Abdominal aortic aneurysms occur in up to 9% of adults older than 65 years of age, and the rupture of these aneurysms accounts for about 15,000 deaths in the United States annually.1 Approximately 33,000 patients undergo repair of abdominal aortic aneurysms each year, with associated illness, death, and health care costs. Smoking is a major risk factor. Although aggressive management of hypertension and hyperlipidemia is recommended in patients with abdominal aortic aneurysms, therapies for these conditions have little effect on aneurysm growth and rupture. In this context, a recent study by Satoh and colleagues2 is welcome because it sheds new light on how abdominal aortic aneurysms form and thereby rekindles interest in a therapy that was shown to protect against experimental aneurysm formation more than a decade ago.
Epidemiologic and pathological studies have yielded few clues to the cause of abdominal aortic aneurysms. Although abdominal aortic aneurysms frequently occur in patients with atherosclerosis and the two disease processes share several common risk factors, atherosclerotic lesions are predominantly intimal in location, whereas the media and adventitia are primarily involved in aneurysms. The hallmark pathologic feature of atherosclerosis is foam-cell formation, whereas aneurysms are typified by intense oxidative stress, inflammation, matrix degradation, and apoptosis of smooth-muscle cells.3 Inhibitors of matrix metalloproteinase are currently being tested in human aneurysmal disease, but their efficacy may be limited, in part because they are directed toward only one aspect of the disease process; thus, targeting factors that contribute to multiple aspects of the pathologic process of aneurysms would probably be of greater benefit. Studies in animal models have shown that angiotensin II induces vascular oxidative stress, inflammation, matrix degradation, and apoptosis of smooth-muscle cells and contributes experimentally to aneurysm formation.4 Studies in humans also suggest a role for angiotensin II in the pathogenesis of the disease. However, the cellular mechanisms that link these pathologic processes together to produce abdominal aortic aneurysms remain to be elucidated.
Satoh et al. examined the role of cyclophilin A (encoded by peptidyl-prolyl isomerase A [Ppia]) in abdominal aortic aneurysms induced by the infusion of angiotensin II in mice with hyperlipidemia, an established model of aneurysm formation. 4 Cyclophilin A, a member of the highly conserved and widely expressed family of proteins called the immunophilins, is the intracellular target of the immunosuppressive drug cyclosporine. Cyclosporine binds to cyclophilin A and forms a ternary complex with calcineurin, thereby inhibiting its activity. Inhibition of calcineurin blocks translocation of the nuclear factor of activated T cells to the nucleus, which in turn inhibits the transcription of inflammatory genes and T-cell activation. This inhibition forms the basis of the well-known immunomodulatory effects of cyclosporine. However, immunophilins also possess peptidyl-prolyl cis-trans-isomerase enzymatic activity. The peptidyl-prolyl cis-trans-isomerase domain of cyclophilin A facilitates binding to CD147, also known as an inducer of extracellular-matrix metalloproteinase. This binding causes CD147 to translocate to the cell surface, where it plays a critical role in stimulating matrix-metalloproteinase activity, leading to matrix degradation. This observation, coupled with reports that cyclophilin A is secreted by smooth-muscle cells in response to reactive oxygen species and triggers vascular inflammatory responses (Fig. 1), prompted the investigators to examine its role in the formation of abdominal aortic aneurysms.
Figure 1. A Model of Cyclophilin A and Abdominal Aortic Aneurysms.
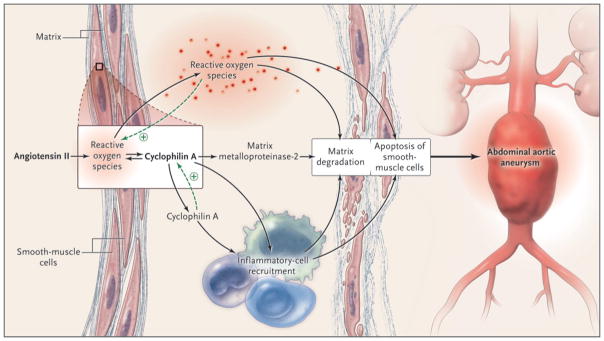
A recent study by Satoh et al.2 showed that angiotensin II, through induction of reactive oxygen species, induces cyclophilin A in smooth-muscle cells; this, in turn, triggers recruitment of inflammatory cells, activation of matrix metalloproteinase-2, and production of reactive oxygen species. Together, these factors lead to matrix degradation, apoptosis of smooth-muscle cells, and consequently, aneurysm formation. Cyclophilin A, itself induced by reactive oxygen species, acts through autocrine and paracrine mechanisms to up-regulate production of reactive oxygen species; this is indicative of a positive feedback loop.
Deletion of Ppia in mice, which led to the absence of cyclophilin A in aortic tissues, prevented the formation of abdominal aortic aneurysms in response to infusion of angiotensin II. Aortic inflammation, oxidative stress, and matrix degradation were also markedly reduced by deletion of Ppia. Mice lacking cyclophilin A were completely protected against aortic rupture leading to sudden death, which occurred in 35% of control animals. In a series of experiments involving bone marrow transplantation, as well as experiments in which cyclophilin A was selectively overexpressed in smooth-muscle cells, the authors showed that expression of cyclophilin A in smooth-muscle cells, rather than bone marrow–derived cells (e.g., macrophages and T cells), is crucial to the development of abdominal aortic aneurysms.
One of the most interesting aspects of the study is that the investigators examined the angiotensin II–induced activity of matrix metalloproteinase-2 in smooth-muscle cells derived from different regions of the murine aorta. They observed the highest level of activity in smooth-muscle cells isolated from the suprarenal aorta, the preferential site of aneurysm formation in mice (as compared with the infrarenal aorta in humans), and they further showed that the deletion of Ppia abolished angiotensin II–triggered induction of matrix metalloproteinase-2 in smooth-muscle cells. Finally, to provide relevance to human aneurysmal disease, the authors showed that angiotensin II caused the release of cyclophilin A and the activation of matrix metalloproteinase-2 in smooth-muscle cells derived from human abdominal aortic aneurysms. Treatment with cyclosporine blocked angiotensin II–induced activation of metalloproteinase-2 in the smooth-muscle cells in humans.
Inflammation has long been known to contribute to the pathogenesis of abdominal aortic aneurysms. A study described more than a decade ago5 showed that treatment with cyclosporine, known at the time for inactivating cyclophilin A in immune cells, attenuated the formation of abdominal aortic aneurysms in the rat model of elastase infusion. The authors of that study pointed out that the immunosuppressive effects of cyclosporine would probably preclude its use in patients with aneurysms. Satoh et al. have now identified cyclophilin A in smooth-muscle cells as an important link among oxidative stress, inflammation, and matrix degradation in the pathogenesis of abdominal aortic aneurysms. The development of an agent that selectively inactivates cyclophilin A in smooth-muscle cells or that blocks binding of cyclophilin A to CD147 could prove to be a fruitful approach to forestalling the growth and rupture of aneurysms.
Acknowledgments
Dr. Weintraub reports receiving grant support from Baxter and Pfizer.
References
- 1.Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–9. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh K, Nigro P, Matoba T, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–56. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–5. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–12. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrin PB, Baumgartner N, Anidjar S, Chejfec G, Mrkvicka R. Inflammatory aspects of experimental aneurysms: effect of methylprednisolone and cyclosporine. Ann N Y Acad Sci. 1996;800:74–88. doi: 10.1111/j.1749-6632.1996.tb33300.x. [DOI] [PubMed] [Google Scholar]


