significant changes in the total glycerophospholipid content of detergent-free lipid rafts isolated from Neuro-2a cells after 3 days of ISP-1 treatment (Figure 6B).
In BHK/MRP1 cells, ISP-1 treatment did not result in significant changes in the total glycerophospholipid content (Figure 6C) or in the content of PA, PC, PE, PG, PI or PS (Figure 6H). Concerning fatty acid composition of glycerophospholipids, some changes were observed: several PEs, PGs and, more prominently, PIs were significantly reduced upon ISP-1 treatment (see Supplementary Table S1). This concerned mostly longer-chain fatty acid species.
The cholesterol content of Neuro-2a was not affected by ISP-1 treatment (Figure 6D). The cholesterol content of Neuro-2a-derived detergent-free lipid rafts showed an increase in ISP-1-treated cells, but this was not significant (Figure 6E).
Residual sphingolipids after ISP-1 treatment have a different fatty acid profile compared with control cells
We also analysed the fatty acid composition of the sphingolipids that remain after ISP-1 treatment. First, the fatty acid composition of sphingolipids in control Neuro-2a cells was relatively simple. In glycolipids, approx. 90 % was contributed by C16:0, C24:0 and C24:1 species. In SM, approx. 80 % were C16:0 species (Figure 7). A comparable distribution was observed in BHK/MRP1 cells (results not shown). Secondly, ISP-1 treatment differentially affected fatty acid species in both Neuro-2a and BHK/MRP1 cells. Residual sphingolipids were relatively rich in short-chain species and relatively poor in C24:0 and C24:1 species (Table 1). This pattern was, to a certain extent, reflected in Neuro-2a-derived detergent-free lipid rafts (Table 1).
Figure 7. Fatty acid composition in sphingolipids of Neuro-2a cells.
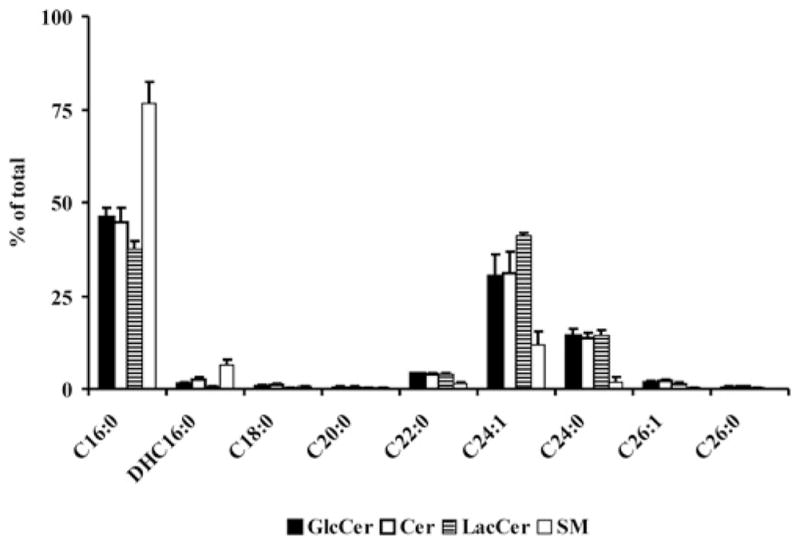
The fatty acid profile of sphingolipids in Neuro-2a cells was analysed by LC–ESI–MS/MS. Results are means + S.D. for three independent experiments.
Table 1. Fatty acid composition of sphingolipids from Neuro-2a or BHK/MRP1 cells or detergent-free lipid rafts derived from Neuro-2a cells after treatment with ISP-1.
The effect of ISP-1 treatment (3 days of incubation with 0.5 μM ISP-1) on the fatty acid profile of sphingolipids in Neuro-2a and BHK/MRP1 cells as well as Neuro-2a-derived detergent-free lipid rafts was established by LC–ESI–MS/MS. Fold indicates the ratio of the amount of a given sphingolipid in ISP-1-treated cells (or detergent-free lipid rafts) divided by the amount in control cells, with a value of 1 indicating no effect of ISP-1 treatment. Results are means for three independent experiments. Values in bold are those where ISP-1 and control are significantly (P < 0.05) different as determined using a Student’s t test. n.d. means not detectable under ISP-1 conditions.
| Sphingolipid | Neuro-2a cells
|
Neuro-2a lipid rafts
|
BHK/MRP1 cells
|
|||
|---|---|---|---|---|---|---|
| Fold | P | Fold | P | Fold | P | |
| GlcCer C16:0 | 1.70 | 3.2 × 10−4 | 1.54 | 2.7 × 10−2 | 1.75 | 1.4 × 10−5 |
| GlcCer C24:1 | 0.37 | 5.2 × 10−3 | n.d. | – | 0.38 | 8.2 × 10−5 |
| GlcCer C24:0 | 0.41 | 1.4 × 10−3 | n.d. | – | 0.37 | 3.6 × 10−4 |
| Cer C16:0 | 1.56 | 1.4 × 10−3 | 1.26 | 2.8 × 10−1 | 1.43 | 5.0 × 10−3 |
| Cer C24:1 | 0.43 | 5.5 × 10−3 | 0.09 | 5.0 × 10−4 | 0.58 | 1.6 × 10−3 |
| Cer C24:0 | 0.46 | 1.8 × 10−3 | 0.72 | 2.0 × 10−1 | 0.54 | 1.3 × 10−2 |
| LacCer C16:0 | 1.42 | 1.6 × 10−2 | 1.06 | 6.3 × 10−1 | 1.20 | 8.2 × 10−2 |
| LacCer C24:1 | 0.71 | 1.2 × 10−3 | 1.31 | 5.7 × 10−2 | 0.64 | 4.7 × 10−3 |
| LacCer C24:0 | 0.76 | 3.2 × 10−2 | 0.38 | 6.3 × 10−2 | 0.86 | 2.3 × 10−1 |
| SM C16:0 | 1.13 | 6.6 × 10−2 | 1.15 | 3.9 × 10−1 | 1.08 | 2.7 × 10−4 |
| SM C24:1 | 0.44 | 4.0 × 10−2 | 0.37 | 9.1 × 10−3 | 0.43 | 4.2 × 10−5 |
| SM C24:0 | 1.95 | 9.5 × 10−1 | 0.12 | 8.2 × 10−2 | 0.67 | 4.1 × 10−4 |
Depletion of sphingolipids does not affect MRP1 efflux activity or its localization in DRMs/detergent-free lipid rafts
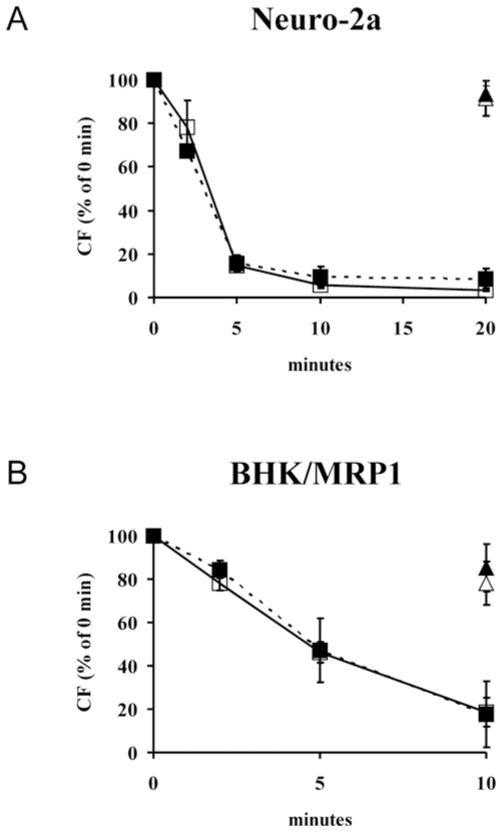
We next determined whether the DRM/lipid raft localization as well as the function of MRP1, a drug-efflux pump active in both Neuro-2a and BHK/MRP1 cells, were affected by depletion of sphingolipids. This protein was chosen in view of its relevance to MDR, its association with Lubrol-based DRMs and the potential modulation of its activity by sphingolipids. To study its function, we tested MRP1-mediated carboxyfluorescein efflux activity. Untreated or ISP-1-treated Neuro-2a cells were loaded with CFDA. Efflux activity was determined on the basis of fluorescence retention after cells were incubated at 37 °C. The MRP1 inhibitor MK571 was used as a positive control for inhibition of MRP1-mediated efflux activity. MRP1-efflux activity was very similar in ISP-1-treated cells compared with control (Figure 8A). Thus depletion of sphingolipids using ISP-1 did not affect MRP1 efflux activity. Accordingly, there was no effect on the DRM (Figures 3A and 3B and see Supplementary Figure S2A) or detergent-free lipid raft (Figures 5A and 5B) localization of the ABC transporter, as indicated by a very similar gradient distribution profile compared with that in control cells. Similarly, in BHK/MRP1 cells, the absence of an effect of ISP-1 on MRP-1-mediated efflux (Figure 8B) correlated with an unchanged gradient distribution of MRP1 (Figure 5C and see Supplementary Figure S2B).
Figure 8. ISP-1 treatment does not affect MRP1-mediated efflux activity of Neuro-2a or BHK/MRP1 cells.
After ISP-1 treatment, Neuro-2a (A) or BHK/MRP1 (B) cells were loaded with CFDA (0.5 μM). Retention of fluorescence was determined by flow cytometric analysis at several time points after cells were incubated at 37 °C and is expressed as the percentage of the value at zero time. □, Control; ■, ISP-1. MK571 (20 μM) was used as a positive control for MRP1-efflux inhibition.△, Control + MK571; ▲, ISP-1 + MK571. Results are means ± S.D. for three independent experiments. No significant differences between ISP-1 treated and control cells were found using Student’s t test.
Combined ISP-1/serine palmitoyltransferase RNAi treatment or long-term ISP-1 treatment does not further reduce sphingolipid levels
We have made several attempts to reduce even further the cellular sphingolipid levels. First, in Neuro-2a cells, we combined ISP-1 treatment for 3 days with RNAi directed against SPTLC1 or SPTLC2 or both to down-regulate the first enzyme in sphingolipid biosynthesis. Down-regulation was effective as indicated by residual levels of 67.3 ± 26.0 %(n = 3) LCB1 for RNAi directed against SPTLC1 and 27.7 ± 18.0 % (n = 3) LCB2 for RNAi directed against SPTLC2. Combined use of siRNA for SPTLC1 and SPTLC2 resulted in levels of 70.8 ± 7.6 %(n = 3) LCB1 and 42.7 ± 11.4 % (n = 3) LCB2 (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/430/bj4300519add.htm). However, none of these treatments combined with ISP-1 reduced the level of sphingolipids further as compared with ISP-1 treatment, but rather resulted in higher residual levels (see Supplementary Figure S4 at http://www.BiochemJ.org/bj/430/bj4300519add.htm). Secondly, we incubated Neuro-2a and BHK/MRP1 cells for 7 days in the presence of ISP-1. The final day of this treatment was performed in medium without serum, to prevent uptake of sphingolipids by the cells from the medium. Under these conditions, we did not observe further reduction of the level of sphingolipids as compared with ISP-1 treatment for 3 days (see Supplementary Figure S4). The viability of the cells started to be affected with this very long-term ISP-1 treatment (see Supplementary Figure S5 at http://www.BiochemJ.org/bj/430/bj4300519add.htm). Given that sphingolipids were not reduced further and side effects started to become apparent, we decided not to pursue further analysis of lipid rafts and MRP1 localization and function under these conditions.
DISCUSSION
Lipid rafts are subdomains of the plasma membrane that contain high concentrations of cholesterol and sphingolipids. They appear to be small in size, but together may constitute a relatively large fraction of the plasma membrane [29,30]. The high content of glycosphingolipids and SM in DRMs gave rise to two different models for lipid raft formation. The first model points out the importance of the relatively long length and high saturation of the acyl chains of glycosphingolipids and SM for raft formation. This allows close packing of the lipids, resulting in a high melting temperature (Tm). Self-aggregates of sphingolipids form a separate phase that is less fluid (liquid-ordered) than the bulk liquid-disordered phospholipids. Cholesterol is recruited to these aggregates, due to its ability to pack tightly with lipids of high Tm [2,3]. According to the second model, lipid rafts are primarily clusters of glycosphingolipids and SM held together through hydrogen-bonding between glycosphingolipid headgroups and close packing of the sphingolipids. Cholesterol fills up the gaps between the bulky heads of the glycosphingolipids [30].
Interestingly, although glycosphingolipids are enriched in lipid rafts, they do not appear to be essential for the formation of these membrane domains [31]. It was shown that glycosphingolipid-deficient GM95 melanoma cells had similar amounts of DRMs compared with control cells. The loss of glycosphingolipid mass in these cells due to mutation of the gene encoding GCS was exactly compensated for by an increase in SM mass (M. van Riezen, J.W. Kok and Merrill, Jr, A.H., unpublished work). Glycosphingolipids in DRMs of GM95 cells had been replaced by SM [31]. In the present study, we show for the first time that, even under conditions when both glycosphingolipids and SM, in fact all sphingolipids, are efficiently depleted from Neuro-2a cells during long-term (72 h) ISP-1 treatment, these cells still have similar amounts (protein content) of DRMs compared with control cells. In addition, three established lipid raft markers, Src, Cav-1 and flotillin, were associated with lipid fractions to the same extent in control and ISP-1-treated cells. In this respect, it is important to note that our sphingolipid-depletion procedure resulted not only in efficient reduction of the total cellular pools of these lipids, but was also highly effective in sphingolipid depletion from DRMs. This leads to the conclusion that DRMs can be isolated from cells which are severely depleted of sphingolipids. Thus a minor amount of sphingolipids appears to be sufficient for the integrity of lipid rafts as defined by detergent isolation. Moreover, formation of new lipid rafts seems to occur in cells with low levels of sphingolipid synthesis since new lipid raft formation is bound to occur during the long-term (72 h) ISP-1 treatment. We made several attempts to decrease even further sphingolipid levels below 10 % of control (Neuro-2a cells). This was done by either combining pharmacological inhibition of SPT using ISP-1 with down-regulation of SPT using RNAi or by lengthening the incubation time with ISP-1 to 7 days, with the last day being in combination with serum-free medium. Both procedures did not further reduce sphingolipid levels beyond those obtained after 3 days of ISP-1 incubation. Thus depletion of sphingolipids to approx. 10 %of control, as occurred in Neuro-2a cells, appeared to be the maximum possible effect. For BHK/MRP1 cells, this was approx. 20 %of control.
The use of detergents to isolate lipid rafts has been the subject of extensive discussion and criticism [19,32–35]. It has been suggested that detergents induce artefacts, e.g. reorganization of molecules during the process of lipid raft isolation. Therefore we additionally employed an established detergent-free method for lipid raft isolation [7]. We first characterized the gradients and showed that fractions 1 and 2 were highly enriched in cholesterol and sphingolipids, hallmarks of lipid rafts. As in the case of DRMs, detergent-free lipid rafts could also be readily isolated from cells after severe depletion of sphingolipids, as indicated by a similar protein profile in the gradients of control and ISP-1-treated cells. Moreover, the gradient distributions of cholesterol and phospholipids were also indistinguishable between control and ISP-1-treated cells. Finally, the classical lipid raft markers Src, Cav-1 and flotillin displayed similar associations to lipid raft fractions in control and ISP-1-treated cells. Thus, in addition to DRMs, detergent-free lipid rafts can be readily isolated from sphingolipid-depleted cells and have very similar properties, except for the levels of sphingolipids. We have shown that these properties of DRMs and detergent-free lipid rafts are not confined to or specific for a single cell type, i.e. Neuro-2a cells. Similar results were obtained with a different cell type, i.e. BHK/MRP1 cells.
It is conceivable that the glycerophospholipid composition had changed in cells and/or lipid rafts with low sphingolipid levels after ISP-1 treatment as a compensation mechanism for the loss of sphingolipids. If this were the case, we would expect to find increased levels of long-chain saturated fatty acids in glycerophospholipids, since lipids with these fatty acids are considered to be enriched in lipid rafts. In addition, changes in headgroup composition of glycerophospholipids could have occurred. However, detailed analysis of glycerophospholipid composition by LC–MS showed that there were no changes in headgroup composition of or the total amount of glycerophospholipids after 3 days of ISP-1 treatment in Neuro-2a or BHK/MRP1 cells or in lipid rafts derived from Neuro-2a cells. We did observe several changes in the fatty acid profile of glycerophospholipids in both Neuro-2a and BHK/MRP1 cells. Most of these changes appeared to be decreases of specific glycerophospholipid species with no clear similarity between the two cell types. These are not likely to be compensatory for the loss of sphingolipids in lipid rafts. In fact, detergent-free lipid rafts derived from Neuro-2a cells displayed a reduction of several PA species, which was hardly observed in whole cells. More interestingly as a potential compensatory mechanism, detergent-free lipid rafts displayed a highly significant relative increase in PC (C32:0). This represents PC species with C16:0 fatty acids or a combination of C14:0 and C18:0 fatty acids. Thus saturated, but short-chain rather than long-chain, fatty acids in glycerophospholipids are favoured and this suggests matching with the fatty acid composition of sphingolipids. The latter is skewed towards C16:0 under control conditions and much more in the residual sphingolipid pool in ISP-1-treated cells.
One of the best-characterized MDR mechanisms is the overexpression of energy-dependent drug-efflux proteins of the ABC transporter protein superfamily, which prevent intracellular drug accumulation. Pgp (or ABCB1) and MRP1 (or ABCC1) are the most widely studied among these proteins. Both ABC transporters are known to depend on their direct lipid environment for optimal functioning [36–38]. Lavie et al. [9] were the first to show the association of an ABC transporter with a membrane domain in intact cells. They found that a substantial fraction of Pgp was located in Cav-1-containing Triton X-100-based DRMs in Pgp-overexpressing cells. On the other hand, it was shown that Pgp and MRP1 were not associated with caveolae in two human MDR tumour cell lines [11]. Both MRP1 in HT29col MDR tumour cells and Pgp in 2780AD MDR tumour cells were found to be enriched in membrane domains defined by their insolubility in the non-ionic detergent Lubrol. It was shown previously that inhibition of GCS and hence depletion of glycosphingolipids did not affect MRP1 efflux activity in HT29col cells [39] or in human neuroblastoma cells [18]. As noted above, other sphingolipids, probably SM and Cer, can compensate for the loss of glycosphingolipids under the conditions of GCS inhibition. In the present study, we have shown for the first time that extensive depletion of all sphingolipid classes, including Cer and SM, does not affect MRP1 efflux activity.
These results are in line with a recent analysis of the lipid composition of Lubrol- and Triton X-100-based DRMs. Sphingolipids were less enriched in Lubrol-based DRMs compared with Triton X-100-based DRMs. Instead, Lubrol-based DRMs contained relatively large amounts of the phospholipids PE and PS [40]. A layered raft model was proposed in which Lubrol-based DRMs consist of a highly sphingolipid-enriched Triton X-100-insoluble core, surrounded by a Triton X-100-soluble region, which contains relatively high levels of cholesterol and specific aminophospholipids and harbours most of the DRM-associated ABC transporter molecules [41,42]. Thus sphingolipids are more enriched in ABC transporter-poor subdomains of DRMs and hence do not affect ABC transporter function. A similar situation may occur for detergent-free lipid rafts, which are likely to consist of a larger membrane area than Triton X-100-based DRMs. According to the same model, detergent-free lipid rafts also have this Triton X-100 insoluble core [41,42].
In conclusion, we have shown that highly efficient depletion of sphingolipids does not abrogate the ability to isolate DRMs or detergent-free lipid rafts from cells. DRM and lipid raft fractions showed similar protein, cholesterol and phospholipid profiles in sphingolipid-depleted and control cells, whereas the gradient distributions of classical lipid raft markers were indistinguishable between the two. The fatty acid profile of the remaining sphingolipids as well as that of the glycerophospholipid PC shows an increase in highly saturated C16 species in detergent-free lipid rafts, whereas the glycerophospholipid headgroup composition is unchanged. Moreover, lipid raft localization as well as efflux function of the ABC transporter MRP1 were not affected by sphingolipid depletion. This uncouples MRP1 function not only from glycosphingolipids, but from all sphingolipid species.
Supplementary Material
Abbreviations used
- ABC
ATP-binding cassette
- BHK
baby hamster kidney
- Cav-1
caveolin-1
- Cer
ceramide
- CFDA
5-carboxyfluorescein diacetate
- DRM
detergent-resistant membrane
- ECL
enhanced chemiluminescence
- ESI
electrospray ionization
- FBS
fetal bovine serum
- GlcCer
glucosylceramide
- GCS
glucosylceramide synthase
- HBSS
Hanks balanced salt solution
- HPTLC
high-performance TLC
- LacCer
lactosylceramide
- LC
liquid chromatography
- LCB
long-chain base subunit
- MDR
multidrug resistance
- MRP1
multidrug-resistance-related protein 1
- MS/MS
tandem MS
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphati-dylethanolamine
- PG
phosphatidylglycerol
- Pgp
P-glycoprotein
- PI
phosphatidylinositol
- PNS
post-nuclear supernatant
- PS
phosphatidylserine
- RNAi
RNA interference
- siRNA
small interfering RNA
- SM
sphingomyelin
- SPTLC
serine palmitoyltransferase long-chain base subunit
Footnotes
AUTHOR CONTRIBUTION
Karin Klappe designed experiments, carried out the majority of the experimental work and contributed to the discussion. Anne-Jan Dijkhuis researched data and contributed to the discussion. Ina Hummel researched data and contributed to the discussion. Annie van Dam and Hjalmar Permentier designed and performed the LC–ESI–MS/MS on sphingolipids. Pavlina Ivanova, Stephen Milne, David Myers and Alex Brown designed and performed the LC–ESI–MS/MS on glycerophospholipids. Jan Kok conceived and designed experiments, analysed the results and wrote the paper. All authors critically reviewed the paper.
References
- 1.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 3.Brown DA. Structure and function of membrane rafts. Int J Med Microbiol. 2002;291:433–437. doi: 10.1078/1438-4221-00150. [DOI] [PubMed] [Google Scholar]
- 4.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.London E, Brown DA. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 6.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA. 2003;100:5795–5800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Pike L. Rafts defined: a report on the Keystone Symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem. 1998;273:32380–32383. doi: 10.1074/jbc.273.49.32380. [DOI] [PubMed] [Google Scholar]
- 10.Demeule M, Jodoin J, Gingras D, Béliveau R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- 11.Hinrichs JWJ, Klappe K, Hummel I, Kok JW. ATP-binding cassette transporters are enriched in non-caveolar detergent-insoluble glycosphingolipid-enriched membrane domains (DIGs) in human multidrug-resistant cancer cells. J Biol Chem. 2004;279:5734–5738. doi: 10.1074/jbc.M306857200. [DOI] [PubMed] [Google Scholar]
- 12.Radeva G, Perabo J, Sharom FJ. P-glycoprotein is localized in intermediate-density membrane microdomains distinct from classical lipid rafts and caveolar domains. FEBS J. 2005;272:4924–4937. doi: 10.1111/j.1742-4658.2005.04905.x. [DOI] [PubMed] [Google Scholar]
- 13.Eckford PD, Sharom FJ. Interaction of the P-glycoprotein multidrug efflux pump with cholesterol: effects on ATPase activity, drug binding and transport. Biochemistry. 2008;47:13686–13698. doi: 10.1021/bi801409r. [DOI] [PubMed] [Google Scholar]
- 14.Troost J, Lindenmaier H, Haefeli WE, Weiss J. Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol Pharmacol. 2004;66:1332–1339. doi: 10.1124/mol.104.002329. [DOI] [PubMed] [Google Scholar]
- 15.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim Biophys Acta. 2002;1585:172–178. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
- 16.Sietsma H, Veldman RJ, Kok JW. The involvement of sphingolipids in multidrug resistance. J Membr Biol. 2001;181:153–162. doi: 10.1007/s00232-001-0033-1. [DOI] [PubMed] [Google Scholar]
- 17.Plo I, Lehne G, Beckstrom KJ, Maestre N, Bettaieb A, Laurent G, Lautier D. Influence of ceramide metabolism on P-glycoprotein function in immature acute myeloid leukemia KG1a cells. Mol Pharmacol. 2002;62:304–312. doi: 10.1124/mol.62.2.304. [DOI] [PubMed] [Google Scholar]
- 18.Dijkhuis AJ, Klappe K, Kamps W, Sietsma H, Kok JW. Gangliosides do not affect ABC transporter function in human neuroblastoma cells. J Lipid Res. 2006;47:1187–1194. doi: 10.1194/jlr.M500518-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology. 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- 20.Fridberg A, Olsen CL, Nakayasu ES, Tyler KM, Almeida LC, Engman DM. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J Cell Sci. 2008;121:522–535. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 21.Chang X, Hou Y, Riordan RJ. ATPase activity of purified multidrug resistance-associated protein. J Biol Chem. 1997;272:30962–30968. doi: 10.1074/jbc.272.49.30962. [DOI] [PubMed] [Google Scholar]
- 22.Lisanti MP, Tang Z, Scherer PE, Sargiacomo M. Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 1995;250:655–668. doi: 10.1016/0076-6879(95)50103-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Chem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 26.Gamble W, Vaughan M, Kruth HS, Avignan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- 27.Böttcher CJF, van Gent CM, Pries C. A rapid and sensitive submicro phosphorus determination. Anal Chim Acta. 1961;24:203–204. [Google Scholar]
- 28.Carmichael J, Degraff WG, Gazdar AF, Minna JD, Mitchel JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 29.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 30.Simons K, Ikonen I. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 31.Ostermeyer AG, Beckrich BT, Ivarson KA, Grove KE, Brown DA. Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells. J Biol Chem. 1999;274:34459–34466. doi: 10.1074/jbc.274.48.34459. [DOI] [PubMed] [Google Scholar]
- 32.Munro S. Lipid rafts: elusive or illusive. Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta P, Baird B, Holowka D. Lipid rafts, fluid/fluid phase separation, and their relevance to plasma membrane structure and function. Semin Cell Dev Biol. 2007;18:583–590. doi: 10.1016/j.semcdb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grzybek M, Kozubek A, Dubielecka P, Sikorski F. Rafts: the current picture. Folia Histochem Cytobiol. 2005;43:3–10. [PubMed] [Google Scholar]
- 35.Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J. 2002;83:2693–2701. doi: 10.1016/S0006-3495(02)75278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudeja PK, Anderson KM, Harris JS, Buckingham L, Coon JS. Reversal of multidrug resistance phenotype by surfactants: relationship to membrane lipid fluidity. Arch Biochem Biophys. 1995;319:309–315. doi: 10.1006/abbi.1995.1298. [DOI] [PubMed] [Google Scholar]
- 37.Sinicrope FA, Dudeja PK, Bissonette BM, Safa AR, Brasitus TA. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. J Biol Chem. 1992;267:24995–2500. [PubMed] [Google Scholar]
- 38.Romsicki Y, Sharom FJ. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry. 1999;38:6887–6896. doi: 10.1021/bi990064q. [DOI] [PubMed] [Google Scholar]
- 39.Klappe K, Hinrichs JWJ, Kroesen BJ, Sietsma H, Kok JW. MRP1 and glucosylceramide are coordinately over expressed and enriched in rafts during multidrug resistance acquisition in colon cancer cells. Int J Cancer. 2004;110:511–522. doi: 10.1002/ijc.20140. [DOI] [PubMed] [Google Scholar]
- 40.Hinrichs JWJ, Klappe K, van Riezen M, Kok JW. Drug resistance-associated changes in sphingolipids and ABC transporters occur in different regions of membrane domains. J Lipid Res. 2005;46:2367–2376. doi: 10.1194/jlr.M500070-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Hinrichs JWJ, Klappe K, Kok JW. Rafts as missing link between multidrug resistance and sphingolipid metabolism. J Membr Biol. 2005;203:57–64. doi: 10.1007/s00232-004-0733-4. [DOI] [PubMed] [Google Scholar]
- 42.Klappe K, Hummel I, Hoekstra D, Kok JW. Lipid dependence of ABC transporter localization and function. Chem Phys Lipids. 2009;161:57–64. doi: 10.1016/j.chemphyslip.2009.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.