Abstract
We report the synthesis and MR relevant properties of CyPic3A, a heptadentate chelator that forms ternary Gd(III) complexes of hydration state q = 2. [Gd(CyPic3A)(H2O)2]− affords an r1 value of 5.70 mM−1s−1 at 1.41 T and 310 K and displays thermodynamic stability and kinetic inertness comparable to FDA approved MR imaging probes.
Magnetic resonance imaging (MRI) exploits the 1H NMR signal of endogenous water to image tissue. Image contrast can be generated by weighting the data acquisition to differences in the longitudinal (T1) relaxation times of water, which reflect local tissue environment and are influenced by several variables. The presence of a paramagnetic species represents one variable that strongly influences nuclear relaxation and can reduce T1-times significantly.1,2 Accordingly, contrast is often enhanced through the addition of a paramagnetic MR probe. The efficacy of such probes is measured in terms of relaxivity (r1), defined by the resultant increase in 1/T1 scaled by probe concentration in mM.
Due to strong paramagnetism (S = 7/2) and slow electronic relaxation (T1e), complexes of Gd(III) are ideal for generating T1 contrast.1 Clinical usage of Gd(III)-based probes has become routine and is widely considered very safe in patients with normal renal function. In fact, roughly one third of all clinical scans are enhanced using paramagnetic agents. However, large dosages are required to obtain meaningful contrast (0.1 mmol/kg patient) using most approved probes (r1 = 3–4 mM−1s−1 at 0.47 T, 310 K).1,3 In light of this, probes displaying enhanced relaxivity represent desirable and oft sought targets in molecular imaging.
Clinically utilized Gd(III) imaging probes comprise nonacoordinate, ternary complexes where the Gd(III) ion is held within an octadentate polyaminocarboxylate ligand and coordinated by one rapidly exchanging water ligand. Regarding r1 optimization at field strengths used clinically, there are three modular parameters readily available to the chemist: the rate of inner-sphere water exchange (kex = 1/τm; τm = mean residency time of the H2O ligand), the rotational correlation time of the complex (τR) and the hydration number of the Gd(III) ion (q). The property τm is dictated by the ligand frame and choice of donor groups, and the manifestations of commonly used functional groups on τm have been explored in detail.4,5 The optimal range of τm values depends on both τR and magnetic field,6,7 however 10 < τm < 30 ns is optimal across all field strengths. Increasing τR can afford tremendous r1 enhancement at relatively low field strength (<1.5 T).6 This strategy has met with much success by either multimerization8 or through covalent or non-covalent conjugation to macromolecular entities.9,10
Modulation of the hydration number q can significantly affect the r1 of Gd(III) complexes, and r1 tends to scale with this parameter independent of field. However, an increase in q requires a reduction in available ligand donors and can come at the cost of reduced thermodynamic stability and kinetic inertness with respect to transchelation of the Gd(III) ion. Therefore, one must be judicious in ligand design. A handful of q > 1 Gd(III) complexes featuring hexa- and heptadentate ligands have been prepared and evaluated,11–16 with several examples highlighted in Figure 1 (See SI for additional examples). These ligands are highly pre-organized and are designed for maximal stability given the reduction in ligand denticity (< 8).
Figure 1.
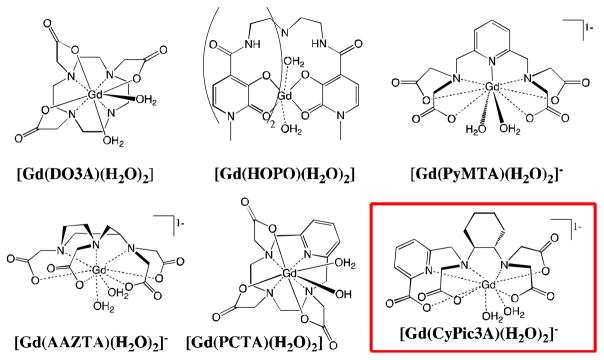
Previously studied Gd(III) complexes of hydration state q = 2 and [Gd(CyPic3A)(H2O)2]−, bottom right.
Herein, we introduce the heptadentate ligand, CyPic3A, designed to chelate Gd(III) in a stable fashion while allowing for a hydration state of q > 1 (Figure 1, highlighted). [Gd(CyPic3A)(H2O)2]− exhibits high relaxivity and maintains stability and inertness comparable to clinically used probes. The synthesis and evaluation of the physical properties of [Gd(CyPic3A)(H2O)2]− are discussed below.
In the design of CyPic3A, stabilizing structural factors were sought to compensate for the reduced denticity. In this regard, trans-1,2-diaminocyclohexane was incorporated into the ligand framework. Exchanging 1,2-diaminoethane for this rigidified linkage has been shown to increase the stability and inertness of lanthanide(III) complexes significantly.17,18 The bidentate 2-picolinate functionality is similarly stabilizing.19,20 These two moieties were fused to comprise the ligand backbone.
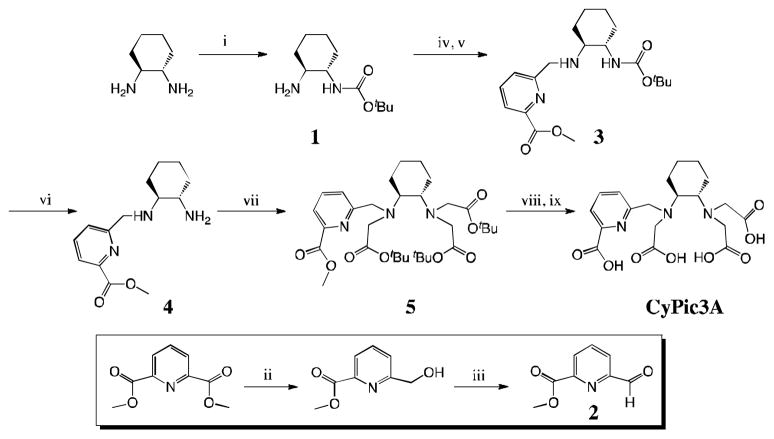
Coupling of mono-BOC protected trans-1,2-diaminocyclohexane (1)21 and methyl 6-formylpyridine-2-carboxylate (2)22 (prepared in one and two steps, respectively) via reductive amination cleanly gives the BOC-protected backbone 3 in high yield. Deprotection of 3 followed by alkylation of the two amines affords O-protected CyPic3A (5). O-deprotection yields the ligand, isolated as CyPic3A•2TFA. In total, this straightforward synthesis requires seven steps, each affording high yields and few requiring chromatography (SI). Complex formation occurs rapidly in water upon addition of GdCl3•6H2O followed by adjustment of the pH to 6.5. We note that 2,6-pyridinedicarboxylate, from which 2 is derived, is a highly modular synthon.23,24 Thus, one could envision the preparation of bifunctional analogues of CyPic3A.
The bis(aqua) hydration state of [Gd(CyPic3A)(H2O)2]− was confirmed by analogy through luminescence lifetime experiments on the Eu(III) congener measured in H2O vs. D2O.25,26 Following excitation at 396 nm, we measured the first order rate constant for the decay of the emission intensity at 616 nm. There is an empirical relationship that relates the difference in luminescence decay rates measured in H2O and D2O to the hydration number.27 We determined q = 2.09 using this method.
The mean residency time of the coordinated H2O molecules was also measured by recording the 17O transverse relaxation (T2) times of solvent H2O between 278 and 363 K in the presence and absence of [Gd(CyPic3A)(H2O)2]−. The reduced relaxation rate (1/T2r) is this relaxation rate difference normalized to the mole fraction of water coordinated to the Gd(III). A four-parameter fit to this data yields the water exchange kinetic parameters and an estimate of the electronic relaxation time (T1e).28 The water residency time at 310 K (τm310) was determined to be 14 ± 1 ns, and this very short residency time is optimal for relaxivity applications (See SI for plot of 1/T2r vs temperature, table of fitted parameters, and comparison to other Gd complexes).
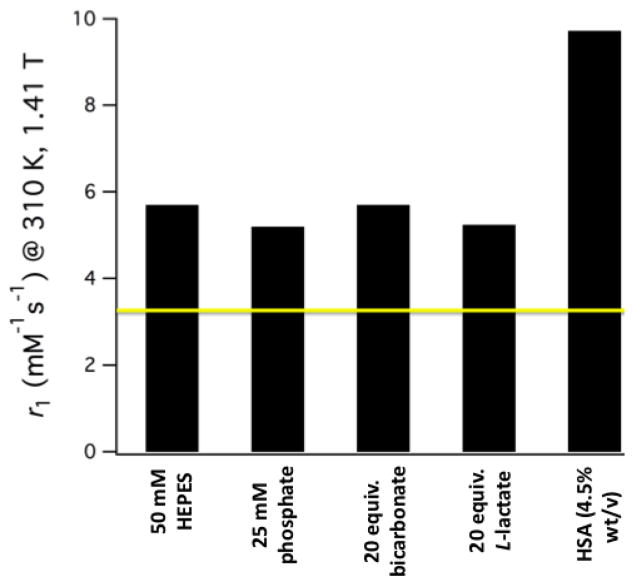
The relaxivity of [Gd(CyPic3A)(H2O)2]− is consistent with two inner-sphere water ligands. At 310 K in pH 7.4 50 mM HEPES buffer, r1 = 5.70 mM−1s−1 at 1.41 T (7.53 mM−1s−1 at 298 K). This is 75% higher than [Gd(DTPA)(H2O)]2− measured under these conditions (see SI for detailed table of r1 values). To probe the effects of potentially coordinating anions, r1 was measured in the presence of 20 equiv. carbonate or L-lactate and also in 25 mM phosphate buffer (pH 7.4). The results are summarized in Figure 2. The r1 values across these conditions indicate that the water coordination sites remain resistant to endogenously encountered bidentate anions known to significantly decrease the r1 of q = 2 Gd(III) complexes such as Gd(DO3A).29 Relaxivity was also measured to be 8.90 mM−1s−1 in bovine blood plasma at 310 K and a value of 9.73 mM−1s−1 was recorded in the presence of 4.5% w/v human serum albumin (HSA) at 1.41 T (12.03 mM−1s−1 at 0.47 T). Separation of free and HSA-bound [Gd(CyPic3A)(H2O)2]− by ultrafiltration (5,000 Da cut-off) followed by quantification of filtrate Gd content by ICP-MS revealed 8% of [Gd(CyPic3A)(H2O)2]− associated with HSA. The large r1 boost provided by the 8% protein bound complex suggests that an r1 of 51 and 71 mM−1s−1 at 1.41 and 0.47 T, respectively, could potentially be achieved through full macromolecular association.
Figure 2.
Relaxivity values recorded for [Gd(CyPic3A)(H2O)2]− at pH 7.4, 310 K in different media. The yellow line corresponds to the measured r1 of [Gd(DTPA)(H2O)]2− (3.26 mM−1s−1) in 50 mM HEPES.
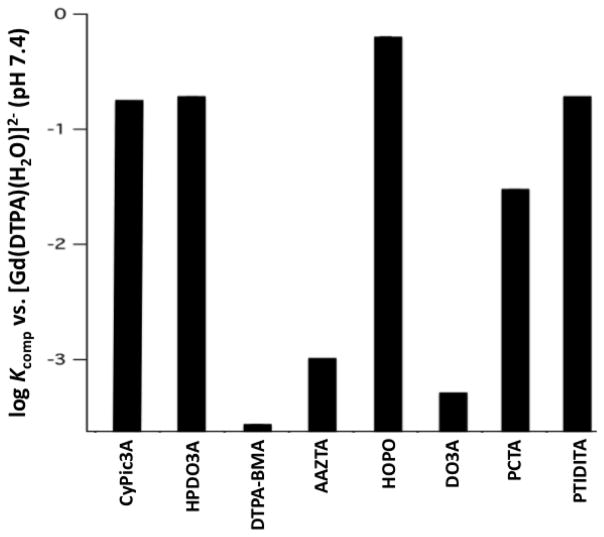
As a means of assessing thermodynamic stability relative to other Gd(III)-complexes, [Gd(CyPic3A)(H2O)2]− was challenged with DTPA at pH 7.4. CyPic3A possesses a strong chromophore at 280 nm and the relative distributions of complex and free ligand are easily monitored by LC-MS. From this information we could deduce equilibrium constants (Kcomp) for the competition reaction described in Equation 1,30 where L = DTPA and L′ = challenging chelator. We obtained a Kcomp of 0.17 at pH 7.4. In other words, the affinity of CyPic3A for Gd(III) is about 5-fold lower than DTPA at pH 7.4. Figure 3 compares this result to calculated Kcomp values for ligands (L′) of complexes used clinically in MRI30–32 and previously characterized q = 2 Gd(III) complexes (see SI for a complete list of structures).31,33–35 The complex [Gd(CyPic3A)(H2O)2]− is of comparable stability to (ProHance®) and >600-fold more stable [Gd(HP-DO3A)(H2O)] (Omniscan®). CyPic3A also forms than [Gd(DTPA-BMA)(H2O)] more stable complexes than many of the q = 2 complexes (See SI for detailed table and comparison against the angiography probe MS-325). Thus, it appears that structural factors considered in the design of CyPic3A compensate for the stability penalty incurred by reduction of available donors.
Figure 3.
Equilibrium (Kcomp) constant of [Gd(DTPA)(H2O)]2− upon challenge with CyPic3A and calculated Kcomp for challenge with select FDA approved probes (HP-DO3A, DTPA-BMA) and prior reported heptadentate chelators (AAZTA, HOPO, DO3A, PCTA, PTDITA).
| (1) |
Kinetic inertness must also be taken into account in the evaluation of potential probes. In this regard, [Gd(CyPic3A)(H2O)2]− was challenged with 1 equiv. [ZnPO4]− as a slurry in pH 7.0 phosphate buffer at 310 K (SI). Several clinically utilized Gd(III) complexes have been benchmarked relative to one another in this manner.36 As Gd(III) replaced by Zn(II) precipitates from the solution as GdPO4, the relaxation rate 1/T1 decreases. In this method, the time required to reach 80% of the initial 1/T1 value was utilized as an index of kinetic inertness. We measured a time to 80% of the initial 1/T1 of 95 min for [Gd(CyPic3A)(H2O)]−, 124 min for [Gd(DTPA-BMA)(H2O)] and 383 min for [Gd(DTPA)(H2O)]2−. The observed kinetic inertness compares well to acyclic clinically used Gd(III) complexes.
In conclusion, we have designed and evaluated a new ligand framework, CyPic3A, capable of forming ternary complexes with Gd(III) featuring two coordinated waters. The two bound waters afford favorable r1 and appear to be impervious to displacement by endogenously encountered bidentate ions such as phosphate, carbonate and lactate. Despite the heptadentate nature of the ligand, CyPic3A forms Gd(III) complexes with thermodyanamic stability and kinetic inertness comparable to some FDA approved probes employing octadentate ligands. CyPic3A holds promise regarding the development of highly efficient imaging probes amenable to use across a wide range of magnetic fields.
Supplementary Material
Scheme 1.
Synthesis of CyPic3A. (i) BOC2O, dioxane; (ii) NaBH4, MetOH, 0° C; (iii) SeO2, dioxane,Δ; (iv) 2, RT; (v) NaBH4, 0° C; (vi) TFA/CH2Cl2; (vii) tert-butyl bromoacetate, KI, DIPEA, (viii) LiOH, H2O/THF; (ix) TFA.
Acknowledgments
This work was supported by the National Institutes of Health (award EB009062 to P.C., and a post-doctoral fellowship, CA009592, to E.M.G.)
Footnotes
Electronic Supplementary Information (ESI) available: Synthesis and experimental details. See DOI: 10.1039/b000000x/
Notes and references
- 1.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 2.Lauffer RB. Chem Rev. 1987;87:901. [Google Scholar]
- 3.Caravan P. Chem Soc Rev. 2006;35:512. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 4.Helm L, Merbach AE. Chem Rev. 2005;105:1923. doi: 10.1021/cr030726o. [DOI] [PubMed] [Google Scholar]
- 5.Dumas S, Jacques V, Sun WC, Troughton JS, Welch JT, Chasse JM, Willich-Schmitt H, Caravan P. Invest Radiol. 2010;45:600. doi: 10.1097/RLI.0b013e3181ee5a9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caravan P, Farrar CT, Frullano L, Uppal R. Contrast Media Mol Imag. 2009:89. doi: 10.1002/cmmi.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boros E, Polasek M, Zhang Z, Caravan P. J Am Chem Soc. 2012;134:19858. doi: 10.1021/ja309187m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mastarone DJ, Harrison VSR, Eckermann AL, Parigi G, Luchinat C, Meade TJ. J Am Chem Soc. 2011;133:5329. doi: 10.1021/ja1099616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villaraza AJL, Bumb A, Brechbiel MW. Chem Rev. 2010;110:2921. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caravan P. Acc Chem Res. 2009;42:851. doi: 10.1021/ar800220p. [DOI] [PubMed] [Google Scholar]
- 11.Aime S, Calabi L, Cavallotti C, Gianolio E, Giovenzana GB, Losi P, Maiocchi A, Palmisano G, Sisti M. Inorg Chem. 2004;43:7588. doi: 10.1021/ic0489692. [DOI] [PubMed] [Google Scholar]
- 12.Pellagatti L, Zhang J, Drahoš B, Villette S, Suzenet F, Guillaumet G, Petoud S, Tóth E. Chem Commun. 2008:6951. doi: 10.1039/b817343e. [DOI] [PubMed] [Google Scholar]
- 13.Datta A, Raymond KN. Acc Chem Res. 2009;42:938. doi: 10.1021/ar800250h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messeri D, Lowe MP, Parker D, Botta M. Chem Commun. 2001:2742. [Google Scholar]
- 15.Aime S, Botta M, Crich SG, Giovenzana G, Pagliarin R, Sisti M, Terreno E. Magn Reson Chem. 1998;36:S200. [Google Scholar]
- 16.Baranyai Z, Tei L, Giovenzana GB, Kálmán FK, Botta M. Inorg Chem. 2012;51:2597. doi: 10.1021/ic202559h. [DOI] [PubMed] [Google Scholar]
- 17.McMurry TJ, Pippin CG, Wu C, Deal KA, Brechbiel MW, Mirzadeh S, Gansow OA. J Med Chem. 1998;41:3546. doi: 10.1021/jm980152t. [DOI] [PubMed] [Google Scholar]
- 18.Thakur P, Conca JL, Van de Burgt LJ, Choppin GR. J Coord Chem. 2009;62:3719. [Google Scholar]
- 19.Nonat AM, Gateau C, Fries PH, Helm L, Mazzanti M. Eur J Inorg Chem. 2012;51:2049. [Google Scholar]
- 20.Balogh E, Mato-Iglesias M, Platas-Iglesias C, Tóth E, Djanashvili K, Peters JA, de Blas A, Rodríguez-Blas T. Inorg Chem. 2006;45:8719. doi: 10.1021/ic0604157. [DOI] [PubMed] [Google Scholar]
- 21.Garimella PD, Datta A, Romanini DW, Raymond KN, Francis MB. J Am Chem Soc. 2011;133:14704. doi: 10.1021/ja204516p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platas-Iglesias C, Mato-Iglesias M, Djanashvili K, Muller RN, Vander Elst L, Peters JA, de Blas A, Rodríguez-Blas T. Chem Eur J. 2004;10:3579. doi: 10.1002/chem.200306031. [DOI] [PubMed] [Google Scholar]
- 23.Syper L, Kloc K, Mochowski J. Tetrahedron. 1980;36:123. [Google Scholar]
- 24.Kotera M, Lehn JM, Vigneron JP. Tetrahedron. 1995;51:1953. [Google Scholar]
- 25.Horrocks WD, Sudnick DR. Acc Chem Res. 1981;14:342. [Google Scholar]
- 26.Manus LM, Strauch RC, Hung AH, Eckermann AL, Meade TJ. Anal Chem. 2012;84:6278. doi: 10.1021/ac300527z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, de Sousa AS, Williams JAG, Woods MJ. Chem Soc Perkin Trans 2. 1999:493. [Google Scholar]
- 28.Caravan P, Parigi G, Chasse JM, Cloutier NJ, Ellison JJ, Lauffer RB, Luchinat C, McDermid SA, Spiller M, McMurry TJ. Inorg Chem. 2007;46:6632. doi: 10.1021/ic700686k. [DOI] [PubMed] [Google Scholar]
- 29.Polasek M, Caravan P. Inorg Chem. 2013;52:4084. doi: 10.1021/ic400227k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caravan P, Comuzzi C, Crooks W, McMurry TJ, Choppin GR, Woulfe SR. Inorg Chem. 2001;40:2170. doi: 10.1021/ic001117r. [DOI] [PubMed] [Google Scholar]
- 31.Kumar K, Chang CA, Francesconi L, Dischino DD, Malley MF, Gougoutas JZ, Tweedle MF. Inorg Chem. 1994;33:3567. [Google Scholar]
- 32.Moriggi L, Cannizzo C, Prestinari C, Berrière F, Helm L. Inorg Chem. 2008;47:8357. doi: 10.1021/ic800512k. [DOI] [PubMed] [Google Scholar]
- 33.Baranyai Z, Uggeri F, Giovenzana GB, Bényei A, Brücher E, Aime S. Chem Eur J. 2009;15:1696. doi: 10.1002/chem.200801803. [DOI] [PubMed] [Google Scholar]
- 34.Doble DMJ, Melchior M, O’Sullivan B, Siering C, Xu J, Pierrre VC, Raymond KN. Inorg Chem. 2003;42:4930. doi: 10.1021/ic026240s. [DOI] [PubMed] [Google Scholar]
- 35.Delgado R, Qunitino S, Teixeira M, Zhang A. J Chem Soc Dalton Trans. 1997:55. [Google Scholar]
- 36.Laurent S, Vander Elst L, Henoumon C, Muller RN. Contrast Media Mol Imag. 2010;5:305. doi: 10.1002/cmmi.388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.